A Novel Method for the Early Detection of Single Circulating, Metastatic and Self-Seeding Cancer Cells in Orthotopic Breast Cancer Mouse Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Establishing Stable Luciferase-Labelled Cell Lines
2.3. Luciferase Activity Quantification and Cell Titration
2.4. In Vivo Experiments
2.5. Ex Vivo Luciferase Assay for Detection and Quantification of Luciferase-Labelled Tumor Cells
2.6. Statistical Analysis
3. Results
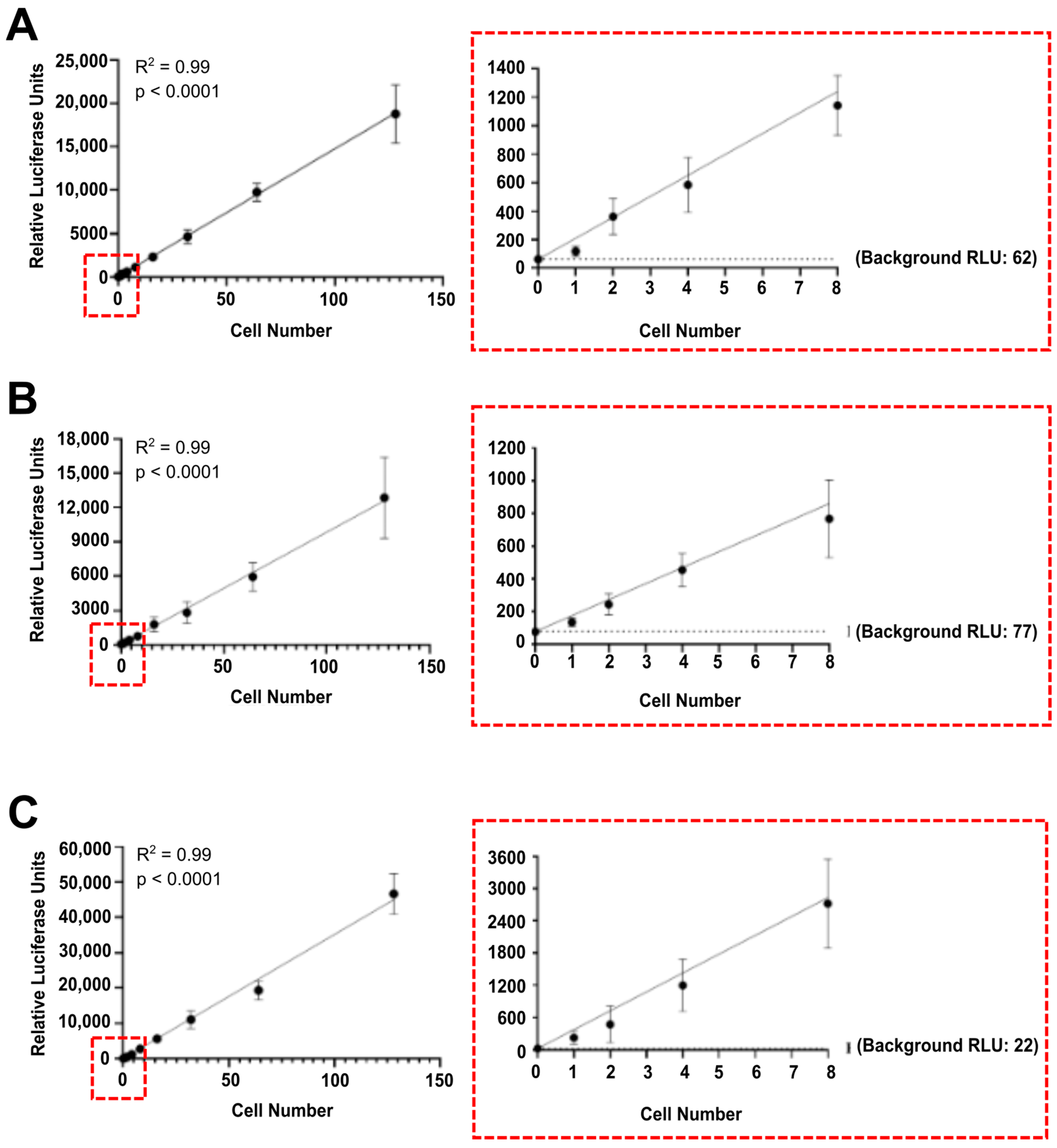
3.1. Luciferase Activity Is Detected In Vitro in Single Luciferase-Labelled Tumour Cells
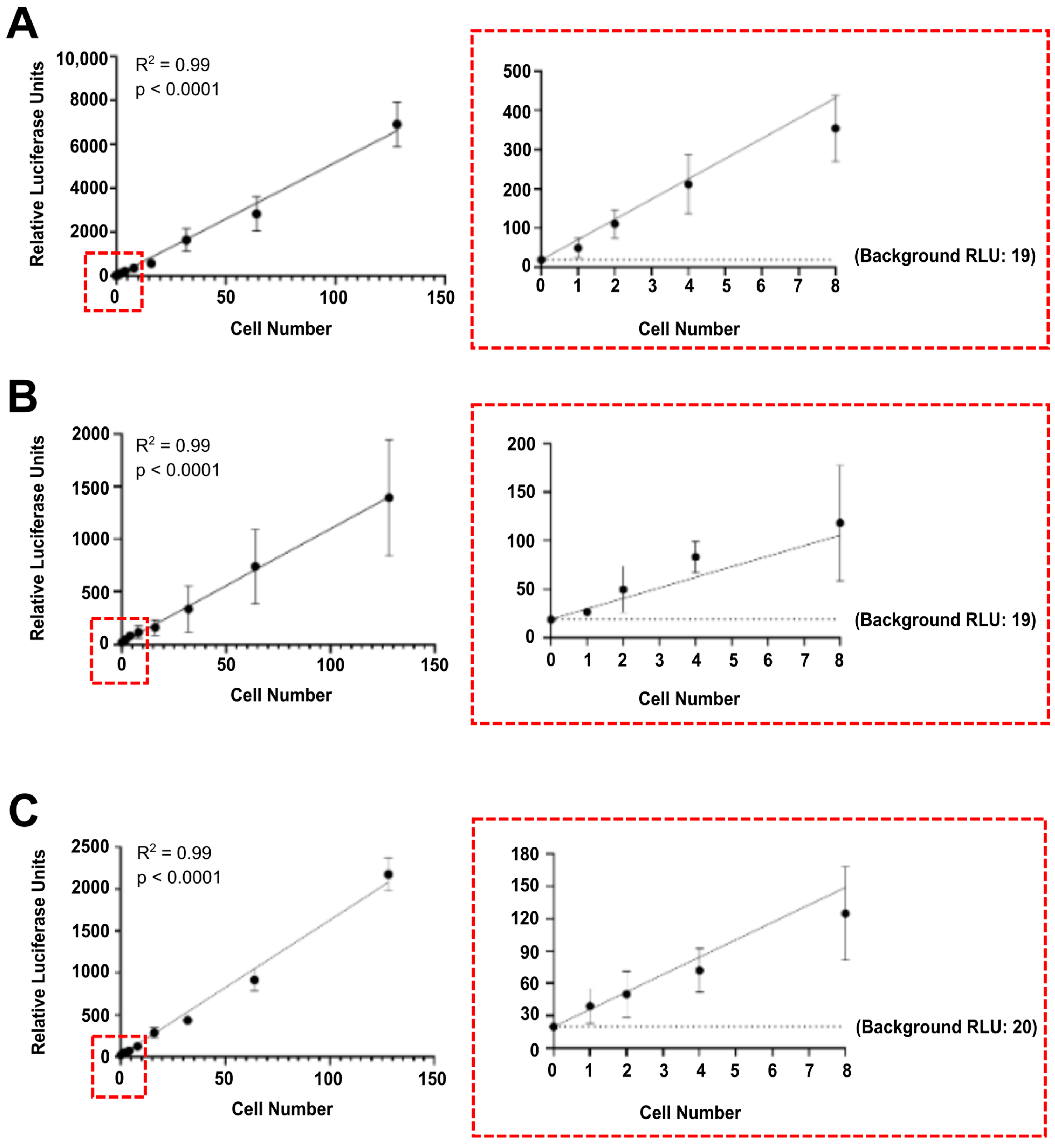
3.2. Tumour Cells Spiked in Mouse Blood and Organ Samples Are Detected at Single Cell Level
3.3. Luciferase-Labelling Enables the Detection of CTCs, Multiorgan Metastasis and Tumour Self-Seeding in an MDA-MB-231 Orthotopic Breast Cancer Model
3.4. Luciferase-Labelling Allows Ex Vivo Quantification of the Dissemination of Poorly Metastatic MCF7 Breast Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.D.; Neal, J.W.; Winslow, M.M. Dissecting metastasis using preclinical models and methods. Nat. Rev. Cancer 2023, 23, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Fonseca Teixeira, A.; Wu, S.; Luwor, R.; Zhu, H.-J. A New Era of Integration between Multiomics and Spatio-Temporal Analysis for the Translation of EMT towards Clinical Applications in Cancer. Cells 2023, 12, 2740. [Google Scholar] [CrossRef] [PubMed]
- Bouchalova, P.; Bouchal, P. Current methods for studying metastatic potential of tumor cells. Cancer Cell Int. 2022, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, A.; Christofori, G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006, 8, 1–11. [Google Scholar] [CrossRef]
- Rampetsreiter, P.; Casanova, E.; Eferl, R. Genetically modified mouse models of cancer invasion and metastasis. Drug Discov. Today: Dis. Models 2011, 8, 67–74. [Google Scholar] [CrossRef]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; Ten Dijke, P.; Zhu, H.J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Harney, A.S.; Arwert, E.N.; Entenberg, D.; Wang, Y.; Guo, P.; Qian, B.-Z.; Oktay, M.H.; Pollard, J.W.; Jones, J.G.; Condeelis, J.S. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage–Derived VEGFA. Cancer Discov. 2015, 5, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Borriello, L.; Coste, A.; Traub, B.; Sharma, V.P.; Karagiannis, G.S.; Lin, Y.; Wang, Y.; Ye, X.; Duran, C.L.; Chen, X.; et al. Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nat. Commun. 2022, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Borriello, L.; Karagiannis, G.S.; Duran, C.L.; Coste, A.; Oktay, M.H.; Entenberg, D.; Condeelis, J.S. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur. J. Cell Biol. 2020, 99, 151098. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Pollok, K.; Rinnenthal, J.-L.; Oehme, L.; Günther, R.; Spiecker, H.; Radbruch, H.; Gerhard, J.; Sporbert, A.; Cseresnyes, Z.; et al. High-Resolution Intravital Microscopy. PLoS ONE 2012, 7, e50915. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.H.; Najiminaini, M.; Zhang, Y.M.; Omidi, P.; Carson, J.J.L. Multispectral intravital microscopy for simultaneous bright-field and fluorescence imaging of the microvasculature. Appl. Microsc. 2021, 51, 12. [Google Scholar] [CrossRef]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of circulating tumor cells: Opportunities and challenges. Biomark. Res. 2022, 10, 58. [Google Scholar] [CrossRef]
- Batth, I.S.; Mitra, A.; Rood, S.; Kopetz, S.; Menter, D.; Li, S. CTC analysis: An update on technological progress. Transl. Res. 2019, 212, 14–25. [Google Scholar] [CrossRef]
- Hamza, B.; Miller, A.B.; Meier, L.; Stockslager, M.; Ng, S.R.; King, E.M.; Lin, L.; DeGouveia, K.L.; Mulugeta, N.; Calistri, N.L.; et al. Measuring kinetics and metastatic propensity of CTCs by blood exchange between mice. Nat. Commun. 2021, 12, 5680. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.-H.; Hsieh, Y.-C.; Huang, L.-C.; Lin, C.-Y.; Hsu, K.-W.; Hsieh, W.-S.; Chi, W.-M.; Lee, C.-H. A rapid and quantitative method to detect human circulating tumor cells in a preclinical animal model. BMC Cancer 2017, 17, 440. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Huo, L.; Fan, Y.; Wang, R.; Scott, A.W.; Pizzi, M.P.; Yao, X.; Shao, S.; Ma, L.; Da Silva, M.S.; et al. A new intronic quantitative PCR method led to the discovery of transformation from human ascites to murine malignancy in a mouse model. Front. Oncol. 2023, 13, 1062424. [Google Scholar] [CrossRef] [PubMed]
- Entenberg, D.; Oktay, M.H.; Condeelis, J.S. Intravital imaging to study cancer progression and metastasis. Nat. Rev. Cancer 2023, 23, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Babes, L.; Yipp, B.G.; Senger, D.L. Intravital Microscopy of the Metastatic Pulmonary Environment. Methods Mol. Biol. 2023, 2614, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.A.; Grek, C.L.; Ghatnekar, G.S.; Yeh, E.S. Evaluation of Lung Metastasis in Mouse Mammary Tumor Models by Quantitative Real-time PCR. J. Vis. Exp. 2016, 107, e53329. [Google Scholar] [CrossRef]
- Liu, S.; González-Prieto, R.; Zhang, M.; Geurink, P.P.; Kooij, R.; Iyengar, P.V.; van Dinther, M.; Bos, E.; Zhang, X.; Le Dévédec, S.E.; et al. Deubiquitinase Activity Profiling Identifies UCHL1 as a Candidate Oncoprotein That Promotes TGFβ-Induced Breast Cancer Metastasis. Clin. Cancer Res. 2020, 26, 1460–1473. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Cheng, J.L.; Farhoodi, H.P.; Toledano, M.; Yu, C.C.; Tierra, B.; Hildebrand, L.; Liu, L.; Liao, M.J.; Cho, J.; et al. Combinatorial targeting of cancer bone metastasis using mRNA engineered stem cells. eBioMedicine 2019, 45, 39–57. [Google Scholar] [CrossRef]
- Hao, M.; Lu, P.; Sotropa, S.; Manupati, K.; Yeo, S.K.; Guan, J.L. In vivo CRISPR knockout screen identifies p47 as a suppressor of HER2+ breast cancer metastasis by regulating NEMO trafficking and autophagy flux. Cell Rep. 2024, 43, 113780. [Google Scholar] [CrossRef]
- Chan, J.M.; Quintanal-Villalonga, Á.; Gao, V.R.; Xie, Y.; Allaj, V.; Chaudhary, O.; Masilionis, I.; Egger, J.; Chow, A.; Walle, T.; et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell 2021, 39, 1479–1496.e1418. [Google Scholar] [CrossRef] [PubMed]
- Solaimuthu, B.; Hayashi, A.; Khatib, A.; Shaul, Y.D. Monitoring Breast Cancer Growth and Metastatic Colony Formation in Mice using Bioluminescence. J. Vis. Exp. 2021, 177, e63060. [Google Scholar] [CrossRef]
- Lim, E.; Modi, K.; Christensen, A.; Meganck, J.; Oldfield, S.; Zhang, N. Monitoring tumor metastases and osteolytic lesions with bioluminescence and micro CT imaging. J. Vis. Exp. 2011, 50, e2775. [Google Scholar] [CrossRef]
- Nakayama, J.; Saito, R.; Hayashi, Y.; Kitada, N.; Tamaki, S.; Han, Y.; Semba, K.; Maki, S.A. High Sensitivity In Vivo Imaging of Cancer Metastasis Using a Near-Infrared Luciferin Analogue seMpai. Int. J. Mol. Sci. 2020, 21, 7896. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Ware, T.M.B.; Luwor, R.B.; Zhu, H.-J. A New Systemic Disease Mouse Model for Glioblastoma Capable of Single-Tumour-Cell Detection. Cells 2024, 13, 192. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Y.; Ware, T.; Iaria, J.; Ten Dijke, P.; Zhu, H.J. Reactivation of BMP signaling by suboptimal concentrations of MEK inhibitor and FK506 reduces organ-specific breast cancer metastasis. Cancer Lett. 2020, 493, 41–54. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Wang, Y.; Iaria, J.; ten Dijke, P.; Zhu, H.-J. Simultaneously targeting extracellular vesicle trafficking and TGF-β receptor kinase activity blocks signaling hyperactivation and metastasis. Signal Transduct. Target. Ther. 2023, 8, 456. [Google Scholar] [CrossRef]
- Hourani, T.; Eivazitork, M.; Balendran, T.; Mc. Lee, K.; Hamilton, J.A.; Zhu, H.-J.; Iaria, J.; Morokoff, A.P.; Luwor, R.B.; Achuthan, A.A. Signaling pathways underlying TGF-β mediated suppression of IL-12A gene expression in monocytes. Mol. Immunol. 2024, 166, 101–109. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Luu, M.; Iaria, J.; Sutherland, J.M.; McLaughlin, E.A.; Zhu, H.-J.; Loveland, K.L. Activin and BMP Signalling in Human Testicular Cancer Cell Lines, and a Role for the Nucleocytoplasmic Transport Protein Importin-5 in Their Crosstalk. Cells 2023, 12, 1000. [Google Scholar] [CrossRef]
- Sanchez, V.E.; Lynes, J.P.; Walbridge, S.; Wang, X.; Edwards, N.A.; Nwankwo, A.K.; Sur, H.P.; Dominah, G.A.; Obungu, A.; Adamstein, N.; et al. GL261 luciferase-expressing cells elicit an anti-tumor immune response: An evaluation of murine glioma models. Sci. Rep. 2020, 10, 11003. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.K.; Mason, J.; Venkatappa, N.; Jochelson, M.S.; Hurt, B.; Nieva, J.; Comen, E.; Norton, L.; Kuhn, P. Spatiotemporal progression of metastatic breast cancer: A Markov chain model highlighting the role of early metastatic sites. NPJ Breast Cancer 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, Q.; Zhang, Y.; Wang, X.; Xu, K.; Yan, K.; Dong, W.; Fan, Q.; Zhang, Y.; Qiu, X. Self-seeding circulating tumor cells promote the proliferation and metastasis of human osteosarcoma by upregulating interleukin-8. Cell Death Dis. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Comen, E.; Norton, L.; Massagué, J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 2011, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Suvilesh, K.N.; Nussbaum, Y.I.; Radhakrishnan, V.; Manjunath, Y.; Avella, D.M.; Staveley-O’Carroll, K.F.; Kimchi, E.T.; Chaudhuri, A.A.; Shyu, C.-R.; Li, G.; et al. Tumorigenic circulating tumor cells from xenograft mouse models of non-metastatic NSCLC patients reveal distinct single cell heterogeneity and drug responses. Mol. Cancer 2022, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mohan, S.C.; Wei, J.; Seki, E.; Liu, M.; Basho, R.; Giuliano, A.E.; Zhao, Y.; Cui, X. Breast cancer liver metastasis: Pathogenesis and clinical implications. Front. Oncol. 2022, 12, 1043771. [Google Scholar] [CrossRef]
- Rashid, N.S.; Grible, J.M.; Clevenger, C.V.; Harrell, J.C. Breast cancer liver metastasis: Current and future treatment approaches. Clin. Exp. Metastasis 2021, 38, 263–277. [Google Scholar] [CrossRef]
- Ji, L.; Cheng, L.; Zhu, X.; Gao, Y.; Fan, L.; Wang, Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer 2021, 21, 238. [Google Scholar] [CrossRef]
- Li, C.; Teixeira, A.F.; Zhu, H.-J.; ten Dijke, P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer 2021, 20, 154. [Google Scholar] [CrossRef]
- Teixeira, A.F.; ten Dijke, P.; Zhu, H.-J. TGF-β Signalling in Cancer: Role and Therapeutic Targeting of the Tumour Stroma. In The Tumor Stroma, 1st ed.; Prakash, J., Ed.; Jenny Stanford Publishing: New York, NY, USA, 2021; pp. 167–231. [Google Scholar]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. A metastasis map of human cancer cell lines. Nature 2020, 588, 331–336. [Google Scholar] [CrossRef]
- Khazaei, Z.; Jarrahi, A.M.; Momenabadi, V.; Ghorat, F.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide Stomach Cancers and Their Relationship with the Human Development Index (Hdi). World Cancer Res. J. 2019, 6, e1257. [Google Scholar]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.K.H.; Rimes, J.S.; Capaldo, B.D.; Vaillant, F.; Mouchemore, K.A.; Pal, B.; Chen, Y.; Surgenor, E.; Murphy, A.J.; Anderson, R.L.; et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat. Commun. 2021, 12, 6920. [Google Scholar] [CrossRef]
- Yamamoto, A.; Huang, Y.; Krajina, B.A.; McBirney, M.; Doak, A.E.; Qu, S.; Wang, C.L.; Haffner, M.C.; Cheung, K.J. Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7. Proc. Natl. Acad. Sci. USA 2023, 120, e2214888120. [Google Scholar] [CrossRef]
- Nugawela, D.; Gorringe, K.L. Targeted therapy for mucinous ovarian carcinoma: Evidence from clinical trials. Int. J. Gynecol. Cancer 2023, 33, 102–108. [Google Scholar] [CrossRef]
- Ho, G.Y.; Kyran, E.L.; Bedo, J.; Wakefield, M.J.; Ennis, D.P.; Mirza, H.B.; Vandenberg, C.J.; Lieschke, E.; Farrell, A.; Hadla, A.; et al. Epithelial-to-Mesenchymal Transition Supports Ovarian Carcinosarcoma Tumorigenesis and Confers Sensitivity to Microtubule Targeting with Eribulin. Cancer Res. 2022, 82, 4457–4473. [Google Scholar] [CrossRef]
- Cheasley, D.; Wakefield, M.J.; Ryland, G.L.; Allan, P.E.; Alsop, K.; Amarasinghe, K.C.; Ananda, S.; Anglesio, M.S.; Au-Yeung, G.; Böhm, M.; et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat. Commun. 2019, 10, 3935. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T. Current status and future outlook for patient-derived cancer models from a rare cancer research perspective. Cancer Sci. 2021, 112, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Obata, R.; Miura, C.; Kiyama, M.; Hama, K.; Nakamura, M.; Amano, Y.; Kojima, S.; Hirano, T.; Maki, S.; et al. Development of simple firefly luciferin analogs emitting blue, green, red, and near-infrared biological window light. Tetrahedron 2013, 69, 3847–3856. [Google Scholar] [CrossRef]
- Hall, M.P.; Woodroofe, C.C.; Wood, M.G.; Que, I.; van’t Root, M.; Ridwan, Y.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Wood, K.V.; et al. Click beetle luciferase mutant and near infrared naphthyl-luciferins for improved bioluminescence imaging. Nat. Commun. 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, L.; Que, I.; Kaijzel, E.; Branchini, B.; Roda, A.; Löwik, C. Sensitive dual color in vivo bioluminescence imaging using a new red codon optimized firefly luciferase and a green click beetle luciferase. PLoS ONE 2011, 6, e19277. [Google Scholar] [CrossRef] [PubMed]
- Zambito, G.; Hall, M.P.; Wood, M.G.; Gaspar, N.; Ridwan, Y.; Stellari, F.F.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Löwik, C.; et al. Red-shifted click beetle luciferase mutant expands the multicolor bioluminescent palette for deep tissue imaging. iScience 2021, 24, 101986. [Google Scholar] [CrossRef]
- Takatsu, K.; Kobayashi, N.; Wu, N.; Janin, Y.L.; Yamazaki, T.; Kuroda, Y. Biophysical analysis of Gaussia luciferase bioluminescence mechanisms using a non-oxidizable coelenterazine. BBA Adv. 2023, 3, 100068. [Google Scholar] [CrossRef]
- Ohmiya, Y.; Hirano, T.; Ohashi, M. The structural origin of the color differences in the bioluminescence of firefly luciferase. FEBS Lett. 1996, 384, 83–86. [Google Scholar] [CrossRef]
- Luwor, R.B.; Baradaran, B.; Taylor, L.E.; Iaria, J.; Nheu, T.V.; Amiry, N.; Hovens, C.M.; Wang, B.; Kaye, A.H.; Zhu, H.J. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene 2013, 32, 2433–2441. [Google Scholar] [CrossRef]
- Zhou, F.; Drabsch, Y.; Dekker, T.J.A.; de Vinuesa, A.G.; Li, Y.; Hawinkels, L.J.A.C.; Sheppard, K.-A.; Goumans, M.-J.; Luwor, R.B.; de Vries, C.J.; et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-β signalling. Nat. Commun. 2014, 5, 3388. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ware, T.M.B.; Iaria, J.; Zhu, H.J. Live Cell Imaging of the TGF- β/Smad3 Signaling Pathway In Vitro and In Vivo Using an Adenovirus Reporter System. J. Vis. Exp. 2018, 137, e57926. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. eBioMedicine 2022, 83. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Nguyen, T.N.A.; Huang, P.S.; Chu, P.Y.; Hsieh, C.H.; Wu, M.H. Recent Progress in Enhanced Cancer Diagnosis, Prognosis, and Monitoring Using a Combined Analysis of the Number of Circulating Tumor Cells (CTCs) and Other Clinical Parameters. Cancers 2023, 15, 5372. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad, M.; Chen, Y.; Iaria, J.; Fonseca Teixeira, A.; Zhu, H.-J. A Novel Method for the Early Detection of Single Circulating, Metastatic and Self-Seeding Cancer Cells in Orthotopic Breast Cancer Mouse Models. Cells 2024, 13, 1166. https://doi.org/10.3390/cells13141166
Murad M, Chen Y, Iaria J, Fonseca Teixeira A, Zhu H-J. A Novel Method for the Early Detection of Single Circulating, Metastatic and Self-Seeding Cancer Cells in Orthotopic Breast Cancer Mouse Models. Cells. 2024; 13(14):1166. https://doi.org/10.3390/cells13141166
Chicago/Turabian StyleMurad, Muhammad, Yanjiang Chen, Josephine Iaria, Adilson Fonseca Teixeira, and Hong-Jian Zhu. 2024. "A Novel Method for the Early Detection of Single Circulating, Metastatic and Self-Seeding Cancer Cells in Orthotopic Breast Cancer Mouse Models" Cells 13, no. 14: 1166. https://doi.org/10.3390/cells13141166





