Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease
Abstract
1. Introduction
2. PVAT: Anatomy and Physiology
3. Extracellular Vesicles (EVs)
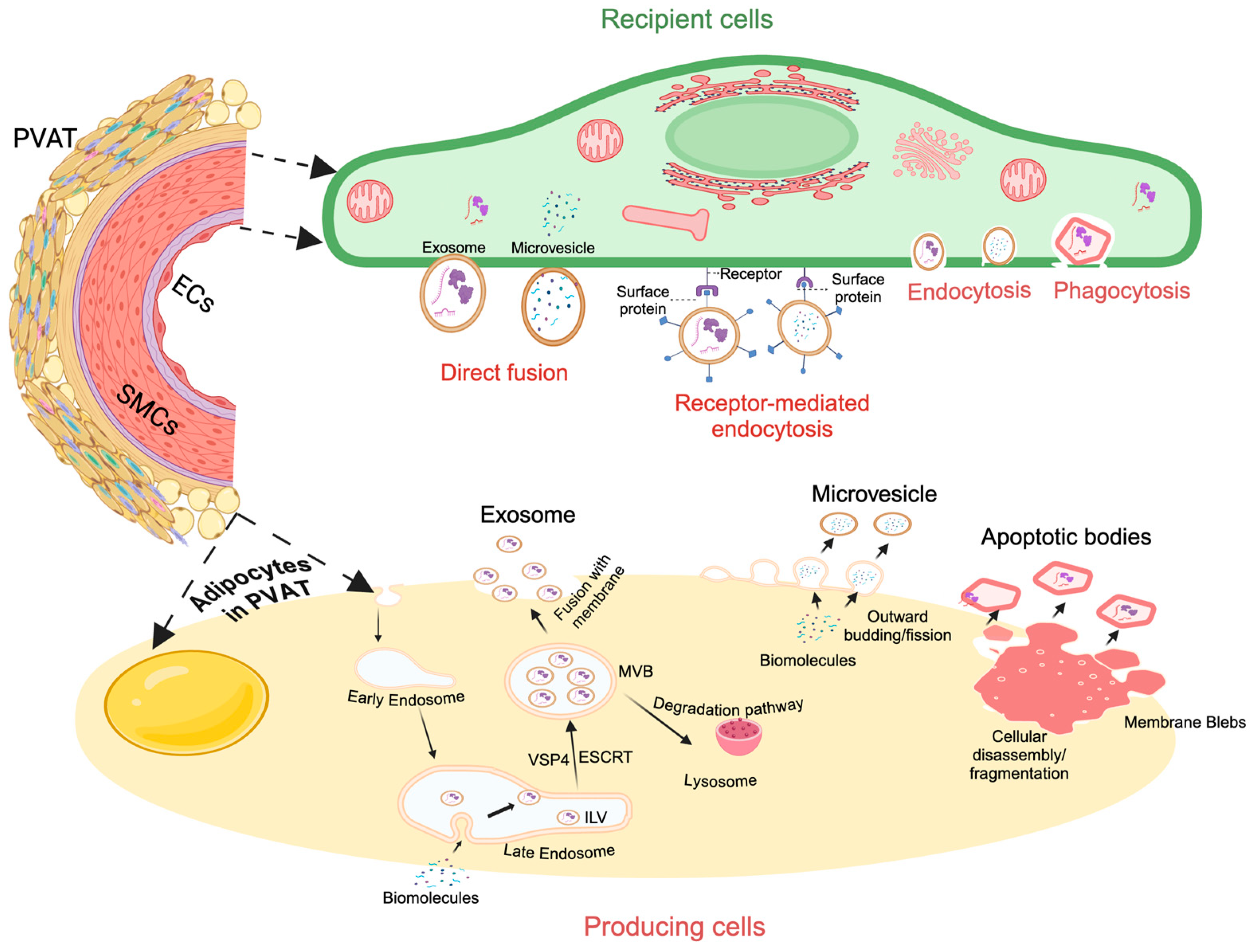
3.1. PVAT-Derived Exosomes and Microvesicles: Biogenesis and Size
3.2. PVAT-Derived Exosomes and Microvesicles: Uptake Pathways and Functions
4. Apoptotic Bodies: Biogenesis, Size, and Function
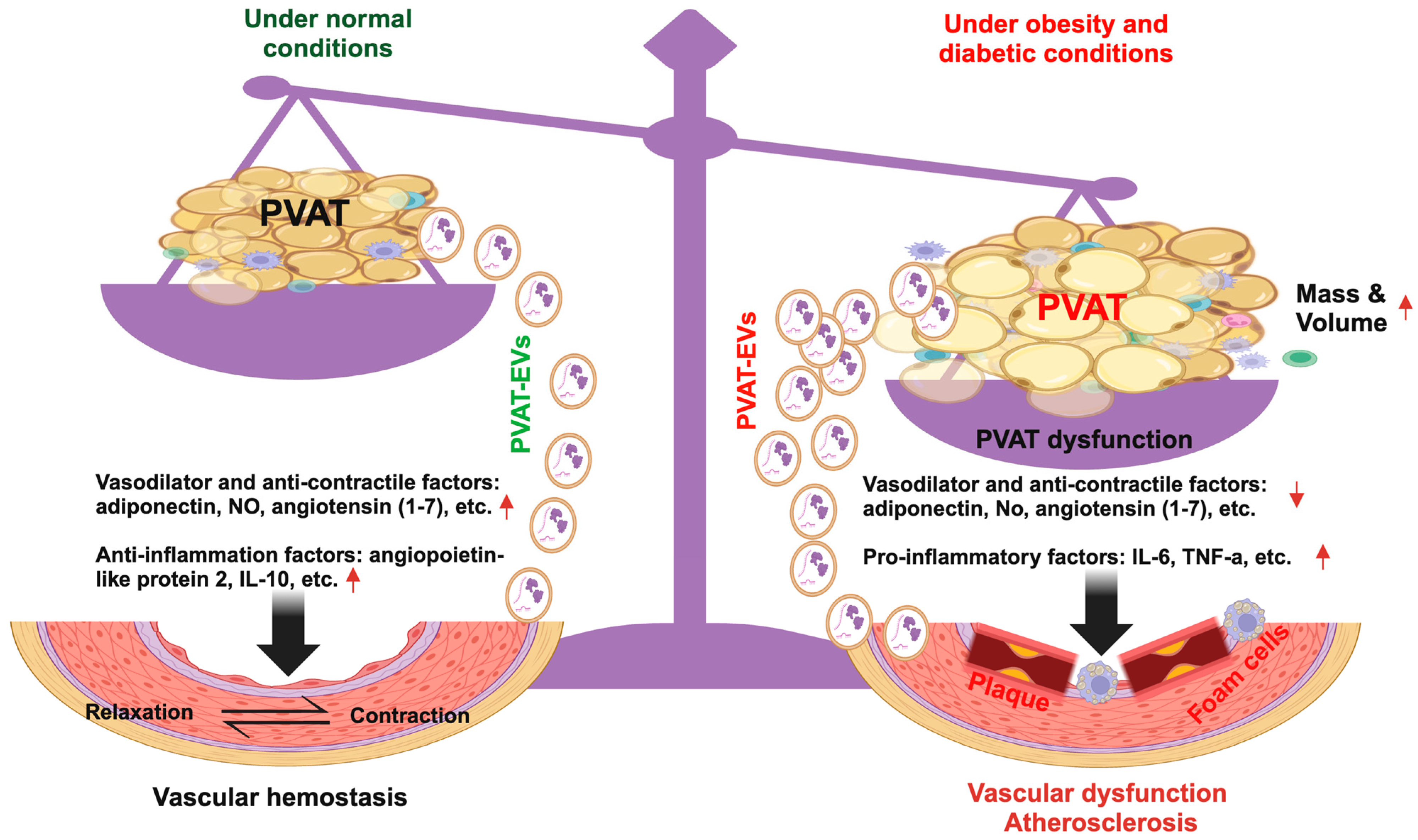
5. PVAT and PVAT-EVs in Obesity and Diabetic-Related Vascular Dysfunction
6. PVAT and PVAT-EVs in the Pathogenesis of Atherosclerosis
7. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Szasz, T.; Webb, R.C. Perivascular adipose tissue: More than just structural support. Clin. Sci. 2011, 122, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Löhn, M.; Dubrovska, G.; Lauterbach, B.; Luft, F.C.; Gollasch, M.; Sharma, A.M. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002, 16, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Wang, Z.; Tian, Y.; Li, C.; Jin, F.; Li, J.; Wang, L. Perivascular brown adipocytes-derived kynurenic acid relaxes blood vessel via endothelium PI3K-Akt-eNOS pathway. Biomed. Pharmacother. 2022, 150, 113040. [Google Scholar] [CrossRef] [PubMed]
- Mitidieri, E.; Turnaturi, C.; Vanacore, D.; Sorrentino, R.; Bianca, R.D.d.V. The Role of Perivascular Adipose Tissue-Derived Hydrogen Sulfide in the Control of Vascular Homeostasis. Antioxid. Redox Signal. 2022, 37, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Ketonen, J.; Shi, J.; Martonen, E.; Mervaala, E. Periadventitial Adipose Tissue Promotes Endothelial Dysfunction via Oxidative Stress in Diet-Induced Obese C57Bl/6 Mice. Circ. J. 2010, 74, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.J.; Massaro, J.M.; Schlett, C.L.; O’Donnell, C.J.; Hoffmann, U.; Fox, C.S. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The Framingham Heart Study. Atherosclerosis 2010, 210, 656–661. [Google Scholar] [CrossRef]
- Marchesi, C.; Ebrahimian, T.; Angulo, O.; Paradis, P.; Schiffrin, E.L. Endothelial Nitric Oxide Synthase Uncoupling and Perivascular Adipose Oxidative Stress and Inflammation Contribute to Vascular Dysfunction in a Rodent Model of Metabolic Syndrome. Hypertension 2009, 54, 1384–1392. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef]
- Deng, Z.-B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose Tissue Exosome-Like Vesicles Mediate Activation of Macrophage-Induced Insulin Resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef]
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Dubrovska, G.; Tsang, S.-Y.; Essin, K.; Luft, F.C.; Huang, Y.; Gollasch, M. Visceral Periadventitial Adipose Tissue Regulates Arterial Tone of Mesenteric Arteries. Hypertension 2004, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J. Dual Modulation of Vascular Function by Perivascular Adipose Tissue and Its Potential Correlation with Adiposity/Lipoatrophy-Related Vascular Dysfunction. Curr. Pharm. Des. 2007, 13, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of Epidemiology and Contribution of Obesity to Cardiovascular Disease. Prog. Cardiovasc. Dis. 2013, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, R.; Pirnes-Karhu, S.; Pietiläinen, K.H.; Pirinen, E. Adipose tissue NAD+-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health. Redox Biol. 2017, 12, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Dalal, S. Lipid metabolism in cancer cachexia. Ann. Palliat. Med. 2019, 8, 13–23. [Google Scholar] [CrossRef]
- Altshuler-Keylin, S.; Shinoda, K.; Hasegawa, Y.; Ikeda, K.; Hong, H.; Kang, Q.; Yang, Y.; Perera, R.M.; Debnath, J.; Kajimura, S. Beige Adipocyte Maintenance Is Regulated by Autophagy-Induced Mitochondrial Clearance. Cell Metab. 2016, 24, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Jiang, L.; Shao, M.; Ye, R.; Zhu, Y.; Gordillo, R.; Ali, A.; Lian, Y.; Holland, W.L.; et al. Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat. Cell Biol. 2015, 17, 1099–1111. [Google Scholar] [CrossRef]
- Lynch, F.M.; Withers, S.B.; Yao, Z.; Werner, M.E.; Edwards, G.; Weston, A.H.; Heagerty, A.M. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H786–H795. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.M.; Bader, M.; Alenina, N.; Santos, R.A.; Gao, Y.-J.; Lu, C. Mas receptors in modulating relaxation induced by perivascular adipose tissue. Life Sci. 2011, 89, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Yearb. Paediatr. Endocrinol. 2018, 15, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Ding, H.; Jiang, M.; Yin, H.; Gollasch, M.; Huang, Y. Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation. Redox Biol. 2023, 62, 102683. [Google Scholar] [CrossRef] [PubMed]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a Biomarker of the Metabolic Syndrome. Circ. J. 2004, 68, 975–981. [Google Scholar] [CrossRef]
- Ahl, S.; Guenther, M.; Zhao, S.; James, R.; Marks, J.; Szabo, A.; Kidambi, S. Adiponectin Levels Differentiate Metabolically Healthy vs Unhealthy Among Obese and Nonobese White Individuals. J. Clin. Endocrinol. Metab. 2015, 100, 4172–4180. [Google Scholar] [CrossRef]
- Drolet, R.; Bélanger, C.; Fortier, M.; Huot, C.; Mailloux, J.; Légaré, D.; Tchernof, A. Fat Depot-specific Impact of Visceral Obesity on Adipocyte Adiponectin Release in Women. Obesity 2009, 17, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Ouchi, N.; Nishida, M.; Arita, Y.; Kumada, M.; Ohashi, K.; Sakai, N.; Shimomura, I.; Kobayashi, H.; et al. Adiponectin Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation 2002, 106, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, L.; Chang, L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm. Mol. Biol. Clin. Investig. 2015, 21, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Turaihi, A.H.; Serné, E.H.; Molthoff, C.F.; Koning, J.J.; Knol, J.; Niessen, H.W.; Goumans, M.J.T.; van Poelgeest, E.M.; Yudkin, J.S.; Smulders, Y.M.; et al. Perivascular Adipose Tissue Controls Insulin-Stimulated Perfusion, Mitochondrial Protein Expression, and Glucose Uptake in Muscle Through Adipomuscular Arterioles. Diabetes 2020, 69, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ueda, K.; Nomura, S.; Ito, K.; Katoh, M.; Katagiri, M.; Yamada, S.; Hashimoto, M.; Zhai, B.; Numata, G.; et al. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nat. Commun. 2022, 13, 5117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise Improves Endothelial Function Associated with Alleviated Inflammation and Oxidative Stress of Perivascular Adipose Tissue in Type 2 Diabetic Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8830537. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Shields, K.J.; Janssen, I.; Hanley, C.; Budoff, M.J.; Barinas-Mitchell, E.; Everson-Rose, S.A.; Powell, L.H.; Matthews, K.A. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J. Clin. Endocrinol. Metab. 2015, 100, 3304–3312. [Google Scholar] [CrossRef]
- Victorio, J.A.; da Costa, R.M.; Tostes, R.C.; Davel, A.P. Modulation of Vascular Function by Perivascular Adipose Tissue: Sex Differences. Curr. Pharm. Des. 2020, 26, 3768–3777. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shang, Q.; Pan, Z.; Bai, Y.; Li, Z.; Zhang, H.; Zhang, Q.; Guo, C.; Zhang, L.; Wang, Q. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes 2018, 67, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB BioAdv. 2021, 3, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Madhyastha, H.; Madhyastha, R.; Rajendran, R.L.; Nakajima, Y.; Watanabe, N.; Velikkakath, A.K.G.; Hong, C.M.; Gopi, R.V.; Muthukalianan, G.K.; et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 2023, 13, 1085057. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Ju, Y.; Bai, H.; Ren, L.; Zhang, L. The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection. Int. J. Mol. Sci. 2021, 22, 9060. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Morel, O.; Jesel, L.; Freyssinet, J.-M.; Toti, F. Cellular Mechanisms Underlying the Formation of Circulating Microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Zwaal, R.F.; Schroit, A.J. Pathophysiologic Implications of Membrane Phospholipid Asymmetry in Blood Cells. Blood 1997, 89, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Chernomordik, L.V.; Melikyan, G.B.; Chizmadzhev, Y.A. Biomembrane fusion: A new concept derived from model studies using two interacting planar lipid bilayers. Biochim. Biophys. Acta. BBA Rev. Biomembr. 1987, 906, 309–352. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, Q.; Liu, P.; Xuan, X.; Du, Y. Experimental immunology Umbilical cord blood-derived dendritic cells loaded with BGC823 tumor antigens and DC-derived exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumor immunity in vitro and in vivo. Cent. Eur. J. Immunol. 2014, 2, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhu, Y.-L.; Zhou, Y.-Y.; Liang, G.-F.; Wang, Y.-Y.; Hu, F.-H.; Xiao, Z.-D. Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tu, C.; Zhang, J.; Wang, J. Inhibition of multiple myeloma-derived exosomes uptake suppresses the functional response in bone marrow stromal cell. Int. J. Oncol. 2019, 54, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef]
- Papini, G.; Furini, G.; Matteucci, M.; Biemmi, V.; Casieri, V.; Di Lascio, N.; Milano, G.; Chincoli, L.R.; Faita, F.; Barile, L.; et al. Cardiomyocyte-targeting exosomes from sulforaphane-treated fibroblasts affords cardioprotection in infarcted rats. J. Transl. Med. 2023, 21, 313. [Google Scholar] [CrossRef]
- Lionetti, V.; Tuana, B.S.; Casieri, V.; Parikh, M.; Pierce, G.N. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur. Heart J. 2018, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Thelen, K.; Ayala-Lopez, N.; Watts, S.W. The distribution and adipogenic potential of perivascular adipose tissue adipocyte progenitors is dependent on sexual dimorphism and vessel location. Physiol. Rep. 2016, 4, e12993. [Google Scholar] [CrossRef] [PubMed]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Schneider, M.; Biemer-Daub, G.; Wied, S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell. Signal. 2011, 23, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cui, B.; Zheng, J.; Yu, C.; Zheng, X.; Yi, L.; Zhang, S.; Wang, K. Platelet-derived microparticles and their cargos: The past, present and future. Asian J. Pharm. Sci. 2024, 19, 100907. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., 3rd; Mackman, N. Microparticles in Hemostasis and Thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef]
- Essola, J.M.; Zhang, M.; Yang, H.; Li, F.; Xia, B.; Mavoungou, J.F.; Hussain, A.; Huang, Y. Exosome regulation of immune response mechanism: Pros and cons in immunotherapy. Bioact. Mater. 2024, 32, 124–146. [Google Scholar] [CrossRef]
- Ling, Z.L.; Combes, V.; Grau, G.E.; King, N.J.C. Microparticles as Immune Regulators in Infectious Disease ? An Opinion. Front. Immunol. 2011, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.; Kalluri, R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rak, J. Extracellular Vesicles-Biomarkers and Effectors of the Cellular Interactome in Cancer. Front. Pharmacol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.N.; Pampliega, O.; Bourdenx, M.; Meissner, W.G.; Bezard, E.; Dehay, B. Exosomes, an Unmasked Culprit in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Y.; Zhong, Y.; Ammar, H.M.; Zhang, P.; Guo, R.; Liu, H.; Cheng, C.F.; Koroscil, T.M.; Chen, Y.F. The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E828–E837. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sigdel, S.; Sawant, H.; Bihl, J.; Wang, J. Exercise-Intervened Endothelial Progenitor Cell Exosomes Protect N2a Cells by Improving Mitochondrial Function. Int. J. Mol. Sci. 2024, 25, 1148. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.; Datta, P.; Singh, Y.P.; Lo, A.; Horchler, S.; Elcheva, I.A.; Ozbolat, I.T.; Ravnic, D.J.; Koduru, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Therapeutic Use and in Bioengineering Applications. Cells 2022, 11, 3366. [Google Scholar] [CrossRef] [PubMed]
- Shekari, F.; Meyfour, A.; Davies, O.G.; Velot, É. Editorial: Mesenchymal stem cell-derived extracellular vesicles: Considerations and therapeutic applications. Front. Cell Dev. Biol. 2024, 12, 1377197. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-X.; Lu, C.-H.; Na, N.; Yin, R.-X.; Huang, F. Endothelial progenitor cell-derived extracellular vesicles: The world of potential prospects for the treatment of cardiovascular diseases. Cell Biosci. 2024, 14, 72. [Google Scholar] [CrossRef]
- Zou, L.; Ma, X.; Lin, S.; Wu, B.; Chen, Y.; Peng, C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp. Ther. Med. 2019, 18, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Meziani, F.; Tesse, A.; Andriantsitohaina, R. Microparticles are vectors of paradoxical information in vascular cells including the endothelium: Role in health and diseases. Pharmacol. Rep. 2008, 60, 75–84. [Google Scholar] [PubMed]
- Tang, H.; Luo, H.; Zhang, Z.; Yang, D. Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential. Cells 2022, 11, 3879. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.; Munro, J.; Rath, N.; AL Zander, S.; Mleczak, A.; Sumpton, D.; Morrice, N.; et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef]
- Trahtemberg, U.; Mevorach, D. Apoptotic Cells Induced Signaling for Immune Homeostasis in Macrophages and Dendritic Cells. Front. Immunol. 2017, 8, 1356. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M. Highlighting diabetes mellitus: The epidemic continues. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef]
- Saeedi, P. A high number of people have CVD, but do not know it. Diabetes Res. Clin. Pract. 2019, 148, 270–271. [Google Scholar] [CrossRef]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation 2022, 145, E722–E759. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am. J. Med. Sci. 2016, 351, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, W.; Wang, J.; Guo, C.; Wang, X.; Wolffe, S.L.; Bodary, P.F.; Eitzman, D.T. Obesity-Induced Endothelial Dysfunction Is Prevented by Deficiency of P-Selectin Glycoprotein Ligand-1. Diabetes 2012, 61, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Henrichot, E.; Juge-Aubry, C.E.; Pernin, A.; Pache, J.C.; Velebit, V.; Dayer, J.M.; Meda, P.; Chizzolini, C.; Meier, C.A. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Fredette, N.C.; Barton, M.; Prossnitz, E.R. Regulation of Vascular Smooth Muscle Tone by Adipose-Derived Contracting Factor. PLoS ONE 2013, 8, e79245. [Google Scholar] [CrossRef] [PubMed]
- Duregotti, E.; Reumiller, C.M.; Mayr, U.; Hasman, M.; Schmidt, L.E.; Burnap, S.A.; Theofilatos, K.; Barallobre-Barreiro, J.; Beran, A.; Grandoch, M.; et al. Reduced secretion of neuronal growth regulator 1 contributes to impaired adipose-neuronal crosstalk in obesity. Nat. Commun. 2022, 13, 7269. [Google Scholar] [CrossRef] [PubMed]
- Rittig, K.; Staib, K.; Machann, J.; Böttcher, M.; Peter, A.; Schick, F.; Claussen, C.; Stefan, N.; Fritsche, A.; Häring, H.-U.; et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia 2008, 51, 2093–2099. [Google Scholar] [CrossRef]
- Police, S.B.; Thatcher, S.E.; Charnigo, R.; Daugherty, A.; Cassis, L.A. Obesity Promotes Inflammation in Periaortic Adipose Tissue and Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1458–1464. [Google Scholar] [CrossRef]
- Bailey-Downs, L.C.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: A paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol. Ser. A Biomed. Sci. Med Sci. 2013, 68, 780–792. [Google Scholar] [CrossRef]
- Bussey, C.E.; Withers, S.B.; Aldous, R.G.; Edwards, G.; Heagerty, A.M. Obesity-Related Perivascular Adipose Tissue Damage Is Reversed by Sustained Weight Loss in the Rat. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadzadeh, R.; Unwin, R.D.; Greenstein, A.S.; Heagerty, A.M. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J. Vasc. Res. 2015, 52, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, K.E.; Wareing, M.; Edwards, G.; Austin, C. Loss of anti-contractile effect of perivascular adipose tissue in offspring of obese rats. Int. J. Obes. 2016, 40, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seiça, R.; Sena, C.M. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2019, 146, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.I.; Bakker, W.; Alta, C.-L.A.; Sipkema, P.; Yudkin, J.S.; Viollet, B.; Richter, E.A.; Smulders, Y.M.; van Hinsbergh, V.W.; Serné, E.H.; et al. Perivascular Adipose Tissue Control of Insulin-Induced Vasoreactivity in Muscle Is Impaired in db/db Mice. Diabetes 2013, 62, 590–598. [Google Scholar] [CrossRef] [PubMed]
- DeVallance, E.; Branyan, K.W.; Lemaster, K.; Olfert, I.M.; Smith, D.M.; Pistilli, E.E.; Frisbee, J.C.; Chantler, P.D. Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFα-and NOX2-dependent pathway. Exp. Physiol. 2018, 103, 590–603. [Google Scholar] [CrossRef]
- Osaki, A.; Kagami, K.; Ishinoda, Y.; Sato, A.; Kimura, T.; Horii, S.; Ito, K.; Toya, T.; Ido, Y.; Namba, T.; et al. Reactive Oxygen Species in the Aorta and Perivascular Adipose Tissue Precedes Endothelial Dysfunction in the Aorta of Mice with a High-Fat High-Sucrose Diet and Additional Factors. Int. J. Mol. Sci. 2023, 24, 6486. [Google Scholar] [CrossRef]
- Takemori, K.; Gao, Y.-J.; Ding, L.; Lu, C.; Su, L.-Y.; An, W.-S.; Vinson, C.; Lee, R.M. Elevated Blood Pressure in Transgenic Lipoatrophic Mice and Altered Vascular Function. Hypertension 2007, 49, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Saxton, S.N.; Toms, L.K.; Aldous, R.G.; Withers, S.B.; Ohanian, J.; Heagerty, A.M. Restoring Perivascular Adipose Tissue Function in Obesity Using Exercise. Cardiovasc. Drugs Ther. 2021, 35, 1291–1304. [Google Scholar] [CrossRef]
- Meziat, C.; Boulghobra, D.; Strock, E.; Battault, S.; Bornard, I.; Walther, G.; Reboul, C. Exercise training restores eNOS activation in the perivascular adipose tissue of obese rats: Impact on vascular function. Nitric Oxide 2019, 86, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Boa, B.C.S.; Yudkin, J.S.; van Hinsbergh, V.W.M.; Bouskela, E.; Eringa, E.C. Exercise effects on perivascular adipose tissue: Endocrine and paracrine determinants of vascular function. Br. J. Pharmacol. 2017, 174, 3466–3481. [Google Scholar] [CrossRef]
- Liao, J.; Yin, H.; Huang, J.; Hu, M. Dysfunction of perivascular adipose tissue in mesenteric artery is restored by aerobic exercise in high-fat diet induced obesity. Clin. Exp. Pharmacol. Physiol. 2021, 48, 697–703. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Sueiro, A.; Couto, I.; Santos, F.; Baltar, J.; Casanueva, F.F.; Pardo, M. Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes. Int. J. Mol. Sci. 2020, 21, 2252. [Google Scholar] [CrossRef]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular vesicles from adipose tissue—A potential role in obesity and type 2 diabetes? Front. Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Huang, L.-F.; Zhao, L.; Zeng, Z.; Wang, X.; Cao, D.; Yang, L.; Ye, Z.; Chen, X.; Liu, B.; et al. Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells. Genes Dis. 2020, 7, 225–234. [Google Scholar] [CrossRef]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta. BBA Proteins Proteom. 2018, 1867, 140172. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Dentelli, P.; Gili, M.; Gallo, S.; Deregibus, C.; Biglieri, E.; Iavello, A.; Santini, E.; Rossi, C.; Solini, A.; et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: Impact on clinical applications. Int. J. Obes. 2016, 40, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.-Y.; Huang, X.-F.; Zhang, D.-D.; Guo, R.-J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.M.; Sullivan, A.E.; Aday, A.W. Atherosclerotic disease: Pathogenesis and approaches to management. Med. Clin. 2023, 107, 793–805. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, Z.; Wang, Y.; Li, X.; Zheng, Y.; Liu, Y. Role of Inflammation in Vascular Disease-Related Perivascular Adipose Tissue Dysfunction. Front. Endocrinol. 2021, 12, 710842. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Antonopoulos, A.S.; Digby, J.; Lee, R.; Reilly, S.; Coutinho, P.; Shirodaria, C.; Sayeed, R.; Petrou, M.; De Silva, R.; et al. Interactions Between Vascular Wall and Perivascular Adipose Tissue Reveal Novel Roles for Adiponectin in the Regulation of Endothelial Nitric Oxide Synthase Function in Human Vessels. Circulation 2013, 127, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Horimatsu, T.; Patel, A.S.; Prasad, R.; Reid, L.E.; Benson, T.W.; Zarzour, A.; Ogbi, M.; Nascimento, T.B.D.; de Chantemele, E.B.; Stansfield, B.K.; et al. Remote Effects of Transplanted Perivascular Adipose Tissue on Endothelial Function and Atherosclerosis. Cardiovasc. Drugs Ther. 2018, 32, 503–510. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Ding, L.L.Q.; Xu, L.; Huang, J.; Zhang, Z.B.; Chen, X.H.; Cheng, Y.W.; Ruan, C.C.; Gao, P.J. Brown adipocyte ADRB3 mediates cardioprotection via suppressing exosomal iNOS. Circ. Res. 2022, 131, 133–147. [Google Scholar] [CrossRef]
- Bitirim, C.V.; Ozer, Z.B.; Aydos, D.; Genc, K.; Demirsoy, S.; Akcali, K.C.; Turan, B. Cardioprotective effect of extracellular vesicles derived from ticagrelor-pretreated cardiomyocyte on hyperglycemic cardiomyocytes through alleviation of oxidative and endoplasmic reticulum stress. Sci. Rep. 2022, 12, 5651. [Google Scholar] [CrossRef]
- Flaherty III, S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W., Jr. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Cao, J.; Ye, C. Exosomes from adipose-derived stem cells promotes VEGF-C-dependent lymphangiogenesis by regulating miRNA-132/TGF-β pathway. Cell. Physiol. Biochem. 2018, 49, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-C. The role of lymphangiogenesis in cardiovascular diseases and heart transplantation. Heart Fail. Rev. 2021, 27, 1837–1856. [Google Scholar] [CrossRef]
- Liu, X.; Oliver, G. The Lymphatic Vasculature in Cardiac Development and Ischemic Heart Disease. Circ. Res. 2023, 132, 1246–1253. [Google Scholar] [CrossRef]
- Maioli, M.; Santaniello, S.; Montella, A.; Bandiera, P.; Cantoni, S.; Cavallini, C.; Bianchi, F.; Lionetti, V.; Rizzolio, F.; Marchesi, I.; et al. Hyaluronan Esters Drive Smad Gene Expression and Signaling Enhancing Cardiogenesis in Mouse Embryonic and Human Mesenchymal Stem Cells. PLoS ONE 2010, 5, e15151. [Google Scholar] [CrossRef]
- Wadey, R.M.; Connolly, K.D.; Mathew, D.; Walters, G.; Rees, D.A.; James, P.E. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis 2019, 283, 19–27. [Google Scholar] [CrossRef]
- Barberio, M.D.; Kasselman, L.J.; Playford, M.P.; Epstein, S.B.; Renna, H.A.; Goldberg, M.; DeLeon, J.; Voloshyna, I.; Barlev, A.; Salama, M.; et al. Cholesterol efflux alterations in adolescent obesity: Role of adipose-derived extracellular vesical microRNAs. J. Transl. Med. 2019, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, F.F.; Shang, Y.Y.; Li, Y.A.; Wang, Z.H.; Han, L.U.; Li, Y.H.; Zhang, L.; Ti, Y.; Zhang, W.; et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE−/− mice. Int. J. Cardiol. 2018, 265, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, J.; Liu, B.; Huang, F.; Li, Y. Exosomes derived from mangiferin-stimulated perivascular adipose tissue ameliorate endothelial dysfunction. Mol. Med. Rep. 2019, 19, 4797–4805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular Adipose-Derived Exosomes Reduce Foam Cell Formation by Regulating Expression of Cholesterol Transporters. Front. Cardiovasc. Med. 2021, 8, 697510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vasc. Pharmacol. 2022, 143, 106968. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef]
| Disease | Type of Change | Effect | Reference |
|---|---|---|---|
| Obesity | Anatomical | Increase in mass | [5,6,95,96,98] |
| Shift from brown to white adipocyte phenotype | [100] | ||
| Type 1 macrophage infiltration | [101] | ||
| Physiological | Loss of vasodilation effects | [102,104,106,108] | |
| Upregulated TNF-⍺, leptin, MIP1-⍺, Downregulated eNOS, adiponectin, PPAR-γ, FABP4, | [102] | ||
| Decreased NO production | [105] | ||
| T2D | Anatomical | Increase in mass | [97] |
| Macrophage infiltration | [107] | ||
| Physiological | Promotion of vasoconstriction rather than vasodilation | [107] | |
| Downregulated adiponectin | [37,108] | ||
| Downregulated IL-10, Upregulated INF-r, TNF-⍺, IL-6 | [37] | ||
| Metabolic syndromes | Anatomical | Loss of vasodilation effects | [7,109,110] |
| Shift from brown to white phenotypes | [109] | ||
| Physiological | Increased ROS production | [7,109,110] | |
| Upregulated TNF-⍺, IL-1β, IL-6, IFN- γ, Downregulated IL-4, IL-5, IL-10, IL-13, adiponectin | [109] |
| EV Source | Disease State | Containing | Taken Up by | Effect | Reference |
|---|---|---|---|---|---|
| Adipose, general | T2D. | N/A | Brain cells | Cause synaptic damage and cognitive impairment. | [116] |
| Obesity | Downregulated vascular endothelial growth factor | Vasculature | Decreased angiogenic potential | [118] | |
| N/A | VAT, liver, muscle | Insulin resistance, differentiation of monocytes to macrophages, production of pro-inflammatory cytokines | [10] | ||
| Human subcutaneous adipose tissue | Obesity | Proteins such as ECHA (a subunit of the trifunctional enzyme), HCDH (hydroxyacyl-coenzyme A dehydrogenase), etc. | Melanoma cells | Fatty acids oxidation | [121] |
| miR-155 | Macrophages | Shift toward M1 phenotype | [67] | ||
| Adipose tissue-derived macrophages | Obesity | miR-155 | N/A | Modulate insulin sensitivity | [40] |
| PVAT | Obesity | miRNA-221 | Vasculature | Stimulate vascular remodeling | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigdel, S.; Udoh, G.; Albalawy, R.; Wang, J. Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease. Cells 2024, 13, 1309. https://doi.org/10.3390/cells13161309
Sigdel S, Udoh G, Albalawy R, Wang J. Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease. Cells. 2024; 13(16):1309. https://doi.org/10.3390/cells13161309
Chicago/Turabian StyleSigdel, Smara, Gideon Udoh, Rakan Albalawy, and Jinju Wang. 2024. "Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease" Cells 13, no. 16: 1309. https://doi.org/10.3390/cells13161309
APA StyleSigdel, S., Udoh, G., Albalawy, R., & Wang, J. (2024). Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease. Cells, 13(16), 1309. https://doi.org/10.3390/cells13161309






