Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Culture of Human Skin Fibroblasts
2.2. Cell Stimulation
2.3. Annexin V/Propidium Iodide Flow Cytometer Assay
2.4. Cell Proliferation Assay
2.5. Cell Morphology and Confluency Assessment
2.6. In Vitro Scratch Assay
2.7. Quantitative PCR
2.8. Western Blotting
2.9. Fluorescence Immunocytochemistry
2.10. Collagen Gel Matrix Contraction Assay
2.11. Statistical Analysis
3. Results
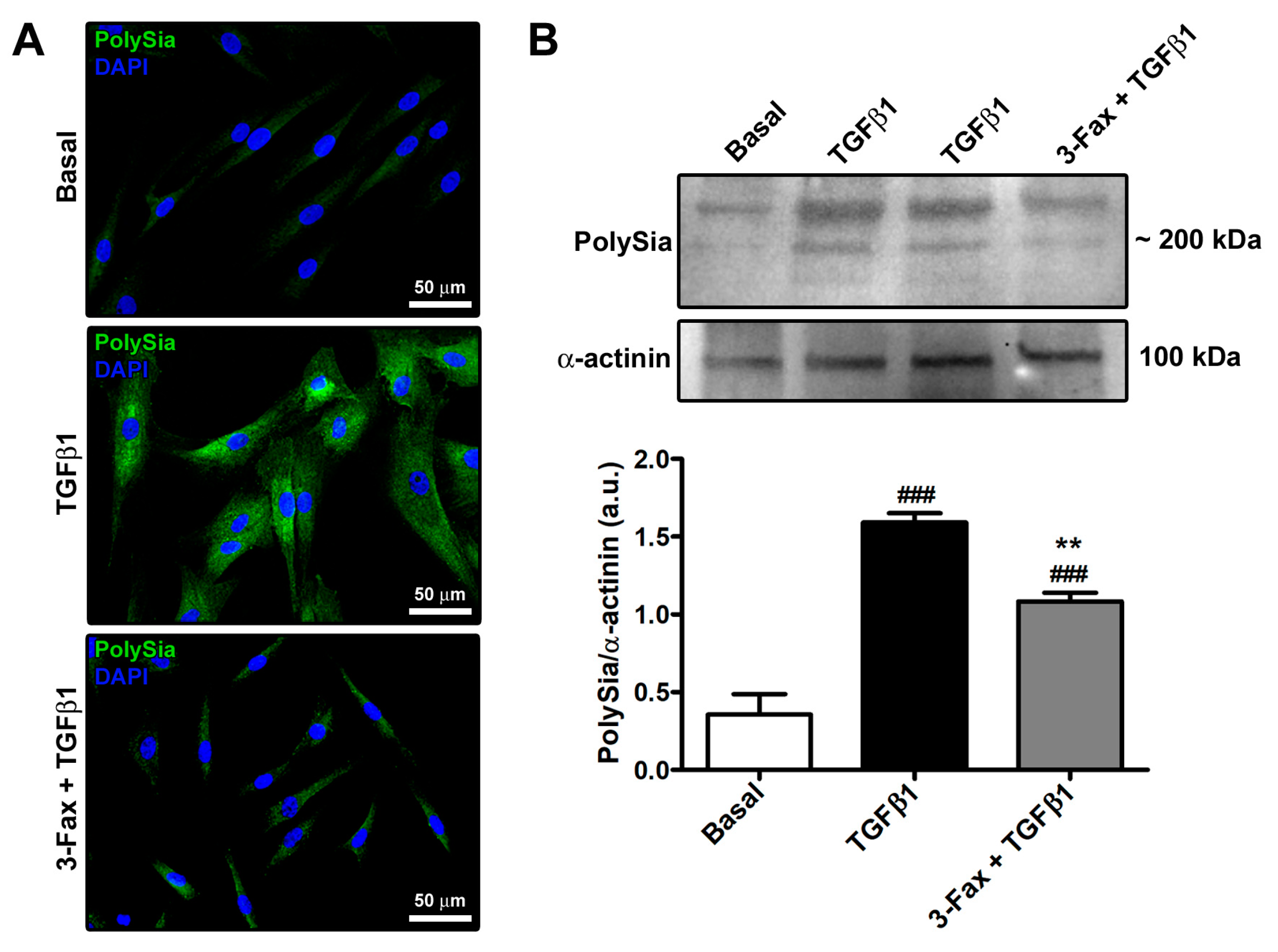
3.1. The Pan-Sialyltransferase Inhibitor 3-Fax Attenuates TGFβ1-Induced PolySia Expression in Human Skin Fibroblasts
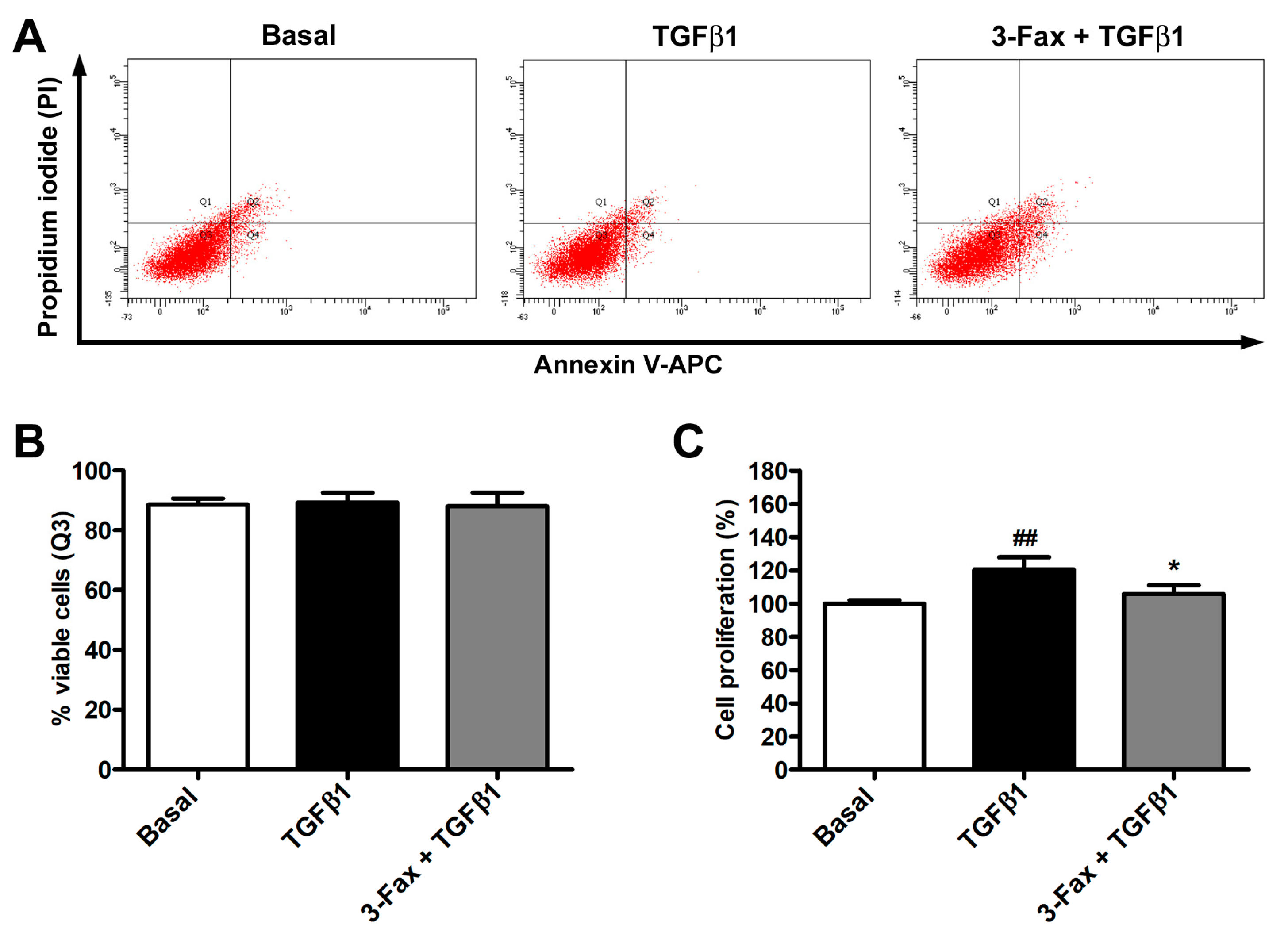
3.2. The Pan-Sialyltransferase Inhibitor 3-Fax Inhibits TGFβ1-Induced Proliferation of Human Skin Fibroblasts
3.3. Cell Confluency and Cell Migratory Capability Determination
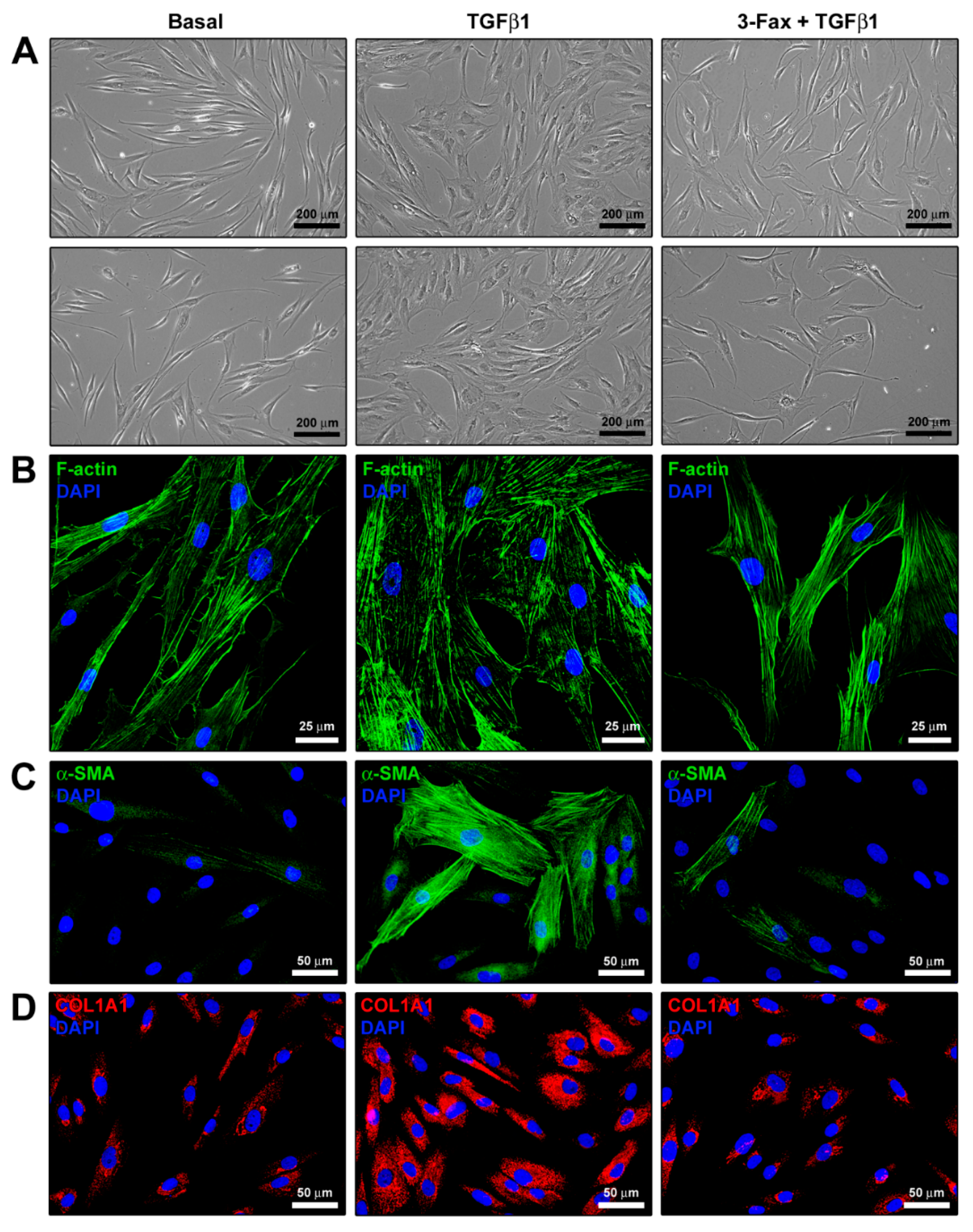
3.4. Cell Morphology and Myofibroblast-Like Phenotype Assessment
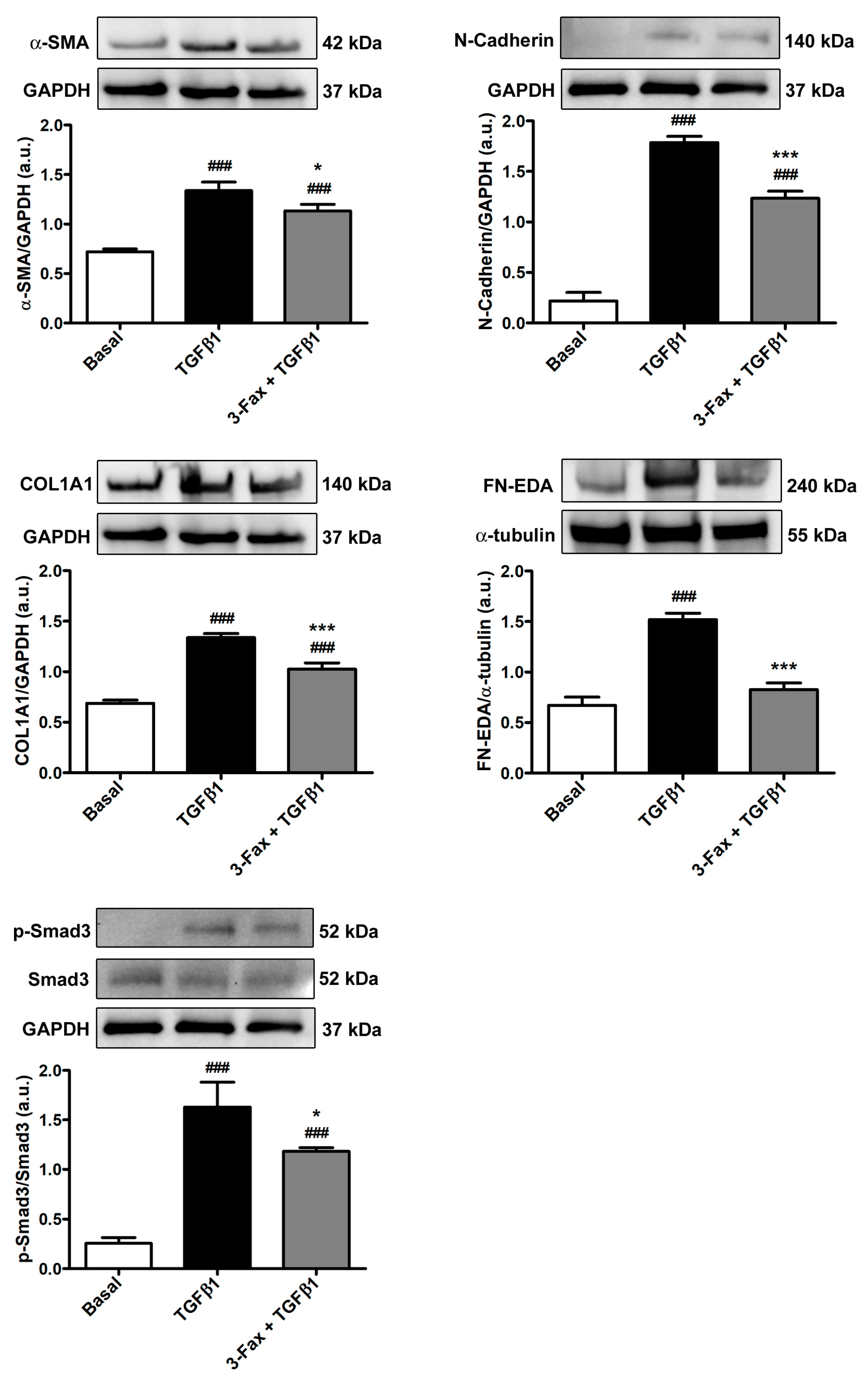
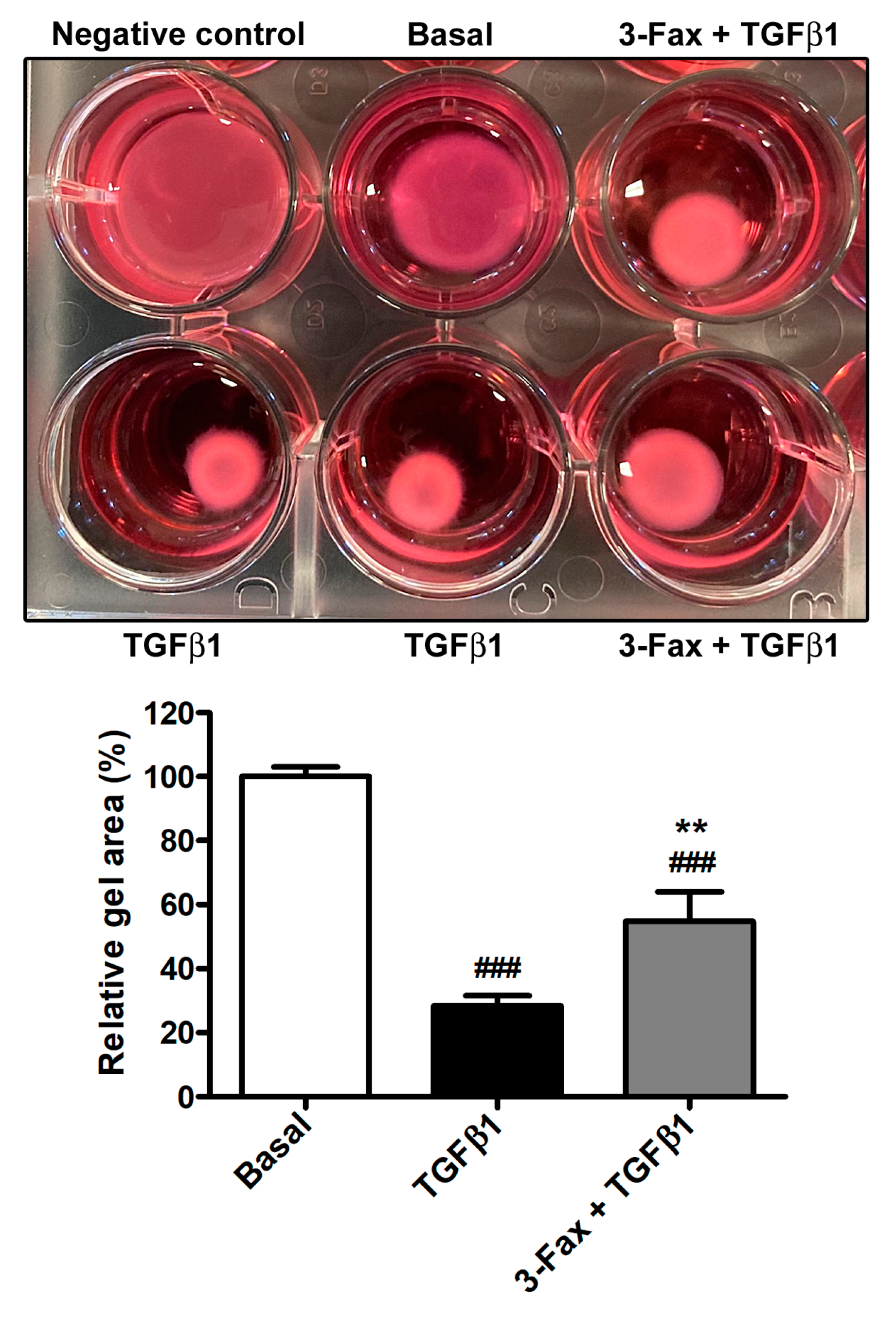
3.5. TGFβ1-Induced Acquisition of Myofibroblast Markers and Contractile Ability by Human Skin Fibroblasts Is Reduced by Preadministration of 3-Fax
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, X.; Lai, Y. Scarring Skin: Mechanisms and Therapies. Int. J. Mol. Sci. 2024, 25, 1458. [Google Scholar] [CrossRef] [PubMed]
- Canady, J.; Karrer, S.; Fleck, M.; Bosserhoff, A.K. Fibrosing Connective Tissue Disorders of the Skin: Molecular Similarities and Distinctions. J. Dermatol. Sci. 2013, 70, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nangole, F.W.; Agak, G.W. Keloid Pathophysiology: Fibroblast or Inflammatory Disorders? JPRAS Open 2019, 22, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wei, J.; Varga, J. Understanding Fibrosis in Systemic Sclerosis: Shifting Paradigms, Emerging Opportunities. Nat. Rev. Rheumatol. 2012, 8, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Bao, S.; Yao, L.; Wen, Z.; Xu, L.; Chen, X.; Guo, S.; Pang, H.; Zhou, Y.; et al. Deciphering the Fibrotic Process: Mechanism of Chronic Radiation Skin Injury Fibrosis. Front. Immunol. 2024, 15, 1338922. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol. Surg. 2017, 43, S3. [Google Scholar] [CrossRef] [PubMed]
- Faour, S.; Farahat, M.; Aijaz, A.; Jeschke, M.G. Fibrosis in Burns: An Overview of Mechanisms and Therapies. Am. J. Physiol. Cell Physiol. 2023, 325, C1545–C1557. [Google Scholar] [CrossRef] [PubMed]
- Ferreli, C.; Gasparini, G.; Parodi, A.; Cozzani, E.; Rongioletti, F.; Atzori, L. Cutaneous Manifestations of Scleroderma and Scleroderma-Like Disorders: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 306–336. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Vadalà, G.; Russo, F.; Pelacchi, F.; Lanotte, A.; Denaro, V. Dupuytren’s Disease Therapy: Targeting the Vicious Cycle of Myofibroblasts? Expert Opin. Ther. Targets 2015, 19, 1677–1687. [Google Scholar] [CrossRef]
- Goussetis, E.; Spiropoulos, A.; Theodosaki, M.; Stefanaki, K.; Petrakou, E.; Graphakos, S. Myofibroblasts Generated in Culture from Sclerotic Skin Lesions of a Patient with Extensive Chronic Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation Are of Recipient Origin. Stem Cells Dev. 2010, 19, 1285–1287. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Coentro, J.Q.; Pugliese, E.; Hanley, G.; Raghunath, M.; Zeugolis, D.I. Current and Upcoming Therapies to Modulate Skin Scarring and Fibrosis. Adv. Drug Deliv. Rev. 2019, 146, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Bochaton-Piallat, M.-L.; Gabbiani, G.; Hinz, B. The Myofibroblast in Wound Healing and Fibrosis: Answered and Unanswered Questions. F1000Research 2016, 5, F1000 Faculty Rev-752. [Google Scholar] [CrossRef] [PubMed]
- Desmoulière, A.; Hinz, B. The Myofibroblast and Giulio Gabbiani: An Inseparable Couple Celebrates Their 50 Years Golden Wedding Anniversary. Wound Repair Regen. 2021, 29, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and Myofibroblast Activation in Normal Tissue Repair and Fibrosis. Nat. Rev. Mol. Cell Biol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound Healing, Fibroblast Heterogeneity, and Fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Lagares, D. Evasion of Apoptosis by Myofibroblasts: A Hallmark of Fibrotic Diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Matucci-Cerinic, M.; Manetti, M. The Role of Pro-Fibrotic Myofibroblasts in Systemic Sclerosis: From Origin to Therapeutic Targeting. Curr. Mol. Med. 2022, 22, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wen, D.; Xu, X.; Zhao, R.; Jiang, F.; Yuan, S.; Zhang, Y.; Gao, Y.; Li, Q. Extracellular Matrix Stiffness—The Central Cue for Skin Fibrosis. Front. Mol. Biosci. 2023, 10, 1132353. [Google Scholar] [CrossRef]
- Do, N.N.; Eming, S.A. Skin Fibrosis: Models and Mechanisms. Curr. Res. Transl. Med. 2016, 64, 185–193. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-Β1—A Truly Transforming Growth Factor in Fibrosis and Immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017. [Google Scholar]
- Varki, A. Glycan-Based Interactions Involving Vertebrate Sialic-Acid-Recognizing Proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic Acid: Biosynthesis, Novel Functions and Applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.; Nathan, J.K.; Stock, C.M.; Chang, P.V.; Muench, M.O.; Nakata, D.; Reader, J.R.; Gip, P.; Golden, K.P.K.; Weinhold, B.; et al. Polysialic Acid, a Glycan with Highly Restricted Expression, Is Found on Human and Murine Leukocytes and Modulates Immune Responses. J. Immunol. 2008, 181, 6850–6858. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Marini, M.; Perna, A.; Tani, A.; Sgambati, E. Sialylation Status and Its Relationship with Morphofunctional Changes in Human Adult Testis during Sexually Mature Life and Aging: A Narrative Review. Acta Histochem. 2024, 126, 152143. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Tani, A.; Manetti, M.; Sgambati, E. Overview of Sialylation Status in Human Nervous and Skeletal Muscle Tissues during Aging. Acta Histochem. 2021, 123, 151813. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Cabello, T.M.; Gutiérrez-Valenzuela, L.D.; Salinas-Marín, R.; López-Guerrero, D.V.; Martínez-Duncker, I. Polysialic Acid in the Immune System. Front. Immunol. 2022, 12, 823637. [Google Scholar] [CrossRef] [PubMed]
- Troy, F.A. Polysialic Acid in Molecular Medicine. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2004; pp. 407–414. ISBN 978-0-12-443710-4. [Google Scholar]
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Ribeiro Morais, G.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic Acid Sustains Cancer Cell Survival and Migratory Capacity in a Hypoxic Environment. Sci. Rep. 2016, 6, 33026. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.A.; Errington, R.J.; Shnyder, S.D.; Smith, P.J.; Patterson, L.H. Polysialyltransferase: A New Target in Metastatic Cancer. Curr. Cancer Drug Targets 2012, 12, 925–939. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic Acid Facilitates Tumor Invasion by Glioma Cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef]
- Thiesler, H.; Küçükerden, M.; Gretenkort, L.; Röckle, I.; Hildebrandt, H. News and Views on Polysialic Acid: From Tumor Progression and Brain Development to Psychiatric Disorders, Neurodegeneration, Myelin Repair and Immunomodulation. Front. Cell Dev. Biol. 2022, 10, 871757. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Kitajima, K. Polysialylation and Disease. Mol. Asp. Med. 2021, 79, 100892. [Google Scholar] [CrossRef] [PubMed]
- Pearce, O.M.T.; Läubli, H. Sialic Acids in Cancer Biology and Immunity. Glycobiology 2016, 26, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Al Saoud, R.; Hamrouni, A.; Idris, A.; Mousa, W.K.; Abu Izneid, T. Recent Advances in the Development of Sialyltransferase Inhibitors to Control Cancer Metastasis: A Comprehensive Review. Biomed. Pharmacother. 2023, 165, 115091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Z.; Lobo, A.; Li, P.-F.; Zhang, Y.-F. Sialylated Glycoproteins and Sialyltransferases in Digestive Cancers: Mechanisms, Diagnostic Biomarkers, and Therapeutic Targets. Crit. Rev. Oncol. Hematol. 2024, 197, 104330. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.J.L.P.; Fu, C.-W.; Li, W.-S. Sialyltransferase Inhibitors for the Treatment of Cancer Metastasis: Current Challenges and Future Perspectives. Molecules 2021, 26, 5673. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.; Derksen, T.; Redmond, D.; Storek, J.; Durand, C.; Gniadecki, R.; Korman, B.; Cohen Tervaert, J.W.; D’Aubeterre, A.; Osman, M.S.; et al. The Cancer-Associated Glycan Polysialic Acid Is Dysregulated in Systemic Sclerosis and Is Associated with Fibrosis. J. Autoimmun. 2023, 140, 103110. [Google Scholar] [CrossRef]
- Macauley, M.S.; Arlian, B.M.; Rillahan, C.D.; Pang, P.-C.; Bortell, N.; Marcondes, M.C.G.; Haslam, S.M.; Dell, A.; Paulson, J.C. Systemic Blockade of Sialylation in Mice with a Global Inhibitor of Sialyltransferases. J. Biol. Chem. 2014, 289, 35149–35158. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global Metabolic Inhibitors of Sialyl- and Fucosyltransferases Remodel the Glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Lucattelli, E.; Innocenti, M.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Manetti, M. A Two-Step Immunomagnetic Microbead-Based Method for the Isolation of Human Primary Skin Telocytes/CD34+ Stromal Cells. Int. J. Mol. Sci. 2020, 21, 5877. [Google Scholar] [CrossRef]
- Andreucci, E.; Fioretto, B.S.; Rosa, I.; Matucci-Cerinic, M.; Biagioni, A.; Romano, E.; Calorini, L.; Manetti, M. Extracellular Lactic Acidosis of the Tumor Microenvironment Drives Adipocyte-to-Myofibroblast Transition Fueling the Generation of Cancer-Associated Fibroblasts. Cells 2023, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Fioretto, B.S.; Romano, E.; Buzzi, M.; Mencucci, R.; Marini, M.; Manetti, M. The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor Β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes. Int. J. Mol. Sci. 2022, 23, 15325. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, B.S.; Rosa, I.; Andreucci, E.; Mencucci, R.; Marini, M.; Romano, E.; Manetti, M. Pharmacological Stimulation of Soluble Guanylate Cyclase Counteracts the Profibrotic Activation of Human Conjunctival Fibroblasts. Cells 2024, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Xu, Y.D.; O’Connor, R.; Duronio, V. Proliferation of Pulmonary Interstitial Fibroblasts Is Mediated by Transforming Growth Factor-Beta1-Induced Release of Extracellular Fibroblast Growth Factor-2 and Phosphorylation of P38 MAPK and JNK. J. Biol. Chem. 2005, 280, 43000–43009. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Thomas, D.W.; Stephens, P.; Enoch, S.; Martin, J.; Steadman, R.; Phillips, A.O. Hyaluronan Facilitates Transforming Growth Factor-Beta1-Mediated Fibroblast Proliferation. J. Biol. Chem. 2008, 283, 6530–6545. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Chen, Y.-G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Tanabe, M.; Date, K.; Sakuda, K.; Sano, K.; Ogawa, H. Sialylation of Vitronectin Regulates Stress Fiber Formation and Cell Spreading of Dermal Fibroblasts via a Heparin-Binding Site. Glycoconj. J. 2016, 33, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.; Sonnenberg, A. Integrin–TGF-β Crosstalk in Fibrosis, Cancer and Wound Healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hong, S.; Dong, L.; Cheng, B.; Lin, L.; Zhao, B.; Chen, Y.-G.; Chen, X. Dynamic Sialylation in Transforming Growth Factor-β (TGF-β)-Induced Epithelial to Mesenchymal Transition. J. Biol. Chem. 2015, 290, 12000–12013. [Google Scholar] [CrossRef]
- Lee, J.; Ballikaya, S.; Schönig, K.; Ball, C.R.; Glimm, H.; Kopitz, J.; Gebert, J. Transforming Growth Factor Beta Receptor 2 (TGFBR2) Changes Sialylation in the Microsatellite Unstable (MSI) Colorectal Cancer Cell Line HCT116. PLoS ONE 2013, 8, e57074. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Sialylation Regulates Myofibroblast Differentiation of Human Skin Fibroblasts. Stem Cell Res. Ther. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; Wassink, M.; De Graaf, A.M.A.; Van Delft, F.L.; Den Brok, M.H.; Adema, G.J. Targeting Aberrant Sialylation in Cancer Cells Using a Fluorinated Sialic Acid Analog Impairs Adhesion, Migration, and in Vivo Tumor Growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Boelaars, K.; Rodriguez, E.; Huinen, Z.R.; Liu, C.; Wang, D.; Springer, B.O.; Olesek, K.; Goossens-Kruijssen, L.; van Ee, T.; Lindijer, D.; et al. Pancreatic Cancer-Associated Fibroblasts Modulate Macrophage Differentiation via Sialic Acid-Siglec Interactions. Commun. Biol. 2024, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Lagares, D.; Hinz, B. Animal and Human Models of Tissue Repair and Fibrosis: An Introduction. Methods Mol. Biol. 2021, 2299, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Romano, E.; Fioretto, B.S.; Guasti, D.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Manetti, M. Scleroderma-like Impairment in the Network of Telocytes/CD34+ Stromal Cells in the Experimental Mouse Model of Bleomycin-Induced Dermal Fibrosis. Int. J. Mol. Sci. 2021, 22, 12407. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nishioka, K. Cellular and Molecular Mechanisms of Bleomycin-Induced Murine Scleroderma: Current Update and Future Perspective. Exp. Dermatol. 2005, 14, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Hunter, C.; Gao, Z.; Chen, H.-M.; Thompson, N.; Wakarchuk, W.; Nitz, M.; Withers, S.G.; Willis, L.M. Attenuation of Polysialic Acid Biosynthesis in Cells by the Small Molecule Inhibitor 8-Keto-Sialic Acid. ACS Chem. Biol. 2023, 18, 41–48. [Google Scholar] [CrossRef]


| Gene | Assay ID | Catalog Number |
|---|---|---|
| FAP | Hs_FAP_1_SG | QT00074963 |
| ACTA2 | Hs_ACTA2_1_SG | QT00088102 |
| COL1A1 | Hs_COL1A1_1_SG | QT00037793 |
| COL1A2 | Hs_COL1A2_1_SG | QT00072058 |
| FN1 | Hs_FN1_1_SG | QT00038024 |
| Primary Antibody | Host Species | Catalog Number | Producer | Dilution |
|---|---|---|---|---|
| anti-polySia | rabbit | RAB00125 | Abnova | 1:1000 |
| anti-α-SMA | mouse | ab7817 | Abcam | 1:300 |
| anti-N-cadherin | rabbit | #13116S | Cell Signaling Technology | 1:1000 |
| anti-COL1A1 | rabbit | #39952 | Cell Signaling Technology | 1:1000 |
| anti-fibronectin | mouse | SAB4200880 | Sigma-Aldrich | 1:1000 |
| anti-p-Smad3 | rabbit | #9520S | Cell Signaling Technology | 1:1000 |
| anti-α-actinin | rabbit | #3134 | Cell Signaling Technology | 1:1000 |
| anti-GAPDH | mouse | ab8245 | Abcam | 1:5000 |
| anti-α-tubulin | rabbit | #2144 | Cell Signaling Technology | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioretto, B.S.; Rosa, I.; Tani, A.; Andreucci, E.; Romano, E.; Sgambati, E.; Manetti, M. Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition. Cells 2024, 13, 1067. https://doi.org/10.3390/cells13121067
Fioretto BS, Rosa I, Tani A, Andreucci E, Romano E, Sgambati E, Manetti M. Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition. Cells. 2024; 13(12):1067. https://doi.org/10.3390/cells13121067
Chicago/Turabian StyleFioretto, Bianca Saveria, Irene Rosa, Alessia Tani, Elena Andreucci, Eloisa Romano, Eleonora Sgambati, and Mirko Manetti. 2024. "Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition" Cells 13, no. 12: 1067. https://doi.org/10.3390/cells13121067
APA StyleFioretto, B. S., Rosa, I., Tani, A., Andreucci, E., Romano, E., Sgambati, E., & Manetti, M. (2024). Blockade of Sialylation with Decrease in Polysialic Acid Levels Counteracts Transforming Growth Factor β1-Induced Skin Fibroblast-to-Myofibroblast Transition. Cells, 13(12), 1067. https://doi.org/10.3390/cells13121067











