Nuclear Receptors and the Hidden Language of the Metabolome
Abstract
:1. Introduction
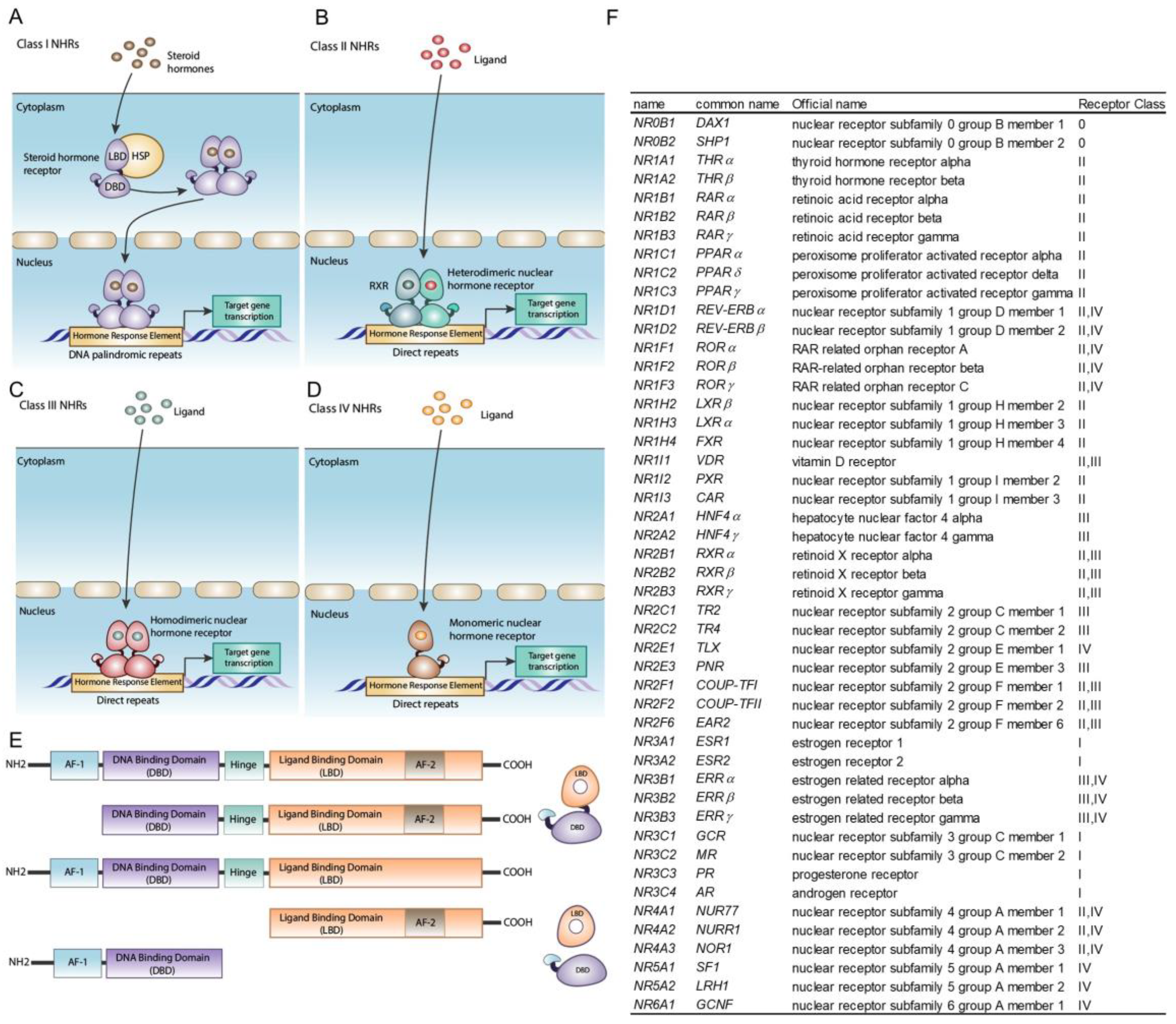
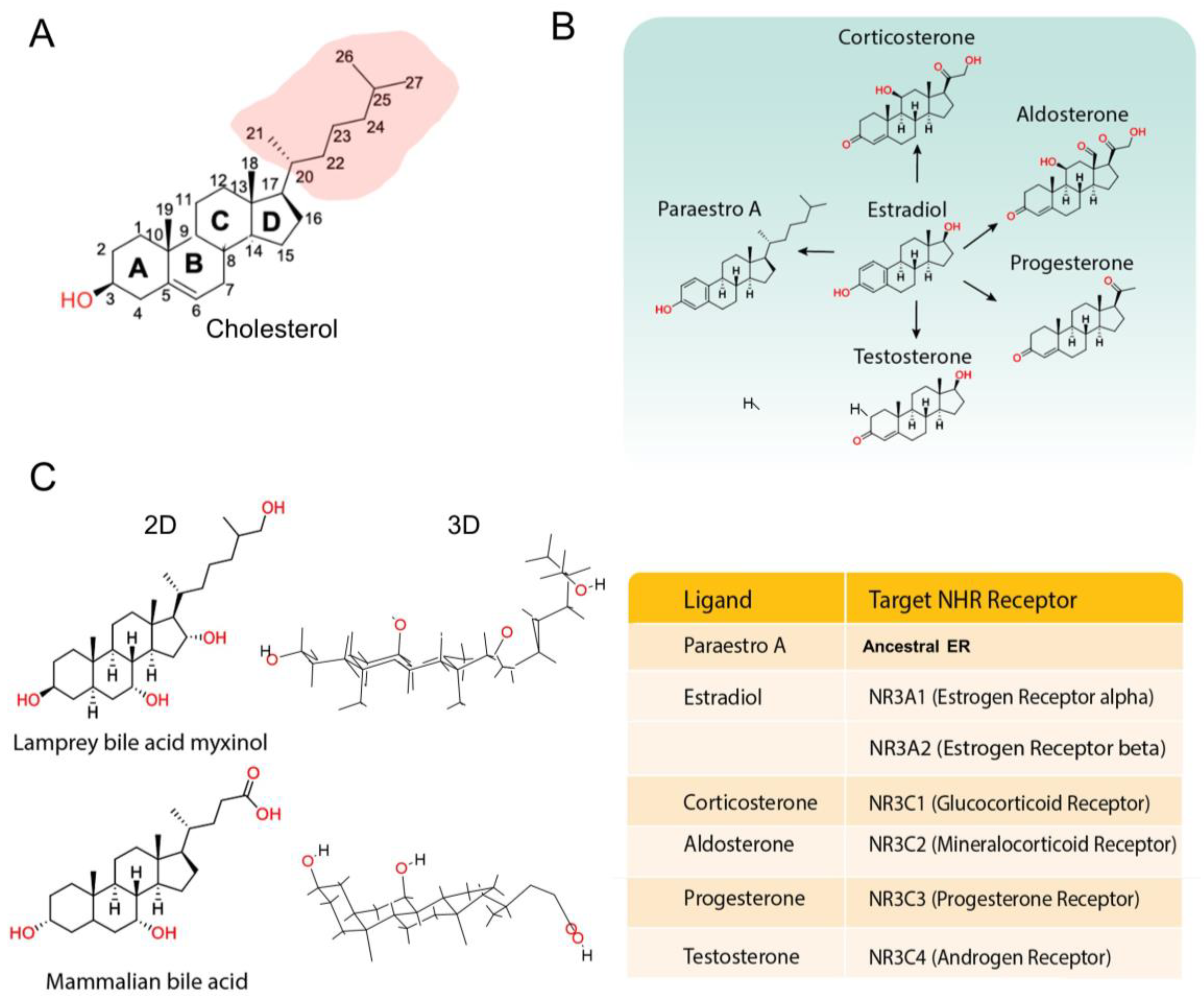
2. The Origins and Functions of NHRs and Their Ligands
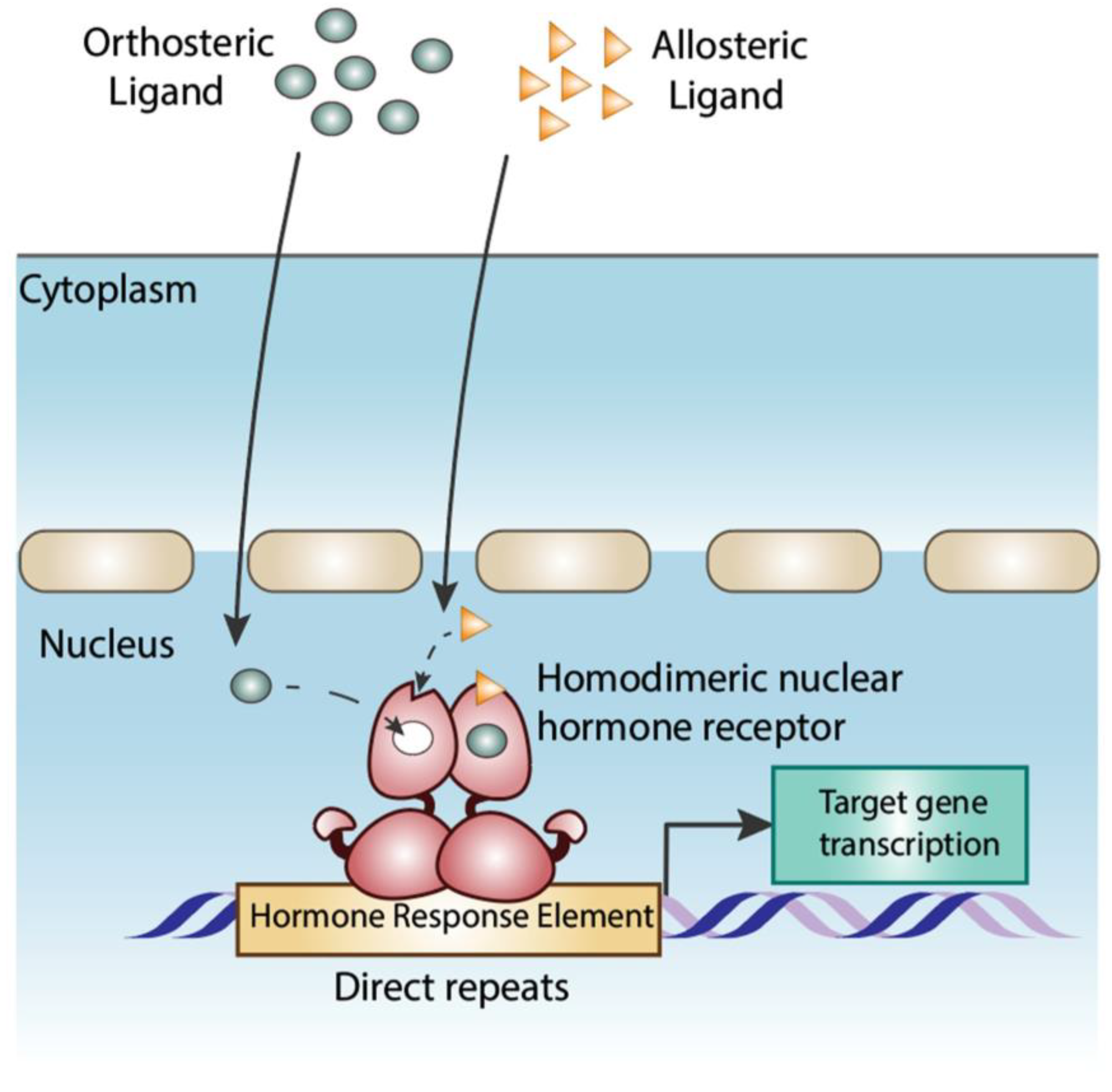
3. Ligand-Receptor Interactions: Determinants of Specificity
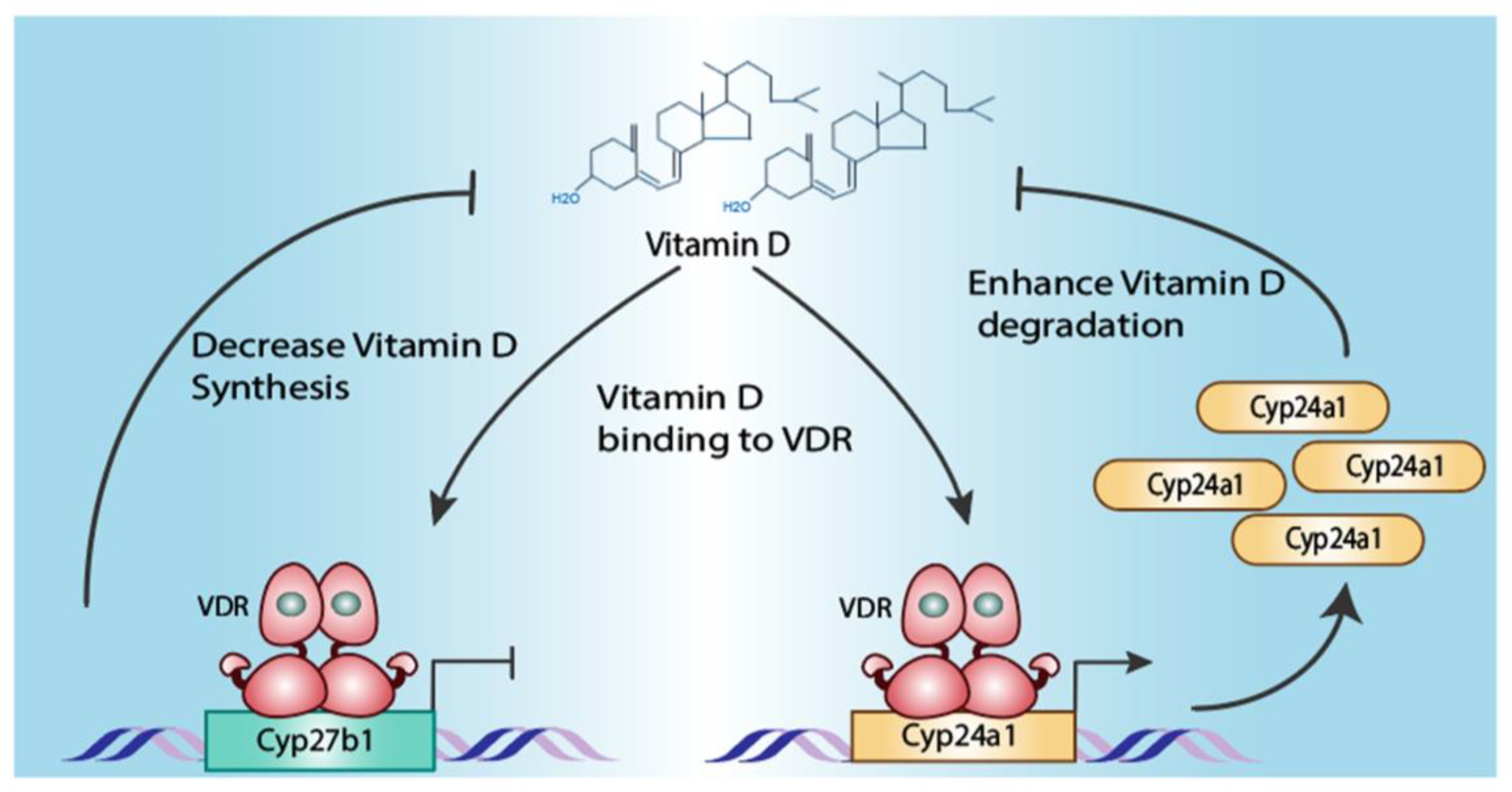
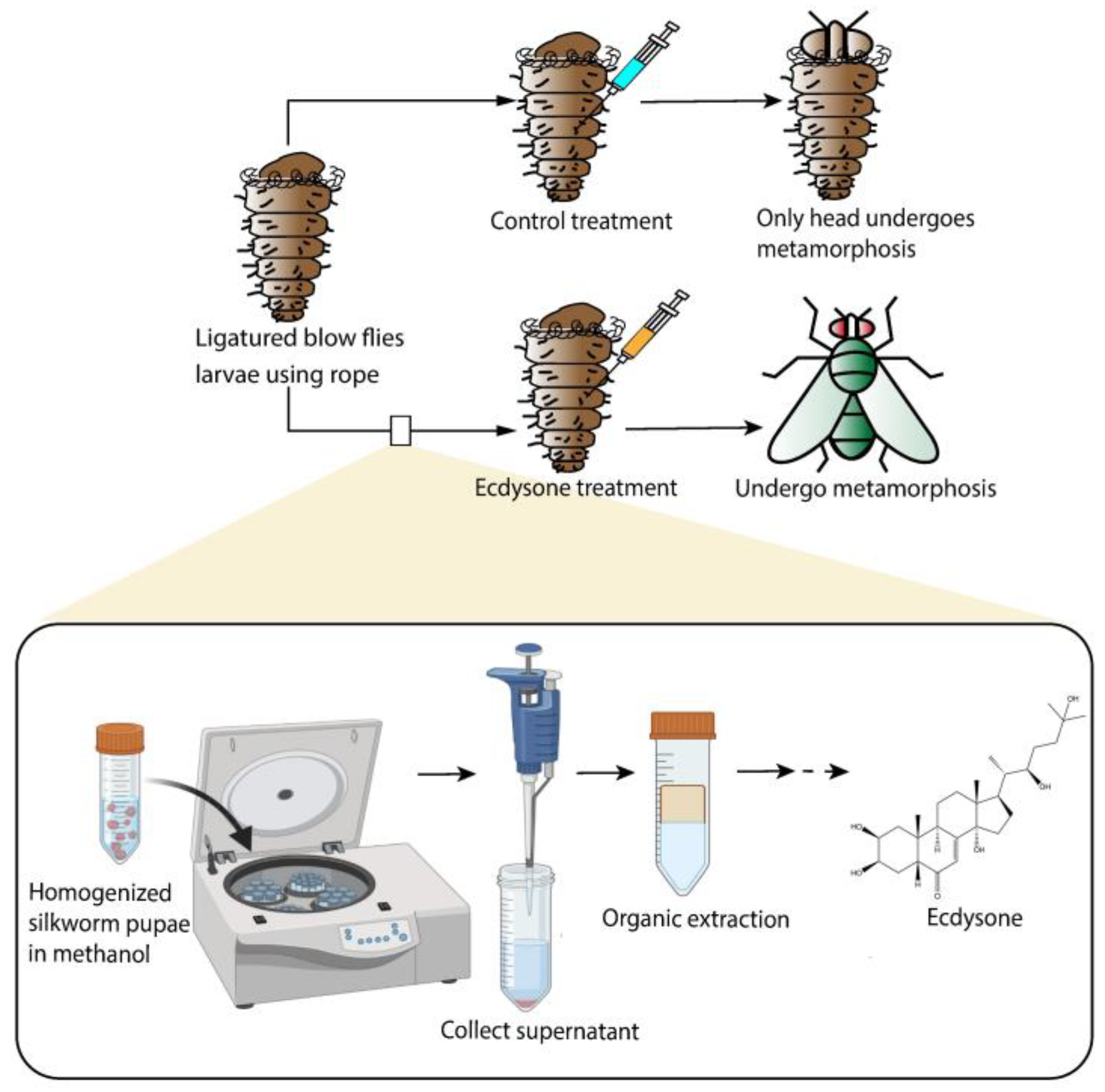
4. Feedback Loops of Ligand Biosynthesis–NHR Regulation
5. Identification of NHR Ligands and Limitations of Present Approaches
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 175, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.J.; Seo, D.E.; Jackson, B.; Ivanova, N.B.; Santori, F.R. Nuclear Hormone Receptors and Their Ligands: Metabolites in Control of Transcription. Cells 2020, 9, 2606. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, Y.; Xia, Y. An overview of aryl hydrocarbon receptor ligands in the Last two decades (2002–2022): A medicinal chemistry perspective. Eur. J. Med. Chem. 2022, 244, 114845. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Stunnenberg, H.G. Mechanisms of transactivation by retinoic acid receptors. BioEssays 1993, 15, 309–315. [Google Scholar] [CrossRef]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.J.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell. Biol. 1992, 12, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Meinke, G.; Sigler, P.B. DNA-binding mechanism of the monomeric orphan nuclear receptor NGFI-B. Nat. Struct. Biol. 1999, 6, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.E.; Fahrner, T.J.; Milbrandt, J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol. Cell. Biol. 1993, 13, 5794–5804. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu. Rev. Physiol. 2010, 72, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Schreihofer, D.A.; Duong, P.; Cunningham, R.L. N-terminal truncations in sex steroid receptors and rapid steroid actions. Steroids 2018, 133, 15–20. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, M.; Schaaf, M.J. Naturally occurring C-terminal splice variants of nuclear receptors. Nucl. Recept. Signal. 2009, 7, e007. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Schmidt, L.J.; Heemers, H.V.; Vessella, R.L.; Tindall, D.J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008, 68, 5469–5477. [Google Scholar] [CrossRef]
- Zanaria, E.; Muscatelli, F.; Bardoni, B.; Strom, T.M.; Guioli, S.; Guo, W.; Lalli, E.; Moser, C.; Walker, A.P.; McCabe, E.R.B.; et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 1994, 372, 635–641. [Google Scholar] [CrossRef]
- Seol, W.; Choi, H.-S.; Moore, D.D. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 1996, 272, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Suehiro, J.-I.; Miyazaki, H.; Minami, T.; Kodama, T.; Miyazono, K.; Watabe, T. The COUP-TFII variant lacking a DNA-binding domain inhibits the activation of the Cyp7a1 promoter through physical interaction with COUP-TFII. Biochem. J. 2013, 452, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Penvose, A.; Keenan, J.L.; Bray, D.; Ramlall, V.; Siggers, T. Comprehensive study of nuclear receptor DNA binding provides a revised framework for understanding receptor specificity. Nat. Commun. 2019, 10, 2514. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Mane-Padros, D.; Bolotin, E.; Jiang, T.; Sladek, F.M. Identification of a binding motif specific to HNF4 by comparative analysis of multiple nuclear receptors. Nucleic Acids Res. 2012, 40, 5343–5356. [Google Scholar] [CrossRef]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The nuclear receptor superfamily: A structural perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef]
- Cotnoir-White, D.; Laperrière, D.; Mader, S. Evolution of the repertoire of nuclear receptor binding sites in genomes. Mol. Cell. Endocrinol. 2011, 334, 76–82. [Google Scholar] [CrossRef]
- Bhimsaria, D.; Rodríguez-Martínez, J.A.; Mendez-Johnson, J.L.; Ghoshdastidar, D.; Varadarajan, A.; Bansal, M.; Daniels, D.L.; Ramanathan, P.; Ansari, A.Z. Hidden modes of DNA binding by human nuclear receptors. Nat. Commun. 2023, 14, 4179. [Google Scholar] [CrossRef]
- van Westen, G.J.P.; Gaulton, A.; Overington, J.P. Chemical, target, and bioactive properties of allosteric modulation. PLoS Comput. Biol. 2014, 10, e1003559. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.W.; Mayne, C.G.; Katzenellenbogen, J.A. Minireview: Not picking pockets: Nuclear receptor alternate-site modulators (NRAMs). Mol. Endocrinol. 2010, 24, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Bolin, S.; Miller, H.; Ng, H.L. RORgamma Structural Plasticity and Druggability. Int. J. Mol. Sci. 2020, 21, 5329. [Google Scholar] [CrossRef]
- Kamaraj, R.; Drastik, M.; Maixnerova, J.; Pavek, P. Allosteric Antagonism of the Pregnane X Receptor (PXR): Current-State-of-the-Art and Prediction of Novel Allosteric Sites. Cells 2022, 11, 2974. [Google Scholar] [CrossRef]
- Krężel, W.; Rühl, R.; de Lera, A.R. Alternative retinoid X receptor (RXR) ligands. Mol. Cell. Endocrinol. 2019, 491, 110436. [Google Scholar] [CrossRef] [PubMed]
- Umetani, M.; Domoto, H.; Gormley, A.K.; Yuhanna, I.S.; Cummins, C.L.; Javitt, N.B.; Korach, K.S.; Shaul, P.W.; Mangelsdorf, D.J. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007, 13, 1185–1192. [Google Scholar] [CrossRef]
- Starkey, N.J.E.; Li, Y.; Drenkhahn-Weinaug, S.K.; Liu, J.; Lubahn, D.B. 27-Hydroxycholesterol Is an Estrogen Receptor beta-Selective Negative Allosteric Modifier of 17beta-Estradiol Binding. Endocrinology 2018, 159, 1972–1981. [Google Scholar] [CrossRef]
- Willems, S.; Gellrich, L.; Chaikuad, A.; Kluge, S.; Werz, O.; Heering, J.; Knapp, S.; Lorkowski, S.; Schubert-Zsilavecz, M.; Merk, D. Endogenous vitamin E metabolites mediate allosteric PPARgamma activation with unprecedented co-regulatory interactions. Cell Chem. Biol. 2021, 28, 1489–1500.e1488. [Google Scholar] [CrossRef]
- Arifi, S.; Marschner, J.A.; Pollinger, J.; Isigkeit, L.; Heitel, P.; Kaiser, A.; Obeser, L.; Höfner, G.; Proschak, E.; Knapp, S.; et al. Targeting the Alternative Vitamin E Metabolite Binding Site Enables Noncanonical PPARgamma Modulation. J. Am. Chem. Soc. 2023, 145, 14802–14810. [Google Scholar] [CrossRef]
- Estébanez-Perpiñá, E.; Arnold, L.A.; Nguyen, P.; Rodrigues, E.D.; Mar, E.; Bateman, R.; Pallai, P.; Shokat, K.M.; Baxter, J.D.; Guy, R.K.; et al. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc. Natl. Acad. Sci. USA 2007, 104, 16074–16079. [Google Scholar] [CrossRef]
- Ciffolilli, S.; Wallert, M.; Bartolini, D.; Krauth, V.; Werz, O.; Piroddi, M.; Sebastiani, B.; Torquato, P.; Lorkowski, S.; Birringer, M.; et al. Human serum determination and in vitro anti-inflammatory activity of the vitamin E metabolite alpha-(13′-hydroxy)-6-hydroxychroman. Free. Radic. Biol. Med. 2015, 89, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Pedersen, K.M.; Bruun, N.H.; Laurberg, P. Narrow individual variations in serum T(4) and T(3) in normal subjects: A clue to the understanding of subclinical thyroid disease. J. Clin. Endocrinol. Metab. 2002, 87, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Metzger, D.; White, J.H.; Chambon, P. The human oestrogen receptor functions in yeast. Nature 1988, 334, 31–36. [Google Scholar] [CrossRef]
- Schena, M.; Yamamoto, K.R. Mammalian glucocorticoid receptor derivatives enhance transcription in yeast. Science 1988, 241, 965–967. [Google Scholar] [CrossRef]
- Schena, M.; Lloyd, A.M.; Davis, R.W. A steroid-inducible gene expression system for plant cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10421–10425. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- Fahrbach, S.E.; Smagghe, G.; Velarde, R.A. Insect nuclear receptors. Annu. Rev. Èntomol. 2012, 57, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.D.; Berg, J.M. Zinc fingers in Caenorhabditis elegans: Finding families and probing pathways. Science 1998, 282, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Sluder, A.E.; Mathews, S.W.; Hough, D.; Yin, V.P.; Maina, C.V. The nuclear receptor superfamily has undergone extensive proliferation and diversification in nematodes. Genome Res. 1999, 9, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Rechavi, M.; Maina, C.V.; Gissendanner, C.R.; Laudet, V.; Sluder, A. Explosive lineage-specific expansion of the orphan nuclear receptor HNF4 in nematodes. J. Mol. Evol. 2005, 60, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sural, S.; Hobert, O. Nematode nuclear receptors as integrators of sensory information. Curr. Biol. 2021, 31, 4361–4366.e4362. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, J.; Zheng, X.; Wang, X. Nematode Pheromones: Structures and Functions. Molecules 2023, 28, 2409. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, J.T.; Eick, G.N.; Larroux, C.; Deshpande, K.; Harms, M.J.; Gauthier, M.E.A.; Ortlund, E.A.; Degnan, B.M.; Thornton, J.W. Protein evolution by molecular tinkering: Diversification of the nuclear receptor superfamily from a ligand-dependent ancestor. PLoS Biol. 2010, 8, e1000497. [Google Scholar] [CrossRef]
- Duda, K.; Chi, Y.-I.; Shoelson, S.E. Structural basis for HNF-4α activation by ligand and coactivator binding. J. Biol. Chem. 2004, 279, 23311–23316. [Google Scholar] [CrossRef]
- Dhe-Paganon, S.; Duda, K.; Iwamoto, M.; Chi, Y.-I.; Shoelson, S.E. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J. Biol. Chem. 2002, 277, 37973–37976. [Google Scholar] [CrossRef]
- Wisely, G.; Davis, R.G.; Thornquest, A.D., Jr.; Johnson, R.; Spitzer, T.; Sefler, A.; Shearer, B.; Moore, J.T.; Miller, A.B.; Willson, T.M.; et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure 2002, 10, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Begovic, E.; Chapman, J.; Putnam, N.H.; Hellsten, U.; Kawashima, T.; Kuo, A.; Mitros, T.; Salamov, A.; Carpenter, M.L.; et al. The Trichoplax genome and the nature of placozoans. Nature 2008, 454, 955–960. [Google Scholar] [CrossRef]
- Coppola, U.; Waxman, J.S. Origin and evolutionary landscape of Nr2f transcription factors across Metazoa. PLoS ONE 2021, 16, e0254282. [Google Scholar] [CrossRef] [PubMed]
- Novotný, J.P.; Chughtai, A.A.; Kostrouchová, M.; Kostrouchová, V.; Kostrouch, D.; Kaššák, F.; Kaňa, R.; Schierwater, B.; Kostrouch, Z. Trichoplax adhaerens reveals a network of nuclear receptors sensitive to 9-cis-retinoic acid at the base of metazoan evolution. PeerJ 2017, 5, e3789. [Google Scholar] [CrossRef]
- Reitzel, A.M.; Macrander, J.; Mane-Padros, D.; Fang, B.; Sladek, F.M.; Tarrant, A.M. Conservation of DNA and ligand binding properties of retinoid X receptor from the placozoan Trichoplax adhaerens to human. J. Steroid Biochem. Mol. Biol. 2018, 184, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Krzyżosiak, A.; Niewiadomska-Cimicka, A.; Rochel, N.; Szeles, L.; Vaz, B.; Wietrzych-Schindler, M.; Álvarez, S.; Szklenar, M.; Nagy, L.; et al. 9-cis-13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice. PLoS Genet. 2015, 11, e1005213. [Google Scholar] [CrossRef]
- Reitzel, A.M.; Tarrant, A.M. Nuclear receptor complement of the cnidarian Nematostella vectensis: Phylogenetic relationships and developmental expression patterns. BMC Evol. Biol. 2009, 9, 230. [Google Scholar] [CrossRef]
- Phelps, C.; Gburcik, V.; Suslova, E.; Dudek, P.; Forafonov, F.; Bot, N.; MacLean, M.; Fagan, R.J.; Picard, D. Fungi and animals may share a common ancestor to nuclear receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 7077–7081. [Google Scholar] [CrossRef]
- Houston, D.R.; Hanna, J.G.; Lathe, J.C.; Hillier, S.G.; Lathe, R. Evidence that nuclear receptors are related to terpene synthases. J. Mol. Endocrinol. 2022, 68, 153–166. [Google Scholar] [CrossRef]
- Tsai, N.-P.; Huq, M.; Gupta, P.; Yamamoto, K.; Kagechika, H.; Wei, L.-N. Activation of testicular orphan receptor 4 by fatty acids. Biochim. Biophys. Acta 2009, 1789, 734–740. [Google Scholar] [CrossRef]
- Xie, S.; Lee, Y.-F.; Kim, E.; Chen, L.-M.; Ni, J.; Fang, L.-Y.; Liu, S.; Lin, S.-J.; Abe, J.-I.; Berk, B.; et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc. Natl. Acad. Sci. USA 2009, 106, 13353–13358. [Google Scholar] [CrossRef]
- Kandel, P.; Semerci, F.; Mishra, R.; Choi, W.; Bajic, A.; Baluya, D.; Ma, L.; Chen, K.; Cao, A.C.; Phongmekhin, T.; et al. Oleic acid is an endogenous ligand of TLX/NR2E1 that triggers hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2023784119. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; de Fabritus, L.; Tao, J.; Saied, E.M.; Lee, H.-J.; Ramazanov, B.R.; Jackson, B.; Burkhardt, D.; Parker, M.; et al. 1-deoxysphingolipids bind to COUP-TF to modulate lymphatic and cardiac cell development. Dev. Cell 2021, 56, 3128–3145.e3115. [Google Scholar] [CrossRef]
- de Urquiza, A.M.; Liu, S.; Sjöberg, M.; Zetterström, R.H.; Griffiths, W.; Sjövall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144. [Google Scholar] [CrossRef]
- Fonseca, E.; Ruivo, R.; Borges, D.; Franco, J.N.; Santos, M.M.; Castro, L.F.C. Of Retinoids and Organotins: The Evolution of the Retinoid X Receptor in Metazoa. Biomolecules 2020, 10, 594. [Google Scholar] [CrossRef]
- Kabeya, N.; Fonseca, M.M.; Ferrier, D.E.K.; Navarro, J.C.; Bay, L.K.; Francis, D.S.; Tocher, D.R.; Castro, L.F.C.; Monroig, Ó. Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 2018, 4, eaar6849. [Google Scholar] [CrossRef]
- Egea, P.F.; Mitschler, A.; Rochel, N.; Ruff, M.; Chambon, P.; Moras, D. Crystal structure of the human RXRalpha ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. EMBO J. 2000, 19, 2592–2601. [Google Scholar] [CrossRef]
- Egea, P.F.; Mitschler, A.; Moras, D. Molecular recognition of agonist ligands by RXRs. Mol. Endocrinol. 2002, 16, 987–997. [Google Scholar] [CrossRef]
- Kruse, S.W.; Suino-Powell, K.; Zhou, X.E.; E Kretschman, J.; Reynolds, R.; Vonrhein, C.; Xu, Y.; Wang, L.; Tsai, S.Y.; Tsai, M.-J.; et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008, 6, e227. [Google Scholar] [CrossRef]
- Zhou, X.E.; Suino-Powell, K.M.; Xu, Y.; Chan, C.-W.; Tanabe, O.; Kruse, S.W.; Reynolds, R.; Engel, J.D.; Xu, H.E. The orphan nuclear receptor TR4 is a vitamin A-activated nuclear receptor. J. Biol. Chem. 2011, 286, 2877–2885. [Google Scholar] [CrossRef]
- Griffett, K.; Bedia-Diaz, G.; Hegazy, L.; de Vera, I.M.S.; Wanninayake, U.S.; Billon, C.; Koelblen, T.; Wilhelm, M.L.; Burris, T.P. The Orphan Nuclear Receptor TLX Is a Receptor for Synthetic and Natural Retinoids. Cell Chem. Biol. 2020, 27, 1272–1284.e1274. [Google Scholar] [CrossRef]
- Eick, G.N.; Colucci, J.K.; Harms, M.J.; Ortlund, E.A.; Thornton, J.W. Evolution of minimal specificity and promiscuity in steroid hormone receptors. PLoS Genet. 2012, 8, e1003072. [Google Scholar] [CrossRef]
- Baker, M.E.; Nelson, D.R.; Studer, R.A. Origin of the response to adrenal and sex steroids: Roles of promiscuity and co-evolution of enzymes and steroid receptors. J. Steroid Biochem. Mol. Biol. 2015, 151, 12–24. [Google Scholar] [CrossRef]
- Poliakov, E.; Soucy, J.; Gentleman, S.; Rogozin, I.B.; Redmond, T.M. Phylogenetic analysis of the metazoan carotenoid oxygenase superfamily: A new ancestral gene assemblage of BCO-like (BCOL) proteins. Sci. Rep. 2017, 7, 13192. [Google Scholar] [CrossRef]
- Sobreira, T.J.P.; Marlétaz, F.; Simões-Costa, M.; Schechtman, D.; Pereira, A.C.; Brunet, F.; Sweeney, S.; Pani, A.; Aronowicz, J.; Lowe, C.J.; et al. Structural shifts of aldehyde dehydrogenase enzymes were instrumental for the early evolution of retinoid-dependent axial patterning in metazoans. Proc. Natl. Acad. Sci. USA 2010, 108, 226–231. [Google Scholar] [CrossRef]
- Albalat, R.; Cañestro, C. Identification of Aldh1a, Cyp26 and RAR orthologs in protostomes pushes back the retinoic acid genetic machinery in evolutionary time to the bilaterian ancestor. Chem. Biol. Interact. 2009, 178, 188–196. [Google Scholar] [CrossRef]
- Belyaeva, O.V.; Chang, C.; Berlett, M.C.; Kedishvili, N.Y. Evolutionary origins of retinoid active short-chain dehydrogenases/reductases of SDR16C family. Chem. Biol. Interact. 2014, 234, 135–143. [Google Scholar] [CrossRef]
- Thornton, J.W.; Need, E.; Crews, D. Resurrecting the ancestral steroid receptor: Ancient origin of estrogen signaling. Science 2003, 301, 1714–1717. [Google Scholar] [CrossRef]
- Markov, G.V.; Gutierrez-Mazariegos, J.; Pitrat, D.; Billas, I.M.L.; Bonneton, F.; Moras, D.; Hasserodt, J.; Lecointre, G.; Laudet, V. Origin of an ancient hormone/receptor couple revealed by resurrection of an ancestral estrogen. Sci. Adv. 2017, 3, e1601778. [Google Scholar] [CrossRef]
- Markov, G.V.; Tavares, R.; Dauphin-Villemant, C.; Demeneix, B.A.; Baker, M.E.; Laudet, V. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc. Natl. Acad. Sci. USA 2009, 106, 11913–11918. [Google Scholar] [CrossRef]
- Santori, F.R. Nuclear hormone receptors put immunity on sterols. Eur. J. Immunol. 2015, 45, 2730–2741. [Google Scholar] [CrossRef]
- Frisch, K.; Alstrup, A.K.O. On the Evolution of Bile Salts and the Farnesoid X Receptor in Vertebrates. Physiol. Biochem. Zoöl. 2018, 91, 797–813. [Google Scholar] [CrossRef]
- Reschly, E.J.; Ai, N.; Ekins, S.; Welsh, W.J.; Hagey, L.R.; Hofmann, A.F.; Krasowski, M.D. Evolution of the bile salt nuclear receptor FXR in vertebrates. J. Lipid Res. 2008, 49, 1577–1587. [Google Scholar] [CrossRef]
- Evans, S.D.; Hughes, I.V.; Gehling, J.G.; Droser, M.L. Discovery of the oldest bilaterian from the Ediacaran of South Australia. Proc. Natl. Acad. Sci. USA 2020, 117, 7845–7850. [Google Scholar] [CrossRef]
- Koelle, M.R.; Talbot, W.S.; Segraves, W.A.; Bender, M.T.; Cherbas, P.; Hogness, D.S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 1991, 67, 59–77. [Google Scholar] [CrossRef]
- Motola, D.L.; Cummins, C.L.; Rottiers, V.; Sharma, K.K.; Li, T.; Li, Y.; Suino-Powell, K.; Xu, H.E.; Auchus, R.J.; Antebi, A.; et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006, 124, 1209–1223. [Google Scholar] [CrossRef]
- Krasowski, M.D.; Ni, A.; Hagey, L.R.; Ekins, S. Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol. Cell. Endocrinol. 2010, 334, 39–48. [Google Scholar] [CrossRef]
- Reinking, J.; Lam, M.M.; Pardee, K.; Sampson, H.M.; Liu, S.; Yang, P.; Williams, S.; White, W.; Lajoie, G.; Edwards, A.; et al. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell 2005, 122, 195–207. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Lone, M.A.; Santos, T.; Alecu, I.; Silva, L.C.; Hornemann, T. 1-Deoxysphingolipids. Biochim. et Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 512–521. [Google Scholar] [CrossRef]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef]
- Wang, Z.; Benoit, G.; Liu, J.; Prasad, S.; Aarnisalo, P.; Liu, X.; Xu, H.; Walker, N.P.C.; Perlmann, T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 2003, 423, 555–560. [Google Scholar] [CrossRef]
- Woo, E.-J.; Jeong, D.G.; Lim, M.-Y.; Kim, S.J.; Kim, K.-J.; Yoon, S.-M.; Park, B.-C.; Ryu, S.E. Structural insight into the constitutive repression function of the nuclear receptor Rev-erbβ. J. Mol. Biol. 2007, 373, 735–744. [Google Scholar] [CrossRef]
- Zhi, X.; Zhou, X.E.; He, Y.; Zechner, C.; Suino-Powell, K.M.; Kliewer, S.A.; Melcher, K.; Mangelsdorf, D.J.; Xu, H.E. Structural insights into gene repression by the orphan nuclear receptor SHP. Proc. Natl. Acad. Sci. USA 2013, 111, 839–844. [Google Scholar] [CrossRef]
- Sablin, E.P.; Woods, A.; Krylova, I.N.; Hwang, P.; Ingraham, H.A.; Fletterick, R.J. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc. Natl. Acad. Sci. USA 2008, 105, 18390–18395. [Google Scholar] [CrossRef]
- de Vera, I.M.S.; Munoz-Tello, P.; Zheng, J.; Dharmarajan, V.; Marciano, D.P.; Matta-Camacho, E.; Giri, P.K.; Shang, J.; Hughes, T.S.; Rance, M.; et al. Defining a Canonical Ligand-Binding Pocket in the Orphan Nuclear Receptor Nurr1. Structure 2018, 27, 66–77.e5. [Google Scholar] [CrossRef]
- Rajan, S.; Jang, Y.; Kim, C.-H.; Kim, W.; Toh, H.T.; Jeon, J.; Song, B.; Serra, A.; Lescar, J.; Yoo, J.Y.; et al. PGE1 and PGA1 bind to Nurr1 and activate its transcriptional function. Nat. Chem. Biol. 2020, 16, 876–886. [Google Scholar] [CrossRef]
- de Vera, I.M.S.; Giri, P.K.; Munoz-Tello, P.; Brust, R.; Fuhrmann, J.; Matta-Camacho, E.; Shang, J.; Campbell, S.; Wilson, H.D.; Granados, J.; et al. Identification of a Binding Site for Unsaturated Fatty Acids in the Orphan Nuclear Receptor Nurr1. ACS Chem. Biol. 2016, 11, 1795–1799. [Google Scholar] [CrossRef]
- Hollenberg, S.M.; Weinberger, C.; Ong, E.S.; Cerelli, G.; Oro, A.; Lebo, R.; Thompson, E.B.; Rosenfeld, M.G.; Evans, R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985, 318, 635–641. [Google Scholar] [CrossRef]
- Green, S.; Walter, P.; Kumar, V.; Krust, A.; Bornert, J.-M.; Argos, P.; Chambon, P. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature 1986, 320, 134–139. [Google Scholar] [CrossRef]
- Giguere, V.; Ong, E.S.; Segui, P.; Evans, R.M. Identification of a receptor for the morphogen retinoic acid. Nature 1987, 330, 624–629. [Google Scholar] [CrossRef]
- Petkovich, M.; Brand, N.J.; Krust, A.; Chambon, P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 1987, 330, 444–450. [Google Scholar] [CrossRef]
- Simons, S.S., Jr.; Thompson, E.B. Dexamethasone 21-mesylate: An affinity label of glucocorticoid receptors from rat hepatoma tissue culture cells. Proc. Natl. Acad. Sci. USA 1981, 78, 3541–3545. [Google Scholar] [CrossRef]
- Simons, S.S., Jr.; Thompson, E.B.; Johnson, D.F. Fluorescent chemoaffinity labeling. Potential application of a new affinity labeling technique to glucocorticoid receptors. Biochemistry 1979, 18, 4915–4922. [Google Scholar] [CrossRef]
- Fischer, E. Einfluss der Configuration auf die Wirkung der Enzyme. Eur. J. Inorg. Chem. 1894, 27, 2985–2993. [Google Scholar] [CrossRef]
- de Jésus-Tran, K.P.; Côté, P.; Cantin, L.; Blanchet, J.; Labrie, F.; Breton, R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006, 15, 987–999. [Google Scholar] [CrossRef]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef]
- Stehlin, C.; Wurtz, J.; Steinmetz, A.; Greiner, E.; Schüle, R.; Moras, D.; Renaud, J. X-ray structure of the orphan nuclear receptor RORbeta ligand-binding domain in the active conformation. EMBO J. 2001, 20, 5822–5831. [Google Scholar] [CrossRef]
- Stehlin-Gaon, C.; Willmann, D.; Zeyer, D.; Sanglier, S.; Van Dorsselaer, A.; Renaud, J.-P.; Moras, D.; Schüle, R. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORβ. Nat. Struct. Mol. Biol. 2003, 10, 820–825. [Google Scholar] [CrossRef]
- Suino-Powell, K.; Xu, Y.; Zhang, C.; Tao, Y.-G.; Tolbert, W.D.; Simons, S.S., Jr.; Xu, H.E. Doubling the size of the glucocorticoid receptor ligand binding pocket by deacylcortivazol. Mol. Cell. Biol. 2008, 28, 1915–1923. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Forman, B.M.; Goode, E.; Chen, J.; E Oro, A.; Bradley, D.J.; Perlmann, T.; Noonan, D.J.; Burka, L.T.; McMorris, T.; Lamph, W.W.; et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995, 81, 687–693. [Google Scholar] [CrossRef]
- Yoshio, A.; Hiroshi, M.; Fumio, N. Correlation of bile acid composition between liver tissue and bile. Clin. Chim. Acta 1983, 133, 125–132. [Google Scholar] [CrossRef]
- Kitareewan, S.; Burka, L.T.; Tomer, K.B.; E Parker, C.; Deterding, L.J.; Stevens, R.D.; Forman, B.M.; E Mais, D.; A Heyman, R.; McMorris, T.; et al. Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol. Biol. Cell 1996, 7, 1153–1166. [Google Scholar] [CrossRef]
- Gudas, L.J. Retinoid metabolism: New insights. J. Mol. Endocrinol. 2022, 69, T37–T49. [Google Scholar] [CrossRef]
- Niederreither, K.; Abu-Abed, S.; Schuhbaur, B.; Petkovich, M.; Chambon, P.; Dollé, P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 2002, 31, 84–88. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Chanda, D.; Park, J.-H.; Choi, H.-S. Molecular basis of endocrine regulation by orphan nuclear receptor Small Heterodimer Partner. Endocr. J. 2008, 55, 253–268. [Google Scholar] [CrossRef]
- Avram, D.; Ishmael, J.E.; Nevrivy, D.J.; Peterson, V.J.; Lee, S.-H.; Dowell, P.; Leid, M. Heterodimeric interactions between chicken ovalbumin upstream promoter-transcription factor family members ARP1 and ear2. J. Biol. Chem. 1999, 274, 14331–14336. [Google Scholar] [CrossRef]
- Berrodin, T.J.; Marks, M.S.; Ozato, K.; Linney, E.; A Lazar, M. Heterodimerization among thyroid hormone receptor, retinoic acid receptor, retinoid X receptor, chicken ovalbumin upstream promoter transcription factor, and an endogenous liver protein. Mol. Endocrinol. 1992, 6, 1468–1478. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef]
- Shaw, N.; Elholm, M.; Noy, N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor β/δ. J. Biol. Chem. 2003, 278, 41589–41592. [Google Scholar] [CrossRef]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef]
- Santori, F.R.; Huang, P.; van de Pavert, S.A.; Douglass, E.F., Jr.; Leaver, D.J.; Haubrich, B.A.; Keber, R.; Lorbek, G.; Konijn, T.; Rosales, B.N.; et al. Identification of Natural RORgamma Ligands that Regulate the Development of Lymphoid Cells. Cell Metab. 2015, 21, 286–298. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.; Takeda, Y.; Janjetovic, Z.; Brożyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168. [Google Scholar] [CrossRef]
- Jin, L.; Martynowski, D.; Zheng, S.; Wada, T.; Xie, W.; Li, Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORγ. Mol. Endocrinol. 2010, 24, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Hao, L.-Y.; Liu, X.; A Lesch, C.; Sanchez, B.M.; Wendling, J.M.; Morgan, R.W.; Aicher, T.D.; Carter, L.L.; et al. Sterol metabolism controls TH17 differentiation by generating endogenous RORγ agonists. Nat. Chem. Biol. 2015, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Kanno, T.; Nakajima, T.; Ikeda, K.; Taketomi, Y.; Yokoyama, S.; Sasamoto, S.; Asou, H.K.; Miyako, K.; Hasegawa, Y.; et al. 1-Oleoyl-lysophosphatidylethanolamine stimulates RORγt activity in T(H)17 cells. Sci. Immunol. 2023, 8, eadd4346. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 451–480. [Google Scholar] [CrossRef]
- Petkovich, M.; Chambon, P. Retinoic acid receptors at 35 years. J. Mol. Endocrinol. 2022, 69, T13–T24. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Zhu, X.H.; Pei, Y.L.; Jackson, D.M.; Holick, M.F. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3-1alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6988–6993. [Google Scholar] [CrossRef]
- Murayama, A.; Takeyama, K.-I.; Kitanaka, S.; Kodera, Y.; Kawaguchi, Y.; Hosoya, T.; Kato, S. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology 1999, 140, 2224–2231. [Google Scholar] [CrossRef]
- Meyer, M.B.; Goetsch, P.D.; Pike, J.W. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J. Biol. Chem. 2010, 285, 15599–15610. [Google Scholar] [CrossRef]
- Zierold, C.; Darwish, H.M.; DeLuca, H.F. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995, 270, 1675–1678. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ozono, K.; Uchida, M.; Yoshimura, M.; Shinki, T.; Suda, T.; Yamamoto, O. Functional assessment of two vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase gene. J. Biol. Chem. 1996, 271, 30381–30385. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.-I.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science 1997, 277, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Hanebuth, E.; Smeyne, R.J.; Everds, N.; Stewart, C.L.; Wehner, J.M.; Curran, T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: Evidence for tissue-specific modulation of receptor function. EMBO J. 1996, 15, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Fraichard, A.; Chassande, O.; Plateroti, M.; Roux, J.P.; Trouillas, J.; Dehay, C.; Legrand, C.; Gauthier, K.; Kedinger, M.; Malaval, L.; et al. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 1997, 16, 4412–4420. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, T.C.; Schmutzler, C.; Meissner, J.; Köhrle, J. The promoter of the human type I 5′-deiodinase gene--mapping of the transcription start site and identification of a DR+4 thyroid-hormone-responsive element. Eur. J. Biochem. 1997, 247, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, H.; Sasaki, S.; Suzuki, S.; Matsushita, A.; Nakamura, H.; Nakamura, H.M.; Hirahara, N.; Kuroda, G.; Iwaki, H.; Ohba, K.; et al. Essential Role of GATA2 in the Negative Regulation of Type 2 Deiodinase Gene by Liganded Thyroid Hormone Receptor β2 in Thyrotroph. PLoS ONE 2015, 10, e0142400. [Google Scholar] [CrossRef] [PubMed]
- Barca-Mayo, O.; Liao, X.-H.; Alonso, M.; Di Cosmo, C.; Hernandez, A.; Refetoff, S.; Weiss, R.E. Thyroid hormone receptor α and regulation of type 3 deiodinase. Mol. Endocrinol. 2011, 25, 575–583. [Google Scholar] [CrossRef]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR α. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Turley, S.D.; Ma, W.; Janowski, B.A.; Lobaccaro, J.M.; Hammer, R.E.; Mangelsdorf, D.J. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 1998, 93, 693–704. [Google Scholar] [CrossRef]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Calkin, A.C.; Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012, 13, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Chappell, P.E.; Lydon, J.P.; Conneely, O.M.; O’Malley, B.W.; Levine, J.E. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 1997, 138, 4147–4152. [Google Scholar] [CrossRef]
- Couse, J.F.; Curtis, S.W.; Washburn, T.F.; Lindzey, J.; Golding, T.S.; Lubahn, D.B.; Smithies, O.; Korach, K.S. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995, 9, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Charest, N.J.; Zhou, Z.-X.; Lubahn, D.B.; Olsen, K.L.; Wilson, E.M.; French, F.S. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol. Endocrinol. 1991, 5, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, W.G.; E Quarmby, V.; A Simental, J.; Joseph, D.R.; Sar, M.; Lubahn, D.B.; Olsen, K.L.; French, F.S.; Wilson, E.M. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J. Biol. Chem. 1990, 265, 8893–8900. [Google Scholar] [CrossRef]
- Brown, T.R.; Lubahn, D.B.; Wilson, E.M.; Joseph, D.R.; French, F.S.; Migeon, C.J. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: Evidence for further genetic heterogeneity in this syndrome. Proc. Natl. Acad. Sci. USA 1988, 85, 8151–8155. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Brown, T.R.; A Simental, J.; Higgs, H.N.; Migeon, C.J.; Wilson, E.M.; French, F.S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc. Natl. Acad. Sci. USA 1989, 86, 9534–9538. [Google Scholar] [CrossRef]
- Murphy, L.; O’Shaughnessy, P.J. Testicular steroidogenesis in the testicular feminized (Tfm) mouse: Loss of 17α-hydroxylase activity. J. Endocrinol. 1991, 131, 443–449. [Google Scholar] [CrossRef]
- Le Goascogne, C.; Sananès, N.; Gouézou, M.; Baulieu, E.E.; Robel, P. Suppressed expression of the cytochrome P45017α protein in the testicular feminized (Tfm) mouse testes. J. Endocrinol. 1993, 139, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; A Blendy, J.; Monaghan, A.P.; Krieglstein, K.; Schmid, W.; Aguzzi, A.; Fantuzzi, G.; Hummler, E.; Unsicker, K.; Schütz, G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995, 9, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Bleich, M.; Schmid, W.; Cole, T.J.; Peters, J.; Watanabe, H.; Kriz, W.; Warth, R.; Greger, R.; Schütz, G. Mineralocorticoid receptor knockout mice: Pathophysiology of Na+ metabolism. Proc. Natl. Acad. Sci. USA 1998, 95, 9424–9429. [Google Scholar] [CrossRef] [PubMed]
- Meinsohn, M.-C.; Smith, O.E.; Bertolin, K.; Murphy, B.D. The Orphan Nuclear Receptors Steroidogenic Factor-1 and Liver Receptor Homolog-1: Structure, Regulation, and Essential Roles in Mammalian Reproduction. Physiol. Rev. 2019, 99, 1249–1279. [Google Scholar] [CrossRef] [PubMed]
- Krylova, I.N.; Sablin, E.P.; Moore, J.; Xu, R.X.; Waitt, G.M.; MacKay, J.A.; Juzumiene, D.; Bynum, J.M.; Madauss, K.; Montana, V.; et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 2005, 120, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wang, J.; Gao, B.; Li, J.; Wu, F.; Zou, J.X.; Xu, J.; Jiang, Y.; Zou, H.; Huang, Z.; et al. RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 2019, 10, 4621. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yang, N.; Zhang, X.; Chen, H.-W. RORγ is a context-specific master regulator of cholesterol biosynthesis and an emerging therapeutic target in cancer and autoimmune diseases. Biochem. Pharmacol. 2022, 196, 114725. [Google Scholar] [CrossRef]
- Erkner, E.; Hentrich, T.; Schairer, R.; Fitzel, R.; Secker-Grob, K.-A.; Jeong, J.; Keppeler, H.; Korkmaz, F.; Schulze-Hentrich, J.M.; Lengerke, C.; et al. The RORγ/SREBP2 pathway is a master regulator of cholesterol metabolism and serves as potential therapeutic target in t(4;11) leukemia. Oncogene 2023, 43, 281–293. [Google Scholar] [CrossRef]
- Merrill, A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Esaki, K.; Sayano, T.; Sonoda, C.; Akagi, T.; Suzuki, T.; Ogawa, T.; Okamoto, M.; Yoshikawa, T.; Hirabayashi, Y.; Furuya, S. L-Serine Deficiency Elicits Intracellular Accumulation of Cytotoxic Deoxysphingolipids and Lipid Body Formation. J. Biol. Chem. 2015, 290, 14595–14609. [Google Scholar] [CrossRef]
- Varshney, R.; Varshney, R.; Das, S.; Das, S.; Trahan, G.D.; Trahan, G.D.; Farriester, J.W.; Farriester, J.W.; Mullen, G.P.; Mullen, G.P.; et al. Neonatal intake of Omega-3 fatty acids enhances lipid oxidation in adipocyte precursors. iScience 2022, 26, 105750. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.C. A method for the decomposition of the proteins of the thyroid, with a description of certain constituents. J. Biol. Chem. 1915, 20, 501–509. [Google Scholar] [CrossRef]
- Kendall, E.C. The isolation in crystalline form for the compound containing iodin, which occurs in the thyroid—Its chemical nature and physiologic activity. JAMA 1915, 64, 2042–2043. [Google Scholar] [CrossRef]
- Harington, C.R.; Barger, G. Chemistry of Thyroxine: Constitution and Synthesis of Thyroxine. Biochem. J. 1927, 21, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.; Clark, A.B. A Contribution to the Study of Keratomalacia among Rats. Biochem. J. 1920, 14, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Karrer, P.; Morf, R.; Schöpp, K. Zur Kenntnis des Vitamins-A aus Fischtranen. Helvetica Chim. Acta 1931, 14, 1036–1040. [Google Scholar] [CrossRef]
- Arens, J.F.; Van Dorp, D.A. Synthesis of some Compounds Possessing Vitamin A Activity. Nature 1946, 157, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, V.B. Memoirs: The Physiology of Ecdysis in Rhodnius Prolixus (Hemiptera). II. Factors controlling Moulting and ‘Metamorphosis’. J. Cell Sci. 1934, s2-77, 191–222. [Google Scholar] [CrossRef]
- Kopeć, S. Studies on the necessity of the brain for the inception of insect metamorphosis. Biol. Bull. 1922, 42, 323–342. [Google Scholar] [CrossRef]
- Becker, E. Uber Versuche Zur Anreicherung Und Physio-Logischer Charakterisierung Des Wirkstoffes Der Puparisierung. Biol. Zentralbl. 1941, 61, 360–388. [Google Scholar]
- Butenandt, A.; Karlson, P. Über die Isolierung eines Metamorphose-Hormons der Insekten in kristallisierter Form. Z. Für Naturforschung B 1954, 9, 389–391. [Google Scholar] [CrossRef]
- Huber, R.; Hoppe, W. Zur Chemie des Ecdysons, VII: Die Kristall- und Molekülstrukturanalyse des Insektenverpuppungshormons Ecdyson mit der automatisierten Faltmolekülmethode. Eur. J. Inorg. Chem. 1965, 98, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Karlson, P.; Hoffmeister, H.; Hummel, H.; Hocks, P.; Spiteller, G. Zur Chemie des Ecdysons, VI: Reaktionen des Ecdysonmoleküls. Eur. J. Inorg. Chem. 1965, 98, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Kerb, U.; Hocks, P.; Wiechert, R.; Furlenmeier, A.; Fürst, A.; Langemann, A.; Waldvogel, G. Die synthese des ecdysons. Tetrahedron Lett. 1966, 7, 1387–1391. [Google Scholar] [CrossRef]
- Siddall, J.B.; Cross, A.D.; Fried, J.H. Steroids. CCXCII.1 Synthetic Studies on Insect Hormones. II. The Synthesis of Ecdysone. J. Am. Chem. Soc. 1966, 88, 862–863. [Google Scholar] [CrossRef]
- Siddall, J.B.; Marshall, J.P.; Bowers, A.; Cross, A.D.; Edwards, J.A.; Fried, J.H. Synthetic Studies on Insect Hormones. I. Synthesis of the Tetracyclic Nucleus of Ecdysone1. J. Am. Chem. Soc. 1966, 88, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Chambon, P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature 1987, 325, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kakidani, H.; Ptashne, M. GAL4 activates gene expression in mammalian cells. Cell 1988, 52, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.; Jin, J.R.; Green, S.; Hollis, M.; Chambon, P. The yeast UASG is a transcriptional enhancer in human HeLa cells in the presence of the GAL4 trans-activator. Cell 1988, 52, 169–178. [Google Scholar] [CrossRef]
- Webster, N.J.; Green, S.; Jin, J.R.; Chambon, P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell 1988, 54, 199–207. [Google Scholar] [CrossRef]
- Klein-Hitpaß, L.; Schorpp, M.; Wagner, U.; Ryffel, G.U. An estrogen-responsive element derived from the 5′ flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell 1986, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Waterman, M.L.; Adlerf, S.; Nelson, C.; Greene, G.L.; Evans, R.M.; Rosenfeld, M.G. A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol. Endocrinol. 1988, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- de Wet, J.R.; Wood, K.V.; DeLuca, M.; Helinski, D.R.; Subramani, S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol. Cell. Biol. 1987, 7, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Ong, E.S.; Dyck, J.A.; Evans, R.M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature 1990, 345, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.A.; Sturzenbecker, L.J.; Kazmer, S.; Bosakowski, T.; Huselton, C.; Allenby, G.; Speck, J.; Ratzeisen, C.; Rosenberger, M.; Lovey, A.; et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR α. Nature 1992, 355, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Heyman, R.A.; Mangelsdorf, D.J.; Dyck, J.A.; Stein, R.B.; Eichele, G.; Evans, R.M.; Thaller, C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 1992, 68, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Banno, A.; Reddy, R.C. Molecular, chemical, and structural characterization of prostaglandin A2 as a novel agonist for Nur77. Biochem. J. 2019, 476, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, S.; Ohkura, N.; Tsukada, T.; Miyagawa, M.; Sugita, Y.; Tsujimoto, G.; Matsumoto, K.; Saito, H.; Hashida, R. Prostaglandin A2 acts as a transactivator for NOR1 (NR4A3) within the nuclear receptor superfamily. Biol. Pharm. Bull. 2005, 28, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Peregrín-Alvarez, J.M.; Sanford, C.; Parkinson, J. The conservation and evolutionary modularity of metabolism. Genome Biol. 2009, 10, R63. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2021, 50, D622–D631. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Bloch, K. The biological synthesis of cholesterol. Science 1965, 150, 19–28. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2017, 58, 2314–2333. [Google Scholar] [CrossRef]
- Hörlein, A.J.; Näär, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Soderstrom, M.; Glass, C.K.; et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef]
- Chen, J.D.; Evans, R.M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 1995, 377, 454–457. [Google Scholar] [CrossRef]
- Glass, C.K.; Franco, R.; Weinberger, C.; Albert, V.R.; Evans, R.M.; Rosenfeld, M.G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature 1987, 329, 738–741. [Google Scholar] [CrossRef]
- Huh, J.R.; Leung, M.W.L.; Huang, P.; Ryan, D.A.; Krout, M.R.; Malapaka, R.R.V.; Chow, J.; Manel, N.; Ciofani, M.; Kim, S.V.; et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 2011, 472, 486–490. [Google Scholar] [CrossRef]
- Hanada, K.; Nishijima, M.; Kiso, M.; Hasegawa, A.; Fujita, S.; Ogawa, T.; Akamatsu, Y. Sphingolipids are essential for the growth of Chinese hamster ovary cells. Restoration of the growth of a mutant defective in sphingoid base biosynthesis by exogenous sphingolipids. J. Biol. Chem. 1992, 267, 23527–23533. [Google Scholar] [CrossRef]
- Wells, G.B.; Lester, R.L. The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J. Biol. Chem. 1983, 258, 10200–10203. [Google Scholar] [CrossRef]
- Crowder, C.M.; Westover, E.J.; Kumar, A.S.; Ostlund, R.E., Jr.; Covey, D.F. Enantiospecificity of cholesterol function in vivo. J. Biol. Chem. 2001, 276, 44369–44372. [Google Scholar] [CrossRef]
- Ozers, M.S.; Ervin, K.M.; Steffen, C.L.; Fronczak, J.A.; Lebakken, C.S.; Carnahan, K.A.; Lowery, R.G.; Burke, T.J. Analysis of ligand-dependent recruitment of coactivator peptides to estrogen receptor using fluorescence polarization. Mol. Endocrinol. 2005, 19, 25–34. [Google Scholar] [CrossRef]
- Usami, M.; Mitsunaga, K.; Ohno, Y. Estrogen receptor binding assay of chemicals with a surface plasmon resonance biosensor. J. Steroid Biochem. Mol. Biol. 2002, 81, 47–55. [Google Scholar] [CrossRef]
- Johnson, B.A.; Wilson, E.M.; Li, Y.; Moller, D.E.; Smith, R.G.; Zhou, G. Ligand-induced stabilization of PPARgamma monitored by NMR spectroscopy: Implications for nuclear receptor activation. J. Mol. Biol. 2000, 298, 187–194. [Google Scholar] [CrossRef]
- Tan, B.; O’dell, D.K.; Yu, Y.W.; Monn, M.F.; Hughes, H.V.; Burstein, S.; Walker, J.M. Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J. Lipid Res. 2010, 51, 112–119. [Google Scholar] [CrossRef]
- Butenandt, A. Über die chemische Untersuchung der Sexualhormone. Angew. Chem. 1931, 44, 905–908. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- David, K.; Dingemanse, E.; Freud, J.; Laqueur, E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Biol. Chem. 1935, 233, 281–283. [Google Scholar] [CrossRef]
- Windaus, A.; Thiele, W. Über die Konstitution des Vitamins D2. Eur. J. Org. Chem. 1936, 521, 160–175. [Google Scholar] [CrossRef]
- Windaus, A.; Schenck, F.; Werder, F.T. Über das antirachitisch wirksame Bestrahlungsprodukt ans 7-Dehydro-cholesterin. Biol. Chem. 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Blunt, J.W.; Tanaka, Y.; DeLuca, H.F. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc. Natl. Acad. Sci. USA 1968, 61, 1503–1506. [Google Scholar] [CrossRef]
- Blunt, J.W.; DeLuca, H.F. The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 1969, 8, 671–675. [Google Scholar] [CrossRef]
- Blunt, J.W.; DeLuca, H.F.; Schnoes, H.K. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry 1968, 7, 3317–3322. [Google Scholar] [CrossRef]
- Lawson, D.E.M.; Wilson, P.W.; Kodicek, E. Metabolism of vitamin D. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem. J. 1969, 115, 269–277. [Google Scholar] [CrossRef]
- Mahanti, P.; Bose, N.; Bethke, A.; Judkins, J.C.; Wollam, J.; Dumas, K.J.; Zimmerman, A.M.; Campbell, S.L.; Hu, P.J.; Antebi, A.; et al. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab. 2014, 19, 73–83. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Anderson, M.T.; Payne, N.; Santori, F.R.; Ivanova, N.B. Nuclear Receptors and the Hidden Language of the Metabolome. Cells 2024, 13, 1284. https://doi.org/10.3390/cells13151284
Chen Y, Anderson MT, Payne N, Santori FR, Ivanova NB. Nuclear Receptors and the Hidden Language of the Metabolome. Cells. 2024; 13(15):1284. https://doi.org/10.3390/cells13151284
Chicago/Turabian StyleChen, Yujie, Matthew Tom Anderson, Nathaniel Payne, Fabio R. Santori, and Natalia B. Ivanova. 2024. "Nuclear Receptors and the Hidden Language of the Metabolome" Cells 13, no. 15: 1284. https://doi.org/10.3390/cells13151284




