Abstract
Preimplantation embryo culture, pivotal in assisted reproductive technology (ART), has lagged in innovation compared to embryo selection advancements. This review examines the persisting gap between in vivo and in vitro embryo development, emphasizing the need for improved culture conditions. While in humans this gap is hardly estimated, animal models, particularly bovines, reveal clear disparities in developmental competence, cryotolerance, pregnancy and live birth rates between in vitro-produced (IVP) and in vivo-derived (IVD) embryos. Molecular analyses unveil distinct differences in morphology, metabolism, and genomic stability, underscoring the need for refining culture conditions for better ART outcomes. To this end, a deeper comprehension of oviduct physiology and embryo transport is crucial for grasping embryo–maternal interactions’ mechanisms. Research on autocrine and paracrine factors, and extracellular vesicles in embryo–maternal tract interactions, elucidates vital communication networks for successful implantation and pregnancy. In vitro, confinement, and embryo density are key factors to boost embryo development. Advanced dynamic culture systems mimicking fluid mechanical stimulation in the oviduct, through vibration, tilting, and microfluidic methods, and the use of innovative softer substrates, hold promise for optimizing in vitro embryo development.
1. Introduction
Although several studies contributed to the design of universal or sequential culture media tailored for preimplantation mammalian embryos [1], in vitro embryo culture was essentially unchanged since the beginning of human in vitro fertilization and is still performed in relatively large media volumes under static conditions [2]. It has been shown that numerous factors and mishandlings during standard culture procedures in these systems can pose a threat to the successful development of embryos [3,4]. However, the fundamental culture conditions persist unchanged, as if the inherent biological constraints on blastocyst development from the produced zygotes had already been reached. In comparison to the few efforts devoted to the improvement of the physico-chemical embryo culture environment, a range of technologies and non-invasive markers have been increasingly developed for selecting embryos endowed with the maximal potential to implant and generate live births [5,6,7,8].
Many researchers acknowledge the significant disparities between the physico-chemical conditions of in vitro preimplantation embryo development and the dynamic environments encountered in vivo as the embryo progresses from fertilization in the Fallopian tube to implantation in the uterus. Despite this recognition, there remains uncertainty regarding the extent to which the developmental potential of in vitro cultured embryos is compromised compared to their in vivo counterparts, particularly in humans with limited data compared to animal models. The primary objective of this paper is to review studies focusing on (1) the characteristics and performance of embryos developed in vivo vs. in vitro; (2) the dynamic physico-chemical conditions experienced by embryos during transit and development within the maternal tract; (3) the standard in vitro culture conditions; and (4) the dynamic culture systems designed in research studies and their effects on embryo development.
2. Natural versus In Vitro Conceptions in the Human
The efficacy of conception in vivo in humans is a highly debated and speculative topic based on relatively few old data. We still do not have definitive estimates of embryo mortality from fertilization to birth during in vivo conception in a fertile women cohort. Moreover, since the earlier marker of pregnancy is represented by a rise in hCG, we have different figures of what occurs from the second week of gestation, i.e., from implantation to birth, but still lack evidence on what has been called the black box of early pregnancy loss that covers the period from fertilization to implantation. Speculative estimates of this currently unmeasurable loss in vivo range from 10 to 70% [9,10,11,12,13].
The best estimates of the efficacy of in vivo conceptions in terms of live births per implantation are provided by three old, well-designed studies showing that about two-thirds of the in vivo conceptions detected by elevated hCG at least 1 week after ovulation result in a live birth [14,15,16]. Since these studies do not measure the pre-implantation embryo loss, embryo mortality from fertilization to birth in the human is still not known. Although speculative estimates of embryo loss before and during implantation range from 30 to 75% [17], it is currently accepted that up to 30% of conceptions do not complete implantation [18]. Therefore, it has been suggested that about 30% of the in vivo conceptions result in a live birth due to a 30% loss before implantation, a further unrecognized 30% loss after implantation but before the missed menstrual period, and a final 10% loss due to clinical miscarriage [18]. The highest estimates of live births during natural conception have been recently provided by Jarvis et al. (2016, 2017) [13,17], who re-analyzed the aggregate data from the three reliable hCG studies. Jarvis hypothesized that the criteria adopted to select fertile women cohorts did not ensure the exclusion of a proportion of sub-fertile or infertile couples. Therefore, he identified sub-cohorts of sub-fertile women to estimate the decline in pregnancy rates across successive cycles. By excluding these sub-cohorts from the initial population of women, it was determined that 40–60% of conceptions resulted in live births when considering only presumably fertile women. While human reproduction in vivo appears relatively inefficient compared to other mammals [19], studies measuring reproductive efficiency on an egg-by-egg basis suggest even lower success in vitro [20,21,22]. However, comparing reproductive efficiency in vitro vs. in vivo requires consideration of several biases. In vivo estimates typically involve unstimulated, fertile women within the appropriate reproductive age range. Conversely, patients undergoing assisted reproduction are sub-fertile and more reproductively aged. According to Patrizio and colleagues (2007) [22], only about 5% of harvested mature oocytes led to live births. However, this analysis was based on 31 cycles involving 26 patients (with a mean age of 37 ± 3.7 years), including 10 cycles with recurrent pregnancy loss, 15 cycles with advanced maternal age, and 6 cycles for unexplained recurrent pregnancy failure. Jones et al. (2010) [23] reported a 6% efficiency of human reproduction in vitro for an age group of 25–29 years and suggested that IVF is only one-fifth as efficient as natural human reproduction. In conclusion, the differences between in vivo and in vitro conceptions in humans are still inconclusive due to the scarcity of data available in literature.
3. Natural versus In Vitro Conceptions in Animal Models
The success of the basic and straightforward embryo culture conditions in assisted reproductive technology (ART) is demonstrated by the birth of over 10 million babies. However, a fundamental yet unanswered question persists: to what extent do current in vitro embryo culture conditions replicate those experienced by embryos during in vivo development? In other words, is there room for improvement in the developmental rates and quality of in vitro-produced embryos to enhance clinical success rates?
While data from human ART are inconclusive, more stringent and unbiased experiments have been conducted using animal models. Studies in the mouse model showed several differences between in vitro-produced (IVP) and in vivo-derived (IVD) embryos. IVP embryos had altered ICM and TE cell numbers, lower hatching rates, increased apoptosis, lower cryotolerance, altered metabolism and oxidative stress, and differential gene expression (Table 1) [24,25,26,27,28,29,30].

Table 1.
Mouse animal model: differences between IVP and IVD embryos. ICM, inner cell mass; TE, trophectoderm; ROS, reactive oxygen species; GSH, reduced glutathione; Mmp-9, matrix metalloproteinase-9.
Bovine is considered a more suitable animal model for humans in many respects.
In vitro embryo culture conditions in both bovines and humans exhibit striking similarities, with many of the technologies initially developed in cattle serving as the foundation for human ART [24]. Furthermore, bovine and human embryo development display more similarities than those observed between human and murine embryonic development [25,26,27,28]. Bovines are excellent models for studying early embryo–maternal communication in humans due to their analogous cycle length, pregnancy duration, usual single offspring, and genetic imprinting patterns [29,30]. Bovine reproduction has been extensively studied for its commercial significance and the lack of ethical constraints that limit experimental studies on human embryos. A unique opportunity for comparing the competence of IVP and IVD bovine embryos arises from data collected in the cattle embryo industry. The 31st annual report of the International Embryo Technology Society (IETS) revealed that over 2 million embryos were collected or produced in cattle in 2021 [31]. Although the number of transferrable IVP embryos exceeded that of IVD embryos for the first time in 2016 [32], accounting for the 79.7% of all transferrable cattle embryos in 2021 [31], the pregnancy and live birth rates of IVP embryos remain lower compared to their in vivo counterparts. On average, pregnancy rates after transfer of IVP or IVD bovine embryos are 40.1% and 64.1%, respectively [33], demonstrating that the competence of IVP embryos consistently lags behind that of IVD embryos [34]. In addition, IVP embryos have a reduced cryotolerance and a higher lipid accumulation compared to IVD embryos [35]. This is also exemplified by the IETS 2021 data showing that frozen IVD embryo transfer is still preferred compared to frozen IVP embryo transfer (60.5% vs. 41.3%) [31]. During development, lipids are used by the mitochondria to increase the ATP production and promote compaction and blastocyst formation [36]. However, lipids can accumulate in the embryo due to an increased uptake from the culture environment [37] or to a decreased activity of mitochondria [38]. Gad et al. (2012) [39] reported that embryos cultured in vitro at the time of embryonic genome activation are particularly affected by a down-regulation of lipid metabolism-related genes compared to embryos developed in vivo. In agreement with those findings, bovine embryos generated by IVM/IVF followed by in vivo culture in the ewe oviduct had similar cryotolerance and pregnancy rates after frozen embryo transfer than IVD embryos [40]. Havlicek et al. (2009) [41] using zygotes produced in vitro and then cultured in vivo also demonstrated that the duration of in vivo culture is crucial for cryotolerance. On the cellular and molecular level, IVP bovine embryos differ from their in vivo counterparts in many respects. Clear differences in morphology and ultrastructure [42,43,44,45], inner cell mass/trophectoderm allocation [46], intercellular connectivity [47], lipid content and profiles [48], energy metabolism [49,50,51], transcriptomic [39,52,53,54], proteomics [55], chromosome aberrations [56,57], and methylation patterns [58] underlie the higher developmental competence of IVD compared to IVP embryos. Rizos et al. (2002) [45] demonstrated that in vitro embryo culture and in vivo development produced similar blastocyst rates, but IVD blastocysts had a dramatically higher competence as shown by the increased survival after cryopreservation. Differences between IVP and IVD bovine embryos are summarized in Table 2.

Table 2.
Bovine animal model: differences between IVP and IVD embryos. ICM, inner cell mass; TE, trophectoderm.
The contribution of the natural environment to the embryo competence is also exemplified by the enhanced quality of IVP embryos when cultured short- or long-term in vivo in homologous or heterologous oviducts [40,41].
In conclusion, data in animal models highlight clear differences between the consequences of in vivo and in vitro environment on embryo developmental competence.
4. Can In Vitro Culture Increase Embryo Aneuploidy?
4.1. Human
Embryo aneuploidy is a major contributor to low human fecundability both in vivo and in vitro. The overall incidence of aneuploidy in newborns following natural conception is less than 1% due to the progressive elimination of aneuploid embryos through pre- and post-implantation loss. Nondisjunction of bivalents and premature separation of sister chromatids during oocyte’s meiosis is responsible for embryo homogeneous aneuploidies which are maternal age-dependent both in vivo and in vitro [59,60]. However, errors during the post-zygotic mitotic divisions give rise to an impressively high incidence of age-independent mosaic embryo aneuploidies [61]. Single blastomere analysis of ART-generated embryos demonstrated that more than 75% of them can be mosaics [62,63]. Although it is still a matter of debate, the aneuploidy levels in the general vs. the ART population are hypothesized to be similar [61]. The observation of average euploidy rates ranging from 39.5 to 82.5 on a population of young oocyte donors in 42 different centers raised concerns on the possibility that controlled ovarian stimulation [64] and/or embryo culture systems can affect the euploidy of ART-generated blastocysts [65]. However, several studies did not find different aneuploidy rates in embryos of unstimulated vs. stimulated patients or in embryos from patients subjected to different doses and length of gonadotropins treatment [65,66,67,68]. The possibility that the culture system affects the embryo aneuploidy rate may indicate that the suboptimal microenvironments experienced by the embryo in vitro might induce perturbations in chromosome segregation during the post-zygotic mitosis. Several studies examined a possible correlation between culture settings and embryo aneuploidy. Culture of sibling oocytes under different extracellular pHs (6 vs. 7.5% CO2 [69]; 6 vs. 7% CO2 [70]) had no impact on blastocyst development but a significantly lower euploidy rate was found at 7 and 7.5% CO2. Different single-step embryo culture media reportedly did not affect the embryo euploidy of sibling oocytes [71]. The possible impact of embryo culture systems on euploidy rates in human ART has been recently reviewed by Swain et al. (2021) [72]. Although some data suggest that the culture system may influence the incidence of post-zygotic mitotic errors, we still do not have conclusive indications in the human.
Despite the fact that the euploidy of embryos is a fundamental prerequisite for successful implantation, the clinical significance of PGT-A is still a matter of debate. Clinical evidence demonstrates that transfer of mosaic embryos can result in healthy live births though with lower implantation rates and increased miscarriage rates [73,74,75,76,77,78]. Although the clinical outcome of mosaic embryos depends on the type of aneuploidy involved, available data indicate that transfer of embryos with less than 50% aneuploid cells have better outcomes compared to embryos with higher levels of mosaicism [74,75,77,78,79,80]. Recent findings suggest that mosaic embryos can undergo a self-correction during development through apoptosis of aneuploid blastomeres [81]. In fact, a recent multicenter study demonstrated that the incidence of mosaicism in births from transfer of mosaic embryos is like that recorded after transfer of euploid embryos [77]. PGT-A analyzes the DNA of 5–10 biopsied trophectoderm cells and we still do not know to what extent mosaicism in trophoectoderm cells is representative of true inner cell mass or fetal mosaicism [82]. Since the live birth rate of single euploid blastocyst transfer is limited to 50–60% [83], blastocyst’s euploidy is not sufficient to predict the embryo competence to implant and result in a healthy live birth. Additional markers such as blastocyst morphological grading [84] and developmental speed [85] must be considered to predict the success rates of euploid blastocyst transfer.
4.2. Animal Models
More rigorous research, relatively unhampered by the ethical limitations and the innumerous confounding variables present in human studies, can be conducted in animal models.
Although performed with older and technically limited procedures, fluorescent in situ hybridization and comparative genomic hybridization studies suggested a higher incidence of whole chromosome aneuploidy in IVP compared to IVD embryos in ovine, equine, and porcine models [86,87,88].
These data were confirmed and extended in recent studies using more robust procedures able to detect the extent and origin of all chromosomal errors. Tsuiko et al. (2017) [57], using the same parents, compared the genome stability of in vivo vs. in vitro conceived bovine embryos through genome-wide single-cell analysis of dissociated blastomeres. Findings showed the presence of at least one chromosomally aberrant blastomere in only 18.8% of the embryos conceived and developed in vivo, compared to 69.2% of IVF embryos and 84.6% of IVM-IVF embryos. Although this study was carried out on a limited number of cleavage-stage embryos, data clearly highlight the involvement of in vitro embryo culture and in vitro maturation on chromosome instability. Similar findings were demonstrated through single nucleotide polymorphism (SNP)-based PGT-A algorithms in the porcine model by Jochems et al. (2023) [89], who observed more errors in in vitro vs. in vivo-conceived blastocysts (79.7% vs. 13.6%). A study in the equine showed that the incidence of micronuclei which are considered indicators of chromosomal aneuploidy was higher in IVP compared to IVD blastocysts [90]. Taken together, animal data indicate a higher genomic instability in embryos produced in vitro compared to their in vivo counterparts corroborating the need for refining current in vitro embryo culture conditions to mimic more closely the in vivo microenvironment and enhance the success and safety of ART.
5. Embryo Transport in the Female Reproductive Tract
During its transport in vivo along the oviduct and the uterus, the embryo undergoes several distinctive biophysical and biochemical interactions that could enhance its ability to reach the blastocyst stage and increase its competence to implant and develop to term, resulting in healthy offspring. Assisted reproduction in humans, farm animals, and studies in animal models that bypass the oviduct to achieve pregnancy have shown that embryo–maternal interactions are indeed dispensable. This may have shifted researchers’ focus away from attempting to more closely mimic the natural environment in which the embryo develops in vivo.
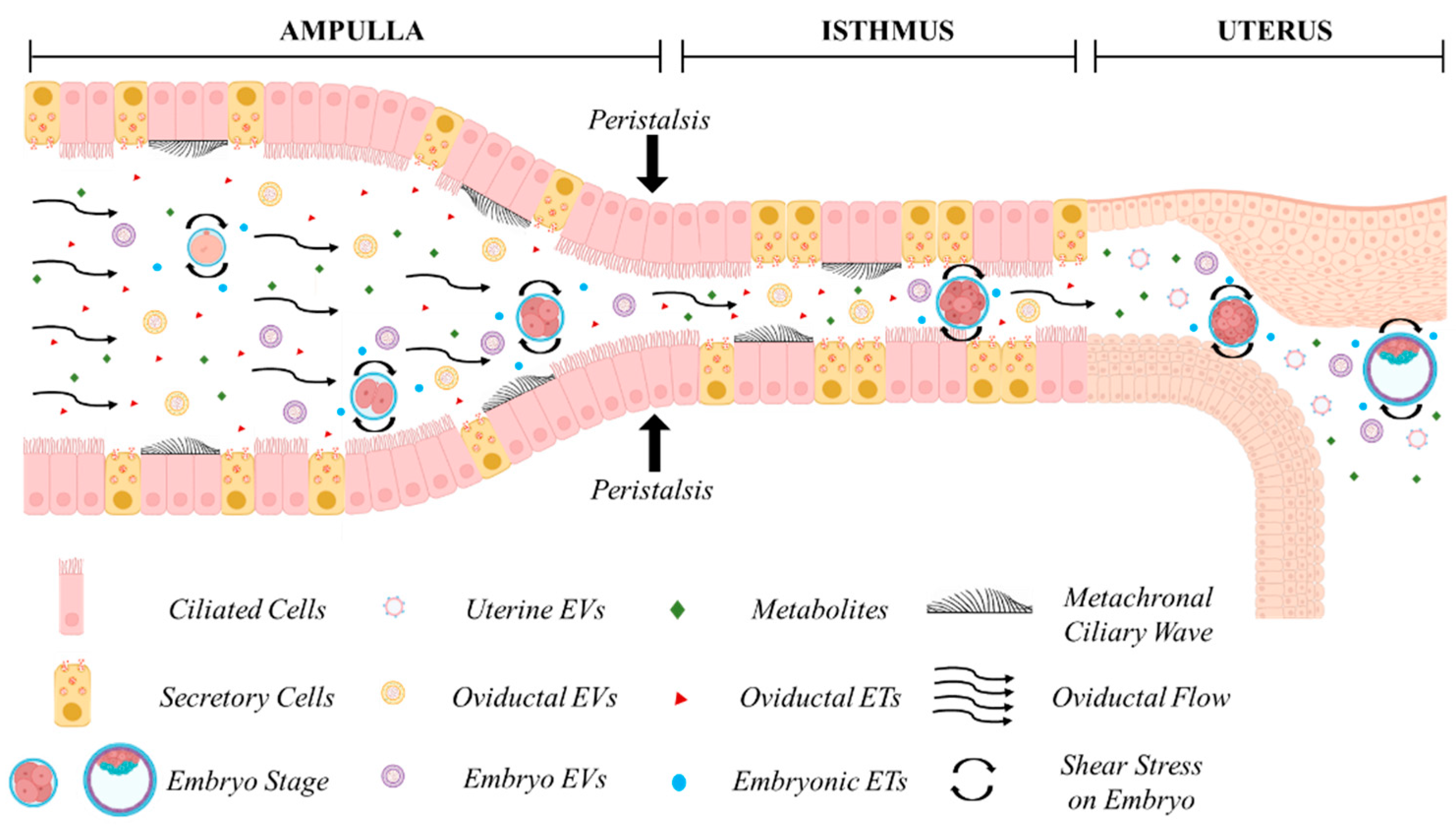
The oviduct (Fallopian tube in humans) (Figure 1) is composed by the infundibulum (fimbria in humans), mostly lined by ciliated epithelial cells, that catches the ovulated cumulus–oocyte complex; the ampulla, site of fertilization, where ciliated cells are more represented than secretory cells; the ampullary isthmic junction that separates the lower ampulla by the isthmus; the isthmus, predominantly lined by secretory epithelial cells, and the utero-tubal junction or the intramural segment that divides the isthmus from the uterus. The mucosa is composed of primary, secondary, and tertiary folds in the ampullary segment and only primary folds in the isthmic segment. The underlying connective tissue separates the mucosa by the smooth muscle which is arranged into an inner longitudinal, a middle circular, and an outer longitudinal layer [91]. The smooth muscle is very thin in the ampulla and thick and composed of multiple layers in the isthmus [92].
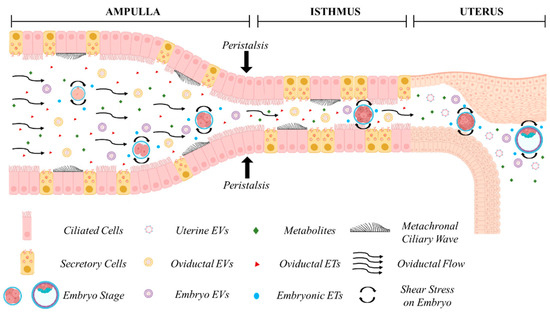
Figure 1.
In vivo embryo development in the maternal tract. Cross-talk between the embryo and the maternal tract. Oviductal, endometrial, and embryonic secretions in the form of embryotropins (ETs) and various signaling factors, enveloped or less in extracellular vesicles (EVs), are concentrated in a confined environment. Secretion of maternal fluids, metachronal ciliary waves, and peristaltic contractions exert biomechanical forces on the developing embryo during its journey through the ampulla, isthmus, and the uterus. Figure created with BioRender.com (accessed on 11 April 2024).
Understanding the dynamics of embryo transport in the oviduct in vivo is crucial for comprehending the mechanisms underlying oviduct–embryo interactions and optimizing embryo culture in vitro. However, most available data across various species are derived from ex vivo studies, with direct in vivo imaging being rare. The duration of embryo residence in the oviduct varies among species. In humans, egg transport duration has been estimated through the recovery of embryos from the Fallopian tube or uterus following surgical sterilization at known times after the LH surge [92]. Embryos spend approximately 80 h in the oviduct [92], with transport occurring in two phases: a slow phase lasting 72 h in the ampulla, where entry into the isthmus is inhibited by a gate that relaxes under progesterone influence, followed by a rapid phase lasting 8 h in the isthmus [93]. It has been assumed that embryos reach the uterine cavity around the 8–12-cell stage, i.e., at the time of compaction, as 8-cell embryos are the latest stage recovered from the Fallopian tube, and 12-cell embryos are the earliest stage observed in the uterus. Studies in other mammals suggest that embryos develop within the oviduct approximately 4 days after ovulation [94], reaching the uterus around the 16-cell stage in bovine [95], 5.5 days in equine [96], and 4 days in mice [97]. In mice, recent in vivo imaging using optical coherence microscopy (OCM) and optical coherence tomography (OCT) through an intravital window has provided new insights into embryo transport, enabling the determination of developmental stages and in vivo volumetric imaging and tracking of oocytes and preimplantation embryos within the native environment of the mouse oviduct [98,99]. Moore et al. (2019) [98] showed the presence of pronuclear zygotes at 0.5 day post-coitum (dpc) in the ampulla, 2-cell embryos at 1.5 dpc were found in the isthmus, and 4–8-cell embryos at 2.5 dpc were located close to the utero-tubal junction. This is consistent with older studies showing that the mouse embryo enters the uterus approximately 72 h post-coitum (0:00–2:00 h of Day 4), in the morula or early blastocyst stage [97].
The passage of the embryo within the oviduct is believed to be facilitated by three different mechanisms, the production of oviductal fluid, the beating of cilia, and the peristaltic contraction of smooth muscle [10] (Figure 1). The contribution of each factor to embryo descent is still matter of debate [100,101,102], and ciliary beating has typically been regarded as the primary propulsive force involved [101,103,104,105,106]. However, while all factors may exert a biophysical influence on the developing embryo, recent studies have underscored the primary role of muscle contraction in embryo transport. Dixon et al. (2009) [107], using video analyses and Spatio-Temporal Maps, suggested that propagating muscular contractions represent the main propulsive force driving the pendular movement of the egg along the mouse oviduct. They also noted that treatment with an L-type Ca2+ channel antagonist suppressed both muscular contractions and egg movement, while ciliary beating remained unaffected. This finding is consistent with the ability of women affected by immotile cilia syndrome to conceive, albeit with reduced efficiency [108,109], and with the fertility of mice deficient in two microRNA clusters lacking cilia but still capable of managing embryo migration from the oviduct into the uterus [110]. Wang and Larina (2020) [99], using in vivo volumetric OCT imaging and tracking of oocytes and embryos in the mouse oviduct, observed unexpected movement patterns, suggesting that egg/embryo transport is more complex than generally assumed. Cumulus–oocyte complexes rotate in groups likely propelled by cilia in the upper ampulla. Groups of fertilized oocytes in the lower ampulla undergo short-distance pulsatile oscillations while descending toward the isthmic-ampullary junction, moving backward when isthmus contracts and forward when it relaxes. At 0.5 days post-coitum (dpc), groups of preimplantation embryos remain stable in the lower ampulla, with average speeds (about 5–70 μm/s) and maximum speeds (20–230 μm/s) depending on the number of embryos present. At 1.5 dpc, embryos in the upper isthmus are relatively spread and individual embryos exhibit very rapid, bi-directional movements (40–740 μm/s) caused by propelling muscular contraction waves. In the lower isthmus, embryos nearing the utero-tubal junctions once again form groups and undergo pulsatile oscillations with reduced speeds (14.7 to 60.0 μm/s). This study highlights that embryos experience different physical forces depending on the timing of transport along the oviduct, which could biomechanically influence embryo development.
6. Embryo–Maternal Tract Interactions
During the descent along the female reproductive tract, the preimplantation embryo communicates bidirectionally with oviductal cells of the ampulla, isthmus, and then with endometrial cells. The beneficial effects of culturing embryos with oviductal cells or with their conditioned media were first demonstrated by Gandolfi and Moor (1987) in the sheep [111] and by Eyestone and First (1989) in cattle [112]. Promotion of embryo development through co-culture with autologous or heterologous oviductal cells, uterine fibroblasts, cumulus cells, and cells extraneous to the maternal reproductive tract has been reported in several mammals and the human as well [113,114,115,116,117,118]. These studies prompted the use of co-culture in clinical embryology in human and domestic ruminants to more closely simulate the interaction with the maternal tract in vitro. Co-culture systems have been hypothesized to improve embryo development through (1) the removal of deleterious components of embryo metabolism and reduction in oxidative stress, and (2) active secretions of embryo-trophic factors [119]. However, co-culture has been discontinued for several reasons, potential transmission of pathogens [120], too complex methodology in the clinical context, introduction of reduced oxygen tension which ameliorates blastocyst formation [121], and uncertainty of the beneficial actions.
Along with the presence of maternal factors, the embryo itself secretes embryotropins that promote embryonic development through an autocrine action [122]. Since the oviduct is a virtual cavity, these putative embryotropins should reach a critical concentration required for their positive action on embryo development. The beneficial effects of culturing embryos in groups rather than singly in a defined culture medium volume was recognized more than 30 years ago and has been putatively attributed to the enhanced concentration of embryo-secreted autocrine and paracrine factors [123]. During in vivo conception, putative signaling factors known as embryotropins or embryokines [124,125] can be secreted by the embryo itself or by the epithelial cells lining the female reproductive tracts, but their exact nature remains elusive. Since embryo development in vitro occurs in the absence of maternally-derived signaling factors, embryotropins secreted by the embryo itself and involved in inter-embryonic communication could be responsible for the positive effects of group culture observed in most mammals studied thus far [123,126,127,128,129]. Embryos can communicate with each other or with neighboring embryos through various signaling factors, including proteins, lipids, neurotransmitters, saccharides, nucleotides, and micro RNAs [122]. Studying the proteins released by embryos, however, is complicated by the low concentration of putative embryotropins compared to albumin and other proteins typically added in high concentrations to embryo culture media. Proteins detected in the culture media may stem from lysed cells, which are commonly found not only in arrested embryos but also in developing embryos [130]. Therefore, secretome studies should be conducted on individually cultured high-quality embryos, ensuring the absence of membrane-damaged cells [131]. Within this framework, several studies indicate that preimplantation embryos secrete various factors that may act through autocrine signaling, as they also express the corresponding receptors [122,132].
Extracellular vesicles (EVs) represent an additional recently discovered mechanism through which embryos may communicate with each other and with the female reproductive tract [133]. EVs are nanoparticles surrounded by a lipid bilayer, physiologically released from cells, which contain bioactive molecules such as lipids, proteins, DNA, mRNA, and miRNAs [134,135]. EVs comprise exosomes, microvesicles, and apoptotic bodies, which are characterized by different biogenesis mechanisms and dimensions [135]. EVs participate in cell–cell communication in various biological processes and are relevant modulators during embryo–maternal communication [136].
EVs released by preimplantation embryos, oviductal, and endometrial cells influence the developmental competence and gene expression of embryos, as well as oviductal/endometrial gene expression and functions [137]. Since the oviduct and uterus are considered virtual cavities in which embryos develop in confined fluid volumes, EVs may be present at high concentrations suitable for acting as bidirectional players in embryo–maternal tract cell–cell communication to regulate developmental competence and implantation in vivo. EVs recovered from the human Fallopian tube have been demonstrated to carry miRNAs that increase murine embryo viability in vitro [138]. Moreover, EVs isolated from media conditioned by primary human endometrial epithelial cells are efficiently internalized by human blastocysts and contain miRNAs directed toward genes involved in several processes related to embryo development [139]. Recent studies attempted to mimic in vivo conditions by sequentially culturing bovine embryos with EVs from oviductal and endometrial cells [140,141]. The addition of isthmus oviductal fluid EVs improved the development and cryotolerance of IVP blastocysts [141], whereas sequential culture with oviductal and endometrial EVs improved blastocyst cryotolerance by altering lipid content and metabolism, possibly in response to EVs’ miRNA contents [140].
While these studies deepen our understanding of embryo–maternal crosstalk during in vivo development, maternal EVs are obviously absent during in vitro embryo production, and the translation of such knowledge to in vitro embryo culture is still distant. Conversely, knowledge of the presence of embryo-derived EVs in vitro could be more readily applied to refine in vitro embryo culture in ART. Recent evidence indicates that embryo EVs may be internalized by the embryo itself, positively affecting its developmental competence. Pavani et al. (2018) [142] demonstrated that embryo EVs are taken up by zona-intact bovine embryos. Moreover, EVs extracted from media conditioned by high-density embryo group culture (25/50 μL) when added to individually cultured embryos increase blastocyst development to rates comparable to group culture, lower apoptotic cell ratios, ultimately demonstrating that EVs act as autocrine embryotropins during embryo culture. However, EVs from high-density group culture failed to increase the blastocyst hatching rates of individually cultured embryos to the same extent as reached under group culture. Generally, the developmental competence of singly cultured embryos is lower compared to group culture in terms of blastocyst cell number, hatching rates, and cryotolerance [143]. In addition, the hatching rates of IVD embryos are much higher than their IVP counterparts [45]. A recent study by the same group [144] isolated EVs from conditioned media of individually cultured embryos that either developed to the blastocyst stage or did not. Among the miRNAs differentially expressed in conditioned media from embryos that successfully developed or less to blastocyst, the authors selected bta-miR-378a-3p for further validation. Supplementation of the embryo culture medium with miR-378a-3p mimic significantly increased blastocyst cell numbers, reduced apoptotic cell numbers, improved hatching rates, and increased the expression of genes involved in embryo development and implantation. Knowledge of EVs derived from embryos may be translated into clinical practice to establish new non-invasive biomarkers for embryo selection in ART [5]. Further research on embryo EVs carrying miRNAs or other molecular cargos is warranted to enhance embryo development and quality and for selecting high-quality embryos [145].
In this scenario, confining single embryos into submicroliter volumes may be the key to success in concentrating autocrine embryotropins to levels similar to those present in vivo or achieved during high-density group embryo culture. However, attempts to reduce the volumes of drops under oil must address (1) nutrient depletion and waste metabolite accumulation; (2) the increase in surface-to-volume ratios that enhance unwanted bidirectional solute exchanges between oil and medium; (3) increased evaporation leading to osmolarity changes [2].
7. In Vitro Embryo Culture: Individual versus Group Culture
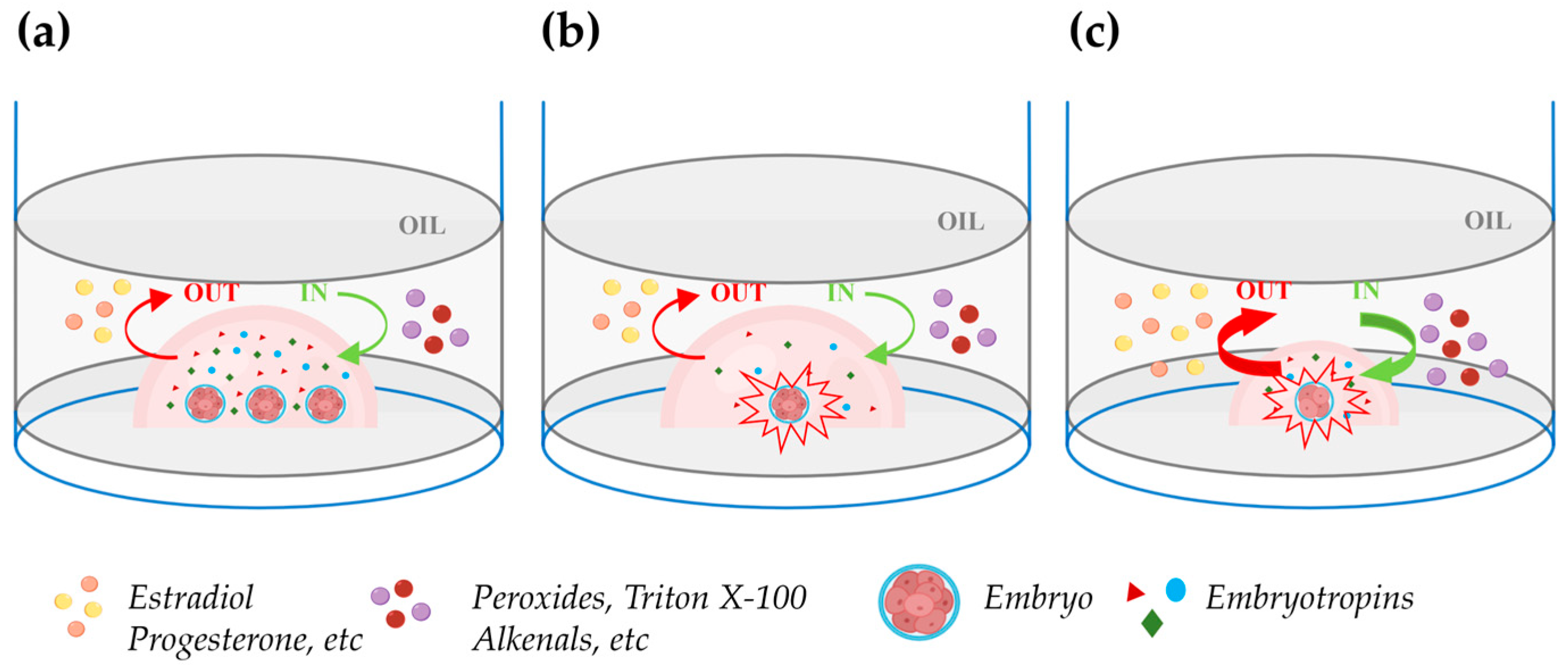
It is well known that embryos benefit from being cultured together at high densities rather than individually in relatively large media volumes (Figure 2a,b). This phenomenon, termed the “group effect”, has been clearly recognized not only in mice, which are poly-ovulatory species, but also in domestic animals, which are largely mono-ovulatory. In multiple species, embryos cultured individually have been reported to exhibit slower cleavage divisions, reduced blastocyst rates and cell numbers, altered ICM-TE cell allocation, reduced hatching, increased apoptosis, altered gene expression, and reduced pregnancy rates [123,126,127,128,129,146] (Data are summarized in Table 3). The reduced blastocyst rates and cell numbers in embryos cultured individually might be explained by the low concentration of positive-effect embryo-secreted signaling molecules (Figure 2b). However, attempts to reduce the volume of drops under oil to achieve a high embryo density during individual culture generally yielded disappointing results.
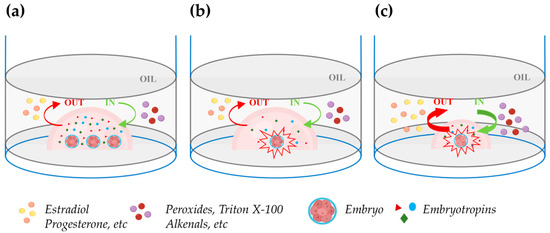
Figure 2.
Impact of embryo density during in vitro culture in drops under oil. (a) Group embryo culture in a defined volume of culture medium improves embryo development due to an enhanced concentration of embryotropins (triangles, circles, and diamonds). The green and red curved arrows, respectively, indicate unwanted in (peroxides, Triton X-100, alkenals, etc.) and out (estradiol, progesterone, etc.) apolar solute exchanges between oil and medium. (b) Individual embryo culture within the same drop volume as in the figure (a): embryo development is compromised due to the reduced concentration of embryotropins. (c) Individual embryo culture in a reduced drop volume: embryotropins are more concentrated than in (b) and the increase in the drop surface exposed to oil relative to the drop volume exacerbates the unwanted in and out exchanges between the oil and medium, impairing embryo development. Figure created with BioRender.com (accessed on 11 April 2024).
Lane and Gardner (1992) [147] cultured single mouse embryos in drops of 5, 10, 20, or 320 μL and reported similar blastocyst rates but higher cell numbers in embryos individually cultured in 10 and 20 μL drops. Although the lower cell numbers in 320 μL of medium could result from low concentration of embryotropins, the same finding in 5 μL drops can be due to the increased surface exposed to oil relative to the drop volume which enhances unwanted damaging bidirectional solute exchanges between oil and medium (Figure 2c). In agreement with this hypothesis, individual culture in 5 μL in glass capillary tubes increased the blastocyst cell number to the same extent as in 10 or 20 μL drops. The beneficial effects of confinement in culture systems that avoid the harmful exchanges between oil and medium in reduced volumes microdrops was also elegantly shown by Thouas et al. (2003) [148] which cultured two mouse embryos in 1 µL glass capillaries achieving blastocyst cell numbers similar to those observed in high-density group culture with 20 zygotes in 10 μL drops under oil. Recently, Travaglione et al. (2024) showed that extreme confinement of individually cultured bovine embryos in ~70 nl microwells improved blastocyst rates compared to both conventional group culture (50 embryos/500 µL) and semi-confined group culture in microwell chambers (16 embryos/60 µL) [149]. Other studies showed that the spacing among embryos in high-density group cultures is critical. Stokes et al. (2005) [127] cultured groups of 16 IVD pig embryos in 20 μL drops at fixed distances and found maximal blastocyst and hatching blastocyst rates when the spacing was 81–160 μm. Lower rates were observed in drops with embryos in direct contact and no hatching blastocysts or blastocysts formed when the spacing was >480 and >640 μm, respectively. Similar findings were reported in the bovine model by Gopichandran and Leese (2006) [150]. Hoelker et al. (2009) [151] showed that group culture of 16 vs. 50 bovine zygotes in 500 μL of medium depresses the blastocyst rates and such effect is reverted when 16 zygotes are cultured in the same volume in a semi-confined well onto the well system.

Table 3.
In vitro embryo culture: differences between individual versus group culture. WOW, Well of the Well.
Table 3.
In vitro embryo culture: differences between individual versus group culture. WOW, Well of the Well.
| Features | Embryo Density/Culture Conditions | Species | References |
|---|---|---|---|
| Blastocyst Rate | 1/25 μL vs. 5 or 10/25 μL (49 vs. >80%) | Mouse | [123] |
| 1/25 μL vs. 1/50 μL (49 vs. 28%) | Mouse | [123] | |
| 1/20 μL vs. 3–6/20 μL vs. 20/20 μL (0 vs. 6 vs. 23%) | Bovine | [126] | |
| 16/20 μL different embryo–embryo distance (165 μm, ~20% vs. direct contact, ~8% vs. >540 μm, 0%) | Bovine | [150] | |
| 16/500 μL vs. 50/500 μL vs. 16/500 μL WOW (21 vs. 32 vs. 31%) | Bovine | [151] | |
| 50/500 μL vs. 16/60 μL MG vs. 1/68 nl MS (28.9 vs. 23.1 vs. 35.8%) | Bovine | [149] | |
| 16/20 μL different embryo–embryo distance (81–160 μm, ~25% vs. >640 μm, 0%) | Porcine | [127] | |
| 1/30 μL vs. 3–4/30 μL (45.2 vs. 55.8%) | Human | [129] | |
| Blastocyst Cell Numbers | 1/25 μL versus 5 or 10/25 μL (34 vs. >60) | Mouse | [123] |
| 1/20 μL vs. 20/20 μL (77 vs. 176) | Bovine | [126] | |
| 16/20 μL different embryo–embryo distance (decreased cell numbers at 240 μm vs. 165 μm) | Bovine | [150] | |
| 16/20 μL different embryo–embryo distance (1–160 μm, 49.9 vs. 401–480 μm, 32) | Porcine | [127] | |
| 1/10 or 20 μL vs. 1/40 μL (75 vs. 60) | Mouse | [147] | |
| 2/1 μL glass capillary vs. 20/10 μL droplet (92 vs. 75) | Mouse | [148] | |
| Blastocyst Hatching | 1/25 μL vs. 5 or 10/25 μL (10.7 vs. 52%) | Mouse | [123] |
| 1/2 μL vs. 10/20 μL (Day 4, 49 vs. 63%) | Mouse | [146] | |
| 2/1 μL glass capillary vs. 20/10 μL droplet (48.3 vs. 3.3%) | Mouse | [148] | |
| Pregnancy Rate | 1/30 μL vs. 3–4/30 μL (30 vs. 60%) | Human | [129] |
| Implantation Rate | 1/20 vs. 2/20 vs. 16/20 (27 vs. 32 vs. 38%) | Mouse | [147] |
As mentioned, mineral oil can be harmful especially when embryos are cultured in small drops due to the increase in the surface exposed to oil relative to the drop volume (Figure 2c). In fact, early studies on oil embryotoxicity showed that mineral oil can absorb apolar solutes from the culture medium like estradiol, progesterone, and androstenedione [152]. A recent study showed that human blastocysts express both the enzymes required to synthesize estradiol and its receptors, and supplementation of embryo culture medium with 8nM estradiol improved formation of blastocyst cavity, blastocyst hatching, and the ICM/TE ratio but only when embryo culture was not performed under oil [153]. Mineral oil can also exert detrimental effects on embryo culture releasing a number of embryotoxic solutes into the culture medium like peroxides, alkenals, aldehydes, Triton X-100, and zinc [154,155,156,157,158]. In summary, high embryo density is the key of success to optimize embryo development but individual culture in drops of reduced volumes under oil is not the right way to increase the embryo density.
Recently, semiconfined individual embryo culture in microwell chambers based on the concept of the well onto the well originally developed by Vajta (2000) [159] has been largely adopted in human ART to follow each embryo individually through time-lapse morphokinetics. Culture of embryos in multiple microwells under one drop of shared medium should allow to concentrate putative, beneficial embryo-secreted factors in each microwell while the shared drop of medium should avoid the depletion of nutrients and the accumulation of waste metabolites. However, such devices preclude the possibility to non-invasively assess the competence of individual embryos through analysis of the spent media. Research in this field in the human and animal models clearly indicates the huge potential of non-invasive assessments on spent media to identify the developmental competence and the genetic status of individual embryos to transfer in a future ART setting [6,7,8].
8. Advanced Culture Systems to Mimic Fluid Mechanical Stimulation on In Vitro Embryo Culture
Recent studies have underscored the critical role of mechanical forces experienced by embryos within the oviduct and their implications for the optimization of in vitro embryo culture [160,161,162,163]. As aforementioned, in the dynamic environment of the oviduct, the embryo encounters a multitude of biomechanical cues and dynamic movements crucial for its transport and development. These include mechanical forces exerted by the oviduct wall, shear stress resulting from oviductal and subsequently intrauterine fluid pressure, as well as constriction from the peristaltic contractions of smooth muscle of the oviduct, and the metachronal ciliary wave of the oviductal cells resulting in a peristaltic-ciliary flow, which propel the embryo towards the uterus [92,104,164,165]. Understanding and replicating these biomechanical microenvironments in vitro have become areas of intense research focus, aiming to ultimately improve ART outcomes. Findings of the effects of advanced culture systems on embryo development in different species are summarized in Table 4.

Table 4.
Advanced culture systems designed to replicate the biomechanical microenvironment of the maternal reproductive tract. ROS, reactive oxygen species; TE, trophectoderm.
Detailed investigations, particularly in the mouse model [186], revealed that to accurately mimic physio-mechanical conditions, sequential and distinct mechanical (i.e., shear stress, compressive strains, pressure) impulses may be necessary. However, early attempts to introduce a dynamic setup with continuous media perfusion at relatively high flow rates (0.1–0.5 µL/h) to culture embryos within microchannels (250 µm deep and 1 mm wide) yielded impaired embryo development, characterized by decreased proportions of morulae and blastocysts, and high abnormal eight-cell embryos compared to static microdevices [166]. Although the precise mechanism underlying this negative effect remains unclear, it could depend on physico-chemical factors including high shear stress on the embryo and/or the continual removal of growth-promoting autocrine factors [123,187]. Previous studies have proved that mouse embryos cultured in oil-suspended microdrops into rotating wall vessels (1.2 dynes/cm2 shear stress) exhibited an elevated susceptibility to shear stress beyond physiological levels in E2.5 embryos (compacted eight-cell/early morula stage) compared to E3.5 embryos (early blastocyst stage) and an up-regulation of stress-signaling pathway constituents. Furthermore, an increased lethality of embryos deprived of zona pellucida was observed after unphysiological shear-stress induction, demonstrating that excessive shear stress impairs embryo development compared with static embryo culture [168]. In this context, defining the magnitude of shear stress acting on human embryos during tubal transport poses a significant challenge, given the variability in reported velocities (average velocities 0.1 µm/s and maximum velocities around 8.6 µm/s) and uncertainties in extrapolating findings from animal models or womens’ oviductal tissue samples [188,189,190,191]. Establishing a safe range and duration of shear stress demands the development of precise, reliable, and controllable engineering systems. For instance, a low shear stress value <1.2 dyn/cm2 is often considered safe [188]. Incorporating a gentle surrounding fluid flow into embryo culture systems is highly complex [192,193]. In this direction, Heo et al. (2010) contributed to the integration of oviductal contractions and ciliary currents developing a micro-funnel with a physiologically-based pulsatile movement. Such a dynamic system improved mouse embryo development in terms of blastocysts hatching (Microfunnel-pulsatile 71 vs. 23% Microfunnel-control and 31% Microdrop-control), mean cell number (Microfunnel-pulsatile 109 + 5 vs. 60 + 3 Microfunnel-control, and 67 + 3 Microdrop-control) and pregnancy rates [169]. Other researchers emulated the in vivo compressive strain and duration of peristaltic constrictions through a micro-modulated syringe pump connected to a membrane-based microfluidic system highlighting the importance of the selection of the correct stimulus amplitude (150 Pa) and duration (2 s) in long-term bovine embryo culture [161]. Other studies have investigated the positive impact of physical stimulation, such as micro-vibration, to simulate the human Fallopian tube metachronal ciliary wave ranging from 5–20 Hz in vivo [194] on embryo development. Mizobe et al. (2010) observed a positive impact of micro-vibration on blastocyst rates when applied during in vitro maturation of pig oocytes [170]. In humans [171], mice [172], and bovines [173], increased blastocyst rates and reduced blastocysts mean cell numbers were observed with 40 Hz and 80 Hz frequencies, respectively. A detrimental effect of micro-vibration (20–42 Hz for 5 s/60 min) was reported on mouse embryos when applied during the early stage of embryo development in comparison with the control static group (47% vs. 56%, respectively) [174]. A recent investigation has suggested that micro-vibration (45 Hz for 5 s/60 min) induces an increased cryotolerance of bovine embryos along with improvements in epigenetic and transcriptional status in blastocysts [175]. A paired randomized controlled trial demonstrated that dynamic culture of human embryos with micro-vibration (42 Hz frequency for 5 min/60 min) did not improve formation of transferrable blastocyst (58.3% vs. 57.1%), aneuploidy rates (20.0% vs. 33.3%), or the blastocyst development rate (67.1% vs. 63.1%) vs. static groups, contrasting previous findings [176]. In striving to replicate mechanical stimuli akin to ciliary beating that move the embryo during transport within the female reproductive tract, a tilting embryo culture system (TECS) has been implemented. This approach demonstrated that estimated shear forces of ~0.7 dyn/mm2 improve blastocyst rates (mouse, TECS vs. control: embryo density 5/50 µL, 59 vs. 46%; embryo density 10/500 µL, 42% vs. 27%), cell numbers (TECS vs. control, mouse: 77 ± 4 vs. 66 ± 4; human: 43 ± 3 vs. 34 ± 3) in both mouse and human embryos [162,177]. An additional study explored the impact of the combined mechanical stimulations including a tilting culture system and straight microchannels (200 µm) with physical constrictions (150 or 160 µm) on bovine early embryo development, demonstrating an enhanced embryo cleavage (constricted vs. straight channel: 56.7 ± 13.7 vs. 23.9 ± 11.0%) with no differences in the blastocyst rates [178]. In the attempt to recapitulate the movements of embryos in the oviduct, a digitalized microfluidic device powered by electrowetting on a dielectric (EWOD), at a relatively low energy, was used to manipulate microculture droplets, demonstrating an enhancement of blastocyst hatching (dynamic vs. static: 50 vs. 21.2%) and the ability to produce normal live births in the mouse model [179]. However, concerns persist regarding the potential impact of the electric field on preimplantation embryos. While some studies suggest that certain levels of electrical stimulation (1.36 kV/cm for 40–60 ms) can enable fertilization in human oocytes [195], others indicate an increase in reactive oxygen species levels, for example in porcine embryos, exposed to electric pulses >1 kV/cm for 30 ms [180]. Another aspect to consider when trying to emulate in vitro the in vivo environment is the compression exerted by the oviductal wall on the embryo. The isthmus lumen diameter is relatively small, with certain sections being comparable to the diameter of a human embryo. Additionally, the uterine epithelium exhibits softer mechanical properties (102–103 Pa) compared to the conventional polystyrene petri dish (1 GPa) with a difference of nearly six orders of magnitude [181,196,197]. Advanced culture systems based on substitutive substrates have been developed in this direction by using static and/or dynamic microfluidic approaches as an alternative to conventional polystyrene petri dishes. Materials with different stiffnesses including polyacrylamide gels [183], agarose gels [182,184], alginate gels [185], PDMS [198] and collagen gels [181] were tested for their suitability for embryo culture, highlighting the positive impact of a low elastic modulus of the culture surface on the early preimplantation embryo developmental rate and allowing the in vitro post-implantation development. Accordingly, studies reported that the 3D type I collagen gels (1 kPa stiffness) culture system showed higher rates of blastocyst (64 ± 9.1 vs. 50 ± 18%), and hatching blastocyst stages (54 ± 25 vs. 21 ± 16%), as well as increased trophectodermal cell numbers (TE, 65 ± 13 vs. 49 ± 12 cells), compared to those cultured in a conventional polystyrene dish [181]. Furthermore, volume of media and oil may also influence forces exerted upon the embryo during various movement schemes, and factors such as friction or impact against platform edges may also be important variables. The use of microfluidic innovative systems has been proposed for customizing in vitro experiments for the manipulation of small volumes in the order of nanoliters to culture single embryos, recapitulating fluid volumes in the oviduct [199]. Microfluidic approaches that reduce volumes compared to the standard microdrop techniques have been demonstrated to have similar behavior in terms of blastocyst attachment (33.22 ± 5.6 vs. 45.64 ± 8.4%) [199] or an enhanced embryo development [167]. Future studies introducing fluid flow to such systems in which autocrine and paracrine factors are concentrated in reduced volumes could lead to higher pregnancy and implantation rates in ART. All these considerations lead us to understand that an optimal embryo culture system should incorporate the capacity to mimic, at a microscale, wide-ranging consistencies, mechanical forces, and manipulation of minute volumes in the nanoliter range, all essential aspects for accurately recapitulating embryo development in vitro and gaining widespread embryo culture implementation [163,167].
9. Conclusions
Current embryo culture systems in ART fail to accurately replicate the biophysical factors and signaling cross-talk that embryos experience in vivo. The consequences of such discrepancies on the developmental competence of in vitro-produced embryos compared to their in vivo counterparts are challenging to discern in human ART. However, research in animal models, particularly in bovine species, more clearly demonstrates the lower developmental competence and higher genomic instability of IVP embryos compared to IVD embryos.
Three main factors of the in vivo environment are currently not replicated in in vitro embryo culture systems: (1) the biophysical conditions experienced by embryos in the maternal tract, such as oviductal fluid flow, metachronal ciliary waves, peristalsis, and the low elastic modulus of biological surfaces in contact with the embryo; (2) oviductal and endometrial soluble embryotropins or other molecular cargos enclosed in extracellular vesicles that have positive effects on embryo development; (3) confinement in sub-microliter volumes that enhances the concentration of embryo-secreted factors beneficial to the embryo.
In animal models, in vitro embryo development is generally improved under high embryo density conditions. However, in human ART, the relatively low number of embryos and the need to monitor the development of individual embryos preclude the possibility of in vitro culture at high embryo densities. Semi-confined culture in well-of-the-well devices has been widely adopted in human ART to allow the collection of morphokinetic data of individual embryos. Nevertheless, such culture systems do not permit the collection of individual embryo spent culture media for non-invasive analysis of embryo developmental competence and euploidy.
Although several research studies indicate that dynamic culture systems can improve embryo developmental competence, we are still far from faithfully recapitulating the high and poorly understood complexity of biophysical factors acting during in vivo embryo development. Nevertheless, the increasing interest of research groups in this field holds promise for establishing a more physiological, confined, and dynamic individual embryo culture system allowing the collection and analysis of non-invasive markers of embryo developmental competence.
Author Contributions
Conceptualization, R.G. and R.T.; writing—original draft, R.G., R.T., V.D.G. and A.C.; investigation, A.T., A.C. and V.D.G.; writing—review and editing, R.G., R.T., A.C., A.T., V.D.G., V.G. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are freely available from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sfontouris, I.A.; Martins, W.P.; Nastri, C.O.; Viana, I.G.R.; Navarro, P.A.; Raine-Fenning, N.; Van Der Poel, S.; Rienzi, L.; Racowsky, C. Blastocyst culture using single versus sequential media in clinical IVF: A systematic review and meta-analysis of randomized controlled trials. J. Assist. Reprod. Genet. 2016, 33, 1261–1272. [Google Scholar] [CrossRef]
- Vajta, G.; Parmegiani, L.; Machaty, Z.; Chen, W.B.; Yakovenko, S. Back to the future: Optimised microwell culture of individual human preimplantation stage embryos. J. Assist. Reprod. Genet. 2021, 38, 2563–2574. [Google Scholar] [CrossRef]
- Swain, J.E.; Cabrera, L.; Xu, X.; Smith, G.D. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod. BioMed. Online 2012, 24, 142–147. [Google Scholar] [CrossRef]
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2015, 22, 2–22. [Google Scholar] [CrossRef]
- Giacomini, E.; Makieva, S.; Murdica, V.; Vago, R.; Viganó, P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2020, 32, 179–184. [Google Scholar] [CrossRef]
- Leaver, M.; Wells, D. Non-invasive preimplantation genetic testing (niPGT): The next revolution in reproductive genetics? Hum. Reprod. Update 2020, 26, 16–42. [Google Scholar] [CrossRef]
- Zmuidinaite, R.; Sharara, F.I.; Iles, R.K. Current Advancements in Noninvasive Profiling of the Embryo Culture Media Secretome. Int. J. Mol. Sci. 2021, 22, 2513. [Google Scholar] [CrossRef]
- Salmerón, A.M.; Abreu, A.C.; Vilches-Ferrón, M.; Fernández, I. Solution NMR in human embryo culture media as an option for assessment of embryo implantation potential. NMR Biomed. 2021, 34, e4536. [Google Scholar] [CrossRef]
- Chard, T. 11 Frequency of implantation and early pregnancy loss in natural cycles. Baillière’s Clin. Obstet. Gynaecol. 1991, 5, 179–189. [Google Scholar] [CrossRef]
- Ezzati, M.; Djahanbakhch, O.; Arian, S.; Carr, B.R. Tubal transport of gametes and embryos: A review of physiology and pathophysiology. J. Assist. Reprod. Genet. 2014, 31, 1337–1347. [Google Scholar] [CrossRef]
- Weinberg, C.R.; Wilcox, A.J. A model for estimating the potency and survival of human gametes in vivo. Biometrics 1995, 51, 405–412. [Google Scholar] [CrossRef]
- Kennedy, T.G. Physiology of implantation. In Proceedings of the 10th World Congress on In Vitro Fertilization and Assisted Reproduction, Vancouver, BC, Canada, 24–28 May 1997; pp. 729–735. [Google Scholar]
- Jarvis, G.E. Estimating limits for natural human embryo mortality. F1000Research 2016, 5, 2083. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef]
- Zinaman, M.J.; Clegg, E.D.; Brown, C.C.; O’Connor, J.; Selevan, S.G. Estimates of human fertility and pregnancy loss. Fertil. Steril. 1996, 65, 503–509. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Wang, L.; Chen, D.; Guang, W.; French, J. Conception, early pregnancy loss, and time to clinical pregnancy: A population-based prospective study. Fertil. Steril. 2003, 79, 577–584. [Google Scholar] [CrossRef]
- Jarvis, G.E. Early embryo mortality in natural human reproduction: What the data say. F1000Research 2017, 5, 2765. [Google Scholar] [CrossRef]
- Macklon, N.S. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef]
- Lubinsky, M. Evolutionary justifications for human reproductive limitations. J. Assist. Reprod. Genet. 2018, 35, 2133–2139. [Google Scholar] [CrossRef]
- Inge, G.B.; Brinsden, P.R.; Elder, K.T. Oocyte number per live birth in IVF: Were Steptoe and Edwards less wasteful? Hum. Reprod. 2005, 20, 588–592. [Google Scholar] [CrossRef]
- Meniru, G.I.; Craft, I.L. Utilization of retrieved oocytes as an index of the efficiency of superovulation strategies for in-vitro fertilization treatment. Hum. Reprod. 1997, 12, 2129–2132. [Google Scholar] [CrossRef]
- Patrizio, P.; Bianchi, V.; Lalioti, M.D.; Gerasimova, T.; Sakkas, D. High rate of biological loss in assisted reproduction: It is in the seed, not in the soil. Reprod. BioMed. Online 2007, 14, 92–95. [Google Scholar] [CrossRef]
- Jones, H.W., Jr.; Oehninger, S.; Bocca, S.; Stadtmauer, L.; Mayer, J. Reproductive efficiency of human oocytes fertilized in vitro. Facts Views Vis. ObGyn 2010, 2, 169–171. [Google Scholar]
- Sirard, M.-A. 40 years of bovine IVF in the new genomic selection context. Reproduction 2018, 156, R1–R7. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, J.; Yang, J.; Liu, X.; Wang, J.; Wang, H.; Ding, X.; Liu, J.; Zhang, Q. Genome-wide detection of copy number variations using high-density SNP genotyping platforms in Holsteins. BMC Genom. 2013, 14, 131. [Google Scholar] [CrossRef]
- Ménézo, Y.J.R.; Hérubel, F. Mouse and bovine models for human IVF*. Reprod. BioMed. Online 2002, 4, 170–175. [Google Scholar] [CrossRef]
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2019, 20, 221–234. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Dong, H.; Luo, O.; Zheng, X.; Obergfell, C.; Tang, Y.; Bi, J.; O’Neill, R.; Ruan, Y.; et al. Transcriptional profiles of bovine in vivo pre-implantation development. BMC Genom. 2014, 15, 756. [Google Scholar] [CrossRef]
- Chen, Z.; Hagen, D.E.; Wang, J.; Elsik, C.G.; Ji, T.; Siqueira, L.G.; Hansen, P.J.; Rivera, R.M. Global assessment of imprinted gene expression in the bovine conceptus by next generation sequencing. Epigenetics 2016, 11, 501–516. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; Machugh, D.E.; Spillane, C.; Magee, D.A. Genomic imprinting effects on complex traits in domesticated animal species. Front. Genet. 2015, 6, 156. [Google Scholar] [CrossRef]
- Viana, J.H. 2021 statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newsl. 2022, 40, 22–40. [Google Scholar]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. BOARD INVITED REVIEW: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Pontes, J.H.F.; Nonato-Junior, I.; Sanches, B.V.; Ereno-Junior, J.C.; Uvo, S.; Barreiros, T.R.R.; Oliveira, J.A.; Hasler, J.F.; Seneda, M.M. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 2009, 71, 690–697. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; da Silva Rascado, T.; Magalhães, L.C.O.; Crocomo, L.F.; de Lima-Neto, J.F.; da Cruz Landim-Alvarenga, F. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef]
- Tarazona, A.; Rodríguez, J.; Restrepo, L.; Olivera-Angel, M. Mitochondrial Activity, Distribution and Segregation in Bovine Oocytes and in Embryos Produced in Vitro. Reprod. Domest. Anim. 2006, 41, 5–11. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Robinson, J.J.; Sinclair, K.D. Developmental consequences of embryo and cell manipulation in mice and farm animals. Reproduction 2001, 122, 507–518. [Google Scholar] [CrossRef]
- Barceló-Fimbres, M.; Seidel, G.E. Effects of either glucose or fructose and metabolic regulators on bovine embryo development and lipid accumulation in vitro. Mol. Reprod. Dev. 2007, 74, 1406–1418. [Google Scholar] [CrossRef]
- Gad, A.; Hoelker, M.; Besenfelder, U.; Havlicek, V.; Cinar, U.; Rings, F.; Held, E.; Dufort, I.; Sirard, M.A.; Schellander, K.; et al. Molecular mechanisms and pathways involved in bovine embryonic genome activation and their regulation by alternative in vivo and in vitro culture conditions. Biol. Reprod. 2012, 87, 100. [Google Scholar] [CrossRef]
- Lazzari, G.; Colleoni, S.; Lagutina, I.; Crotti, G.; Turini, P.; Tessaro, I.; Brunetti, D.; Duchi, R.; Galli, C. Short-term and long-term effects of embryo culture in the surrogate sheep oviduct versus in vitro culture for different domestic species. Theriogenology 2010, 73, 748–757. [Google Scholar] [CrossRef]
- Havlicek, V.; Kuzmany, A.; Cseh, S.; Brem, G.; Besenfelder, U. The Effect of Long-termIn VivoCulture in Bovine Oviduct and Uterus on the Development and Cryo-tolerance ofIn VitroProduced Bovine Embryos. Reprod. Domest. Anim. 2009, 45, 832–837. [Google Scholar] [CrossRef]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef]
- Fair, T.; Lonergan, P.; Dinnyes, A.; Cottell, D.C.; Hyttel, P.; Ward, F.A.; Boland, M.P. Ultrastructure of bovine blastocysts following cryopreservation: Effect of method of blastocyst production. Mol. Reprod. Dev. 2001, 58, 186–195. [Google Scholar] [CrossRef]
- Rizos, D.; Fair, T.; Papadopoulos, S.; Boland, M.P.; Lonergan, P. Developmental, qualitative, and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Mol. Reprod. Dev. 2002, 62, 320–327. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Plourde, D.; Vigneault, C.; Laflamme, I.; Blondin, P.; Robert, C. Cellular and molecular characterization of the impact of laboratory setup on bovine in vitro embryo production. Theriogenology 2012, 77, 1767–1778.e1. [Google Scholar] [CrossRef]
- Boni, R.; Tosti, E.; Roviello, S.; Dale, B. Intercellular communication in in vivo- and in vitro-produced bovine embryos. Biol. Reprod. 1999, 61, 1050–1055. [Google Scholar] [CrossRef][Green Version]
- Janati Idrissi, S.; Le Bourhis, D.; Lefevre, A.; Emond, P.; Le Berre, L.; Desnoës, O.; Joly, T.; Buff, S.; Maillard, V.; Schibler, L.; et al. Lipid profile of bovine grade-1 blastocysts produced either in vivo or in vitro before and after slow freezing process. Sci. Rep. 2021, 11, 11618. [Google Scholar] [CrossRef]
- De Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Gardner, D.K. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar] [CrossRef]
- Khurana, N.K.; Niemann, H. Energy Metabolism in Preimplantation Bovine Embryos Derived In Vitro or In Vivo. Biol. Reprod. 2000, 62, 847–856. [Google Scholar] [CrossRef]
- Driver, A.M.; Peñagaricano, F.; Huang, W.; Ahmad, K.R.; Hackbart, K.S.; Wiltbank, M.C.; Khatib, H. RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genom. 2012, 13, 118. [Google Scholar] [CrossRef]
- Knijn, H.M.; Wrenzycki, C.; Hendriksen, P.J.; Vos, P.L.; Zeinstra, E.C.; van der Weijden, G.C.; Niemann, H.; Dieleman, S.J. In vitro and in vivo culture effects on mRNA expression of genes involved in metabolism and apoptosis in bovine embryos. Reprod. Fertil. Dev. 2005, 17, 775–784. [Google Scholar] [CrossRef]
- Smith, S.L.; Everts, R.E.; Sung, L.Y.; Du, F.; Page, R.L.; Henderson, B.; Rodriguez-Zas, S.L.; Nedambale, T.L.; Renard, J.P.; Lewin, H.A.; et al. Gene expression profiling of single bovine embryos uncovers significant effects of in vitro maturation, fertilization and culture. Mol. Reprod. Dev. 2009, 76, 38–47. [Google Scholar] [CrossRef]
- Banliat, C.; Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Labas, V.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. The proteomic analysis of bovine embryos developed in vivo or in vitro reveals the contribution of the maternal environment to early embryo. BMC Genom. 2022, 23, 839. [Google Scholar] [CrossRef]
- Lonergan, P.; Pedersen, H.G.; Rizos, D.; Greve, T.; Thomsen, P.D.; Fair, T.; Evans, A.; Boland, M.P. Effect of the Post-Fertilization Culture Environment on the Incidence of Chromosome Aberrations in Bovine Blastocysts1. Biol. Reprod. 2004, 71, 1096–1100. [Google Scholar] [CrossRef]
- Tšuiko, O.; Catteeuw, M.; Zamani Esteki, M.; Destouni, A.; Bogado Pascottini, O.; Besenfelder, U.; Havlicek, V.; Smits, K.; Kurg, A.; Salumets, A.; et al. Genome stability of bovine in vivo-conceived cleavage-stage embryos is higher compared to in vitro-produced embryos. Hum. Reprod. 2017, 32, 2348–2357. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Saeed-Zidane, M.; Hoelker, M.; Gebremedhn, S.; Poirier, M.; Pandey, H.O.; Tholen, E.; Neuhoff, C.; Held, E.; Besenfelder, U.; et al. Genome-wide DNA methylation patterns of bovine blastocysts derived from in vivo embryos subjected to in vitro culture before, during or after embryonic genome activation. BMC Genom. 2018, 19, 424. [Google Scholar] [CrossRef]
- Holubcová, Z.; Blayney, M.; Elder, K.; Schuh, M. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 2015, 348, 1143–1147. [Google Scholar] [CrossRef]
- Patel, J.; Tan, S.L.; Hartshorne, G.M.; McAinsh, A.D. Unique geometry of sister kinetochores in human oocytes during meiosis I may explain maternal age-associated increases in chromosomal abnormalities. Biol. Open 2016, 5, 178–184. [Google Scholar] [CrossRef]
- McCoy, R.C. Mosaicism in Preimplantation Human Embryos: When Chromosomal Abnormalities Are the Norm. Trends Genet. 2017, 33, 448–463. [Google Scholar] [CrossRef]
- Starostik, M.R.; Sosina, O.A.; McCoy, R.C. Single-cell analysis of human embryos reveals diverse patterns of aneuploidy and mosaicism. Genome Res. 2020, 30, 814–825. [Google Scholar] [CrossRef]
- Vanneste, E.; Voet, T.; Le Caignec, C.; Ampe, M.; Konings, P.; Melotte, C.; Debrock, S.; Amyere, M.; Vikkula, M.; Schuit, F.; et al. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 2009, 15, 577–583. [Google Scholar] [CrossRef]
- Munné, S.; Alikani, M.; Ribustello, L.; Colls, P.; Martínez-Ortiz, P.A.; McCulloh, D.H. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum. Reprod. 2017, 32, 743–749. [Google Scholar] [CrossRef]
- Labarta, E.; Bosch, E.; Alamá, P.; Rubio, C.; Rodrigo, L.; Pellicer, A. Moderate Ovarian Stimulation Does Not Increase the Incidence of Human Embryo Chromosomal Abnormalities inin VitroFertilization Cycles. J. Clin. Endocrinol. Metab. 2012, 97, E1987–E1994. [Google Scholar] [CrossRef]
- Bosch, E.; Labarta, E.; Kolibianakis, E.; Rosen, M.; Meldrum, D. Regimen of ovarian stimulation affects oocyte and therefore embryo quality. Fertil. Steril. 2016, 105, 560–570. [Google Scholar] [CrossRef]
- Hong, K.H.; Franasiak, J.M.; Werner, M.M.; Patounakis, G.; Juneau, C.R.; Forman, E.J.; Scott, R.T. Embryonic aneuploidy rates are equivalent in natural cycles and gonadotropin-stimulated cycles. Fertil. Steril. 2019, 112, 670–676. [Google Scholar] [CrossRef]
- Irani, M.; Canon, C.; Robles, A.; Maddy, B.; Gunnala, V.; Qin, X.; Zhang, C.; Xu, K.; Rosenwaks, Z. No effect of ovarian stimulation and oocyte yield on euploidy and live birth rates: An analysis of 12,298 trophectoderm biopsies. Hum. Reprod. 2020, 35, 1082–1089. [Google Scholar] [CrossRef]
- Abdala, A.; Elkhatib, I.; Bayram, A.; Arnanz, A.; El-Damen, A.; Lawrenz, B.; Fatemi, H.M.; De Munck, N. Impact of slight variations of extracellular culture media ph (phe) on embryo quality, euploidy rates and mitochondrial dna (mtDNA). Fertil. Steril. 2020, 114, E418. [Google Scholar] [CrossRef]
- Abdala, A.; Elkhatib, I.; Bayram, A.; Arnanz, A.; El-Damen, A.; Melado, L.; Lawrenz, B.; Garrido, N.; Fatemi, H.M.; De Munck, N. Different CO2 settings (6.0% vs. 7.0%) do have an impact on extracellular pH of culture medium (pHe) and euploidy rates rather than on blastocyst development: A sibling oocyte study. J. Assist. Reprod. Genet. 2021, 38, 2915–2923. [Google Scholar] [CrossRef]
- Quinn, M.M.; Marsh, P.; Ribeiro, S.; Simbulan, R.K.; Hickman, C.; Berntsen, J.; Rosen, M.P. Aneuploidy rates and morphokinetic parameters of embryos cultured in distinct culture media: A sibling oocyte study. Hum. Reprod. 2022, 37, 226–234. [Google Scholar] [CrossRef]
- Swain, J.E. Can Culture Media Impact Preimplantation Embryo Aneuploidy? Fertil. Reprod. 2021, 03, 120–124. [Google Scholar] [CrossRef]
- Capalbo, A.; Poli, M.; Rienzi, L.; Girardi, L.; Patassini, C.; Fabiani, M.; Cimadomo, D.; Benini, F.; Farcomeni, A.; Cuzzi, J.; et al. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am. J. Hum. Genet. 2021, 108, 2238–2247. [Google Scholar] [CrossRef]
- Greco, E.; Yakovlev, P.; Kornilov, N.; Vyatkina, S.; Bogdanova, D.; Ermakova, M.; Tarasova, Y.; Tikhonov, A.; Pendina, A.; Biricik, A.; et al. Two clinical case reports of embryonic mosaicism identified with PGT-A persisting during pregnancy as true fetal mosaicism. Hum. Reprod. 2023, 38, 315–323. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Lee, C.-I.; Cheng, E.-H.; Huang, C.-C.; Lee, T.-H.; Shih, H.-H.; Pai, Y.-P.; Chen, Y.-C.; Lee, M.-S. Clinical Outcomes of Single Mosaic Embryo Transfer: High-Level or Low-Level Mosaic Embryo, Does It Matter? J. Clin. Med. 2020, 9, 1695. [Google Scholar] [CrossRef]
- Munné, S.; Blazek, J.; Large, M.; Martinez-Ortiz, P.A.; Nisson, H.; Liu, E.; Tarozzi, N.; Borini, A.; Becker, A.; Zhang, J.; et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil. Steril. 2017, 108, 62–71.e68. [Google Scholar] [CrossRef]
- Viotti, M.; Greco, E.; Grifo, J.A.; Madjunkov, M.; Librach, C.; Cetinkaya, M.; Kahraman, S.; Yakovlev, P.; Kornilov, N.; Corti, L.; et al. Chromosomal, gestational, and neonatal outcomes of embryos classified as a mosaic by preimplantation genetic testing for aneuploidy. Fertil. Steril. 2023, 120, 957–966. [Google Scholar] [CrossRef]
- Viotti, M.; McCoy, R.C.; Griffin, D.K.; Spinella, F.; Greco, E.; Madjunkov, M.; Madjunkova, S.; Librach, C.L.; Victor, A.R.; Barnes, F.L.; et al. Let the data do the talking: The need to consider mosaicism during embryo selection. Fertil. Steril. 2021, 116, 1212–1219. [Google Scholar] [CrossRef]
- Marin, D.; Xu, J.; Treff, N.R. Preimplantation genetic testing for aneuploidy: A review of published blastocyst reanalysis concordance data. Prenat. Diagn. 2021, 41, 545–553. [Google Scholar] [CrossRef]
- Treff, N.R.; Marin, D. The “mosaic” embryo: Misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil. Steril. 2021, 116, 1205–1211. [Google Scholar] [CrossRef]
- Coticchio, G.; Barrie, A.; Lagalla, C.; Borini, A.; Fishel, S.; Griffin, D.; Campbell, A. Plasticity of the human preimplantation embryo: Developmental dogmas, variations on themes and self-correction. Hum. Reprod. Update 2021, 27, 848–865. [Google Scholar] [CrossRef]
- Popovic, M.; Dhaenens, L.; Boel, A.; Menten, B.; Heindryckx, B. Chromosomal mosaicism in human blastocysts: The ultimate diagnostic dilemma. Hum. Reprod. Update 2020, 26, 313–334. [Google Scholar] [CrossRef]
- Cimadomo, D.; Rienzi, L.; Conforti, A.; Forman, E.; Canosa, S.; Innocenti, F.; Poli, M.; Hynes, J.; Gemmell, L.; Vaiarelli, A.; et al. Opening the black box: Why do euploid blastocysts fail to implant? A systematic review and meta-analysis. Hum. Reprod. Update 2023, 29, 570–633. [Google Scholar] [CrossRef]
- Irani, M.; Reichman, D.; Robles, A.; Melnick, A.; Davis, O.; Zaninovic, N.; Xu, K.; Rosenwaks, Z. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil. Steril. 2017, 107, 664–670. [Google Scholar] [CrossRef]
- Irani, M.; O’Neill, C.; Palermo, G.D.; Xu, K.; Zhang, C.; Qin, X.; Zhan, Q.; Clarke, R.N.; Ye, Z.; Zaninovic, N.; et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil. Steril. 2018, 110, 95–102.e1. [Google Scholar] [CrossRef]
- Coppola, G.; Alexander, B.; Di Berardino, D.; St John, E.; Basrur, P.K.; King, W.A. Use of cross-species in-situ hybridization (ZOO-FISH) to assess chromosome abnormalities in day-6 in-vivo- or in-vitro-produced sheep embryos. Chromosome Res. 2007, 15, 399–408. [Google Scholar] [CrossRef][Green Version]
- Hornak, M.; Jeseta, M.; Hanulakova, S.; Rubes, J. A high incidence of chromosome abnormalities in two-cell stage porcine IVP embryos. J. Appl. Genet. 2015, 56, 515–523. [Google Scholar] [CrossRef]
- Rambags, B.P.B.; Krijtenburg, P.J.; Drie, H.F.V.; Lazzari, G.; Galli, C.; Pearson, P.L.; Colenbrander, B.; Stout, T.A.E. Numerical chromosomal abnormalities in equine embryos produced in vivo and in vitro. Mol. Reprod. Dev. 2005, 72, 77–87. [Google Scholar] [CrossRef]
- Jochems, R.; Canedo-Ribeiro, C.; Silvestri, G.; Derks, M.F.L.; Hamland, H.; Zak, L.J.; Knol, E.F.; Handyside, A.H.; Grindflek, E.; Griffin, D.K. Preimplantation Genetic Testing for Aneuploidy (PGT-A) Reveals High Levels of Chromosomal Errors in In Vivo-Derived Pig Embryos, with an Increased Incidence When Produced In Vitro. Cells 2023, 12, 790. [Google Scholar] [CrossRef]
- Ducheyne, K.D.; Rizzo, M.; Cuervo-Arango, J.; Claes, A.; Daels, P.F.; Stout, T.A.E.; de Ruijter-Villani, M. In vitro production of horse embryos predisposes to micronucleus formation, whereas time to blastocyst formation affects likelihood of pregnancy. Reprod. Fertil. Dev. 2019, 31, 1830–1839. [Google Scholar] [CrossRef]
- Kölle, S.; Hughes, B.; Steele, H. Early embryo-maternal communication in the oviduct: A review. Mol. Reprod. Dev. 2020, 87, 650–662. [Google Scholar] [CrossRef]
- Croxatto, H.B. Physiology of gamete and embryo transport through the fallopian tube. Reprod. BioMed. Online 2002, 4, 160–169. [Google Scholar] [CrossRef]
- Gamete Transport. Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Maillo, V.; Sanchez-Calabuig, M.J.; Lopera-Vasquez, R.; Hamdi, M.; Gutierrez-Adan, A.; Lonergan, P.; Rizos, D. Oviductal response to gametes and early embryos in mammals. Reproduction 2016, 152, R127–R141. [Google Scholar] [CrossRef]
- Hunter, R.H.F. Components of oviduct physiology in eutherian mammals. Biol. Rev. 2012, 87, 244–255. [Google Scholar] [CrossRef]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of embryo transport through the mare oviduct. Theriogenology 1991, 36, 823–830. [Google Scholar] [CrossRef]
- Potts, D.M.; Wilson, I.B. The preimplantation conceptus of the mouse at 90 h post coitum. J. Anat. 1967, 102, 1–11. [Google Scholar]
- Moore, E.L.; Wang, S.; Larina, I.V. Staging mouse preimplantation development in vivo using optical coherence microscopy. J. Biophotonics 2019, 12, e201800364. [Google Scholar] [CrossRef]
- Wang, S.; Larina, I. Optical Imaging of the Mammalian Oviduct In Vivo. In Imaging from Cells to Animals In Vivo; CRC Press: Boca Raton, FL, USA, 2020; pp. 267–278. [Google Scholar]
- Besenfelder, U.; Havlicek, V.; Brem, G. Role of the Oviduct in Early Embryo Development. Reprod. Domest. Anim. 2012, 47, 156–163. [Google Scholar] [CrossRef]
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the oviduct in mammalian fertilization. Reproduction 2012, 144, 649–660. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Halbert, S.A.; Tam, P.Y.; Blandau, R.J. Egg Transport in the Rabbit Oviduct: The Roles of Cilia and Muscle. Science 1976, 191, 1052–1053. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef]
- Shi, D.; Komatsu, K.; Uemura, T.; Fujimori, T. Analysis of ciliary beat frequency and ovum transport ability in the mouse oviduct. Genes Cells 2011, 16, 282–290. [Google Scholar] [CrossRef]
- Dixon, R.E.; Hwang, S.J.; Hennig, G.W.; Ramsey, K.H.; Schripsema, J.H.; Sanders, K.M.; Ward, S.M. Chlamydia Infection Causes Loss of Pacemaker Cells and Inhibits Oocyte Transport in the Mouse Oviduct1. Biol. Reprod. 2009, 80, 665–673. [Google Scholar] [CrossRef]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters, “miR-34b/c” and “miR-449”, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, G.; Shornick, L.P.; Roswit, W.T.; Shipley, J.M.; Brody, S.L.; Holtzman, M.J. A Transgenic FOXJ1-Cre System for Gene Inactivation in Ciliated Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2007, 36, 515–519. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; Yan, W. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef]
- Gandolfi, F.; Moor, R.M. Stimulation of early embryonic development in the sheep by co-culture with oviduct epithelial cells. Reproduction 1987, 81, 23–28. [Google Scholar] [CrossRef]
- Eyestone, W.H.; First, N.L. Co-culture of early cattle embryos to the blastocyst stage with oviducal tissue or in conditioned medium. Reproduction 1989, 85, 715–720. [Google Scholar] [CrossRef]
- White, K.L.; Hehnke, K.; Rickords, L.F.; Southern, L.L.; Thompson, D.L., Jr.; Wood, T.C. Early embryonic development in vitro by coculture with oviductal epithelial cells in pigs. Biol. Reprod. 1989, 41, 425–430. [Google Scholar] [CrossRef]
- Tucker, M.J.; Ingargiola, P.E.; Massey, J.B.; Morton, P.C.; Wiemer, K.E.; Wiker, S.R.; Wright, G. Assisted hatching with or without bovine oviductal epithelial cell co-culture for poor prognosis in-vitro fertilization patients. Hum. Reprod. 1994, 9, 1528–1531. [Google Scholar] [CrossRef]
- Wiemer, K.E.; Cohen, J.; Amborski, G.F.; Wright, G.; Wiker, S.; Munyakazi, L.; Godke, R.A. In-vitro development and implantation of human embryos following culture on fetal bovine uterine fibroblast cells. Hum. Reprod. 1989, 4, 595–600. [Google Scholar] [CrossRef]
- Mansour, R.T.; Aboulghar, M.A.; Serour, G.I.; Abbass, A.M. Co-culture of human pronucleate oocytes with their cumulus cells. Hum. Reprod. 1994, 9, 1727–1729. [Google Scholar] [CrossRef]
- Menezo, Y.J.; Guerin, J.F.; Czyba, J.C. Improvement of human early embryo development in vitro by coculture on monolayers of Vero cells. Biol. Reprod. 1990, 42, 301–306. [Google Scholar] [CrossRef]
- Joo, B.S.; Kim, M.K.; Na, Y.J.; Moon, H.S.; Lee, K.S.; Kim, H.D. The mechanism of action of coculture on embryo development in the mouse model: Direct embryo-to-cell contact and the removal of deleterious components. Fertil. Steril. 2001, 75, 193–199. [Google Scholar] [CrossRef]
- Menezo, Y.J.; Servy, E.; Veiga, A.; Hazout, A.; Elder, K. Culture systems: Embryo co-culture. Methods Mol. Biol. 2012, 912, 231–247. [Google Scholar] [CrossRef]
- Melnick, A.P.; Spandorfer, S.D. Embryo coculture: A review. J. Epidemiol. Res. 2015, 2, 15. [Google Scholar] [CrossRef]
- Sciorio, R.; Smith, G.D. Embryo culture at a reduced oxygen concentration of 5%: A mini review. Zygote 2019, 27, 355–361. [Google Scholar] [CrossRef]
- Wydooghe, E.; Vandaele, L.; Heras, S.; De Sutter, P.; Deforce, D.; Peelman, L.; De Schauwer, C.; Van Soom, A. Autocrine embryotropins revisited: How do embryos communicate with each other in vitro when cultured in groups? Biol. Rev. Camb. Philos. Soc. 2017, 92, 505–520. [Google Scholar] [CrossRef]
- Paria, B.C.; Dey, S.K. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth factors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–4760. [Google Scholar] [CrossRef]
- Ealy, A.D.; Speckhart, S.L.; Wooldridge, L.K. Cytokines That Serve as Embryokines in Cattle. Animals 2021, 11, 2313. [Google Scholar] [CrossRef]
- Lonergan, P. Autocrine Embryotropins in Embryo Development: Controlling One’s Own Fate. Biol. Reprod. 2011, 84, 1075–1076. [Google Scholar] [CrossRef]
- Donnay, I.; Van Langendonckt, A.; Auquier, P.; Grisart, B.; Vansteenbrugge, A.; Massip, A.; Dessy, F. Effects of co-culture and embryo number on the in vitro development of bovine embryos. Theriogenology 1997, 47, 1549–1561. [Google Scholar] [CrossRef]
- Stokes, P.J.; Abeydeera, L.R.; Leese, H.J. Development of porcine embryos in vivo and in vitro; evidence for embryo ‘cross talk’ in vitro. Dev. Biol. 2005, 284, 62–71. [Google Scholar] [CrossRef]
- Spindler, R.E.; Wildt, D.E. Quality and Age of Companion Felid Embryos Modulate Enhanced Development by Group Culture1. Biol. Reprod. 2002, 66, 167–173. [Google Scholar] [CrossRef][Green Version]
- Ebner, T.; Shebl, O.; Moser, M.; Mayer, R.B.; Arzt, W.; Tews, G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod. BioMed. Online 2010, 21, 762–768. [Google Scholar] [CrossRef]
- Leidenfrost, S.; Boelhauve, M.; Reichenbach, M.; Güngör, T.; Reichenbach, H.-D.; Sinowatz, F.; Wolf, E.; Habermann, F.A. Cell Arrest and Cell Death in Mammalian Preimplantation Development: Lessons from the Bovine Model. PLoS ONE 2011, 6, e22121. [Google Scholar] [CrossRef]
- Raes, A.; Wydooghe, E.; Pavani, K.C.; Bogado Pascottini, O.; Van Steendam, K.; Dhaenens, M.; Boel, A.; Heras, S.; Heindryckx, B.; Peelman, L.; et al. Cathepsin-L Secreted by High-Quality Bovine Embryos Exerts an Embryotrophic Effect In Vitro. Int. J. Mol. Sci. 2023, 24, 6563. [Google Scholar] [CrossRef]
- O’Neill, C.; Li, Y.; Jin, X.L. Survival Signalling in the Preimplantation Embryo; Springer: New York, NY, USA, 2015; pp. 129–149. [Google Scholar]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial Exosomes/Microvesicles in the Uterine Microenvironment: A New Paradigm for Embryo-Endometrial Cross Talk at Implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Machtinger, R.; Baccarelli, A.A.; Wu, H. Extracellular vesicles and female reproduction. J. Assist. Reprod. Genet. 2021, 38, 549–557. [Google Scholar] [CrossRef]
- Fan, W.; Qi, Y.; Wang, Y.; Yan, H.; Li, X.; Zhang, Y. Messenger roles of extracellular vesicles during fertilization of gametes, development and implantation: Recent advances. Front. Cell Dev. Biol. 2023, 10, 1079387. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Guo, N.; Cai, L.; Wang, M.; Zhu, L.; Li, F.; Jin, L.; Sui, C. Extracellular vesicles from human Fallopian tubal fluid benefit embryo development in vitro. Hum. Reprod. Open 2023, 2023, hoad006. [Google Scholar] [CrossRef]
- Segura-Benítez, M.; Bas-Rivas, A.; Juárez-Barber, E.; Carbajo-García, M.C.; Faus, A.; De Los Santos, M.J.; Pellicer, A.; Ferrero, H. Human blastocysts uptake extracellular vesicles secreted by endometrial cells containing miRNAs related to implantation. Hum. Reprod. 2023, 38, 1547–1559. [Google Scholar] [CrossRef]
- Leal, C.L.V.; Cañón-Beltrán, K.; Cajas, Y.N.; Hamdi, M.; Yaryes, A.; Millán De La Blanca, M.G.; Beltrán-Breña, P.; Mazzarella, R.; Da Silveira, J.C.; Gutiérrez-Adán, A.; et al. Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J. Anim. Sci. Biotechnol. 2022, 13, 116. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.Á.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef]
- Pavani, K.; Hendrix, A.; Van Den Broeck, W.; Couck, L.; Szymanska, K.; Lin, X.; De Koster, J.; Van Soom, A.; Leemans, B. Isolation and Characterization of Functionally Active Extracellular Vesicles from Culture Medium Conditioned by Bovine Embryos In Vitro. Int. J. Mol. Sci. 2018, 20, 38. [Google Scholar] [CrossRef]
- Goovaerts, I.G.; Leroy, J.L.; Jorssen, E.P.; Bols, P.E. Noninvasive bovine oocyte quality assessment: Possibilities of a single oocyte culture. Theriogenology 2010, 74, 1509–1520. [Google Scholar] [CrossRef]
- Pavani, K.C.; Meese, T.; Pascottini, O.B.; Guan, X.; Lin, X.; Peelman, L.; Hamacher, J.; Van Nieuwerburgh, F.; Deforce, D.; Boel, A.; et al. Hatching is modulated by microRNA-378a-3p derived from extracellular vesicles secreted by blastocysts. Proc. Natl. Acad. Sci. USA 2022, 119, e2122708119. [Google Scholar] [CrossRef]
- Hawke, D.C.; Watson, A.J.; Betts, D.H. Extracellular vesicles, microRNA and the preimplantation embryo: Non-invasive clues of embryo well-being. Reprod. BioMed. Online 2021, 42, 39–54. [Google Scholar] [CrossRef]
- Kelley, R.L.; Gardner, D.K. In vitro culture of individual mouse preimplantation embryos: The role of embryo density, microwells, oxygen, timing and conditioned media. Reprod. BioMed. Online 2017, 34, 441–454. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum. Reprod. 1992, 7, 558–562. [Google Scholar] [CrossRef]
- Thouas, G.; Jones, G.; Trounson, A. The ‘GO’ system--a novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction 2003, 126, 161–169. [Google Scholar] [CrossRef]
- Travaglione, A.; Candela, A.; De Gregorio, V.; Genovese, V.; Cimmino, M.; Barbato, V.; Talevi, R.; Gualtieri, R. Individually Cultured Bovine Zygotes Successfully Develop to the Blastocyst Stage in an Extremely Confined Environment. Cells 2024, 13, 868. [Google Scholar] [CrossRef]
- Gopichandran, N.; Leese, H.J. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006, 131, 269–277. [Google Scholar] [CrossRef]
- Hoelker, M.; Rings, F.; Lund, Q.; Ghanem, N.; Phatsara, C.; Griese, J.; Schellander, K.; Tesfaye, D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction 2009, 137, 415–425. [Google Scholar] [CrossRef]
- Miller, K.F.; Pursel, V.G. Absorption of compounds in medium by the oil covering microdrop cultures. Gamete Res. 1987, 17, 57–61. [Google Scholar] [CrossRef]
- Logsdon, D.M.; Churchwell, A.; Schoolcraft, W.B.; Krisher, R.L.; Yuan, Y. Estrogen signaling encourages blastocyst development and implantation potential. J. Assist. Reprod. Genet. 2023, 40, 1003–1014. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y.; Chiba, K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil. Steril. 2007, 88, 741–743. [Google Scholar] [CrossRef]
- Otsuki, J.; Nagai, Y.; Chiba, K. Damage of embryo development caused by peroxidized mineral oil and its association with albumin in culture. Fertil. Steril. 2009, 91, 1745–1749. [Google Scholar] [CrossRef]
- Erbach, G.T.; Bhatnagar, P.; Baltz, J.M.; Biggers, J.D. Zinc is a possible toxic contaminant of silicone oil in microdrop cultures of preimplantation mouse embryos. Hum. Reprod. 1995, 10, 3248–3254. [Google Scholar] [CrossRef]
- Martinez, C.A.; Nohalez, A.; Parrilla, I.; Motas, M.; Roca, J.; Romero, I.; García-GonzáLez, D.L.; Cuello, C.; Rodriguez-Martinez, H.; Martinez, E.A.; et al. The overlaying oil type influences in vitro embryo production: Differences in composition and compound transfer into incubation medium between oils. Sci. Rep. 2017, 7, 10505. [Google Scholar] [CrossRef]
- Morbeck, D.E.; Khan, Z.; Barnidge, D.R.; Walker, D.L. Washing mineral oil reduces contaminants and embryotoxicity. Fertil. Steril. 2010, 94, 2747–2752. [Google Scholar] [CrossRef]
- Vajta, G.; Peura, T.T.; Holm, P.; Páldi, A.; Greve, T.; Trounson, A.O.; Callesen, H. New method for culture of zona-included or zona-free embryos: The Well of the Well (WOW) system. Mol. Reprod. Dev. 2000, 55, 256–264. [Google Scholar] [CrossRef]
- Matsuzaki, S. Mechanobiology of the female reproductive system. Reprod. Med. Biol. 2021, 20, 371–401. [Google Scholar] [CrossRef]
- Bae, C.Y.; Kim, M.S.; Park, J.-K. Mechanical stimulation of bovine embryos in a microfluidic culture platform. BioChip J. 2011, 5, 106–113. [Google Scholar] [CrossRef]
- Matsuura, K.; Hayashi, N.; Kuroda, Y.; Takiue, C.; Hirata, R.; Takenami, M.; Aoi, Y.; Yoshioka, N.; Habara, T.; Mukaida, T.; et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod. BioMed. Online 2010, 20, 358–364. [Google Scholar] [CrossRef]
- Esteves, T.C.; Van Rossem, F.; Nordhoff, V.; Schlatt, S.; Boiani, M.; Le Gac, S. A microfluidic system supports single mouse embryo culture leading to full-term development. RSC Adv. 2013, 3, 26451. [Google Scholar] [CrossRef]
- Ashraf, H.; Siddiqui, A.M.; Rana, M.A. Analysis of the peristaltic-ciliary flow of Johnson-Segalman fluid induced by peristalsis-cilia of the human fallopian tube. Math. Biosci. 2018, 300, 64–75. [Google Scholar] [CrossRef]
- Ashraf, H.; Siddiqui, A.M.; Rana, M.A. Fallopian tube assessment of the peristaltic-ciliary flow of a linearly viscous fluid in a finite narrow tube. Appl. Math. Mech. 2018, 39, 437–454. [Google Scholar] [CrossRef]
- Hickman, D.L.; Beebe, D.J.; Rodriguez-Zas, S.L.; Wheeler, M.B. Comparison of static and dynamic medium environments for culturing of pre-implantation mouse embryos. Comp. Med. 2002, 52, 122–126. [Google Scholar]
- Raty, S.; Walters, E.M.; Davis, J.; Zeringue, H.; Beebe, D.J.; Rodriguez-Zas, S.L.; Wheeler, M.B. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip 2004, 4, 186–190. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, F.; Zhong, W.; Puscheck, E.; Shen, H.; Rappolee, D.A. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol. Reprod. 2006, 75, 45–55. [Google Scholar] [CrossRef]
- Heo, Y.S.; Cabrera, L.M.; Bormann, C.L.; Shah, C.T.; Takayama, S.; Smith, G.D. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum. Reprod. 2010, 25, 613–622. [Google Scholar] [CrossRef]
- Mizobe, Y.; Yoshida, M.; Miyoshi, K. Enhancement of Cytoplasmic Maturation of In Vitro-Matured Pig Oocytes by Mechanical Vibration. J. Reprod. Dev. 2010, 56, 285–290. [Google Scholar] [CrossRef]
- Isachenko, V.; Maettner, R.; Sterzik, K.; Strehler, E.; Kreinberg, R.; Hancke, K.; Roth, S.; Isachenko, E. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod. BioMed. Online 2011, 22, 536–544. [Google Scholar] [CrossRef]
- Hur, Y.S.; Park, J.H.; Ryu, E.K.; Park, S.J.; Lee, J.H.; Lee, S.H.; Yoon, J.; Yoon, S.H.; Hur, C.Y.; Lee, W.D.; et al. Effect of micro-vibration culture system on embryo development. J. Assist. Reprod. Genet. 2013, 30, 835–841. [Google Scholar] [CrossRef]
- Takahashi, M.; Honda, T.; Hatoya, S.; Inaba, T.; Kawate, N.; Tamada, H. Efficacy of mechanical micro-vibration in the development of bovine embryos during in vitro maturation and culture. J. Vet. Med. Sci. 2018, 80, 532–535. [Google Scholar] [CrossRef]
- Asano, Y.; Matsuura, K. Mouse embryo motion and embryonic development from the 2-cell to blastocyst stage using mechanical vibration systems. Reprod. Fertil. Dev. 2014, 26, 733–741. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Joaquim, D.C.; Nociti, R.P.; Macabelli, C.H.; Sampaio, R.V.; Oliveira, A.S.; Pita, M.O.; de Oliveira, R.A.M.; da Silveira, J.C.; Meirelles, F.V.; et al. Micro-vibration results in vitro-derived bovine blastocysts with greater cryotolerance, epigenetic abnormalities, and a massive transcriptional change. Theriogenology 2023, 196, 214–226. [Google Scholar] [CrossRef]
- Juneau, C.R.; Tiegs, A.W.; Franasiak, J.M.; Goodman, L.R.; Whitehead, C.; Patounakis, G.; Scott, R.T., Jr. Embryo’s Natural Motion (enMotion): A paired randomized controlled trial evaluating a dynamic embryo culture system. Fertil. Steril. 2020, 113, 578–586.e571. [Google Scholar] [CrossRef]
- Hara, T.; Matsuura, K.; Kodama, T.; Sato, K.; Kikkawa, Y.; Muneto, T.; Tanaka, J.; Naruse, K. A tilting embryo culture system increases the number of high-grade human blastocysts with high implantation competence. Reprod. BioMed. Online 2013, 26, 260–268. [Google Scholar] [CrossRef]
- Kim, M.S.; Bae, C.Y.; Wee, G.; Han, Y.M.; Park, J.K. A microfluidic in vitro cultivation system for mechanical stimulation of bovine embryos. Electrophoresis 2009, 30, 3276–3282. [Google Scholar] [CrossRef]
- Huang, H.Y.; Shen, H.H.; Tien, C.H.; Li, C.J.; Fan, S.K.; Liu, C.H.; Hsu, W.S.; Yao, D.J. Digital Microfluidic Dynamic Culture of Mammalian Embryos on an Electrowetting on Dielectric (EWOD) Chip. PLoS ONE 2015, 10, e0124196. [Google Scholar] [CrossRef]
- Koo, O.J.; Jang, G.; Kwon, D.K.; Kang, J.T.; Kwon, O.S.; Park, H.J.; Kang, S.K.; Lee, B.C. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology 2008, 70, 1111–1118. [Google Scholar] [CrossRef]
- Kolahi, K.S.; Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.K.; Bloise, E.; Maltepe, E.; Rinaudo, P. Effect of Substrate Stiffness on Early Mouse Embryo Development. PLoS ONE 2012, 7, e41717. [Google Scholar] [CrossRef]
- Peura, T.T.; Vajta, G. A comparison of established and new approaches in ovine and bovine nuclear transfer. Cloning Stem Cells 2003, 5, 257–277. [Google Scholar] [CrossRef]
- Morris, S.A.; Grewal, S.; Barrios, F.; Patankar, S.N.; Strauss, B.; Buttery, L.; Alexander, M.; Shakesheff, K.M.; Zernicka-Goetz, M. Dynamics of anterior–posterior axis formation in the developing mouse embryo. Nat. Commun. 2012, 3, 673. [Google Scholar] [CrossRef]
- BrandãO, D.O.; Maddox-Hyttel, P.; Løvendahl, P.; Rumpf, R.; Stringfellow, D.; Callesen, H. Post Hatching Development: A Novel System for Extended in Vitro Culture of Bovine Embryos. Biol. Reprod. 2004, 71, 2048–2055. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.X.; Gao, H.; Wu, Y.; Fang, Y.; Wu, S.S.; Li, M.J.; Bai, J.H.; Liu, Y.; Evans, A.; et al. A three-dimensional culture system using alginate hydrogel prolongs hatched cattle embryo development in vitro. Theriogenology 2015, 84, 184–192. [Google Scholar] [CrossRef]
- Wang, S.; Larina, I.V. Dynamics of gametes and embryos in the oviduct: What can in vivo imaging reveal? Reproduction 2023, 165, R25–R37. [Google Scholar] [CrossRef]
- Walker, G.M.; Zeringue, H.C.; Beebe, D.J. Microenvironment design considerations for cellular scale studies. Lab Chip 2004, 4, 91–97. [Google Scholar] [CrossRef]
- Hawkins, J.; Miao, X.; Cui, W.; Sun, Y. Biophysical optimization of preimplantation embryo culture: What mechanics can offer ART. Mol. Hum. Reprod. 2021, 27, gaaa087. [Google Scholar] [CrossRef]
- Greenwald, G.S. A study of the transport of ova through the rabbit oviduct. Fertil. Steril. 1961, 12, 80–95. [Google Scholar] [CrossRef]
- Anand, S.; Guha, S.K. Mechanics of transport of the ovum in the oviduct. Med. Biol. Eng. Comput. 1978, 16, 256–261. [Google Scholar] [CrossRef]
- Halbert, S.A.; Becker, D.R.; Szal, S.E. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol. Reprod. 1989, 40, 1131–1136. [Google Scholar] [CrossRef]
- Bavister, B.D. Culture of preimplantation embryos: Facts and artifacts. Hum. Reprod. Update 1995, 1, 91–148. [Google Scholar] [CrossRef]
- Wheeler, M.B.; Beebe, D.J.; Walters, E.M.; Raty, S. Microfluidic technology for in vitro embryo production. In Proceedings of the 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology. Proceedings (Cat. No.02EX578), Madison, WI, USA, 2–4 May 2002; pp. 104–108. [Google Scholar] [CrossRef]
- Weström, L.; Mårdh, P.A.; Mecklenburg, C.V.; Håkansson, C.H. Studies on ciliated epithelia of the human genital tract. II. The mucociliary wave pattern of fallopian tube epithelium. Fertil. Steril. 1977, 28, 955–961. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.W.; Blaszcyzk, A.; Grifo, J.A.; Ozil, J.; Haberman, E.; Adler, A.; Krey, L.C. Electrical activation and in vitro development of human oocytes that fail to fertilize after intracytoplasmic sperm injection. Fertil. Steril. 1999, 72, 509–512. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Thie, M.; Röspel, R.; Dettmann, W.; Benoit, M.; Ludwig, M.; Gaub, H.E.; Denker, H.W. Interactions between trophoblast and uterine epithelium: Monitoring of adhesive forces. Hum. Reprod. 1998, 13, 3211–3219. [Google Scholar] [CrossRef]
- Walters, E.M.; Clark, S.G.; Beebe, D.J.; Wheeler, M.B. Mammalian embryo culture in a microfluidic device. Methods Mol. Biol. 2004, 254, 375–382. [Google Scholar] [CrossRef]
- Mancini, V.; McKeegan, P.J.; Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A.; Picton, H.M.; Pensabene, V. A new microfluidic concept for successful in vitro culture of mouse embryos. bioRxiv 2020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).