Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen Sponge Matrix Preparation
2.2. Cell Experiments
2.3. Staining Technique
2.3.1. Phosphotungstic Acid
2.3.2. Lead Acetate and Phosphotungstic Acid
2.3.3. Lanthanoides and Phosphotungstic Acid
2.4. Micro-CT Image Acquisition
2.5. Photography of the Unstained Scaffolds
2.6. Volume Rendering and Processing
2.7. Intensity Level Analysis and Statistics
3. Results
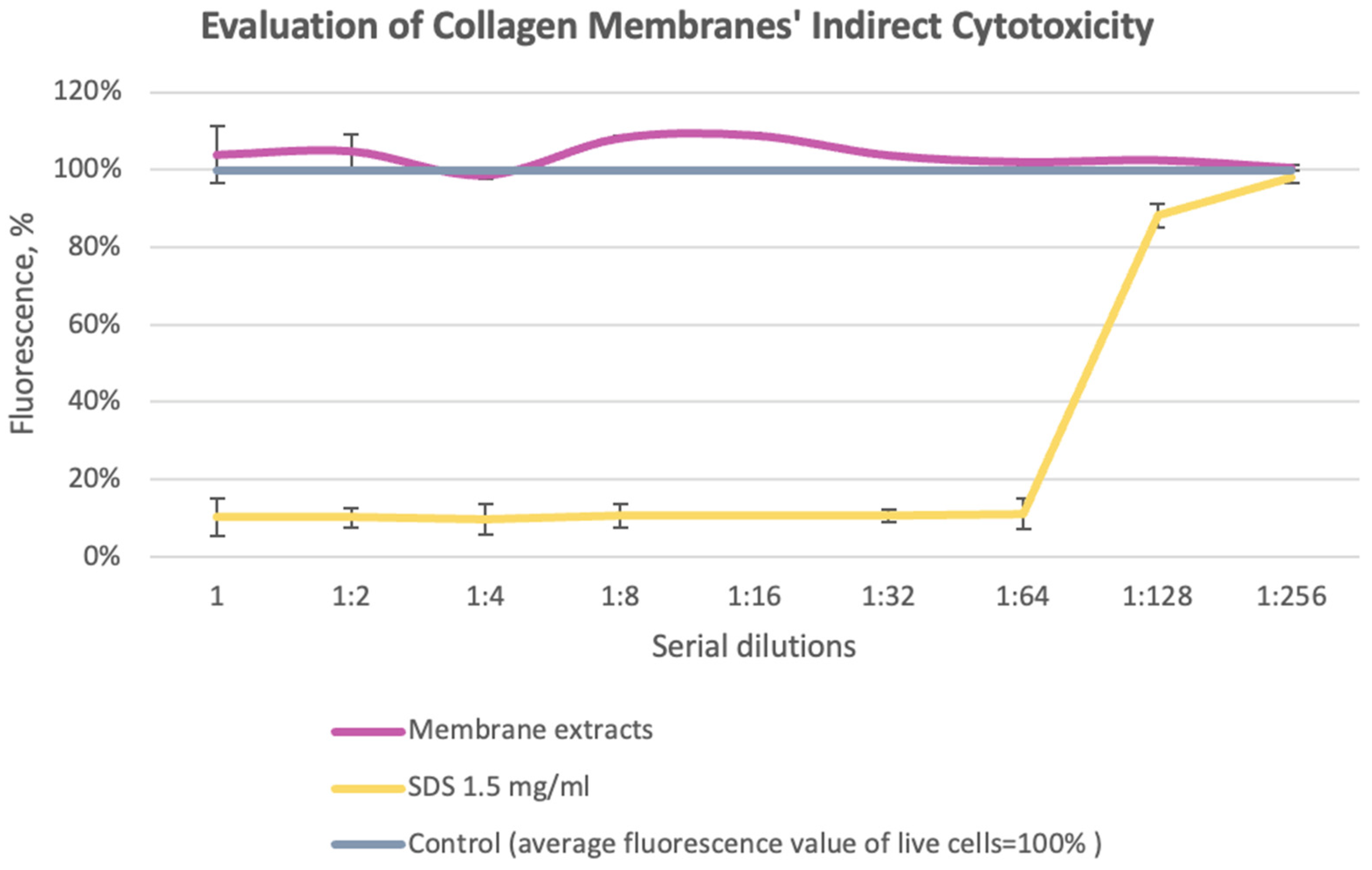
3.1. Evaluation of Collagen Sponges’ Indirect Cytotoxicity
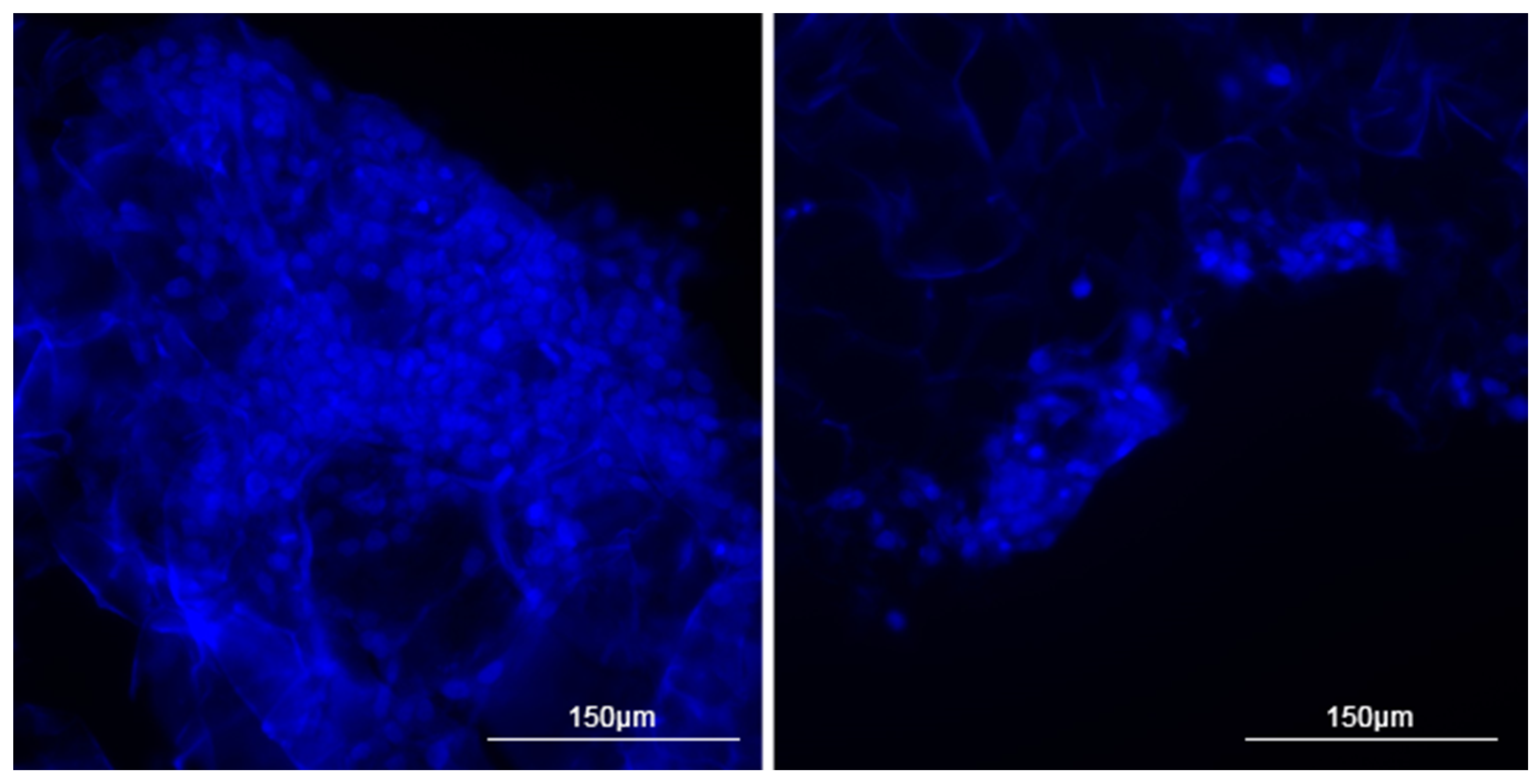
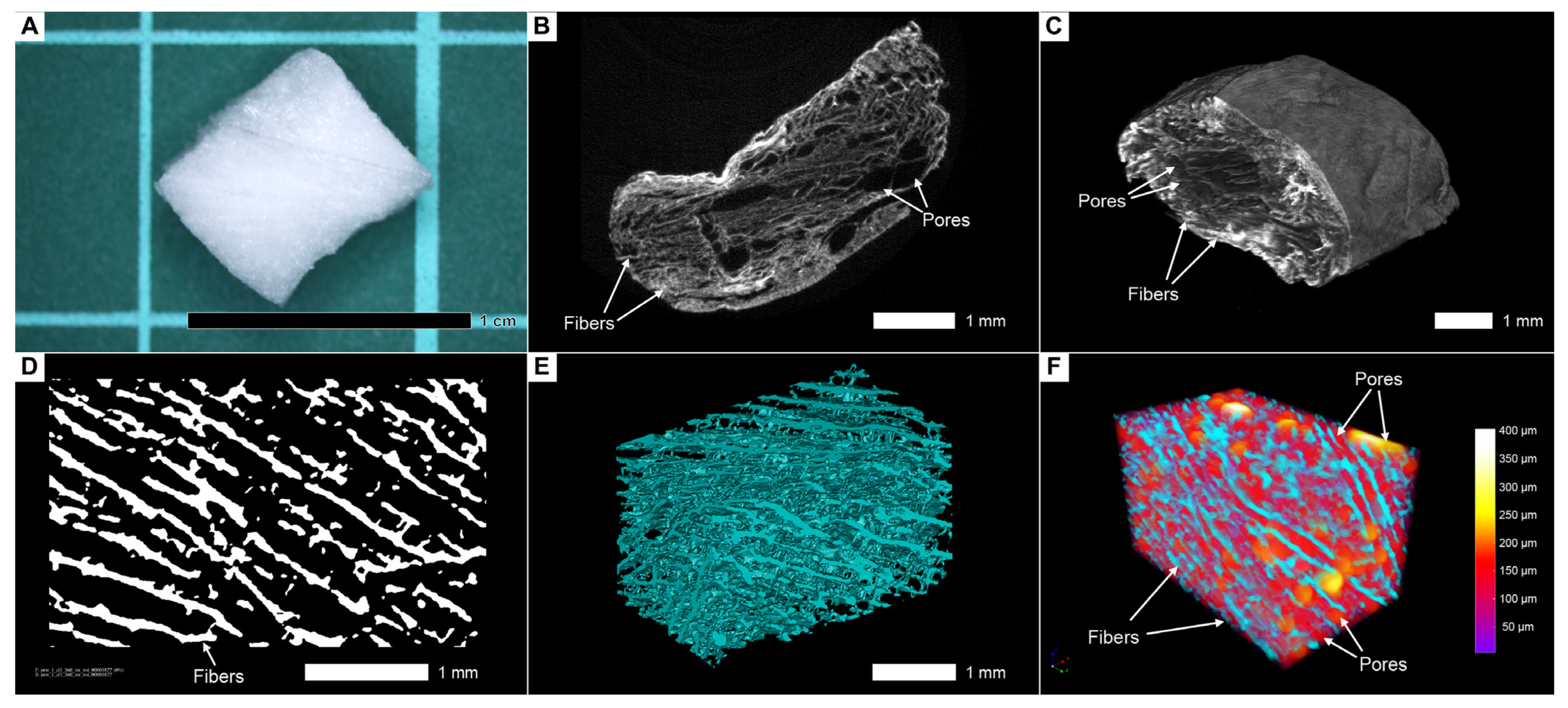
3.2. Two-Dimensional Visualization of Collagen Scaffolds Seeded with Cells
3.3. Contrast Enhancement of the Scaffolds Is a Prerequisite for Analysis of Their Native Internal Structure in Aqueous Conditions
3.4. Lead Acetate in Combination with PTA Is not Suitable for Contrasting Cells and Scaffolds
3.5. The Combination of Lanthanoids and Phosphotungstic Acid Does Not Allow for Adequate Separation of Cells and Collagen Fibers
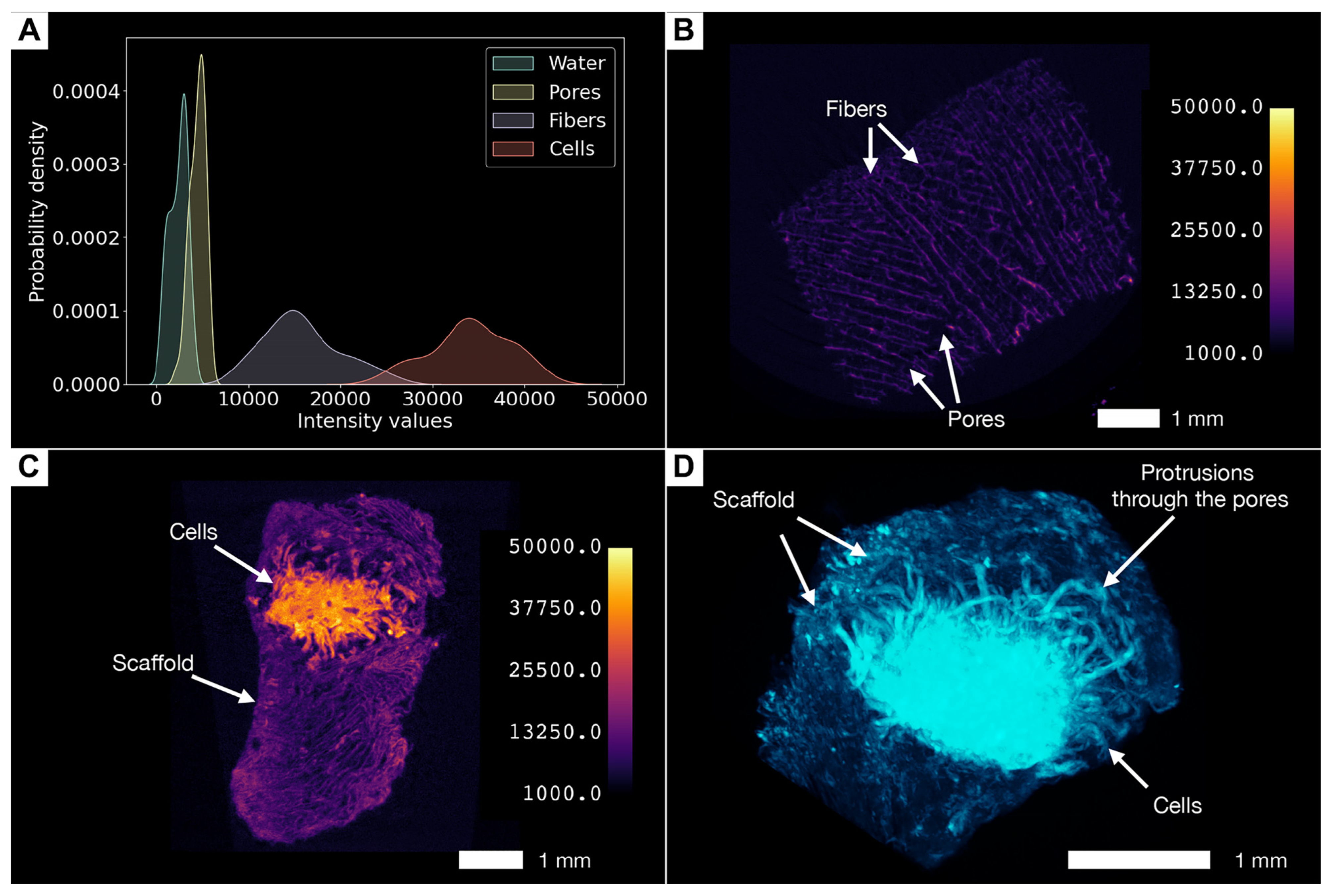
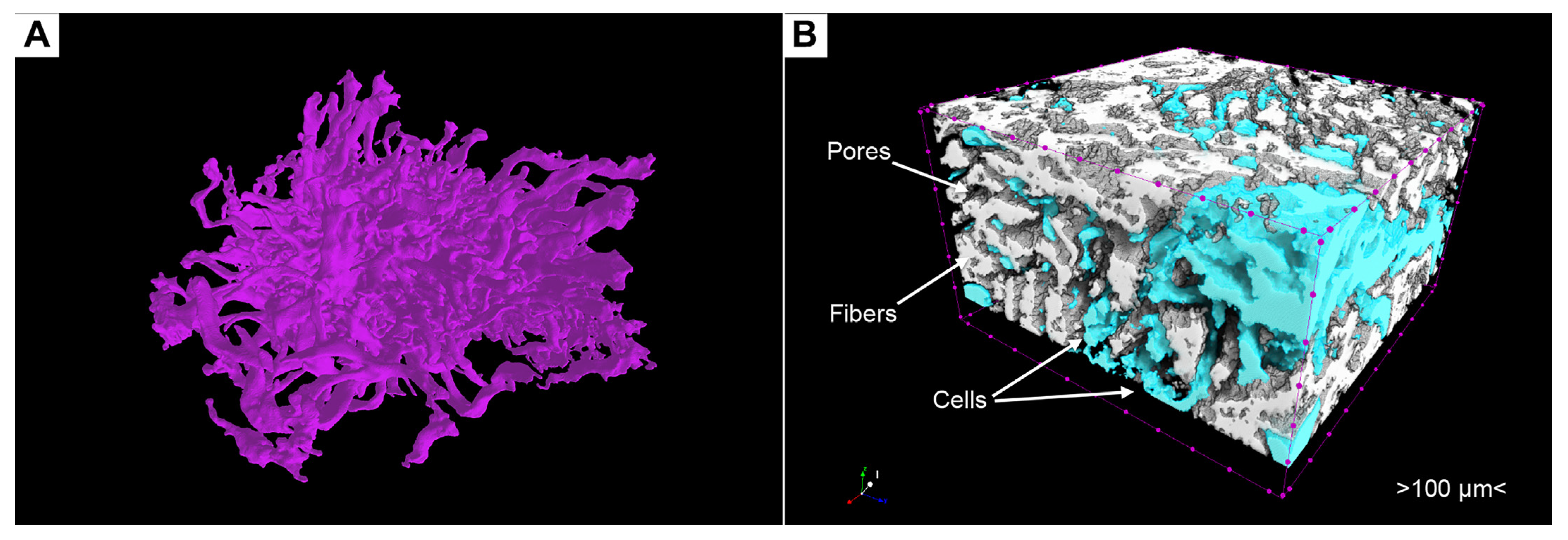
3.6. Phosphotungstic Acid Treatment Enables Characterization of Pore Network and Simultaneous Visualization of Cell Masses and Collagen Scaffolds
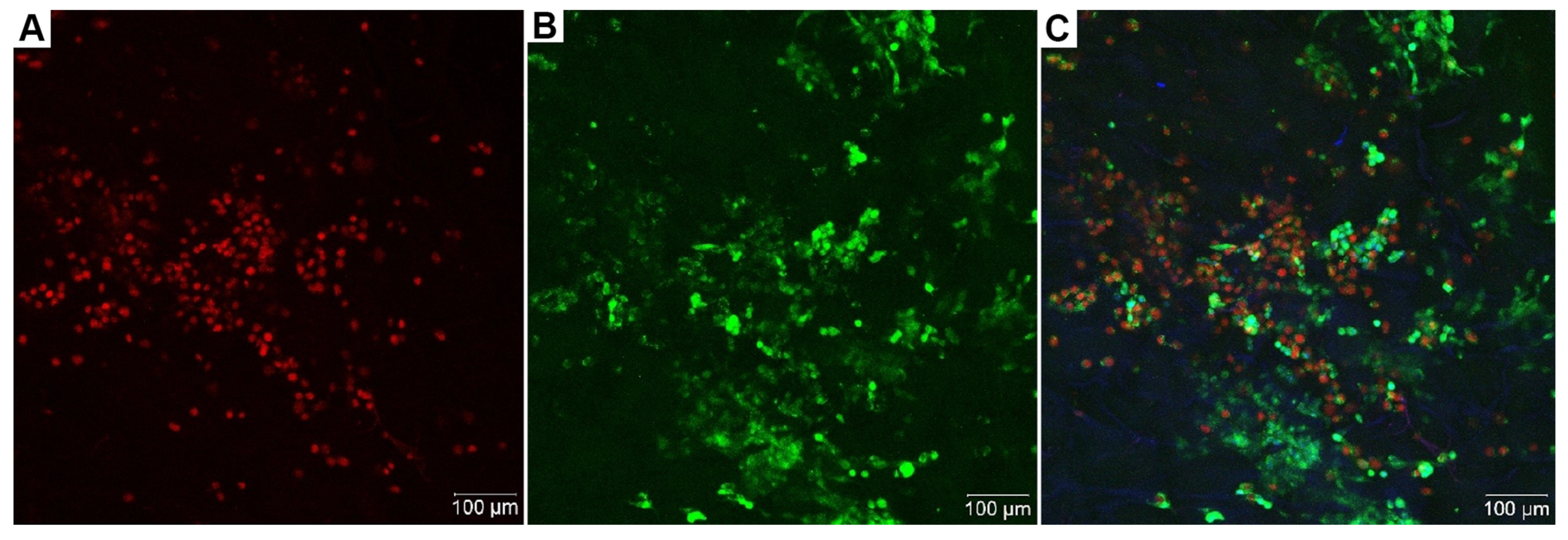
3.7. Phosphotungstic Acid Does Not Allow Distinguishing of Live or Dead Cells
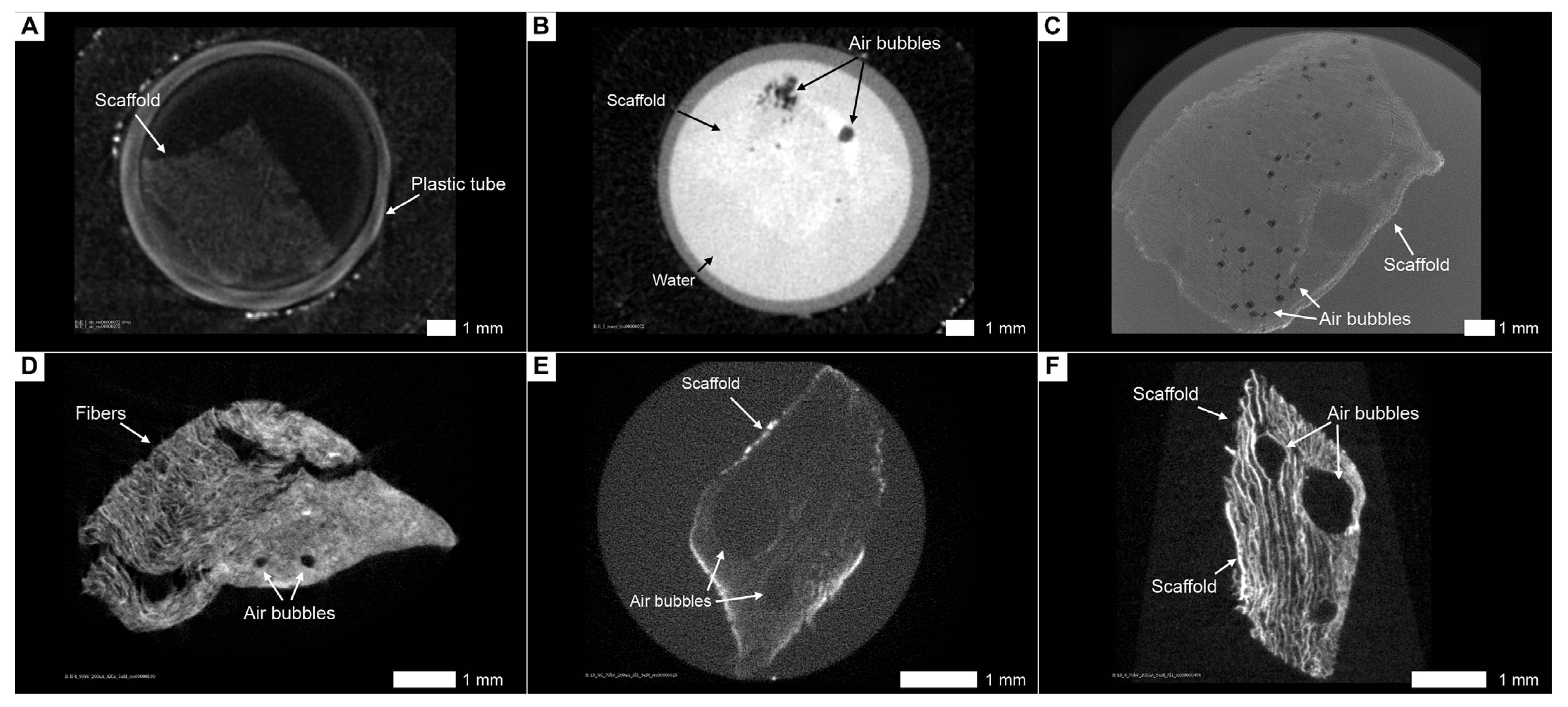
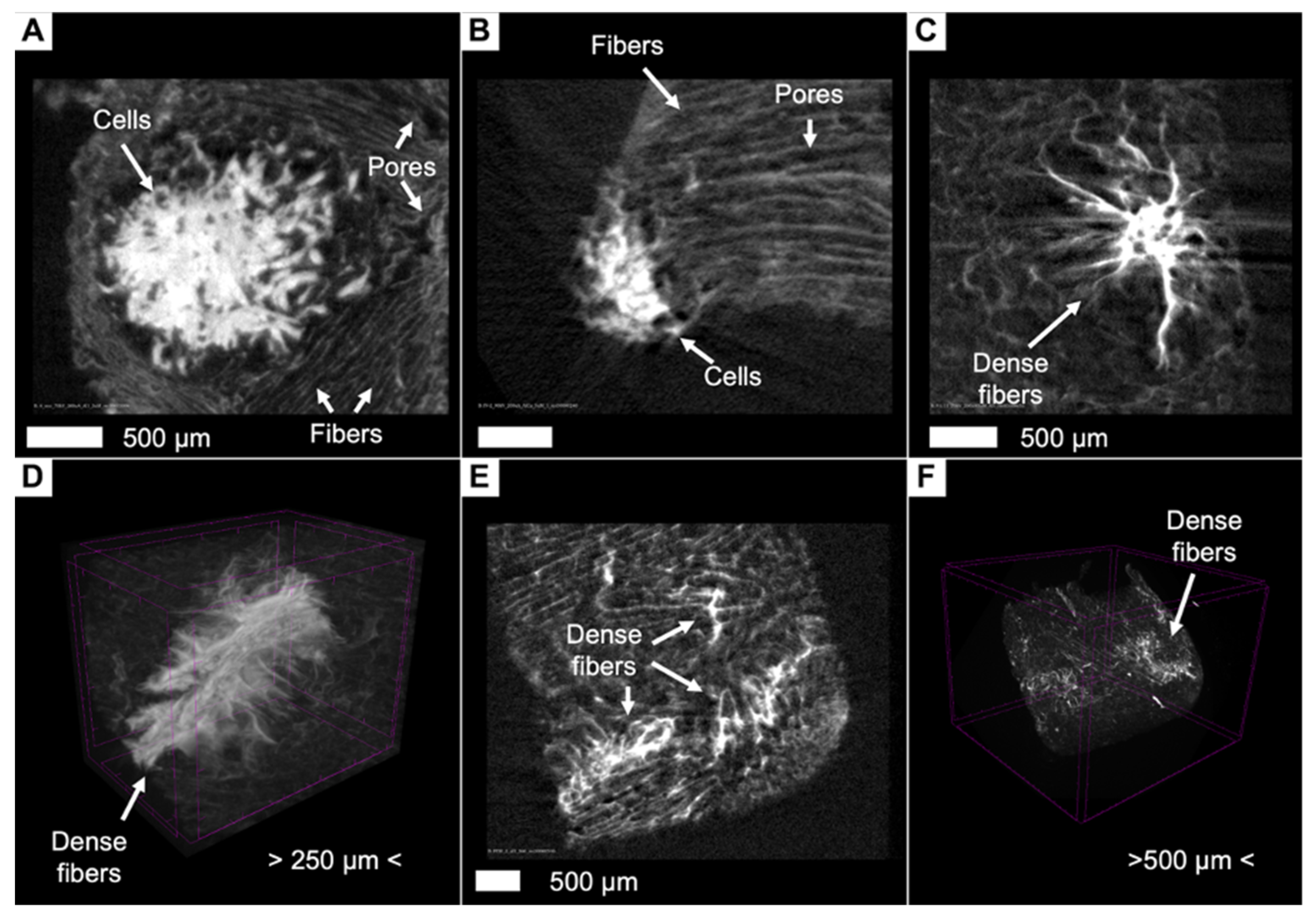
3.8. Artifacts and Pseudopositive Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. In Fibrous Proteins: Design, Synthesis, and Assembly; Ling, S., Ed.; Springer: New York, NY, USA, 2021; pp. 17–25. [Google Scholar]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.L.; Shekhter, A.B.; Istranov, L.P.; Istranova, E.V.; Rudenko, T.G.; Guller, A.E.; Aboyants, R.K.; Timashev, P.S.; Butnaru, D.V. Bioresorbable collagen materials in surgery: 50 years of success. Sechenov Med. J. 2020, 11, 59–70. [Google Scholar] [CrossRef]
- Patil, V.A.; Masters, K.S. Engineered Collagen Matrices. Bioengineering 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, E.Y.; Skorova, E.Y.; Chudakova, D.A.; Anikin, V.B.; Reshetov, I.V.; Mynbaev, O.A.; Petersen, E.V. The Matrix-Dependent 3D Spheroid Model of the Migration of Non-Small Cell Lung Cancer: A Step towards a Rapid Automated Screening. Front. Mol. Biosci. 2021, 8, 610407. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Eva, F.; Zuzana, B.; Michala, R.; Paolo, B.; Martin, C.; Eva, K.; Evzen, A. Lucie Kubínová Analysis and Three-Dimensional Visualization of Collagen in Artificial Scaffolds Using Nonlinear Microscopy Techniques. J. Biomed. Opt. 2010, 15, 066011. [Google Scholar] [CrossRef]
- Fouquet, C.; Gilles, J.-F.; Heck, N.; Dos Santos, M.; Schwartzmann, R.; Cannaya, V.; Morel, M.-P.; Davidson, R.S.; Trembleau, A.; Bolte, S. Improving Axial Resolution in Confocal Microscopy with New High Refractive Index Mounting Media. PLoS ONE 2015, 10, e0121096. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Larina, I.V. 8-High-Resolution Imaging Techniques in Tissue Engineering. In Monitoring and Evaluation of Biomaterials and Their Performance In Vivo; Narayan, R.J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 151–180. ISBN 978-0-08-100603-0. [Google Scholar]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Reilly, W.M.; Obara, C.J. Advances in Confocal Microscopy and Selected Applications. In Confocal Microscopy: Methods and Protocols; Brzostowski, J., Sohn, H., Eds.; Springer: New York, NY, USA, 2021; pp. 1–35. ISBN 978-1-07-161402-0. [Google Scholar]
- Zhou, X.; Zhao, R.; Yanamandra, A.K.; Hoth, M.; Qu, B. Light-Sheet Scattering Microscopy to Visualize Long-Term Interactions Between Cells and Extracellular Matrix. Front. Immunol. 2022, 13, 828634. [Google Scholar] [CrossRef]
- Deng, C.X.; Hong, X.; Stegemann, J.P. Ultrasound Imaging Techniques for Spatiotemporal Characterization of Composition, Microstructure, and Mechanical Properties in Tissue Engineering. Tissue Eng. Part. B Rev. 2016, 22, 311–321. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT—A Digital 3D Microstructural Voyage into Scaffolds: A Systematic Review of the Reported Methods and Results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Olăreț, E.; Stancu, I.-C.; Iovu, H.; Serafim, A. Computed Tomography as a Characterization Tool for Engineered Scaffolds with Biomedical Applications. Materials 2021, 14, 6763. [Google Scholar] [CrossRef]
- Kwon, K.A.; Bax, D.V.; Shepherd, J.H.; Cameron, R.E.; Best, S.M. Avoiding Artefacts in MicroCT Imaging of Collagen Scaffolds: Effect of Phosphotungstic Acid (PTA)-Staining and Crosslink Density. Bioact. Mater. 2022, 8, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Vladimirov, G.; Kuryanova, A.; Gafarova, E.; Tkachev, S.; Kosheleva, N.; Istranova, E.; Istranov, L.; Efremov, Y.; Novikov, I.; et al. A Defined Road to Tracheal Reconstruction: Laser Structuring and Cell Support for Rapid Clinic Translation. Stem Cell Res. Ther. 2022, 13, 317. [Google Scholar] [CrossRef]
- Tkachev, S.Y.; Mitrin, B.I.; Karnaukhov, N.S.; Sadyrin, E.V.; Voloshin, M.V.; Maksimov, A.Y.; Goncharova, A.S.; Lukbanova, E.A.; Zaikina, E.V.; Volkova, A.V.; et al. Visualization of Different Anatomical Parts of the Enucleated Human Eye Using X-ray Micro-CT Imaging. Exp. Eye Res. 2021, 203, 108394. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.; Leszczyński, B.; Kołodziej, T.; Kubicz, E.; Przybyło, M.; Stępień, E. X-ray Microtomography as a New Approach for Imaging and Analysis of Tumor Spheroids. Micron 2020, 137, 102917. [Google Scholar] [CrossRef]
- Kogan, M.I.; Popov, I.V.; Kirichenko, E.Y.; Mitrin, B.I.; Sadyrin, E.V.; Kulaeva, E.D.; Popov, I.V.; Kulba, S.N.; Logvinov, A.K.; Akimenko, M.A.; et al. X-ray Micro-Computed Tomography in the Assessment of Penile Cavernous Fibrosis in a Rabbit Castration Model. Andrology 2021, 9, 1467–1480. [Google Scholar] [CrossRef]
- Iberite, F.; Piazzoni, M.; Guarnera, D.; Iacoponi, F.; Locarno, S.; Vannozzi, L.; Bolchi, G.; Boselli, F.; Gerges, I.; Lenardi, C.; et al. Soft Perfusable Device to Culture Skeletal Muscle 3D Constructs in Air. ACS Appl. Bio Mater. 2023, 6, 2712–2724. [Google Scholar] [CrossRef]
- Xu, J.; Vecstaudza, J.; Wesdorp, M.A.; Labberté, M.; Kops, N.; Salerno, M.; Kok, J.; Simon, M.; Harmand, M.-F.; Vancíková, K.; et al. Incorporating Strontium Enriched Amorphous Calcium Phosphate Granules in Collagen/Collagen-Magnesium-Hydroxyapatite Osteochondral Scaffolds Improves Subchondral Bone Repair. Mater. Today Bio 2024, 25, 100959. [Google Scholar] [CrossRef]
- Balachandran Megha, K.; Syama, S.; Padmalayathil Sangeetha, V.; Vandana, U.; Oyane, A.; Valappil Mohanan, P. Development of a 3D Multifunctional Collagen Scaffold Impregnated with Peptide LL-37 for Vascularised Bone Tissue Regeneration. Int. J. Pharm. 2024, 652, 123797. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Power, R.N.; Genoud, K.J.; Matheson, A.; González-Vázquez, A.; Costard, L.; Eichholz, K.; Pitacco, P.; Hallegouet, T.; Chen, G.; et al. A Multifunctional Scaffold for Bone Infection Treatment by Delivery of microRNA Therapeutics Combined With Antimicrobial Nanoparticles. Adv. Mater. 2024, 36, 2307639. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.; Van Loo, D.; Cornillie, P.; Brabant, L.; Van Hoorebeke, L. An Exploratory Study of Contrast Agents for Soft Tissue Visualization by Means of High Resolution X-ray Computed Tomography Imaging. J. Microsc. 2013, 250, 21–31. [Google Scholar] [CrossRef]
- de Bournonville, S.; Vangrunderbeeck, S.; Kerckhofs, G. Contrast-Enhanced MicroCT for Virtual 3D Anatomical Pathology of Biological Tissues: A Literature Review. Contrast Media Mol. Imaging 2019, 2019, 8617406. [Google Scholar] [CrossRef] [PubMed]
- Glancy, S.B.; Morris, H.D.; Ho, V.B.; Klarmann, G.J. Optimal Agents for Visualizing Collagen Tissue Microarchitecture Using Contrast-Enhanced MicroCT. Pharmaceuticals 2023, 16, 1719. [Google Scholar] [CrossRef]
- Mizutani, R.; Takeuchi, A.; Uesugi, K.; Takekoshi, S.; Osamura, R.Y.; Suzuki, Y. Microtomographic Analysis of Neuronal Circuits of Human Brain. Cereb. Cortex 2010, 20, 1739–1748. [Google Scholar] [CrossRef]
- Müller, M.; Kimm, M.A.; Ferstl, S.; Allner, S.; Achterhold, K.; Herzen, J.; Pfeiffer, F.; Busse, M. Nucleus-Specific X-ray Stain for 3D Virtual Histology. Sci. Rep. 2018, 8, 17855. [Google Scholar] [CrossRef] [PubMed]
- Metscher, B. A Simple Nuclear Contrast Staining Method for microCT-Based 3D Histology Using Lead(II) Acetate. J. Anat. 2021, 238, 1036–1041. [Google Scholar] [CrossRef]
- Busse, M.; Müller, M.; Kimm, M.A.; Ferstl, S.; Allner, S.; Achterhold, K.; Herzen, J.; Pfeiffer, F. Three-Dimensional Virtual Histology Enabled through Cytoplasm-Specific X-ray Stain for Microscopic and Nanoscopic Computed Tomography. Proc. Natl. Acad. Sci. USA 2018, 115, 2293–2298. [Google Scholar] [CrossRef]
- Nemetschek, T.; Riedl, H.; Jonak, R. Topochemistry of the Binding of Phosphotungstic Acid to Collagen. J. Mol. Biol. 1979, 133, 67–83. [Google Scholar] [CrossRef]
- Missbach-Guentner, J.; Pinkert-Leetsch, D.; Dullin, C.; Ufartes, R.; Hornung, D.; Tampe, B.; Zeisberg, M.; Alves, F. 3D Virtual Histology of Murine Kidneys—High Resolution Visualization of Pathological Alterations by Micro Computed Tomography. Sci. Rep. 2018, 8, 1407. [Google Scholar] [CrossRef]
- Hildebrand, T.; Nogueira, L.; Sunde, P.T.; Ørstavik, D.; Glasmacher, B.; Haugen, H.J. Contrast-Enhanced Nano-CT Reveals Soft Dental Tissues and Cellular Layers. Int. Endod. J. 2021, 54, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Hanly, A.; Johnston, R.D.; Lemass, C.; Jose, A.; Tornifoglio, B.; Lally, C. Phosphotungstic Acid (PTA) Preferentially Binds to Collagen- Rich Regions of Porcine Carotid Arteries and Human Atherosclerotic Plaques Observed Using Contrast Enhanced Micro-Computed Tomography (CE-µCT). Front. Physiol. 2023, 14, 1057394. [Google Scholar] [CrossRef]
- Palmroth, A.; Pitkänen, S.; Hannula, M.; Paakinaho, K.; Hyttinen, J.; Miettinen, S.; Kellomäki, M. Evaluation of Scaffold Microstructure and Comparison of Cell Seeding Methods Using Micro-Computed Tomography-Based Tools. J. R. Soc. Interface 2020, 17, 20200102. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Muja, N.; Marelli, B.; Nazhat, S.N. Real Time Responses of Fibroblasts to Plastically Compressed Fibrillar Collagen Hydrogels. Biomaterials 2011, 32, 4761–4772. [Google Scholar] [CrossRef]
- Subbot, A.; Kondratieva, S.; Novikov, I.; Gogoleva, N.; Kozlova, O.; Chebotar, I.; Gazizova, G.; Ryabova, A.; Vorontsova, M.; Kikawada, T.; et al. Life-on-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells. Biology 2021, 10, 607. [Google Scholar] [CrossRef]
- Turunen, M.J.; Khayyeri, H.; Guizar-Sicairos, M.; Isaksson, H. Effects of Tissue Fixation and Dehydration on Tendon Collagen Nanostructure. J. Struct. Biol. 2017, 199, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Oosterlaken, B.M.; Friedrich, H.; de With, G. The Effects of Washing a Collagen Sample Prior to TEM Examination. Microsc. Res. Tech. 2022, 85, 412–417. [Google Scholar] [CrossRef]
- Dey, P. Connective Tissue Stain: Principle and Procedure. In Basic and Advanced Laboratory Techniques in Histopathology and Cytology; Dey, P., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 101–111. ISBN 978-981-19661-6-3. [Google Scholar]
- Silverman, L.; Glick, D. The Reactivity and Staining of Tissue Proteins with Phosphotungstic Acid. J. Cell Biol. 1969, 40, 761–767. [Google Scholar] [CrossRef]
- Meek, M.C.; Best, S.; Cameron, R. The Effects of Despeckling Filters on Pore Size Measurements in Collagen Scaffold Micro-CT Data. J. Microsc. 2021, 284, 142–156. [Google Scholar] [CrossRef]
- Reedy, C.L.; Reedy, C.L. High-Resolution Micro-CT with 3D Image Analysis for Porosity Characterization of Historic Bricks. Herit. Sci. 2022, 10, 83. [Google Scholar] [CrossRef]
- Shkarin, R.; Shkarin, A.; Shkarina, S.; Cecilia, A.; Surmenev, R.A.; Surmeneva, M.A.; Weinhardt, V.; Baumbach, T.; Mikut, R. Quanfima: An Open Source Python Package for Automated Fiber Analysis of Biomaterials. PLoS ONE 2019, 14, e0215137. [Google Scholar] [CrossRef] [PubMed]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing Forces: Architectural Control of Mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, C.; Hyun, J.K.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer Cell Angiogenic Capability Is Regulated by 3D Culture and Integrin Engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Schimmel, N.; Al-Ameen, M.A.; Ghosh, G. Breast Cancer Cells Mechanosensing in Engineered Matrices: Correlation with Aggressive Phenotype. J. Mech. Behav. Biomed. Mater. 2016, 61, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, R.; Takeuchi, A.; Uesugi, K.; Ohyama, M.; Takekoshi, S.; Osamura, R.Y.; Suzuki, Y. Three-Dimensional Microtomographic Imaging of Human Brain Cortex. Brain Res. 2008, 1199, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, A.; Madi, K.; Disney, C.M.; Courtois, L.; Jupe, A.; Zhang, F.; Bodey, A.J.; Lee, P.; Rau, C.; Robinsona, I.K.; et al. Phase-Contrast 3D Tomography of HeLa Cells Grown in PLLA Polymer Electrospun Scaffolds Using Synchrotron X-rays Bhartiya A. J. Synchrotron Radiat. 2020, 27, 158–163. [Google Scholar] [CrossRef]
- Brown, G.E.; Sutton, S.R.; Calas, G. User Facilities around the World. Elements 2006, 2, 9–14. [Google Scholar] [CrossRef]
- Ohnishi, T.; Teplov, A.; Kawata, N.; Ibrahim, K.; Ntiamoah, P.; Firat, C.; Haneishi, H.; Hameed, M.; Shia, J.; Yagi, Y. Three-Dimensional Vessel Segmentation in Whole-Tissue and Whole-Block Imaging Using a Deep Neural Network: Proof-of-Concept Study. Am. J. Pathol. 2021, 191, 463–474. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Average internal porosity, % | 58 |
| Min pore size, µm | 150 |
| Max pore size, µm | 250 |
| Min volume of segmented cells, mm3 | 0.2 |
| Max volume of segmented cells, mm3 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachev, S.; Chepelova, N.; Galechyan, G.; Ershov, B.; Golub, D.; Popova, E.; Antoshin, A.; Giliazova, A.; Voloshin, S.; Efremov, Y.; et al. Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment. Cells 2024, 13, 1234. https://doi.org/10.3390/cells13151234
Tkachev S, Chepelova N, Galechyan G, Ershov B, Golub D, Popova E, Antoshin A, Giliazova A, Voloshin S, Efremov Y, et al. Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment. Cells. 2024; 13(15):1234. https://doi.org/10.3390/cells13151234
Chicago/Turabian StyleTkachev, Sergey, Natalia Chepelova, Gevorg Galechyan, Boris Ershov, Danila Golub, Elena Popova, Artem Antoshin, Aliia Giliazova, Sergei Voloshin, Yuri Efremov, and et al. 2024. "Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment" Cells 13, no. 15: 1234. https://doi.org/10.3390/cells13151234
APA StyleTkachev, S., Chepelova, N., Galechyan, G., Ershov, B., Golub, D., Popova, E., Antoshin, A., Giliazova, A., Voloshin, S., Efremov, Y., Istranova, E., & Timashev, P. (2024). Three-Dimensional Cell Culture Micro-CT Visualization within Collagen Scaffolds in an Aqueous Environment. Cells, 13(15), 1234. https://doi.org/10.3390/cells13151234








