Identification of Poliovirus Receptor-like 3 Protein as a Prognostic Factor in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Profiling of Immune-Related Genes in TNBC Patients
2.2. Cell Line and Culture
2.3. Drug Treatments
2.4. Cell Viability
2.5. Generation of Human TNBC Cell Lines Constitutively Overexpressing EZH2
2.6. Generation of Human TNBC Cell Lines Silenced for EZH2
2.7. Total RNA Extraction, cDNA Synthesis, and Quantitative RT-qPCR Analyses
2.8. Computational Analysis for Regulation of PVRL3 Expression
2.9. Statistical Analyses
3. Results
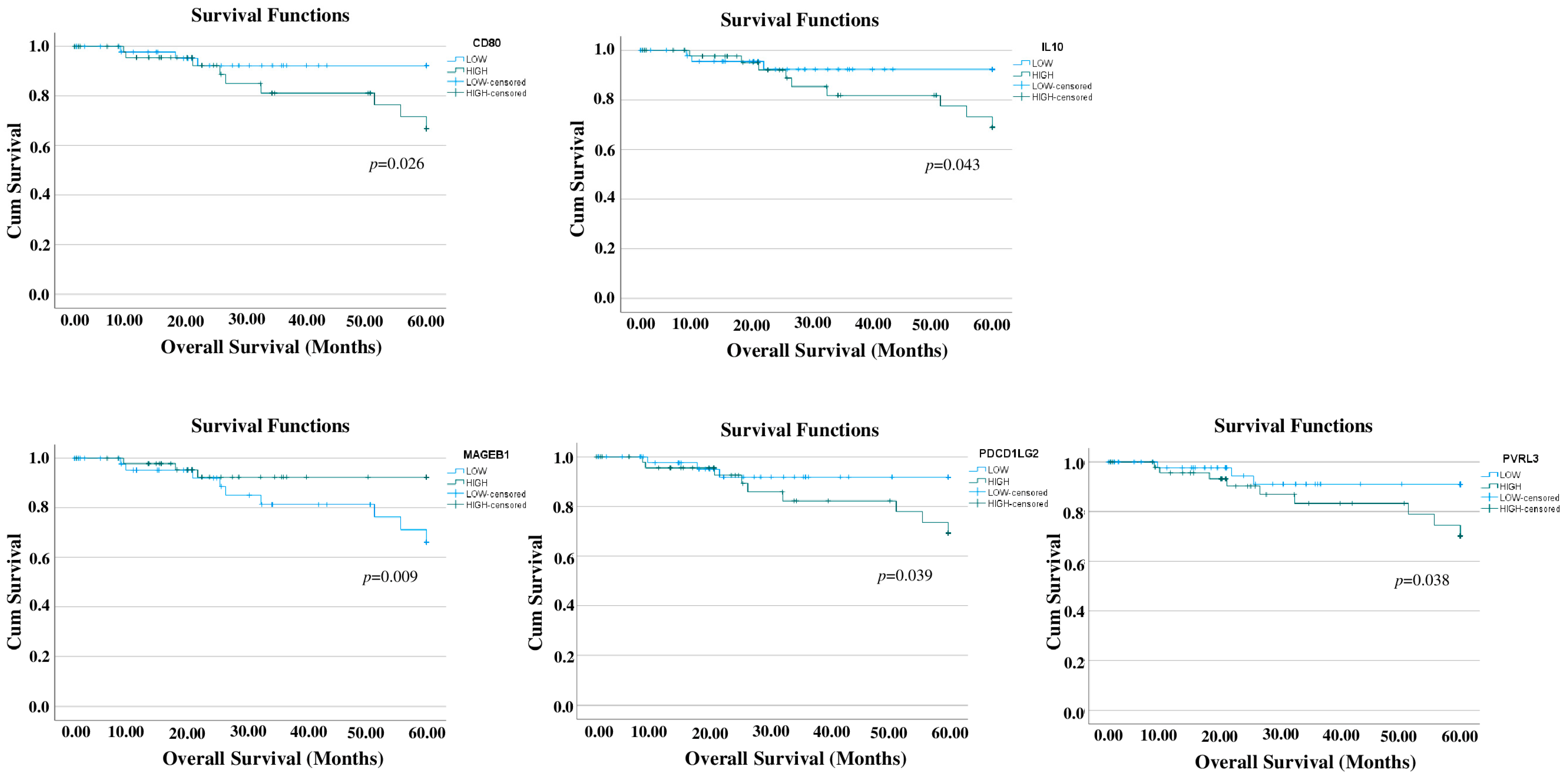
3.1. Prognostic Relevance of Immune-Related Genes in TNBC
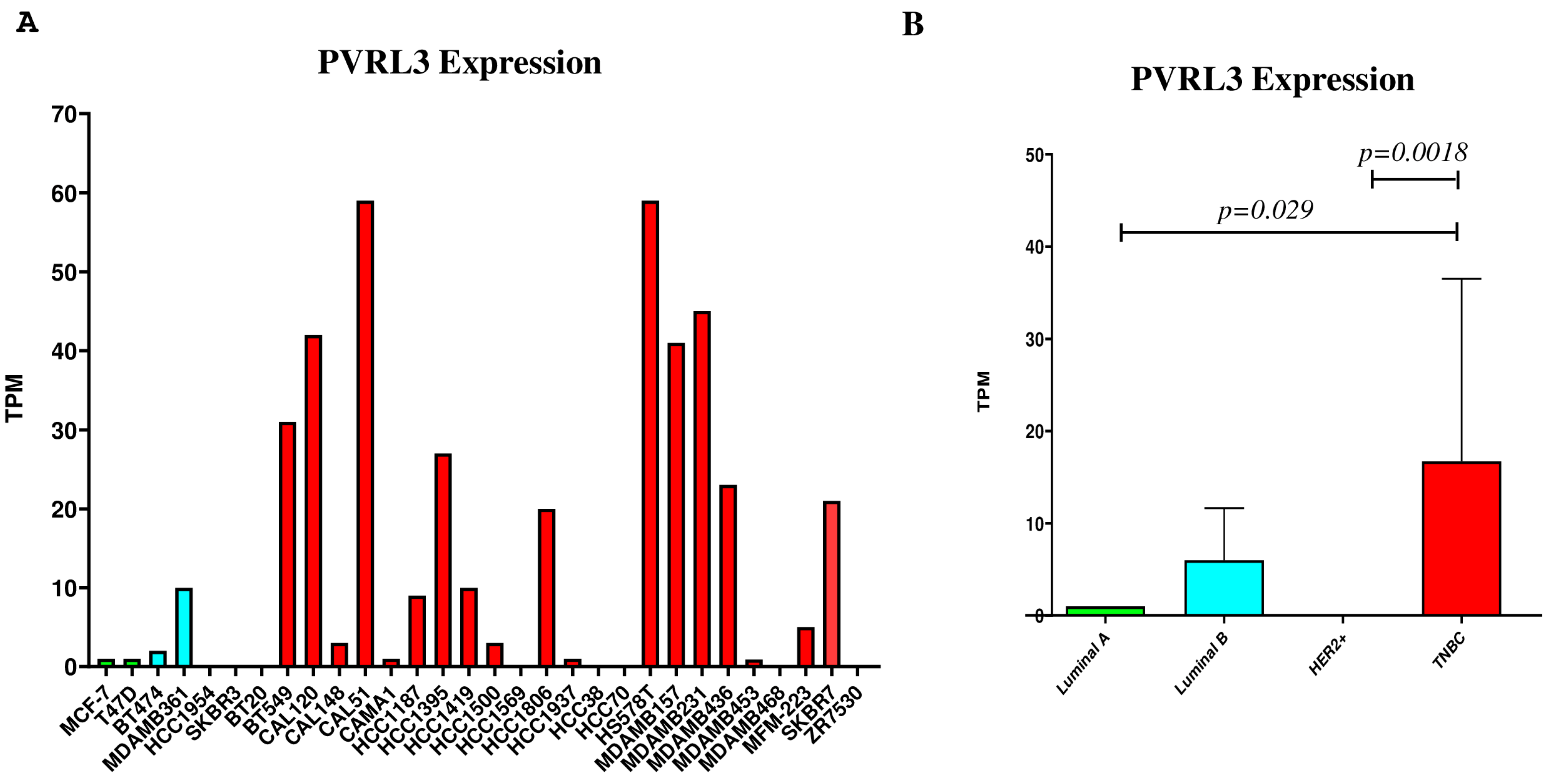
3.2. PVRL3 Gene Expression Is Higher in TNBC Cell Lines
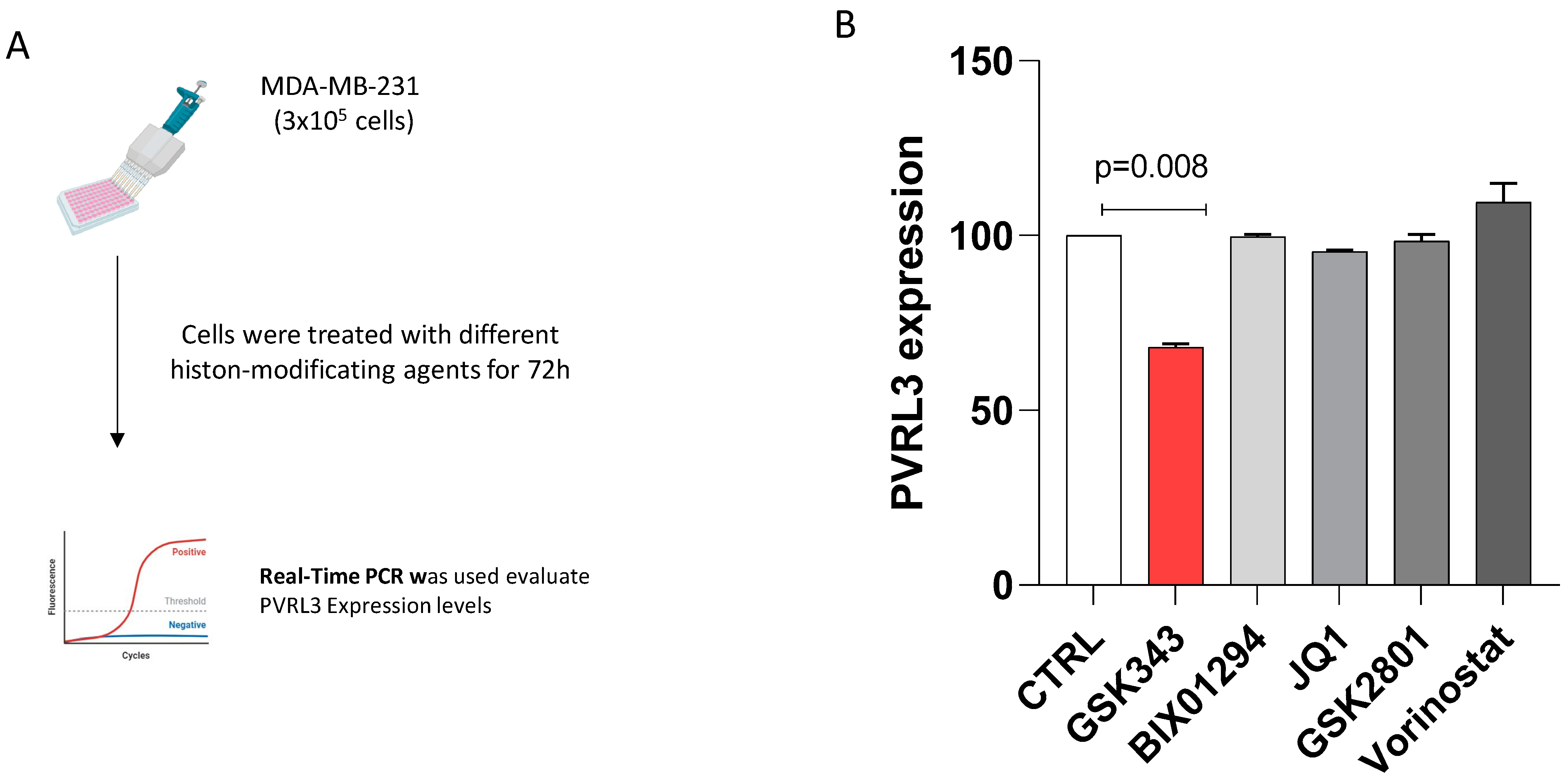
3.3. The Inhibition of EZH2 Catalytic Activity Modulates PVRL3 Expression in MDA-MB-231 Cells
3.4. PVRL3 Expression Is Modulated by EZH2
3.5. Computational Analysis Identifies EZH2 as a Regulator Factor of PVRL3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef] [PubMed]
- Won, K.; Spruck, C. Triple-negative Breast Cancer Therapy: Current and Future Perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-Negative Breast Cancer: Current Treatment Strategies and Factors of Negative Prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Huppert, L.A.; Gumusay, O.; Rugo, H.S. Emerging Treatment Strategies for Metastatic Triple-Negative Breast Cancer. Ther. Adv. Med. Oncol. 2022, 14, 175883592210869. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef] [PubMed]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.-R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387.e19. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Tang, H.; Meng, X.; Zheng, Q. Molecular Mechanisms of Immunotherapy Resistance in Triple-Negative Breast Cancer. Front. Immunol. 2023, 14, 1153990. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint Inhibitors in Triple-negative Breast Cancer (TNBC): Where to Go from Here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef]
- Maniwa, Y.; Nishio, W.; Okita, Y.; Yoshimura, M. Expression of Nectin 3: Novel Prognostic Marker of Lung Adenocarcinoma. Thorac. Cancer 2012, 3, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hong, X.; Li, K.; Li, Y.; Li, Y.; He, S.; Zhang, P.; Li, J.; Li, Q.; Liang, Y.; et al. ZNF582 Hypermethylation Promotes Metastasis of Nasopharyngeal Carcinoma by Regulating the Transcription of Adhesion Molecules Nectin-3 and NRXN3. Cancer Commun. 2020, 40, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Si, X.; Wang, J.; Yang, A.; Qin, T.; Yang, Y. Nectin-3 Is a New Biomarker That Mediates the Upregulation of MMP2 and MMP9 in Ovarian Cancer Cells. Biomed. Pharmacother. 2019, 110, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Suhovskih, A.V.; Kashuba, V.I.; Klein, G.; Grigorieva, E.V. Prostate Cancer Cells Specifically Reorganize Epithelial Cell-Fibroblast Communication through Proteoglycan and Junction Pathways. Cell Adh. Migr. 2017, 11, 39–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, B.; Zhong, C.; Lang, Q.; Liang, Z.; Zhang, Y.; Zhao, X.; Yu, Y.; Zhang, H.; Xu, F.; Tian, Y. Poliovirus Receptor (PVR)-like Protein Cosignaling Network: New Opportunities for Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gong, Y.; Meng, H.; Li, C.; Xue, L. Symphony of Epigenetic and Metabolic Regulation—Interaction between the Histone Methyltransferase EZH2 and Metabolism of Tumor. Clin. Epigenet. 2020, 12, 72. [Google Scholar] [CrossRef]
- Dardis, G.J.; Wang, J.; Simon, J.M.; Wang, G.G.; Baldwin, A.S. An EZH2-NF-ΚB Regulatory Axis Drives Expression of pro-Oncogenic Gene Signatures in Triple Negative Breast Cancer. iScience 2023, 26, 107115. [Google Scholar] [CrossRef]
- Sun, S.; Yu, F.; Xu, D.; Zheng, H.; Li, M. EZH2, a Prominent Orchestrator of Genetic and Epigenetic Regulation of Solid Tumor Microenvironment and Immunotherapy. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188700. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; Luo, Y.; Tong, S.; Liu, Y. EZH2: The Roles in Targeted Therapy and Mechanisms of Resistance in Breast Cancer. Biomed. Pharmacother. 2024, 175, 116624. [Google Scholar] [CrossRef]
- Yu, W.; Liu, N.; Song, X.; Chen, L.; Wang, M.; Xiao, G.; Li, T.; Wang, Z.; Zhang, Y. EZH2: An Accomplice of Gastric Cancer. Cancers 2023, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhou, Y.; Luo, Y.; Qiu, J.; Xiao, Y.; Zou, J.; Zhang, Y.; Liu, X.; Yang, X.; Gou, K.; et al. Epigenetic Regulation in Cancer Therapy: From Mechanisms to Clinical Advances. MedComm-Oncology 2024, 3, e59. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.-C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive Analyses of Tumor Immunity: Implications for Cancer Immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Bramanti, A.; Ciurleo, R.; Basile, M.; Pennisi, M.; Bella, R.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P. Profiling of Inhibitory Immune Checkpoints in Glioblastoma: Potential Pathogenetic Players. Oncol. Lett. 2020, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef] [PubMed]
- Huuhtanen, J.; Kasanen, H.; Peltola, K.; Lönnberg, T.; Glumoff, V.; Brück, O.; Dufva, O.; Peltonen, K.; Vikkula, J.; Jokinen, E.; et al. Single-Cell Characterization of Anti–LAG-3 and Anti–PD-1 Combination Treatment in Patients with Melanoma. J. Clin. Investig. 2023, 133, e164809. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Swain, D.; Kushwaha, P.P.; Brahmbhatt, S.; Gupta, K.; Sundi, D.; Gupta, S. Melanoma Antigen Family A (MAGE A) as Promising Biomarkers and Therapeutic Targets in Bladder Cancer. Cancers 2024, 16, 246. [Google Scholar] [CrossRef]
- Li, S.; Shi, X.; Li, J.; Zhou, X. Pathogenicity of the MAGE Family (Review). Oncol. Lett. 2021, 22, 844. [Google Scholar] [CrossRef]
- Ai, H.; Yang, H.; Li, L.; Ma, J.; Liu, K.; Li, Z. Cancer/Testis Antigens: Promising Immunotherapy Targets for Digestive Tract Cancers. Front. Immunol. 2023, 14, 1190883. [Google Scholar] [CrossRef]
- Takai, Y.; Ikeda, W.; Ogita, H.; Rikitake, Y. The Immunoglobulin-Like Cell Adhesion Molecule Nectin and Its Associated Protein Afadin. Annu. Rev. Cell Dev. Biol. 2008, 24, 309–342. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; C Gonzalez, L.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone Methylation: A Dynamic Mark in Health, Disease and Inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Yamagishi, M.; Uchimaru, K. Targeting EZH2 in Cancer Therapy. Curr. Opin. Oncol. 2017, 29, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.G. No Easy Way Out for EZH2: Its Pleiotropic, Noncanonical Effects on Gene Regulation and Cellular Function. Int. J. Mol. Sci. 2020, 21, 9501. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Nouruzi, S.; Ganguli, D.; Namekawa, T.; Thaper, D.; Linder, S.; Karaoğlanoğlu, F.; Omur, M.E.; Kim, S.; Kobelev, M.; et al. An Androgen Receptor Switch Underlies Lineage Infidelity in Treatment-Resistant Prostate Cancer. Nat. Cell Biol. 2021, 23, 1023–1034. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.; Woo, D.-H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of EZH2 Activates STAT3 Signaling via STAT3 Methylation and Promotes Tumorigenicity of Glioblastoma Stem-like Cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef]
- Zheng, M.; Cao, M.; Luo, X.; Li, L.; Wang, K.; Wang, S.; Wang, H.; Tang, Y.; Tang, Y.; Liang, X. EZH2 Promotes Invasion and Tumour Glycolysis by Regulating STAT3 and FoxO1 Signalling in Human OSCC Cells. J. Cell. Mol. Med. 2019, 23, 6942–6954. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Liu, L.-C.; Ye, H.-Y.; Wu, J.-Y.; Yu, Y.-L. EZH2 Promotes Migration and Invasion of Triple-Negative Breast Cancer Cells via Regulating TIMP2-MMP-2/-9 Pathway. Am. J. Cancer Res. 2018, 8, 422–434. [Google Scholar]
- Zhang, R.; Li, X.; Liu, Z.; Wang, Y.; Zhang, H.; Xu, H. EZH2 Inhibitors-Mediated Epigenetic Reactivation of FOSB Inhibits Triple-Negative Breast Cancer Progress. Cancer Cell Int. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Okabe, H.; Sakamoto, Y.; Hayashi, H.; Hashimoto, D.; Yokoyama, N.; Sakamoto, K.; Kuroki, H.; Mima, K.; Nitta, H.; et al. Enhancer of Zeste Homolog 2 (EZH2) Promotes Progression of Cholangiocarcinoma Cells by Regulating Cell Cycle and Apoptosis. Ann. Surg. Oncol. 2013, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-N.; Chai, Z.-T.; Zhu, X.-D.; Zhang, N.; Zhan, D.-H.; Ye, B.-G.; Wang, C.-H.; Qin, C.-D.; Zhao, Y.-M.; Zhu, W.-P.; et al. MicroRNA-26a Suppresses Epithelial-Mesenchymal Transition in Human Hepatocellular Carcinoma by Repressing Enhancer of Zeste Homolog 2. J. Hematol. Oncol. 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Ge, X.; Wang, X.; Xu, X.; Zhang, Z.; Seng, J.; Cao, Z.; Gu, Y.; Han, M. EZH2 Contributes To Cisplatin Resistance In Breast Cancer By Epigenetically Suppressing MiR-381 Expression. OncoTargets Ther. 2019, 12, 9627–9637. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qi, J.; Xiong, J.; Jiang, L.; Cui, D.; He, J.; Chen, P.; Li, L.; Wu, C.; Ma, T.; et al. Epigenetic Co-Deregulation of EZH2/TET1 Is a Senescence-Countering, Actionable Vulnerability in Triple-Negative Breast Cancer. Theranostics 2019, 9, 761–777. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, J.; Sun, Y.; Wang, J.; Ren, C.; Banerjee, S.; Ouyang, L.; Wang, Y. Targeting EZH2 for Cancer Therapy: From Current Progress to Novel Strategies. Eur. J. Med. Chem. 2022, 238, 114419. [Google Scholar] [CrossRef]


| Target Transcript | Primer Name | Sequence (5′->3′) | Product Length (bp) |
|---|---|---|---|
| PVRL3 | PVRL3_F | GCAGTTCACCATCCCCAATATG | 162 |
| PVRL3_R | TCCAAGCGGGAATGTAACAGC | ||
| EZH2 | EZH2_F | AATCAGAGTACATGCGACTGAGA | 141 |
| EZH2_R | GCTGTATCCTTCGCTGTTTCC | ||
| GAPDH | GAPDH_F | AGAAGGCTGGGGCTCATTTG | 258 |
| GAPDH_R | AGGGGCCATCCACAGTCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, G.M.; Mangano, K.; Caponnetto, S.; Fagone, P.; Nicoletti, F. Identification of Poliovirus Receptor-like 3 Protein as a Prognostic Factor in Triple-Negative Breast Cancer. Cells 2024, 13, 1299. https://doi.org/10.3390/cells13151299
Leone GM, Mangano K, Caponnetto S, Fagone P, Nicoletti F. Identification of Poliovirus Receptor-like 3 Protein as a Prognostic Factor in Triple-Negative Breast Cancer. Cells. 2024; 13(15):1299. https://doi.org/10.3390/cells13151299
Chicago/Turabian StyleLeone, Gian Marco, Katia Mangano, Salvatore Caponnetto, Paolo Fagone, and Ferdinando Nicoletti. 2024. "Identification of Poliovirus Receptor-like 3 Protein as a Prognostic Factor in Triple-Negative Breast Cancer" Cells 13, no. 15: 1299. https://doi.org/10.3390/cells13151299
APA StyleLeone, G. M., Mangano, K., Caponnetto, S., Fagone, P., & Nicoletti, F. (2024). Identification of Poliovirus Receptor-like 3 Protein as a Prognostic Factor in Triple-Negative Breast Cancer. Cells, 13(15), 1299. https://doi.org/10.3390/cells13151299









