Skin Telocytes Could Fundament the Cellular Mechanisms of Wound Healing in Platelet-Rich Plasma Administration
Abstract
:1. Introduction
2. PRP Plasma Biochemistry and Active Factors
3. The Composition of the Platelets’ α-Granules
3.1. Growth Factors
3.1.1. Platelet-Derived Growth Factor (PDGF), AA-AB-BB
3.1.2. Transforming Growth Factor β (TGFβ)
3.1.3. Vascular Endothelial Growth Factor (VEGF)
3.1.4. Epidermal Growth Factor (EGF)
3.1.5. Fibroblast Growth Factor (FGF)
3.1.6. Connective Tissue Growth Factor (CTGF)
3.1.7. Insulin-like Growth Factor (IGF-1)
3.1.8. Hepatocyte Growth Factor (HGF)
3.1.9. Keratinocyte Growth Factor/Fibroblast Growth Factor-7 (KGF/FGF-7)
3.1.10. Angiopoietin-1 (ANG-1)
3.2. Chemokines
3.2.1. Growth-Regulated Protein Alpha (CXCL1/GRO α/KC/CINC-1)
3.2.2. Platelet Factor 4/Chemokine (C-X-C Motif) Ligand 4 (PF4/CXCL4)
3.2.3. Epithelial-Cell-Derived Neutrophil-Activating Peptide-78 (ENA-78)/C-X-C Motif Chemokine 5 (CXCL5)
3.2.4. C-X-C Motif Chemokine 7 (CXCL7)/Neutrophil-Activating Peptide 2 (NAP-2)
3.2.5. C-X-C Motif Chemokine 8 (CXCL8)/Interleukin-8 (IL-8)
3.2.6. Stromal-Cell-Derived Factor 1 α/C-X-C Motif Chemokine 12 (SDF-1α/CXCL12)
3.2.7. Monocyte Chemoattractant Protein (MCP-1)/Chemokine C-C Ligand-2 (CCL2)
3.2.8. Macrophage Inflammatory Protein-1α (MIP-1α)/Chemokine C-C Ligand-3 (CCL3)
3.2.9. Chemokine C-C Ligand-5 (CCL5)/Regulated on Activation, Normal T-Cell Expressed and Secreted (RANTES)
3.3. Adhesion Molecules
3.3.1. Fibrinogen
3.3.2. Thrombospondin
3.3.3. The von Willebrand Factor (vWF)
3.4. Integral Membrane Proteins
3.4.1. Integrin αIIbβ3
3.4.2. Glycoprotein (GP)Ib-IX-V Complex
3.4.3. Triggering Receptor Expressed on Myeloid Cells (TREM)-like Transcript 1 (TLT-1)
3.4.4. P-Selectin
3.5. Coagulants and Anticoagulants
3.5.1. Platelet Factor V
3.5.2. Factor IX (Christmas Factor)
3.5.3. Factor XIII
3.5.4. Growth Arrest Specific-6 (Gas6)
3.6. Antimicrobial Agents
3.7. Inflammatory/Immune Agents
4. Composition of Platelets’ Dense δ-Granules
4.1. Nucleotides
4.1.1. Adenosine Triphosphate (ATP)
4.1.2. Adenosine Diphosphate (ADP)
4.1.3. Cyclic Adenosine Monophosphate (cAMP)
4.1.4. Uridine Triphosphate (UTP)
4.1.5. Guanosine-5′-Triphosphate (GTP)
4.2. Bioactive Amines
4.2.1. Serotonin (5-hydroxytryptamine)
4.2.2. Histamine
4.2.3. Phosphates (Polyphosphate, Pyrophosphate)
4.3. Ions
5. The Composition of the Platelet’s Lysosomes
6. Platelet T-Granules
7. The Physiological Process of Skin Repair/Regeneration
8. Cellular and Biochemical Milieu of Skin Repair/Regeneration Scene
8.1. Neutrophils
8.2. Macrophages
8.3. Mast Cells
8.4. Langerhans Cells
8.5. Dendritic Epithelial T-Cells (DETCs)
8.6. Invariant Natural Killer Cells (iNKTs)
9. Dermal TCs: A Distinct Cell Population with a Promising Skin Regenerative Potential
10. Dermal Telocytes’ Involvement in the Course of a Few Dermatologic Pathologies
11. Variable Results of PRP on Treated Skin
12. PRP Treatment Limitations, Contraindications, and Practical Considerations
13. Limitations of PRP Therapy
14. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Gawaz, M.; Vogel, S. Platelets in Tissue Repair: Control of Apoptosis and Interactions with Regenerative Cells. Blood 2013, 122, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, Y.-T.; Fan, J. Exosomal Mediators in Sepsis and Inflammatory Organ Injury: Unraveling the Role of Exosomes in Intercellular Crosstalk and Organ Dysfunction. Mil. Med. Res. 2024, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Li, R.; Wang, S.; Xu, Y.; Wang, Y.; Zhang, H.; Zhou, Y.; Zhang, X.; Chen, X.; et al. Assessing the Role of Programmed Cell Death Signatures and Related Gene TOP2A in Progression and Prognostic Prediction of Clear Cell Renal Cell Carcinoma. Cancer Cell Int. 2024, 24, 164. [Google Scholar] [CrossRef]
- An, N.; Chen, Z.; Zhao, P.; Yin, W. Extracellular Vesicles in Sepsis: Pathogenic Roles, Organ Damage, and Therapeutic Implications. Int. J. Med. Sci. 2023, 20, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Effah, C.Y.; Ding, X.; Drokow, E.K.; Li, X.; Tong, R.; Sun, T. Bacteria-Derived Extracellular Vesicles: Endogenous Roles, Therapeutic Potentials and Their Biomimetics for the Treatment and Prevention of Sepsis. Front. Immunol. 2024, 15, 1296061. [Google Scholar] [CrossRef] [PubMed]
- Crisci, A.; De Crescenzo, U.; Crisci, M. Platelet-Rich Concentrates (L-PRF, PRP) in Tissue Regeneration: Control of Apoptosis and Interactions with Regenerative Cells. J. Clin. Mol. Med. 2018, 1, 1000116. [Google Scholar] [CrossRef]
- Ludwig, N.; Hilger, A.; Zarbock, A.; Rossaint, J. Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation. Cells 2022, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, M.R.; Guglielmini, G.; Battiston, M.; Momi, S.; Lombardi, E.; Miller, E.C.; De Zanet, D.; Mazzucato, M.; Gresele, P.; De Marco, L. Visualization of Nitric Oxide Production by Individual Platelets during Adhesion in Flowing Blood. Blood 2015, 125, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhu, Z.; Zheng, Y.; Wan, W.; Manole, C.G.; Zhang, Q. Skin Telocytes versus Fibroblasts: Two Distinct Dermal Cell Populations. J. Cell. Mol. Med. 2015, 19, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Gherghiceanu, M.; Ceafalan, L.C.; Hinescu, M.E. Dermal Telocytes: A Different Viewpoint of Skin Repairing and Regeneration. Cells 2022, 11, 3903. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Cismaşiu, V.; Gherghiceanu, M.; Popescu, L.M. Experimental Acute Myocardial Infarction: Telocytes Involvement in Neo-Angiogenesis. J. Cell. Mol. Med. 2011, 15, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-Granules: Basic Biology and Clinical Correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, L.J.; Italiano, J.E. Unlocking the Molecular Secrete(s) of α-Granule Biogenesis. Arter. Thromb. Vasc. Biol. 2018, 38, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Woods, V.M.A.; Latorre-Rey, L.J.; Schenk, F.; Rommel, M.G.E.; Moritz, T.; Modlich, U. Targeting Transgenic Proteins to Alpha Granules for Platelet-Directed Gene Therapy. Mol. Ther. Nucleic Acids 2022, 27, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Heemskerk, J.W.M.; Baaten, C.C.F.M.J. Platelet Membrane Receptor Proteolysis: Implications for Platelet Function. Front. Cardiovasc. Med. 2021, 7, 608391. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S.; Kim, S. Shedding Light on the Cell Biology of Platelet-Derived Extracellular Vesicles and Their Biomedical Applications. Life 2023, 13, 1403. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Boggio, E.; Gigliotti, C.L.; Stoppa, I.; Sutti, S.; Giordano, M.; Dianzani, U.; Rolla, R. Platelets, Protean Cells with All-Around Functions and Multifaceted Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 4565. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.; Wang, P.; Young, L.R.; Schwake, M.; Saftig, P.; Weng, X.; Meng, Y.; Neculai, D.; Marks, M.S.; Gonzales, L.; et al. Impaired Lysosomal Integral Membrane Protein 2-Dependent Peroxiredoxin 6 Delivery to Lamellar Bodies Accounts for Altered Alveolar Phospholipid Content in Adaptor Protein-3-Deficient Pearl Mice. J. Biol. Chem. 2016, 291, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, G.; Somogyvári, M.; Csermely, P.; Sőti, C. Lysosome-Related Organelles Promote Stress and Immune Responses in C. Elegans. Commun. Biol. 2023, 6, 936. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.; van der Sluijs, P. Platelet Secretory Behaviour: As Diverse as the Granules … or Not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Pokrovskaya, I.D.; Yadav, S.; Rao, A.; McBride, E.; Kamykowski, J.A.; Zhang, G.; Aronova, M.A.; Leapman, R.D.; Storrie, B. 3D Ultrastructural Analysis of A-granule, Dense Granule, Mitochondria, and Canalicular System Arrangement in Resting Human Platelets. Res. Pract. Thromb. Haemost. 2020, 4, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W. Release of α-Granule Contents during Platelet Activation. Platelets 2022, 33, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Lee, S.H.; Park, J.; Nam, B.; Kim, H.; Youn, J.; Lee, S.; Kim, T.-J.; Sung, I.-H.; Choi, S.H.; et al. Platelet-Derived Growth Factor B Is a Key Element in the Pathological Bone Formation of Ankylosing Spondylitis. J. Bone Miner. Res. 2020, 38, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Zhang, Z.-B.; Lan, B.-D.; Lin, J.-R.; Chen, X.-H.; Kong, L.-R.; Xu, L.; Ruan, C.-C.; Gao, P.-J. PDGF-D Activation by Macrophage-Derived UPA Promotes AngII-Induced Cardiac Remodeling in Obese Mice. J. Exp. Med. 2021, 218, e20210252. [Google Scholar] [CrossRef] [PubMed]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.-C.; Davies, M.J.; Siebuhr, A.S. Dermal Fibroblasts Have Different Extracellular Matrix Profiles Induced by TGF-β, PDGF and IL-6 in a Model for Skin Fibrosis. Sci. Rep. 2020, 10, 17300. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Junior, D.M.; Tsirigoti, C.; Lim, S.K.; Heldin, C.-H.; Moustakas, A. Extracellular Vesicles and Transforming Growth Factor β Signaling in Cancer. Front. Cell Dev. Biol. 2022, 10, 849938. [Google Scholar] [CrossRef] [PubMed]
- Sonker, A.; Dubey, A.; Bhatnagar, A.; Chaudhary, R. Platelet Growth Factors from Allogeneic Platelet-Rich Plasma for Clinical Improvement in Split-Thickness Skin Graft. Asian J. Transfus. Sci. 2015, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Karolczak, K.; Watala, C. Blood Platelets as an Important but Underrated Circulating Source of TGFβ. Int. J. Mol. Sci. 2021, 22, 4492. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Zheng, T.; Zhang, Y.; Liu, H.; Wang, X.; Tang, X.; Zhao, B.; Liu, P. Torin 1 Alleviates Impairment of TFEB-Mediated Lysosomal Biogenesis and Autophagy in TGFBI (p.G623_H626del)-Linked Thiel-Behnke Corneal Dystrophy. Autophagy 2022, 18, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, P.-K.; Bae, S.M.; Son, H.-N.; Thoudam, D.S.; Kim, J.-E.; Lee, B.-H.; Park, R.-W.; Kim, I.-S. Transforming Growth Factor-β–Induced Protein (TGFBIp/β Ig-H3) Activates Platelets and Promotes Thrombogenesis. Blood 2009, 114, 5206–5215. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, A.; Okitaka, M.; Takagi, S.; Takami, M.; Sato, S.; Nishio, M.; Okumura, S.; Fujita, N. A Critical Role of Platelet TGF-β Release in Podoplanin-Mediated Tumour Invasion and Metastasis. Sci. Rep. 2017, 7, 42186. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pronk, E.; van Dijk, C.; Bian, Y.; Feyen, J.; van Tienhoven, T.; Yildirim, M.; Pisterzi, P.; de Jong, M.M.E.; Bastidas, A.; et al. A Single-Cell Taxonomy Predicts Inflammatory Niche Remodeling to Drive Tissue Failure and Outcome in Human AML. Blood Cancer Discov. 2023, 4, 394–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, M.; Wang, Y.; Li, C.; Zeng, L.; Wang, Y.; Xiao, M.; Chen, X.; Geng, S.; Lai, P.; et al. Mesenchymal Stem Cells Reversibly De-Differentiate Myofibroblasts to Fibroblast-like Cells by Inhibiting the TGF-β-SMAD2/3 Pathway. Mol. Med. 2023, 29, 59. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.-J.; ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Sun, Y.; Shimokado, A.; Muragaki, Y. The Roles of Mitogen-Activated Protein Kinase Pathways in TGF-β-Induced Epithelial-Mesenchymal Transition. J. Signal Transduct. 2012, 2012, 289243. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming Growth Factor-beta 1 (TGF-β1) Induces Angiogenesis through Vascular Endothelial Growth Factor (VEGF)-mediated Apoptosis. J. Cell. Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, H.; Ning, L.; Wu, K.; Limbu, S.M.; Shi, Q.; Qin, C.; Wen, X. The Transforming Growth Factor Beta (TGF-β/Smads) Pathway Regulates Collagen Synthesis and Deposition in Swim Bladder of Chu’s Croaker (Nibea Coibor) Stimulated by Proline. Aquaculture 2022, 558, 738360. [Google Scholar] [CrossRef]
- Fontemaggi, G. Non-coding RNA Regulatory Networks in Post-transcriptional Regulation of VEGFA in Cancer. IUBMB Life 2023, 75, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular Endothelial Growth Factor. Arter. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The Role of VEGF in Cancer-Induced Angiogenesis and Research Progress of Drugs Targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Guo, M.; He, G.-H.; Xi, K.-H.; Zhou, M.-Y.; Shi, R.-Y.; Chen, G.-Q. VEGF-Induced Nrdp1 Deficiency in Vascular Endothelial Cells Promotes Cancer Metastasis by Degrading Vascular Basement Membrane. Oncogene 2024, 43, 1836–1851. [Google Scholar] [CrossRef] [PubMed]
- Yarjoo, S.; Siampour, H.; Khalilipour, M.; Sajedi, R.H.; Bagheri, H.; Moshaii, A. Gold Nanostructure-Enhanced Immunosensing: Ultra-Sensitive Detection of VEGF Tumor Marker for Early Disease Diagnosis. Sci. Rep. 2024, 14, 10450. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, B.; Shahosseini, Z.; Baniasadi, V. Production of Human Epidermal Growth Factor Using Adenoviral Based System. Res. Pharm. Sci. 2016, 11, 43–48. [Google Scholar] [PubMed]
- Zeng, F.; Harris, R.C. Epidermal Growth Factor, from Gene Organization to Bedside. Semin. Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.B.S.; Lago, C.A.P.; Almeida, R.d.A.C.; Barbirato, D.d.S.; Vasconcelos, B.C.d.E. Biological and Cellular Properties of Advanced Platelet-Rich Fibrin (A-PRF) Compared to Other Platelet Concentrates: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 25, 482. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Prudovsky, I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Teven, C.M.; Farina, E.M.; Rivas, J.; Reid, R.R. Fibroblast Growth Factor (FGF) Signaling in Development and Skeletal Diseases. Genes. Dis. 2014, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Czyz, M. Fibroblast Growth Factor Receptor Signaling in Skin Cancers. Cells 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, L. Cyclic Adenosine Monophosphate Regulates Connective Tissue Growth Factor Expression in Myocardial Fibrosis after Myocardial Infarction. J. Int. Med. Res. 2021, 49, 030006052110155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.-K.; Zhang, G.; Zhang, B.-T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef] [PubMed]
- Arnott, J.A.; Lambi, A.G.; Mundy, C.; Hendesi, H.; Pixley, R.A.; Owen, T.A.; Safadi, F.F.; Popoff, S.N. The Role of Connective Tissue Growth Factor (CTGF/CCN2) in Skeletogenesis. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Hall-Glenn, F.; Lyons, K.M. Roles for CCN2 in Normal Physiological Processes. Cell. Mol. Life Sci. 2011, 68, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Schenkl, C.; Schrepper, A.; Heyne, E.; Doenst, T.; Schwarzer, M. The IGF-1R Inhibitor NVP-AEW541 Causes Insulin-Independent and Reversible Cardiac Contractile Dysfunction. Biomedicines 2022, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Ndandala, C.B.; Zhou, Q.; Li, Z.; Guo, Y.; Li, G.; Chen, H. Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus Ovatus: Molecular Cloning, Characterization and Gene Expression. Int. J. Mol. Sci. 2024, 25, 2499. [Google Scholar] [CrossRef]
- Kiernan, K.; Alwarawrah, Y.; Nichols, A.G.; Danzaki, K.; MacIver, N.J. Insulin and IGF-1 Have Both Overlapping and Distinct Effects on CD4+ T Cell Mitochondria, Metabolism, and Function. Sci. Rep. 2024, 14, 4331. [Google Scholar] [CrossRef]
- Del Amo, C.; Fernández-San Argimiro, X.; Cascajo-Castresana, M.; Perez-Valle, A.; Madarieta, I.; Olalde, B.; Andia, I. Wound-Microenvironment Engineering through Advanced-Dressing Bioprinting. Int. J. Mol. Sci. 2022, 23, 2836. [Google Scholar] [CrossRef] [PubMed]
- Mayfosh, A.J.; Nguyen, T.K.; Hulett, M.D. The Heparanase Regulatory Network in Health and Disease. Int. J. Mol. Sci. 2021, 22, 11096. [Google Scholar] [CrossRef] [PubMed]
- Matwiejuk, M.; Myśliwiec, H.; Chabowski, A.; Flisiak, I. An Overview of Growth Factors as the Potential Link between Psoriasis and Metabolic Syndrome. J. Clin. Med. 2023, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T.-H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 21, 49. [Google Scholar] [CrossRef]
- Li, J.-F.; Duan, H.-F.; Wu, C.-T.; Zhang, D.-J.; Deng, Y.; Yin, H.-L.; Han, B.; Gong, H.-C.; Wang, H.-W.; Wang, Y.-L. HGF Accelerates Wound Healing by Promoting the Dedifferentiation of Epidermal Cells through β1-Integrin/ILK Pathway. Biomed. Res. Int. 2013, 2013, 470418. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.; Thao, D.; Thuoc, T. An Overview on Keratinocyte Growth Factor: From the Molecular Properties to Clinical Applications. Protein Pept. Lett. 2014, 21, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Q.-F.; Li, J.-Y.; Liu, X.-J.; Li, K.-C.; Zhong, Y.-Q.; Wu, D.; Wang, Q.; Lu, Y.-J.; Bao, L.; et al. Fibroblast Growth Factor 7 Is a Nociceptive Modulator Secreted via Large Dense-Core Vesicles. J. Mol. Cell Biol. 2015, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sonar, S.A.; Watanabe, M.; Nikolich, J.Ž. Disorganization of Secondary Lymphoid Organs and Dyscoordination of Chemokine Secretion as Key Contributors to Immune Aging. Semin. Immunol. 2023, 70, 101835. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Choi, H.; Oh, D.; Kim, M.; Cai, L.; Jawad, A.; Kim, S.; Lee, J.; Hyun, S.-H. Supplementation with Fibroblast Growth Factor 7 during in Vitro Maturation of Porcine Cumulus-Oocyte Complexes Improves Oocyte Maturation and Early Embryonic Development. Front. Vet. Sci. 2023, 10, 1250551. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.R.; Smith, M.P.; Francavilla, C. Fibroblast Growth Factor Receptors (FGFRs) and Noncanonical Partners in Cancer Signaling. Cells 2021, 10, 1201. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Kaur, G.; Kumar, P.; Singh, T.G. Therapeutic Targeting of Angiopoietins in Tumor Angiogenesis and Cancer Development. Biochem. Biophys. Res. Commun. 2023, 687, 149130. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.; Noviyanti, R.; Fijnheer, R.; de Groot, P.G.; Trianty, L.; Mudaliana, S.; Roest, M.; Syafruddin, D.; van der Ven, A.; de Mast, Q. Platelet Activation Determines Angiopoietin-1 and VEGF Levels in Malaria: Implications for Their Use as Biomarkers. PLoS ONE 2013, 8, e64850. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Bartlett, C.S.; Zheng, C.; Bigwarfe, T.; Grant, J.M.; MacDougall, M.; Berger, V.; Kerr, S.; Qian, H.S.; McHugh, M.; et al. Effect of Novel Biotherapeutic Elevating Angiopoietin 1 on Progression of Diabetic Nephropathy in Diabetic/Obese Mice. J. Pharmacol. Exp. Ther. 2022, 382, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Kim, J.; Cooke, V.G.; Wu, C.-C.; Sugimoto, H.; Gu, C.; De Palma, M.; Kalluri, R.; LeBleu, V.S. Targeting Vascular Pericytes in Hypoxic Tumors Increases Lung Metastasis via Angiopoietin-2. Cell Rep. 2015, 10, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Yu, S.; Yin, J.; Liu, D.; Zhuo, M.; Li, X. Role of Angiopoietin/Tie2 System in Sepsis: A Potential Therapeutic Target. Clin. Appl. Thromb./Hemost. 2024, 30. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.D.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 Protects the Adult Vasculature against Plasma Leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Flad, H.-D.; Brandt, E. Platelet-Derived Chemokines: Pathophysiology and Therapeutic Aspects. Cell. Mol. Life Sci. 2010, 67, 2363–2386. [Google Scholar] [CrossRef] [PubMed]
- Collinson, R.J.; Mazza-Parton, A.; Fuller, K.A.; Linden, M.D.; Erber, W.N.; Guo, B.B. Gene Expression of CXCL1 (GRO-α) and EGF by Platelets in Myeloproliferative Neoplasms. Hemasphere 2020, 4, e490. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Warkentin, T.E. Platelet Factor 4 Triggers Thrombo-inflammation by Bridging Innate and Adaptive Immunity. Int. J. Lab. Hematol. 2023, 45, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Alhanshani, A.A. Heparin Induced Thrombocytopenia—Pathophysiology, Diagnosis and Treatment: A Narrative Review. Int. J. Gen. Med. 2023, 16, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.M.; Padmanabhan, A. Heparin-Induced Thrombocytopenia. Arter. Thromb. Vasc. Biol. 2020, 41, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.A.; Rauova, L.; Poncz, M. Role of the Platelet Chemokine Platelet Factor 4 (PF4) in Hemostasis and Thrombosis. Thromb. Res. 2010, 125, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Gray, J.W. Chemokine Signaling in Cancer-Stroma Communications. J. Cell Commun. Signal. 2021, 15, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Greene, M.I.; Zhu, Z.; Zhang, H. Structural Features and PF4 Functions That Occur in Heparin-Induced Thrombocytopenia (HIT) Complicated by COVID-19. Antibodies 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Krauel, K.; Pötschke, C.; Weber, C.; Kessler, W.; Fürll, B.; Ittermann, T.; Maier, S.; Hammerschmidt, S.; Bröker, B.M.; Greinacher, A. Platelet Factor 4 Binds to Bacteria, Inducing Antibodies Cross-Reacting with the Major Antigen in Heparin-Induced Thrombocytopenia. Blood 2011, 117, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, N.; Yang, Z.; Li, H.; Zheng, H.; Yang, J.; Chen, Y.; Zhao, X.; Mei, J.; Shi, H.; et al. Role of CXCL5 in Regulating Chemotaxis of Innate and Adaptive Leukocytes in Infected Lungs Upon Pulmonary Influenza Infection. Front. Immunol. 2021, 12, 785457. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, W.; Sun, A.; Wei, Z.; Lin, Q. The Role of CXC Chemokines in Cancer Progression. Cancers 2022, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; Li, D.; Guo, X.; Zhou, Z.; Qi, M.; Wang, G.; Wang, F. The Abundance and Function of Neutrophils in the Endometriosis Systemic and Pelvic Microenvironment. Mediat. Inflamm. 2023, 2023, 1481489. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Sepuru, K.; Rajarathnam, K. Structural Basis of Native CXCL7 Monomer Binding to CXCR2 Receptor N-Domain and Glycosaminoglycan Heparin. Int. J. Mol. Sci. 2017, 18, 508. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Sepuru, K.M.; Sawant, K.V.; Rajarathnam, K. Platelet-Derived Chemokine CXCL7 Dimer Preferentially Exists in the Glycosaminoglycan-Bound Form: Implications for Neutrophil–Platelet Crosstalk. Front. Immunol. 2017, 8, 1248. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil Chemoattractant Receptors in Health and Disease: Double-Edged Swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The Chemokines CXCL8 and CXCL12: Molecular and Functional Properties, Role in Disease and Efforts towards Pharmacological Intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Chen, L.; Ren, M.; Li, S.; Liu, T.; Chen, H.; Yu, H.; Sun, Y. CXCL8 Promotes Endothelial-to-Mesenchymal Transition of Endothelial Cells and Protects Cells from Erastin-Induced Ferroptosis via CXCR2-Mediated Activation of the NF-ΚB Signaling Pathway. Pharmaceuticals 2023, 16, 1210. [Google Scholar] [CrossRef] [PubMed]
- Schall, N.; Daubeuf, F.; Marsol, C.; Gizzi, P.; Frossard, N.; Bonnet, D.; Galzi, J.-L.; Muller, S. A Selective Neutraligand for CXCL12/SDF-1α With Beneficial Regulatory Functions in MRL/Lpr Lupus Prone Mice. Front. Pharmacol. 2021, 12, 752194. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, S.; Mohammadi-Asl, J.; Far, M.A.J.; Asnafi, A.A.; Dehuri, F.; Tavakolifar, Y.; Saki, N. Is There a Relationship Between CXCR4 Gene Expression and Prognosis of Immune Thrombocytopenia in Children? Indian J. Hematol. Blood Transfus. 2017, 33, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Zhang, A.; Lathi, R.B.; Huddleston, H.G. Platelet-Rich Plasma Infusion as an Adjunct Treatment for Persistent Thin Lining in Frozen Embryo Transfer Cycles: First US Experience Report. J. Assist. Reprod. Genet. 2024, 41, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Chatterjee, M.; Borst, O.; Muller, K.; Stellos, K.; Mack, A.F.; Bongartz, A.; Bigalke, B.; Langer, H.; Schwab, M.; et al. Expression of Stromal Cell-Derived Factor-1 Receptors CXCR4 and CXCR7 on Circulating Platelets of Patients with Acute Coronary Syndrome and Association with Left Ventricular Functional Recovery. Eur. Heart J. 2014, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Y.; Zu, J.; Zhuang, J.; Xu, G.; Yan, J.; Liu, X. Stromal Cell-Derived Factor (SDF)-1α and Platelet-Rich Plasma Enhance Bone Regeneration and Angiogenesis Simultaneously in Situ in Rabbit Calvaria. J. Mater. Sci. Mater. Med. 2021, 32, 125. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, D.; Penn, B.M.; Penn, M.S. SDF-1 for Cardiac Repair. In Stem Cell and Gene Therapy for Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2016; pp. 401–407. [Google Scholar]
- Camps, J.; Rodríguez-Gallego, E.; García-Heredia, A.; Triguero, I.; Riera-Borrull, M.; Hernández-Aguilera, A.; Luciano-Mateo, F.; Fernández-Arroyo, S.; Joven, J. Paraoxonases and Chemokine (C–C Motif) Ligand-2 in Noncommunicable Diseases. Adv. Clin. Chem. 2014, 63, 247–308. [Google Scholar] [PubMed]
- Yoshimura, T.; Li, C.; Wang, Y.; Matsukawa, A. The Chemokine Monocyte Chemoattractant Protein-1/CCL2 Is a Promoter of Breast Cancer Metastasis. Cell. Mol. Immunol. 2023, 20, 714–738. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 Alpha)/CCL3: As a Biomarker. Gen. Methods Biomark. Res. Their Appl. 2015, 223, 223–249. [Google Scholar]
- Baba, T.; Mukaida, N. Role of Macrophage Inflammatory Protein (MIP)-1α/CCL3 in Leukemogenesis. Mol. Cell. Oncol. 2014, 1, e29899. [Google Scholar] [CrossRef] [PubMed]
- Marín Oyarzún, C.P.; Glembotsky, A.C.; Goette, N.P.; Lev, P.R.; De Luca, G.; Baroni Pietto, M.C.; Moiraghi, B.; Castro Ríos, M.A.; Vicente, A.; Marta, R.F.; et al. Platelet Toll-Like Receptors Mediate Thromboinflammatory Responses in Patients with Essential Thrombocythemia. Front. Immunol. 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Deshauer, C.; Morgan, A.M.; Ryan, E.O.; Handel, T.M.; Prestegard, J.H.; Wang, X. Interactions of the Chemokine CCL5/RANTES with Medium-Sized Chondroitin Sulfate Ligands. Structure 2015, 23, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P. Platelet Alpha-Granular Fibrinogen. Platelets 1992, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, J.; Wang, M.; Liu, Y. New Insights of Platelet Endocytosis and Its Implication for Platelet Function. Front. Cardiovasc. Med. 2024, 10, 8170. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.G.; Flaumenhaft, R. Localization of VAMP Isoforms in Platelets Reveals Separate Granule Populations with Distinct Functions. Blood 2010, 116, 2015. [Google Scholar] [CrossRef]
- Swinkels, M.; Hordijk, S.; Bürgisser, P.E.; Slotman, J.A.; Carter, T.; Leebeek, F.W.G.; Jansen, A.J.G.; Voorberg, J.; Bierings, R. Quantitative Super-Resolution Imaging of Platelet Degranulation Reveals Differential Release of von Willebrand Factor and von Willebrand Factor Propeptide from Alpha-Granules. J. Thromb. Haemost. 2023, 21, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Gkouvi, A.; Tsiogkas, S.G.; Bogdanos, D.P.; Gika, H.; Goulis, D.G.; Grammatikopoulou, M.G. Proteomics in Patients with Fibromyalgia Syndrome: A Systematic Review of Observational Studies. Curr. Pain. Headache Rep. 2024, 28, 565–586. [Google Scholar] [CrossRef] [PubMed]
- Aburima, A.; Berger, M.; Spurgeon, B.E.J.; Webb, B.A.; Wraith, K.S.; Febbraio, M.; Poole, A.W.; Naseem, K.M. Thrombospondin-1 Promotes Hemostasis through Modulation of CAMP Signaling in Blood Platelets. Blood 2021, 137, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Mereuta, O.M.; Agarwal, T.; Ghozy, S.; Dai, D.; Arul, S.; Brinjikji, W.; Kallmes, D.F.; Kadirvel, R. Shell Versus Core Architecture and Biology of Thrombi in Acute Ischemic Stroke: A Systematic Review. Clin. Appl. Thromb./Hemost. 2023, 29, 10760296231213632. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Vanhoorelbeke, K.; De Meyer, S.F. Von Willebrand Factor and Platelet Glycoprotein Ib: A Thromboinflammatory Axis in Stroke. Front. Immunol. 2019, 10, 2881. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Swieringa, F.; de Laat, B.; de Groot, P.G.; Roest, M.; Heemskerk, J.W.M. Reversible Platelet Integrin AIIbβ3 Activation and Thrombus Instability. Int. J. Mol. Sci. 2022, 23, 12512. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.L.; Alwis, I.; Smythe, R.; Yuan, Y.; Jackson, S.P. Megakaryocyte Buds Are Distinct from Microvesicles and Likely to Represent Platelet Precursors. Blood Adv. 2023, 7, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, M.; Janus-Bell, E.; Schaff, M.; Gachet, C.; Mangin, P. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers 2017, 9, 133. [Google Scholar] [CrossRef]
- Li, R.; Emsley, J. The Organizing Principle of the Platelet Glycoprotein Ib–IX–V Complex. J. Thromb. Haemost. 2013, 11, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G.; Schlesinger, M. The GPIb-IX Complex on Platelets: Insight into Its Novel Physiological Functions Affecting Immune Surveillance, Hepatic Thrombopoietin Generation, Platelet Clearance and Its Relevance for Cancer Development and Metastasis. Exp. Hematol. Oncol. 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Schmoker, A.M.; Perez Pearson, L.M.; Cruz, C.; Colon Flores, L.G.; Branfeild, S.; Pagán Torres, F.D.; Fonseca, K.; Cantres, Y.M.; Salgado Ramirez, C.A.; Melendez, L.M.; et al. Defining the TLT-1 Interactome from Resting and Activated Human Platelets. J. Proteom. 2020, 215, 103638. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.G. WPBs and α-Granules: More and More Look-Alike? Blood 2019, 133, 2634–2636. [Google Scholar] [CrossRef] [PubMed]
- Pluthero, F.G.; Kahr, W.H.A. Evaluation of Human Platelet Granules by Structured Illumination Laser Fluorescence Microscopy. Platelets 2023, 34, 2157808. [Google Scholar] [CrossRef] [PubMed]
- Stolla, M.C.; Leyens, K.; Catherman, S.C.; McGrath, K.E.; Palis, J. P-Selectin Expression and Platelet Function Are Developmentally Regulated. Blood 2014, 124, 1439. [Google Scholar] [CrossRef]
- Russo, V.; Falco, L.; Tessitore, V.; Mauriello, A.; Catapano, D.; Napolitano, N.; Tariq, M.; Caturano, A.; Ciccarelli, G.; D’Andrea, A.; et al. Anti-Inflammatory and Anticancer Effects of Anticoagulant Therapy in Patients with Malignancy. Life 2023, 13, 1888. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Zarbock, A. Platelets in Leucocyte Recruitment and Function. Cardiovasc. Res. 2015, 107, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, T.; Panitch, A. Endothelial Cells, Neutrophils and Platelets: Getting to the Bottom of an Inflammatory Triangle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef] [PubMed]
- Lehwald, N.; Duhme, C.; Pinchuk, I.; Kirchner, J.; Wieferich, K.; Schmelzle, M.; Jurk, K.; Windmöller, B.A.; Hübner, W.; Homey, B.; et al. Platelets Boost Recruitment of CD133+ Bone Marrow Stem Cells to Endothelium and the Rodent Liver—The Role of P-Selectin/PSGL-1 Interactions. Int. J. Mol. Sci. 2020, 21, 6431. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Moreno, J.A.; Miguel-Batuecas, A.; de Sancha, M.; Liras, A. The Magic of Proteases: From a Procoagulant and Anticoagulant Factor V to an Equitable Treatment of Its Inherited Deficiency. Int. J. Mol. Sci. 2023, 24, 6243. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, R.; Chen, N.; Pang, N.; Li, Y.; Deng, X.; Wang, L.; Luo, M.; Liu, Y.; Hao, H.; et al. Platelet-Derived Factor V Is a Critical Mediator of Arterial Thrombosis. J. Am. Heart Assoc. 2017, 6, e006345. [Google Scholar] [CrossRef] [PubMed]
- Orlova, N.A.; Kovnir, S.V.; Vorobiev, I.I.; Gabibov, A.G. Coagulation Factor IX for Hemophilia B Therapy. Acta Naturae 2012, 4, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Misenheimer, T.M.; Lasarev, M.R.; Kumfer, K.T.; Sheehan, J.P.; Schwartz, B.S. A Novel Factor IXa–Specific Enzyme-Linked Immunosorbent Assay Detects Factor IXa in Human Plasma. Res. Pr. Thromb. Haemost. 2024, 8, 102338. [Google Scholar] [CrossRef] [PubMed]
- Chamarthy, S. Normal Coagulation and Hemostasis. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1544–1552. [Google Scholar]
- Durda, M.A.; Wolberg, A.S.; Kerlin, B.A. State of the Art in Factor XIII Laboratory Assessment. Transfus. Apher. Sci. 2018, 57, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.-Y.; Wang, S.-J. Advances of Coagulation Factor XIII. Chin. Med. J. 2017, 130, 219–223. [Google Scholar] [CrossRef]
- Kucuk, B.; Bingol-Ozakpinar, O.; Demir, M.; Uras, F. Gas6 and TAM Receptors in Mouse Platelets. Blood 2012, 120, 5199. [Google Scholar] [CrossRef]
- Breitenecker, K.; Heiden, D.; Demmer, T.; Weber, G.; Primorac, A.-M.; Hedrich, V.; Ortmayr, G.; Gruenberger, T.; Starlinger, P.; Herndler-Brandstetter, D.; et al. Tumor-Extrinsic Axl Expression Shapes an Inflammatory Microenvironment Independent of Tumor Cell Promoting Axl Signaling in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 4202. [Google Scholar] [CrossRef]
- Zdżalik-Bielecka, D.; Poświata, A.; Kozik, K.; Jastrzębski, K.; Schink, K.O.; Brewińska-Olchowik, M.; Piwocka, K.; Stenmark, H.; Miączyńska, M. The GAS6-AXL Signaling Pathway Triggers Actin Remodeling That Drives Membrane Ruffling, Macropinocytosis, and Cancer-Cell Invasion. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Domínguez, A.S.; Romero-Tlalolini, M.d.l.A.; Torres-Aguilar, H.; Aguilar-Ruiz, S.R. Recent Advances in the Discovery and Function of Antimicrobial Molecules in Platelets. Int. J. Mol. Sci. 2021, 22, 10230. [Google Scholar] [CrossRef] [PubMed]
- CL, K.; Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Khanna, M.; Yadav, S. Antimicrobial Effects of Platelet-Rich Plasma and Platelet-Rich Fibrin: A Scoping Review. Cureus 2023, 15, e51360. [Google Scholar] [CrossRef] [PubMed]
- Portier, I.; Campbell, R.A. Role of Platelets in Detection and Regulation of Infection. Arter. Thromb. Vasc. Biol. 2020, 41, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.S.; Migliari Branco, L.; Franklin, B.S. Regulation of Innate Immune Responses by Platelets. Front. Immunol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Ezzeroug Ezzraimi, A.; Hannachi, N.; Mariotti, A.; Rolain, J.-M.; Camoin-Jau, L. Platelets and Escherichia Coli: A Complex Interaction. Biomedicines 2022, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Wallis, S.; Wolska, N.; Englert, H.; Posner, M.; Upadhyay, A.; Renné, T.; Eggleston, I.; Bagby, S.; Pula, G. A Peptide from the Staphylococcal Protein Efb Binds P-selectin and Inhibits the Interaction of Platelets with Leukocytes. J. Thromb. Haemost. 2022, 20, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Mackman, N. Platelets and Viruses. Platelets 2021, 32, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets Kill Circulating Parasites of All Major Plasmodium Species in Human Malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Kim, S.; Kim, S. An Insight into Recent Advances on Platelet Function in Health and Disease. Int. J. Mol. Sci. 2022, 23, 6022. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.L.; Boyle, J.A.; Di Pietro, S.M. Mechanism of Platelet Dense Granule Biogenesis: Study of Cargo Transport and Function of Rab32 and Rab38 in a Model System. Blood 2012, 120, 4072–4081. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Flaumenhaft, R. The Life Cycle of Platelet Granules. F1000Res 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Signorello, M.G.; Panfoli, I. Platelet Metabolic Flexibility: A Matter of Substrate and Location. Cells 2023, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Ravid, K. Biology of Platelet Purinergic Receptors and Implications for Platelet Heterogeneity. Front. Pharmacol. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Zieger, B. Endothelial Cells and Coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.; Bordet, J.-C.; Eckly, A.; Gachet, C. Platelet δ-Storage Pool Disease: An Update. J. Clin. Med. 2020, 9, 2508. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Gachet, C. P2 Receptors and Platelet Function. Purinergic Signal. 2011, 7, 293–303. [Google Scholar] [CrossRef]
- Ponomarenko, E.A.; Ignatova, A.A.; Polokhov, D.M.; Khismatullina, R.D.; Kurilo, D.S.; Shcherbina, A.; Zharkov, P.A.; Maschan, A.A.; Novichkova, G.A.; Panteleev, M.A. Healthy Pediatric Platelets Are Moderately Hyporeactive in Comparison with Adults’ Platelets. Platelets 2022, 33, 727–734. [Google Scholar] [CrossRef]
- Battinelli, E.M.; Markens, B.A.; Italiano, J.E. Release of Angiogenesis Regulatory Proteins from Platelet Alpha Granules: Modulation of Physiologic and Pathologic Angiogenesis. Blood 2011, 118, 1359–1369. [Google Scholar] [CrossRef]
- Kubacka, M.; Mogilski, S.; Bednarski, M.; Pociecha, K.; Świerczek, A.; Nicosia, N.; Schabikowski, J.; Załuski, M.; Chłoń-Rzepa, G.; Hockemeyer, J.; et al. Antiplatelet Effects of Selected Xanthine-Based Adenosine A2A and A2B Receptor Antagonists Determined in Rat Blood. Int. J. Mol. Sci. 2023, 24, 13378. [Google Scholar] [CrossRef]
- Gündüz, D.; Tanislav, C.; Sedding, D.; Parahuleva, M.; Santoso, S.; Troidl, C.; Hamm, C.; Aslam, M. Uridine Triphosphate Thio Analogues Inhibit Platelet P2Y12 Receptor and Aggregation. Int. J. Mol. Sci. 2017, 18, 269. [Google Scholar] [CrossRef]
- Aslam, M.; Sedding, D.; Koshty, A.; Santoso, S.; Schulz, R.; Hamm, C.; Gündüz, D. Nucleoside Triphosphates Inhibit ADP, Collagen, and Epinephrine-Induced Platelet Aggregation: Role of P2Y1 and P2Y12 Receptors. Thromb. Res. 2013, 132, 548–557. [Google Scholar] [CrossRef]
- Kassouf, N.; Ambily, A.; Watson, S.; Hassock, S.; Authi, H.S.; Srivastava, S.; Watson, S.P.; Authi, K.S. Phosphatidylinositol-3,4,5-Trisphosphate Stimulates Ca2+ Elevation and Akt Phosphorylation to Constitute a Major Mechanism of Thromboxane A2 Formation in Human Platelets. Cell Signal. 2015, 27, 1488–1498. [Google Scholar] [CrossRef]
- Maclean, J.A.; Schoenwaelder, S.M. Serotonin in Platelets. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–119. [Google Scholar]
- Mammadova-Bach, E.; Mauler, M.; Braun, A.; Duerschmied, D. Immuno-Thrombotic Effects of Platelet Serotonin. In Serotonin—A Chemical Messenger between All Types of Living Cells; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Briones-Aranda, A.; Corzo-Gómez, J.; Casique-Aguirre, D.; Megchún-Hernández, M. The Platelet Serotonergic System and the Search for New Biomarkers and Therapeutic Options for Diverse Diseases. In Serotonin—Neurotransmitter and Hormone of Brain, Bowels and Blood [Working Title]; IntechOpen: Houston, TX, USA, 2023. [Google Scholar]
- Rieder, M.; Gauchel, N.; Bode, C.; Duerschmied, D. Serotonin: A Platelet Hormone Modulating Cardiovascular Disease. J. Thromb. Thrombolysis 2021, 52, 42–47. [Google Scholar] [CrossRef]
- Jiang, S.Z.; To, J.L.; Hughes, M.R.; McNagny, K.M.; Kim, H. Platelet Signaling at the Nexus of Innate Immunity and Rheumatoid Arthritis. Front. Immunol. 2022, 13, 977828. [Google Scholar] [CrossRef]
- Sage, S.O.; Harper, A.G.S. Calcium Sequestration by Human Platelet Acidic Organelles Is Regulated by the Actin Cytoskeleton and Autocrine 5-Hydroxytryptamine. Cell Calcium 2022, 101, 102522. [Google Scholar] [CrossRef]
- Weitz, J.I.; Fredenburgh, J.C. Platelet Polyphosphate: The Long and the Short of It. Blood 2017, 129, 1574–1575. [Google Scholar] [CrossRef]
- Docampo, R. Advances in the Cellular Biology, Biochemistry, and Molecular Biology of Acidocalcisomes. Microbiol. Mol. Biol. Rev. 2024, 88, e0004223. [Google Scholar] [CrossRef]
- Bernhard, E.; Nitschke, Y.; Khursigara, G.; Sabbagh, Y.; Wang, Y.; Rutsch, F. A Reference Range for Plasma Levels of Inorganic Pyrophosphate in Children Using the ATP Sulfurylase Method. J. Clin. Endocrinol. Metab. 2022, 107, 109–118. [Google Scholar] [CrossRef]
- Fitch-Tewfik, J.L.; Flaumenhaft, R. Platelet Granule Exocytosis: A Comparison with Chromaffin Cells. Front. Endocrinol. 2013, 4, 77. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Sharda, A. Platelet Secretion. In Platelets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–370. [Google Scholar]
- Feng, M.; Hechler, B.; Adam, F.; Gachet, C.; Eckly, A.; Kauskot, A.; Denis, C.V.; Bryckaert, M.; Bobe, R.; Rosa, J.-P. ADP Receptor P2Y12 Is the Capstone of the Cross-Talk between Ca2+ Mobilization Pathways Dependent on Ca2+ ATPases Sarcoplasmic/Endoplasmic Reticulum Type 3 and Type 2b in Platelets. Res. Pr. Thromb. Haemost. 2023, 7, 100004. [Google Scholar] [CrossRef]
- Karampini, E.; Bierings, R.; Voorberg, J. Orchestration of Primary Hemostasis by Platelet and Endothelial Lysosome-Related Organelles. Arter. Thromb. Vasc. Biol. 2020, 40, 1441–1453. [Google Scholar] [CrossRef]
- Södergren, A.L.; Svensson Holm, A.-C.B.; Ramström, S.; Lindström, E.G.; Grenegård, M.; Öllinger, K. Thrombin-Induced Lysosomal Exocytosis in Human Platelets Is Dependent on Secondary Activation by ADP and Regulated by Endothelial-Derived Substances. Platelets 2016, 27, 86–92. [Google Scholar] [CrossRef]
- Koupenova, M.; Livada, A.C.; Morrell, C.N. Platelet and Megakaryocyte Roles in Innate and Adaptive Immunity. Circ. Res. 2022, 130, 288–308. [Google Scholar] [CrossRef]
- Thon, J.N.; Peters, C.G.; Machlus, K.R.; Aslam, R.; Rowley, J.; Macleod, H.; Devine, M.T.; Fuchs, T.A.; Weyrich, A.S.; Semple, J.W.; et al. T Granules in Human Platelets Function in TLR9 Organization and Signaling. J. Cell Biol. 2012, 198, 561–574. [Google Scholar] [CrossRef]
- Khan, A.B.; Siddiqui, U.; Fatima, S.; Rehman, A.A.; Jairajpuri, M.A. Naringin Binds to Protein Disulfide Isomerase to Inhibit Its Activity and Modulate the Blood Coagulation Rates: Implications in Controlling Thrombosis. Int. J. Biol. Macromol. 2023, 252, 126241. [Google Scholar] [CrossRef]
- Seifert, A.W.; Monaghan, J.R.; Voss, S.R.; Maden, M. Skin Regeneration in Adult Axolotls: A Blueprint for Scar-Free Healing in Vertebrates. PLoS ONE 2012, 7, e32875. [Google Scholar] [CrossRef]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef]
- Yokoyama, H.; Maruoka, T.; Aruga, A.; Amano, T.; Ohgo, S.; Shiroishi, T.; Tamura, K. Prx-1 Expression in Xenopus Laevis Scarless Skin-Wound Healing and Its Resemblance to Epimorphic Regeneration. J. Investig. Dermatol. 2011, 131, 2477–2485. [Google Scholar] [CrossRef]
- Bohaud, C.; Johansen, M.D.; Jorgensen, C.; Ipseiz, N.; Kremer, L.; Djouad, F. The Role of Macrophages During Zebrafish Injury and Tissue Regeneration Under Infectious and Non-Infectious Conditions. Front. Immunol. 2021, 12, 707824. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Chen, N.; Quan, Y.; Chen, M.; Lu, Y.; Yang, L.; Wang, S.; Chen, F.; Xu, Y.; Shen, M.; Zeng, H.; et al. Melanocortin/MC5R Axis Regulates the Proliferation of Hematopoietic Stem Cells in Mice after Ionizing Radiation Injury. Blood Adv. 2023, 7, 3199–3212. [Google Scholar] [CrossRef]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The Functional Organization of Cutaneous Low-Threshold Mechanosensory Neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Calcium: A Potential Central Regulator in Wound Healing in the Skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef]
- van der Vliet, A.; Janssen-Heininger, Y.M.W. Hydrogen Peroxide as a Damage Signal in Tissue Injury and Inflammation: Murderer, Mediator, or Messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef]
- Su, Y.; Richmond, A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv. Wound Care 2015, 4, 631–640. [Google Scholar] [CrossRef]
- Gillitzer, R.; Goebeler, M. Chemokines in Cutaneous Wound Healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [CrossRef]
- Othman, A.; Sekheri, M.; Filep, J.G. Roles of Neutrophil Granule Proteins in Orchestrating Inflammation and Immunity. FEBS J. 2022, 289, 3932–3953. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-Derived Granule Cargoes: Paving the Way for Tumor Growth and Progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Zhou, Y.; Bréchard, S. Neutrophil Extracellular Vesicles: A Delicate Balance between Pro-Inflammatory Responses and Anti-Inflammatory Therapies. Cells 2022, 11, 3318. [Google Scholar] [CrossRef]
- Immler, R.; Nadolni, W.; Bertsch, A.; Morikis, V.; Rohwedder, I.; Masgrau-Alsina, S.; Schroll, T.; Yevtushenko, A.; Soehnlein, O.; Moser, M.; et al. The Voltage-Gated Potassium Channel KV1.3 Regulates Neutrophil Recruitment during Inflammation. Cardiovasc. Res. 2022, 118, 1289–1302. [Google Scholar] [CrossRef]
- Domon, H.; Nagai, K.; Maekawa, T.; Oda, M.; Yonezawa, D.; Takeda, W.; Hiyoshi, T.; Tamura, H.; Yamaguchi, M.; Kawabata, S.; et al. Neutrophil Elastase Subverts the Immune Response by Cleaving Toll-Like Receptors and Cytokines in Pneumococcal Pneumonia. Front. Immunol. 2018, 9, 732. [Google Scholar] [CrossRef]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The Paradox of the Neutrophil’s Role in Tissue Injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- He, L.; Marneros, A.G. Macrophages Are Essential for the Early Wound Healing Response and the Formation of a Fibrovascular Scar. Am. J. Pathol. 2013, 182, 2407–2417. [Google Scholar] [CrossRef]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The Role of Macrophages in Skin Homeostasis. Pflug. Arch. 2017, 469, 455–463. [Google Scholar] [CrossRef]
- Dash, S.P.; Gupta, S.; Sarangi, P.P. Monocytes and Macrophages: Origin, Homing, Differentiation, and Functionality during Inflammation. Heliyon 2024, 10, e29686. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Wang, H.-T.; Lin, P.-T.; Chuang, J.-H.; Yang, M.-Y. Macrophage Inflammatory Protein-1 Alpha, a Potential Biomarker for Predicting Left Atrial Remodeling in Patients with Atrial Fibrillation. Front. Cardiovasc. Med. 2021, 8, 784792. [Google Scholar] [CrossRef]
- Georgakis, M.K.; van der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; de Jager, S.C.A.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.G.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate with Plaque Vulnerability. Arter. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef]
- Goren, I.; Allmann, N.; Yogev, N.; Schürmann, C.; Linke, A.; Holdener, M.; Waisman, A.; Pfeilschifter, J.; Frank, S. A Transgenic Mouse Model of Inducible Macrophage Depletion. Am. J. Pathol. 2009, 175, 132–147. [Google Scholar] [CrossRef]
- Mirza, R.; DiPietro, L.A.; Koh, T.J. Selective and Specific Macrophage Ablation Is Detrimental to Wound Healing in Mice. Am. J. Pathol. 2009, 175, 2454–2462. [Google Scholar] [CrossRef]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential Roles of Macrophages in Diverse Phases of Skin Repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Ma, Z.; Iwashina, T.; Tredget, E.E. Systemic Depletion of Macrophages in the Subacute Phase of Wound Healing Reduces Hypertrophic Scar Formation. Wound Repair Regen. 2016, 24, 644–656. [Google Scholar] [CrossRef]
- Hu, M.S.; Walmsley, G.G.; Barnes, L.A.; Weiskopf, K.; Rennert, R.C.; Duscher, D.; Januszyk, M.; Maan, Z.N.; Hong, W.X.; Cheung, A.T.M.; et al. Delivery of Monocyte Lineage Cells in a Biomimetic Scaffold Enhances Tissue Repair. JCI Insight 2017, 2, e96260. [Google Scholar] [CrossRef]
- Godwin, J.W.; Pinto, A.R.; Rosenthal, N.A. Macrophages Are Required for Adult Salamander Limb Regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9415–9420. [Google Scholar] [CrossRef]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and Sustained Induction of Chemokines during Impaired Wound Healing in the Genetically Diabetic Mouse: Prolonged Persistence of Neutrophils and Macrophages during the Late Phase of Repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Khanna, S.; Biswas, S.; Shang, Y.; Collard, E.; Azad, A.; Kauh, C.; Bhasker, V.; Gordillo, G.M.; Sen, C.K.; Roy, S. Macrophage Dysfunction Impairs Resolution of Inflammation in the Wounds of Diabetic Mice. PLoS ONE 2010, 5, e9539. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Zou, B.; Yang, D.; Lu, Y.; Zhang, X.; Zhang, C.; Li, Y.; Zhu, C. Macrophage Regulation in Vascularization upon Regeneration and Repair of Tissue Injury and Engineered Organ Transplantation. Fundam. Res. 2024; in press. [Google Scholar] [CrossRef]
- Di Nardo, A.; Yamasaki, K.; Dorschner, R.A.; Lai, Y.; Gallo, R.L. Mast Cell Cathelicidin Antimicrobial Peptide Prevents Invasive Group A Streptococcus Infection of the Skin. J. Immunol. 2008, 180, 7565–7573. [Google Scholar] [CrossRef]
- Li, H.; Niu, J.; Wang, X.; Niu, M.; Liao, C. The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics 2023, 15, 2278. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Y.; Bernard, J.J.; MacLeod, D.T.; Cogen, A.L.; Moss, B.; Di Nardo, A. Skin Mast Cells Protect Mice against Vaccinia Virus by Triggering Mast Cell Receptor S1PR2 and Releasing Antimicrobial Peptides. J. Immunol. 2012, 188, 345–357. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Wulff, B.C. The Importance of Mast Cells in Dermal Scarring. Adv. Wound Care 2014, 3, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Younan, G.; Suber, F.; Xing, W.; Shi, T.; Kunori, Y.; Åbrink, M.; Pejler, G.; Schlenner, S.M.; Rodewald, H.-R.; Moore, F.D.; et al. The Inflammatory Response after an Epidermal Burn Depends on the Activities of Mouse Mast Cell Proteases 4 and 5. J. Immunol. 2010, 185, 7681–7690. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, H.; Dong, X.; Wang, Z.; Yang, Z.; Shang, Q.; Wang, Q. Regulation of Inflammation during Wound Healing: The Function of Mesenchymal Stem Cells and Strategies for Therapeutic Enhancement. Front. Pharmacol. 2024, 15, 1345779. [Google Scholar] [CrossRef] [PubMed]
- Wulff, B.C.; Parent, A.E.; Meleski, M.A.; DiPietro, L.A.; Schrementi, M.E.; Wilgus, T.A. Mast Cells Contribute to Scar Formation during Fetal Wound Healing. J. Investig. Dermatol. 2012, 132, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Wulff, B.C.; Wilgus, T.A. Mast Cell Activity in the Healing Wound: More than Meets the Eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-S.; Nam, J.-H.; Noh, K.-E.; Lim, D.-S. Dendritic Cell-Based Immunotherapy: The Importance of Dendritic Cell Migration. J. Immunol. Res. 2024, 2024, 7827246. [Google Scholar] [CrossRef] [PubMed]
- Malissen, B.; Tamoutounour, S.; Henri, S. The Origins and Functions of Dendritic Cells and Macrophages in the Skin. Nat. Rev. Immunol. 2014, 14, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, N.; Li, J.; Li, J.; Zhu, W.; Kuang, Y.; Chen, X.; Peng, C. The Role of Langerhans Cells in Epidermal Homeostasis and Pathogenesis of Psoriasis. J. Cell. Mol. Med. 2020, 24, 11646–11655. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Malissen, B.; Mantovani, A.; De Baetselier, P.; Van Ginderachter, J.A. Regulation and Function of the E-Cadherin/Catenin Complex in Cells of the Monocyte-Macrophage Lineage and DCs. Blood 2012, 119, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, A.; Veenstra, J.; Ozog, D.; Mi, Q.-S. The Roles of Skin Langerhans Cells in Immune Tolerance and Cancer Immunity. Vaccines 2022, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, J.; Meller, S.; Conrad, C.; Di Nardo, A.; Homey, B.; Lauerma, A.; Arai, N.; Gallo, R.L.; DiGiovanni, J.; Gilliet, M. Plasmacytoid Dendritic Cells Sense Skin Injury and Promote Wound Healing through Type I Interferons. J. Exp. Med. 2010, 207, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Hornero, R.; Idoyaga, J. Plasmacytoid Dendritic Cells: A Dendritic Cell in Disguise. Mol. Immunol. 2023, 159, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Keyes, B.E.; Liu, S.; Asare, A.; Naik, S.; Levorse, J.; Polak, L.; Lu, C.P.; Nikolova, M.; Pasolli, H.A.; Fuchs, E. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell 2016, 167, 1323–1338.e14. [Google Scholar] [CrossRef] [PubMed]
- Witherden, D.A.; Watanabe, M.; Garijo, O.; Rieder, S.E.; Sarkisyan, G.; Cronin, S.J.F.; Verdino, P.; Wilson, I.A.; Kumanogoh, A.; Kikutani, H.; et al. The CD100 Receptor Interacts with Its Plexin B2 Ligand to Regulate Epidermal Γδ T Cell Function. Immunity 2012, 37, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, J. Properties of Immature and Mature Dendritic Cells: Phenotype, Morphology, Phagocytosis, and Migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.M.; Cauvi, G.; Witherden, D.A.; Havran, W.L. A Keratinocyte-Responsive Γδ TCR Is Necessary for Dendritic Epidermal T Cell Activation by Damaged Keratinocytes and Maintenance in the Epidermis. J. Immunol. 2004, 172, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Havran, W.L.; Jameson, J.M. Epidermal T Cells and Wound Healing. J. Immunol. 2010, 184, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.L.; Nagy, N.; Kaber, G.; Barlow, G.L.; Ramesh, A.; Xie, B.J.; Linde, M.H.; Haddock, N.L.; Lester, C.A.; Tran, Q.-L.; et al. Hyaluronan Synthesis Inhibition Impairs Antigen Presentation and Delays Transplantation Rejection. Matrix Biol. 2021, 96, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Satoh, M.; Yoshino, K.; Ishimori, N. Recent Advances Regarding the Potential Roles of Invariant Natural Killer T Cells in Cardiovascular Diseases with Immunological and Inflammatory Backgrounds. Int. Immunol. 2024, 36, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Papadopoulos, M.; Pant, H.; Tumes, D.J. The Role of Invariant T Cells in Inflammation of the Skin and Airways. Semin. Immunopathol. 2019, 41, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Kawakami, K.; Ritsu, M.; Kanno, E.; Suzuki, A.; Kamimatsuno, R.; Takagi, N.; Miyasaka, T.; Ishii, K.; Imai, Y.; et al. Contribution of Invariant Natural Killer T Cells to Skin Wound Healing. Am. J. Pathol. 2015, 185, 3248–3257. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Kawakami, K.; Kanno, E.; Suzuki, A.; Takagi, N.; Yamamoto, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Invariant NKT Cells Promote Skin Wound Healing by Preventing a Prolonged Neutrophilic Inflammatory Response. Wound Repair Regen. 2017, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-Dependent Inflammation, Fibrogenesis, and Collagenolysis Regulates Extracellular Matrix Remodeling and Tensile Strength during Cutaneous Wound Healing. Matrix Biol. 2019, 75–76, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The IL-4/IL-13 Axis in Skin Fibrosis and Scarring: Mechanistic Concepts and Therapeutic Targets. Arch. Dermatol. Res. 2020, 312, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Saw, V.P.J.; Schmidt, E.; Offiah, I.; Galatowicz, G.; Zillikens, D.; Dart, J.K.G.; Calder, V.L.; Daniels, J.T. Profibrotic Phenotype of Conjunctival Fibroblasts from Mucous Membrane Pemphigoid. Am. J. Pathol. 2011, 178, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.; Kubo, T.; Kiya, K.; Kawai, K.; Matsuzaki, S.; Kobayashi, D.; Fujiwara, T.; Katayama, T.; Hosokawa, K. Periostin Is Induced by IL-4/IL-13 in Dermal Fibroblasts and Promotes RhoA/ROCK Pathway-Mediated TGF-Β1 Secretion in Abnormal Scar Formation. J. Plast. Surg. Hand Surg. 2019, 53, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Min, M.S.; Mazori, D.R.; Lee, M.S.; Merola, J.F.; Vleugels, R.A.; Cobos, G.; LaChance, A.H. Successful Treatment of Keloids and Hypertrophic Scars with Systemic and Intralesional Dupilumab. J. Drugs Dermatol. 2023, 22, 1220–1222. [Google Scholar] [CrossRef]
- Wong, V.W.; Paterno, J.; Sorkin, M.; Glotzbach, J.P.; Levi, K.; Januszyk, M.; Rustad, K.C.; Longaker, M.T.; Gurtner, G.C. Mechanical Force Prolongs Acute Inflammation via T-cell-dependent Pathways during Scar Formation. FASEB J. 2011, 25, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Faussone-Pellegrini, M.-S. TELOCYTES—A Case of Serendipity: The Winding Way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Bei, Y. Decoding Telocytes. Telocytes Connect. Cells 2016, 913, 23–39. [Google Scholar]
- Aleksandrovych, V.; Pasternak, A.; Basta, P.; Sajewicz, M.; Walocha, J.A.; Gil, K. Telocytes: Facts, Speculations and Myths (Review Article). Folia Med. Cracov. 2017, 57, 5–22. [Google Scholar] [PubMed]
- Vannucchi, M.G. Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which? Int. J. Mol. Sci. 2022, 23, 8435. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, H.; Zhu, Z. Telocytes and Endometriosis. Arch. Gynecol. Obs. 2022, 307, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.E.-Z.A. The Cellular Architecture of the Primo Vascular System. J. Acupunct. Meridian Stud. 2022, 15, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Nardini, P.; Fioretto, B.S.; Guasti, D.; Romano, E.; Sgambati, E.; Marini, M.; Manetti, M. Immunohistochemical and Ultrastructural Identification of Telocytes in the Lamina Propria of Human Vaginal Mucosa. Acta Histochem. 2023, 125, 152094. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G. The Telocytes: Ten Years after Their Introduction in the Scientific Literature. An Update on Their Morphology, Distribution, and Potential Roles in the Gut. Int. J. Mol. Sci. 2020, 21, 4478. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, Y.; Nagasawa, T. Identification of Microenvironmental Niches for Hematopoietic Stem Cells and Lymphoid Progenitors—Bone Marrow Fibroblastic Reticular Cells with Salient Features. Int. Immunol. 2021, 33, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrovych, V.; Gil, A.; Poniatowski, A. Notes about Telocytes and Immunity. Folia Medica Cracoviensia 2022, 62, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ma, Q.; Meng, X.; Pan, Y.; Li, Y.; Wang, J.; Liu, Y.; Yang, P. Interstitial Cell Dysregulation in Allergic Contact Dermatitis: A Morphodynamic Study of Novel Interstitial Cell Telocytes. Microsc. Microanal. 2023, 29, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Chunhua, L.; Xuebing, B.; Yonghong, S.; Min, Y.; Haixiang, H.; Jianming, Y.; Zhenwei, Z.; Qiusheng, C. Distribution and Ultrastructural Features of Telocytes in the Pars Distalis of the Rat Pituitary Gland. Microsc. Microanal. 2023, 29, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Mei, L.; Shi, Y.; Huang, H.; Guo, Y.; Liang, C.; Yang, M.; Wu, R.; Zhang, Y.; Chen, Q. The Cellular Mechanism of Acupuncture for Ulcerative Colitis Based on the Communication of Telocytes. Microsc. Microanal. 2023, 29, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, L.; Chen, B.; Lin, S.; Gu, J.; Tan, L.; Lin, M. Telocytes Protect against Lung Tissue Fibrosis through Hexokinase 2-dependent Pathway by Secreting Hepatocyte Growth Factor. Clin. Exp. Pharmacol. Physiol. 2023, 50, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Mei, L.; Zhang, Y.; Liang, C.; Bai, X.; Zhang, Z.; Shi, Y.; Chen, Q. The Potential of Berberine to Target Telocytes in Rabbit Heart. Planta Med. 2023, 90, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Sayed, R.K.A.; Mokhtar, D.M. Structural and Immunohistochemical Characterization of Pancreas of Molly Fish (Poecilia sphenops), with a Special Reference to Its Immune Role. Microsc. Res. Tech. 2023, 86, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.d.P.; Carrasco, J.L.; Madrid, J.F.; Díaz-Flores, L. Telocytes/CD34+ Stromal Cells in the Normal, Hyperplastic, and Adenomatous Human Parathyroid Glands. Int. J. Mol. Sci. 2023, 24, 12118. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Guo, J.; Zhang, S.; Wu, H.; Chen, Y.-G.; Wang, J.; Li, B.; Liu, H. A Stromal Lineage Maintains Crypt Structure and Villus Homeostasis in the Intestinal Stem Cell Niche. BMC Biol. 2023, 21, 169. [Google Scholar] [CrossRef]
- Bugajska, J.; Berska, J.; Pasternak, A.; Sztefko, K. Biliary Amino Acids and Telocytes in Gallstone Disease. Metabolites 2023, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhafeez, H.H.; Rutland, C.S.; Soliman, S.A. Morphology of Migrating Telocytes and Their Potential Role in Stem Cell Differentiation during Cartilage Development in Catfish (Clarias gariepinus). Microsc. Res. Tech. 2023, 86, 1108–1121. [Google Scholar] [CrossRef] [PubMed]
- Dama, G.; Hu, X.; Yan, Y.; Li, Y.; Li, H.; Yang, F.; Liu, Y.; Lin, J. Identification and Protective Role of CD34+ Stromal Cells/Telocytes in Experimental Autoimmune Encephalomyelitis (EAE) Mouse Spleen. Histochem. Cell Biol. 2023, 160, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yuan, L.; Chen, S.; Zhang, Y.; Ma, X.; Xing, Y.; Song, J. Morphological and Histochemical Identification of Telocytes in Adult Yak Epididymis. Sci. Rep. 2023, 13, 5295. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Godoy, J.M.; Pereira de Godoy, A.C.; Guerreiro Godoy, M.d.F.; de Santi Neto, D. Synthesis and Physiological Remodeling of CD34 Cells in the Skin Following the Reversal of Fibrosis through Intensive Treatment for Lower Limb Lymphedema: A Case Report. Dermatopathology 2023, 10, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Flores, A.; Cassarino, D.S. Type 2 Dermal Dendrocytes Are Telocytes and So Should They Be Called. Am. J. Dermatopathol. 2023, 45, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Hu, Q.; Wang, Y.; Jiang, M.; Cui, Z.; Guo, R.; Liu, L.; Chen, F.; Hu, H.; Zhao, G. Smooth Muscle Cells, Interstitial Cells and Neurons in the Gallbladder (GB): Functional Syncytium of Electrical Rhythmicity and GB Motility (Review). Int. J. Mol. Med. 2023, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.d.P.; Palmas, M.; Carrasco, J.L.; Madrid, J.F.; Díaz-Flores, L. Delimiting CD34+ Stromal Cells/Telocytes Are Resident Mesenchymal Cells That Participate in Neovessel Formation in Skin Kaposi Sarcoma. Int. J. Mol. Sci. 2023, 24, 3793. [Google Scholar] [CrossRef] [PubMed]
- Henrot, P.; Blervaque, L.; Dupin, I.; Zysman, M.; Esteves, P.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Cellular Interplay in Skeletal Muscle Regeneration and Wasting: Insights from Animal Models. J. Cachexia Sarcopenia Muscle 2023, 14, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Q.-W.; Sun, Y.; Chen, X.-F. The Emerging Role of Extracellular Vesicles in the Testis. Hum. Reprod. 2023, 38, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Hussein, M.R. Telocytes in Cutaneous Biology: A Reappraisal. Actas Dermosifiliogr. 2023, 114, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Babadag, S.; Çelebi-Saltik, B. A Cellular Regulator of the Niche: Telocyte. Tissue Barriers 2023, 11, 2131955. [Google Scholar] [CrossRef] [PubMed]
- Abe, S. Behavior and Functional Roles of CD34+ Mesenchymal Cells in Mammalian Testes. Int. J. Mol. Sci. 2022, 23, 9585. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Ibba-Manneschi, L.; Guasti, D.; Perigli, G.; Faussone-Pellegrini, M.; Manetti, M. Morphologic Evidence of Telocytes in Human Thyroid Stromal Tissue. J. Cell. Mol. Med. 2022, 26, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M. Molecular Morphology and Function of Stromal Cells. Int. J. Mol. Sci. 2021, 22, 13422. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Mirancea, N.; Mănoiu, V.S.; Vâlcu, M.; Nicolescu, M.I.; Păduraru, D. Skin Telocytes. Ann. Anat.—Anat. Anz. 2012, 194, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Soare, C.; Ceafalan, L.C.; Voiculescu, V.M. Platelet-Rich Plasma in Dermatology: New Insights on the Cellular Mechanism of Skin Repair and Regeneration. Life 2023, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Lucattelli, E.; Innocenti, M.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Manetti, M. A Two-Step Immunomagnetic Microbead-Based Method for the Isolation of Human Primary Skin Telocytes/CD34+ Stromal Cells. Int. J. Mol. Sci. 2020, 21, 5877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, L.; Zhang, R.; Jin, H.; Shi, H. Ultrastructural and Immunohistochemical Characteristics of Telocytes in Human Scalp Tissue. Sci. Rep. 2020, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Mirancea, N.; Moroşanu, A.-M.; Mirancea, G.-V.; Juravle, F.D.; Mănoiu, V.S. Infrastructure of the Telocytes from Tumor Stroma in the Skin Basal and Squamous Cell Carcinomas. Rom. J. Morphol. Embryol. 2013, 54, 1025–1037. [Google Scholar] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Pino García, M.; González, M.; Díaz-Flores, L.; Francisco Madrid, J. Telocytes as a Source of Progenitor Cells in Regeneration and Repair through Granulation Tissue. Curr. Stem Cell Res. Ther. 2016, 11, 395–403. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Ruffo, M.; Rosa, I.; Faussone-Pellegrini, M.S.; Matucci-Cerinic, M.; Ibba-Manneschi, L. Evidence for Progressive Reduction and Loss of Telocytes in the Dermal Cellular Network of Systemic Sclerosis. J. Cell. Mol. Med. 2013, 17, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Cismasiu, V.B.; Radu, E.; Popescu, L.M. MiR-193 Expression Differentiates Telocytes from Other Stromal Cells. J. Cell. Mol. Med. 2011, 15, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.G.; Marinescu, B.G.; Marta, D.; Nicolescu, M.I. Areas of Cartilaginous and Osseous Metaplasia After Experimental Myocardial Infarction in Rats. Anat. Rec. 2019, 302, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Cucu, I.; Nicolescu, M.I.; Busnatu, Ș.S.; Manole, C.G. Dynamic Involvement of Telocytes in Modulating Multiple Signaling Pathways in Cardiac Cytoarchitecture. Int. J. Mol. Sci. 2022, 23, 5769. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Rodríguez-Rodriguez, R.; Hernández-León, N.; Díaz-Flores, L.; Carrasco, J.L. Cd34+ Stromal Cells/Telocytes in Normal and Pathological Skin. Int. J. Mol. Sci. 2021, 22, 7342. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Romano, E.; Fioretto, B.S.; Guasti, D.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Manetti, M. Scleroderma-like Impairment in the Network of Telocytes/CD34+ Stromal Cells in the Experimental Mouse Model of Bleomycin-Induced Dermal Fibrosis. Int. J. Mol. Sci. 2021, 22, 12407. [Google Scholar] [CrossRef]
- Matei, A.E.; Distler, J.H.W. Response to: ‘In Search for the Ideal Anatomical Composition of Vascularised Human Skin Equivalents for Systemic Sclerosis Translational Research: Should We Recruit the Telocytes?’ By Manetti and Matucci-Cerinic. Ann. Rheum. Dis. 2021, 80, e150. [Google Scholar] [CrossRef] [PubMed]
- Tey, R.V.; Haldankar, P.; Joshi, V.R.; Raj, R.; Maradi, R. Variability in Platelet-Rich Plasma Preparations Used in Regenerative Medicine: A Comparative Analysis. Stem Cells Int. 2022, 2022, 3852898. [Google Scholar] [CrossRef] [PubMed]
- Trevisson, B.; Becerro-de-Bengoa-Vallejo, R.; Sevillano, D.; González, N.; Losa-Iglesias, M.; López-López, D.; Alou, L. Age-based Inter-subject Variability in Platelet and White Blood Cell Concentrations of Platelet-rich Plasma Prepared Using a New Application to Blood Separation System. Int. Wound J. 2022, 19, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Zahir, H.; Dehghani, B.; Yuan, X.; Chinenov, Y.; Kim, C.; Burge, A.; Bandhari, R.; Nemirov, D.; Fava, P.; Moley, P.; et al. In Vitro Responses to Platelet-Rich-Plasma Are Associated with Variable Clinical Outcomes in Patients with Knee Osteoarthritis. Sci. Rep. 2021, 11, 11493. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Pundkar, A.; Shrivastava, S.; Chandanwale, R.; Jaiswal, A.M. A Comprehensive Review on Platelet-Rich Plasma Activation: A Key Player in Accelerating Skin Wound Healing. Cureus 2023, 15, e48943. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Chauhan, K.; Shetty, V.; Dashore, S.; Bhatia, S. Platelet-Rich Plasma in Aesthetics. Indian Dermatol. Online J. 2021, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Buontempo, M.; Alhanshali, L.; Shapiro, J.; Sicco, K.; Garshick, M. Platelet-Rich Plasma Applications, The Past 5 Years: A Review Article. EMJ Dermatol. 2023. [Google Scholar] [CrossRef]
- Maisel-Campbell, A.L.; Ismail, A.; Reynolds, K.A.; Poon, E.; Serrano, L.; Grushchak, S.; Farid, C.; West, D.P.; Alam, M. A Systematic Review of the Safety and Effectiveness of Platelet-Rich Plasma (PRP) for Skin Aging. Arch. Dermatol. Res. 2020, 312, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, J.; Zheng, H.; Zou, C.; Yu, Z.; Wu, Z.; Zhang, J. Study of Platelet-rich Plasma Application for Skin and Plastic Surgery in Recent 20 Years: A Bibliometric Analysis. J. Cosmet. Dermatol. 2023, 22, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, Y.-B.; Ha, C.-W.; Roh, Y.J.; Park, J.-G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Orthop. J. Sports Med. 2021, 9, 232596712110119. [Google Scholar] [CrossRef] [PubMed]
- Phoebe, L.K.W.; Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Wu, R.; Wong, S.; Wan, J.; Yi, K. Use of Platelet Rich Plasma for Skin Rejuvenation. Ski. Res. Technol. 2024, 30, e13714. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Pawde, A.M. Variables Affecting the Potential Efficacy of Platelet-Rich Plasma in Dermatology. J. Am. Acad. Dermatol. 2021, 84, e47–e48. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.L. Platelet-Rich Plasma for Skin Rejuvenation. Facial Plast. Surg. Clin. N. Am. 2019, 27, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, V.; Venkataram, M. Platelet-Rich Plasma with Microneedling in Androgenetic Alopecia: Study of Efficacy of the Treatment and the Number of Sessions Required. J. Cutan. Aesthet. Surg. 2021, 14, 184–190. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Abdel-Wahab, H.; Hossam, A. Combining Microneedling with Other Minimally Invasive Procedures for Facial Rejuvenation: A Split-face Comparative Study. Int. J. Dermatol. 2018, 57, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Gulati, M. Platelet-Rich Plasma: A Healing Virtuoso. Blood Res. 2016, 51, 3. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Ornetti, P.; Maillet, J.; Noel, É.; Adam, P.; Legré-Boyer, V.; Boyer, T.; Allali, F.; Gremeaux, V.; Kaux, J.-F.; et al. Intra-Articular Injections of Platelet-Rich Plasma in Symptomatic Knee Osteoarthritis: A Consensus Statement from French-Speaking Experts. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3195–3210. [Google Scholar] [CrossRef] [PubMed]
- Yaman, R.; Kinard, T.N. Platelet Rich Plasma: Hope or Hype? Ann. Blood 2022, 7, 6. [Google Scholar] [CrossRef]
- Mallis, P.; Michalopoulos, E.; Panagouli, E.; Dimou, Z.; Sarri, E.F.; Georgiou, E.; Gkioka, V.; Stavropoulos-Giokas, C. Selection Criteria of Cord Blood Units for Platelet Gel Production: Proposed Directions from Hellenic Cord Blood Bank. Comment on Mallis et al. Short Term Results of Fibrin Gel Obtained from Cord Blood Units: A Preliminary in Vitro Study. Bioengineering 2019, 6, 66. Bioengineering 2021, 8, 53. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; McKenzie, M.B.; Rahman, M.M.; Cleland, H. Allogeneic Platelet-Rich Plasma: Is It Safe and Effective for Wound Repair? Eur. Surg. Res. 2021, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oneto, P.; Zubiry, P.R.; Schattner, M.; Etulain, J. Anticoagulants Interfere with the Angiogenic and Regenerative Responses Mediated by Platelets. Front. Bioeng. Biotechnol. 2020, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Eichler, C.; Üner, J.; Thangarajah, F.; Radosa, J.; Zinser, M.; Fischer, L.A.; Puppe, J.; Warm, M.; Malter, W.; Lenz, C. Platelet-Rich Plasma (PRP) in Oncological Patients: Long-Term Oncological Outcome Analysis of the Treatment of Subcutaneous Venous Access Device Scars in 89 Breast Cancer Patients. Arch. Gynecol. Obs. 2022, 306, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Morrison, B.; Tosti, A. Adverse Effects of Platelet-Rich Plasma and Microneedling. J. Am. Acad. Dermatol. 2020, 82, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Izzo, P.; De Intinis, C.; Molle, M.; Polistena, A.; Sibio, S.; Codacci-Pisanelli, M.; Biacchi, D.; Di Cello, P.; Santini, D.; Izzo, L.; et al. Case Report: The Use of PRP in the Treatment of Diabetic Foot: Case Series and a Review of the Literature. Front. Endocrinol. 2023, 14, 1286907. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Kountouri, A.; Psahoulia, F.; Koliou, G.-A.; Lazaris, A.; Michalopoulos, E.; Mallis, P.; Korakas, E.; Eleftheriadou, I.; Balampanis, K.; et al. Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer. J. Clin. Med. 2024, 13, 1310. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Timungpi, R. Platelet Rich Plasma in Treatment of Topical Steroid Damaged Face: A Retrospective Analytical Study. Dermatol. Ther. 2022, 35, e15356. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Ferreira, A.F.; Soares, M.; Santos, S.; Tomé, P.; Machado-Simões, J.; Pais, A.S.; Sousa, A.P.; Paiva, A.; Almeida-Santos, T. Optimization of Platelet-Rich Plasma Preparation for Regenerative Medicine: Comparison of Different Anticoagulants and Resuspension Media. Bioengineering 2024, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting. J. Bone Jt. Surg. 2017, 99, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Behrangi, E.; Akbarzadehpasha, A.; Dehghani, A.; Zare, S.; Ghassemi, M.; Zeinali, R.; Goodarzi, A.; Lotfi, Z. Platelet-rich Plasma as a New and Successful Treatment for Lichen Planopilaris: A Controlled Blinded Randomized Clinical Trial. J. Cosmet. Dermatol. 2024, 23, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Brahs, A.; Dorton, D.; Witfill, K. Platelet-Rich Plasma: A Comprehensive Review of Emerging Applications in Medical and Aesthetic Dermatology. J. Clin. Aesthet. Dermatol. 2021, 14, 44–57. [Google Scholar] [PubMed]
- Coulange Zavarro, A.; De Girolamo, L.; Laver, L.; Sánchez, M.; Tischer, T.; Filardo, G.; Sabatier, F.; Magalon, J. The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine—A Bibliometric Analysis—From the ESSKA Orthobiologic Initiative. Bioengineering 2022, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.C.; Lana, G.L.; Santos, G.S.; Visoni, S.B.C.; Brigagão, R.J.; Santos, N.; Sobreiro, R.; da Cruz Silva Reis, A.; Rodrigues, B.L.; Ferrari, S.; et al. The Biological Role of Platelet Derivatives in Regenerative Aesthetics. Int. J. Mol. Sci. 2024, 25, 5604. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-Y.; Lin, C.-S.; Hu, S.; Chung, W.-H. Progress in the Use of Platelet-Rich Plasma in Aesthetic and Medical Dermatology. J. Clin. Aesthet. Dermatol. 2020, 13, 28–35. [Google Scholar] [PubMed]
- Pixley, J.N.; Cook, M.K.; Singh, R.; Larrondo, J.; McMichael, A.J. A Comprehensive Review of Platelet-Rich Plasma for the Treatment of Dermatologic Disorders. J. Dermatol. Treat. 2023, 34, 2142035. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh Bahri, R.; Maleki, S.; Shafiee, A.; Ghandi, N.; Abedini, R.; Ehsani, A.H.; Ehsani, A.; Razavi, Z. Efficacy and Safety of Platelet-Rich Plasma Therapy in Alopecia Areata Patients: A Systematic Review. Dermatol. Ther. 2023, 2023, 8827644. [Google Scholar] [CrossRef]
- Verma, R.; Kumar, S.; Garg, P.; Verma, Y.K. Platelet-Rich Plasma: A Comparative and Economical Therapy for Wound Healing and Tissue Regeneration. Cell Tissue Bank. 2023, 24, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Acharya, N. Platelet-Rich Plasma: A Promising Regenerative Therapy in Gynecological Disorders. Cureus 2022, 14, e28998. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Chahla, J.; Wordie, S.J.; Shapiro, S.A.; Piuzzi, N.S.; Frank, R.M.; Halbrecht, J.; Okada, K.; Nakamura, N.; Mandelbaum, B.; et al. Regulatory and Ethical Aspects of Orthobiologic Therapies. Orthop. J. Sports Med. 2022, 10, 232596712211016. [Google Scholar] [CrossRef] [PubMed]
- Codorean, I.-B.; Cernat, E.; Popescu, D.; Sinescu, R.D.; Perlea, P. Legal and Ethical Aspects of Platelet-Rich Plasma. Rom. J. Leg. Med. 2017, 25, 405–408. [Google Scholar] [CrossRef]
- Mayoly, A.; Iniesta, A.; Curvale, C.; Kachouh, N.; Jaloux, C.; Eraud, J.; Vogtensperger, M.; Veran, J.; Grimaud, F.; Jouve, E.; et al. Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results. Int. J. Mol. Sci. 2019, 20, 1111. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.M.; Humpe, A. ATMPs: New Challenge for Transfusion Services. Transfus. Med. Hemother. 2022, 49, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Schirmann, A.; Boutin, E.; Faix, A.; Yiou, R. Tolerance and Efficacy of Platelet-Rich Plasma Injections in Peyronie’s Disease: Pilot Study. Progrès En. Urol. 2022, 32, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Shome, S.; Kodieswaran, M.; Dadheech, R.; Chevella, M.; Sensharma, S.; Awasthi, S.; Bandyopadhyay, A.; Mandal, B.B. Recent Advances in Platelet-Rich Plasma and Its Derivatives: Therapeutic Agents for Tissue Engineering and Regenerative Medicine. Prog. Biomed. Eng. 2024, 6, 012004. [Google Scholar] [CrossRef]
- Mehdipour chari, K.; Enderami, S.E.; Mansour, R.N.; Hasanzadeh, E.; Amini Mahabadi, J.; Abazari, M.; Asadi, P.; Hojjat, A. Applications of Blood Plasma Derivatives for Cutaneous Wound Healing: A Mini-Review of Clinical Studies. Regen. Ther. 2024, 27, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in Platelet Rich Plasma-Derived Extracellular Vesicles for Regenerative Medicine: A Systematic-Narrative Review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human Clinical Trial of Allogeneic, Platelet-derived Extracellular Vesicles as a Potential Therapeutic for Delayed Wound Healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Vaidakis, D.; Papapanou, M.; Siristatidis, C.S. Autologous Platelet-Rich Plasma for Assisted Reproduction. Cochrane Database Syst. Rev. 2024, 2024, CD013875. [Google Scholar] [CrossRef]
- Miroshnychenko, O.; Chang, W.; Dragoo, J.L. The Use of Platelet-Rich and Platelet-Poor Plasma to Enhance Differentiation of Skeletal Myoblasts: Implications for the Use of Autologous Blood Products for Muscle Regeneration. Am. J. Sports Med. 2017, 45, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Alhawari, H.; Jafar, H.; Al Soudi, M.; Ameereh, L.A.; Fawaris, M.; Saleh, M.; Aladwan, S.; Younes, N.; Awidi, A. Perilesional Injections of Human Platelet Lysate versus Platelet Poor Plasma for the Treatment of Diabetic Foot Ulcers: A double-blinded Prospective Clinical Trial. Int. Wound J. 2023, 20, 3116–3122. [Google Scholar] [CrossRef] [PubMed]
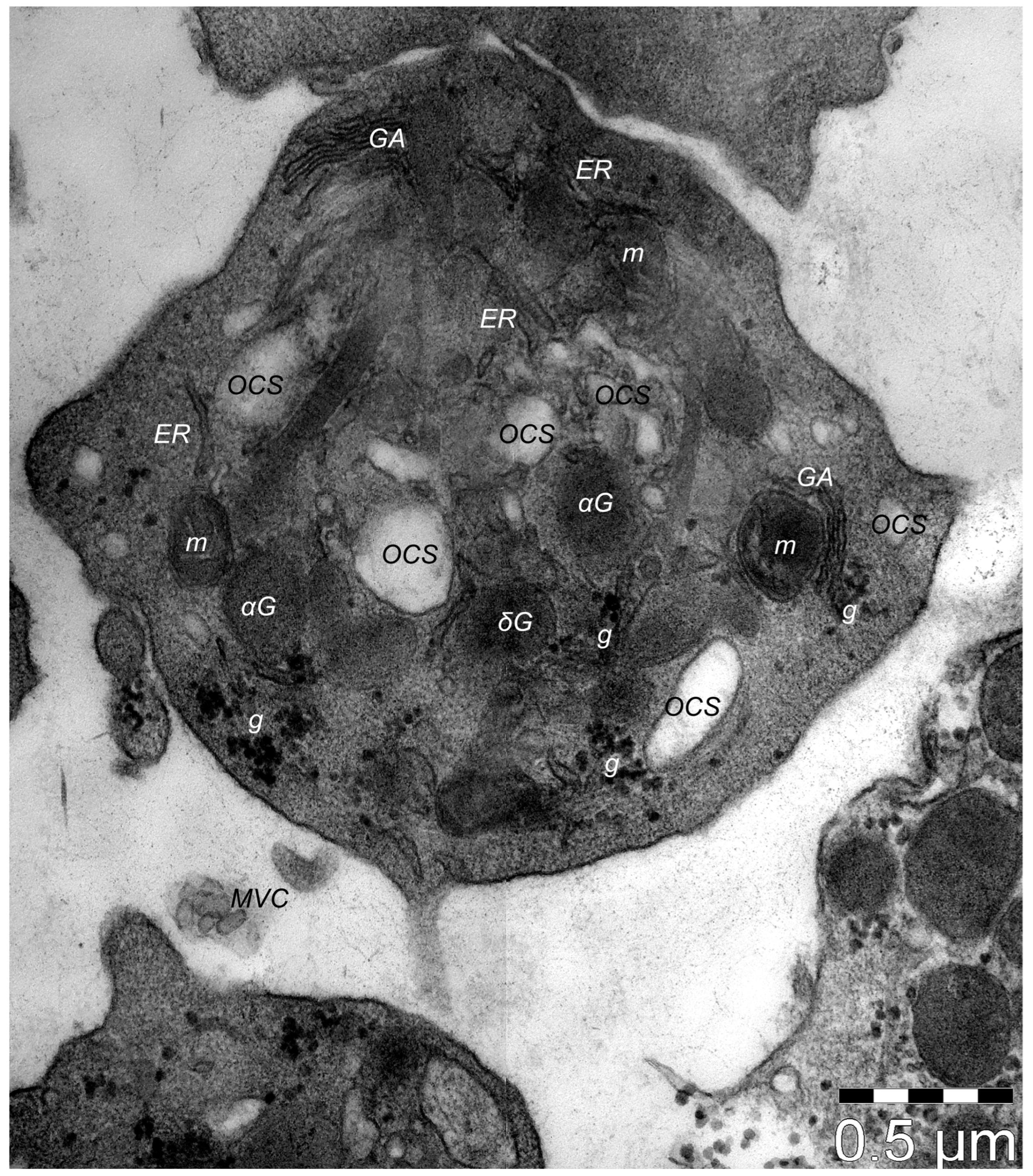
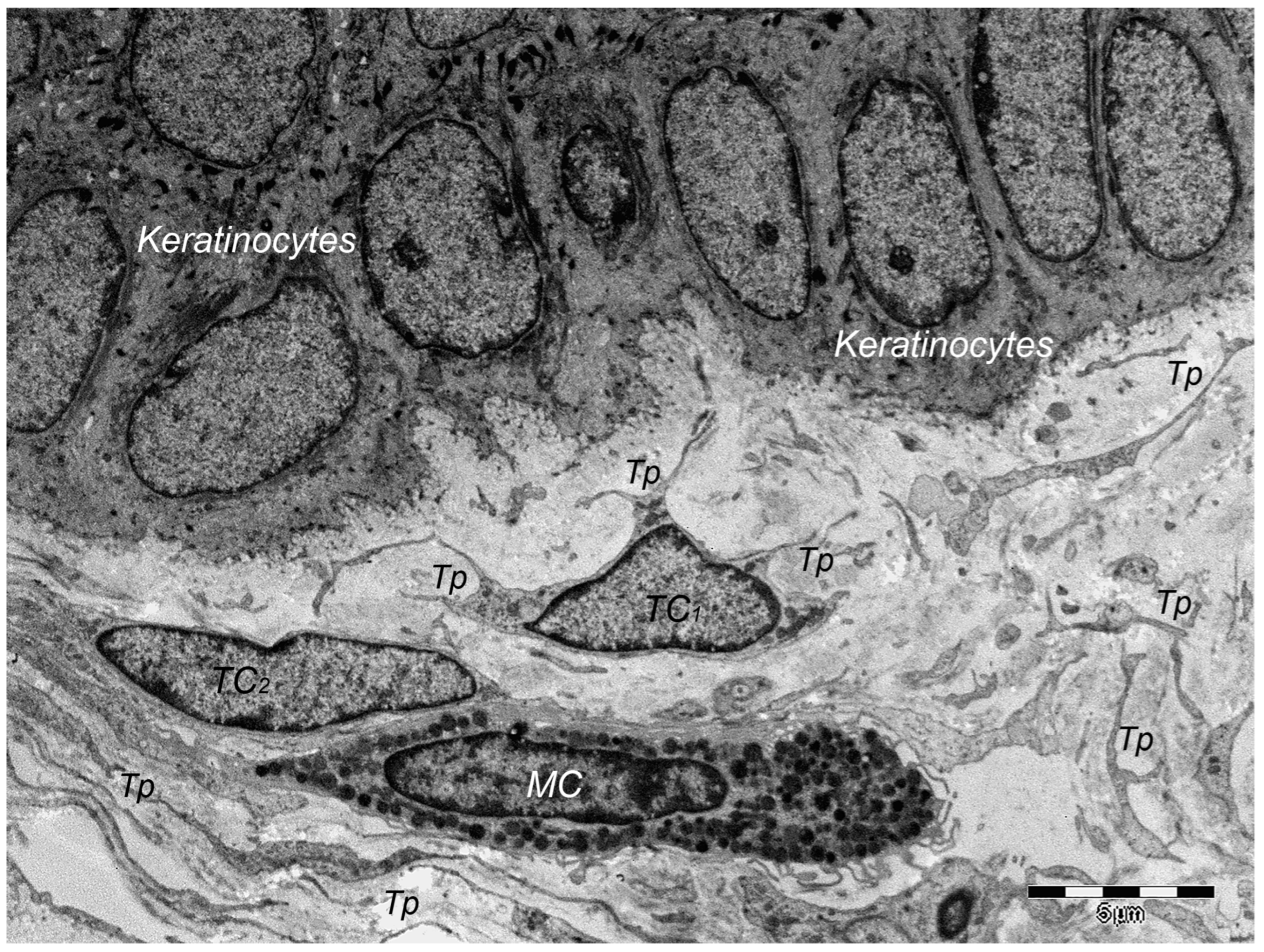

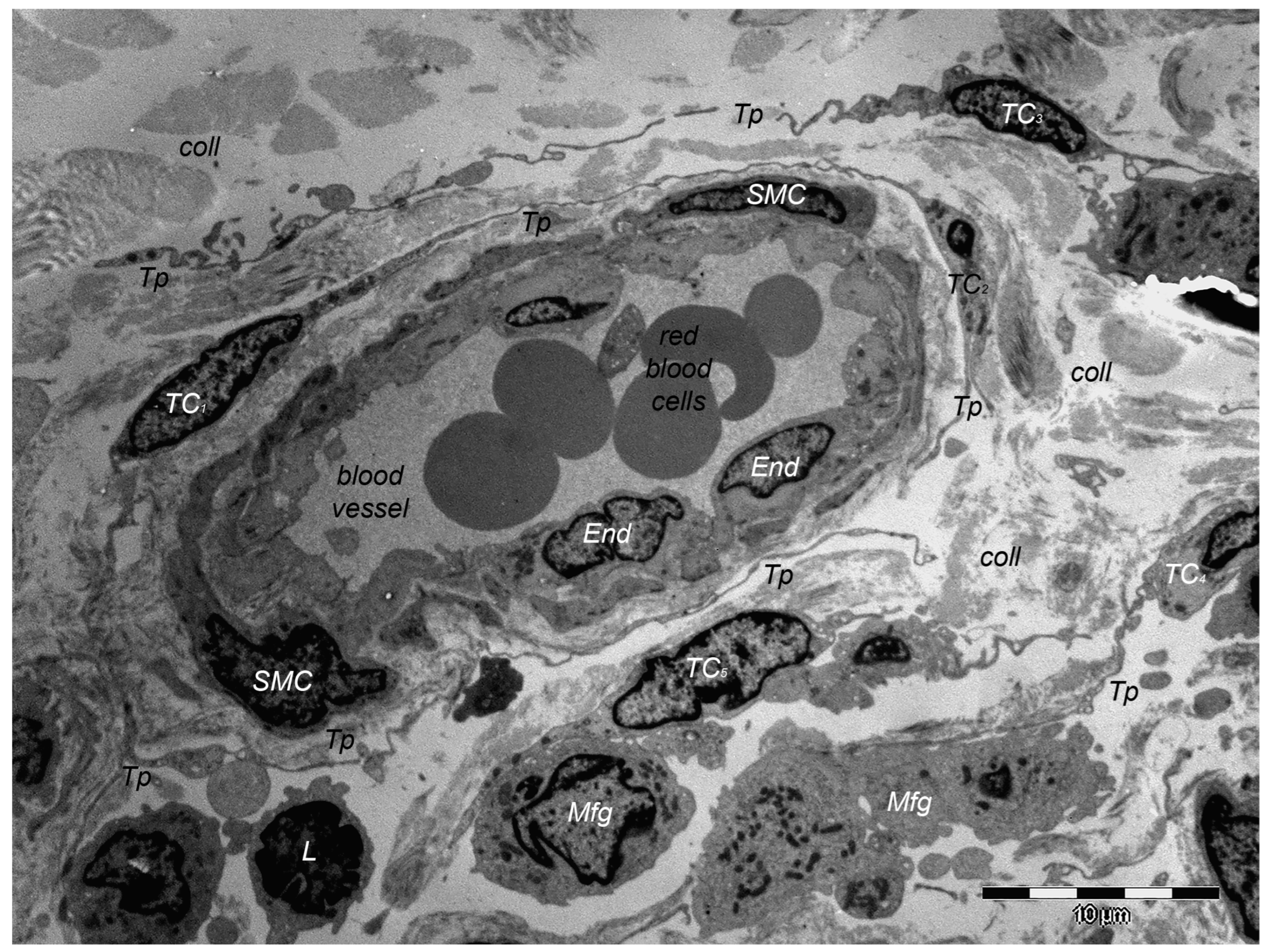
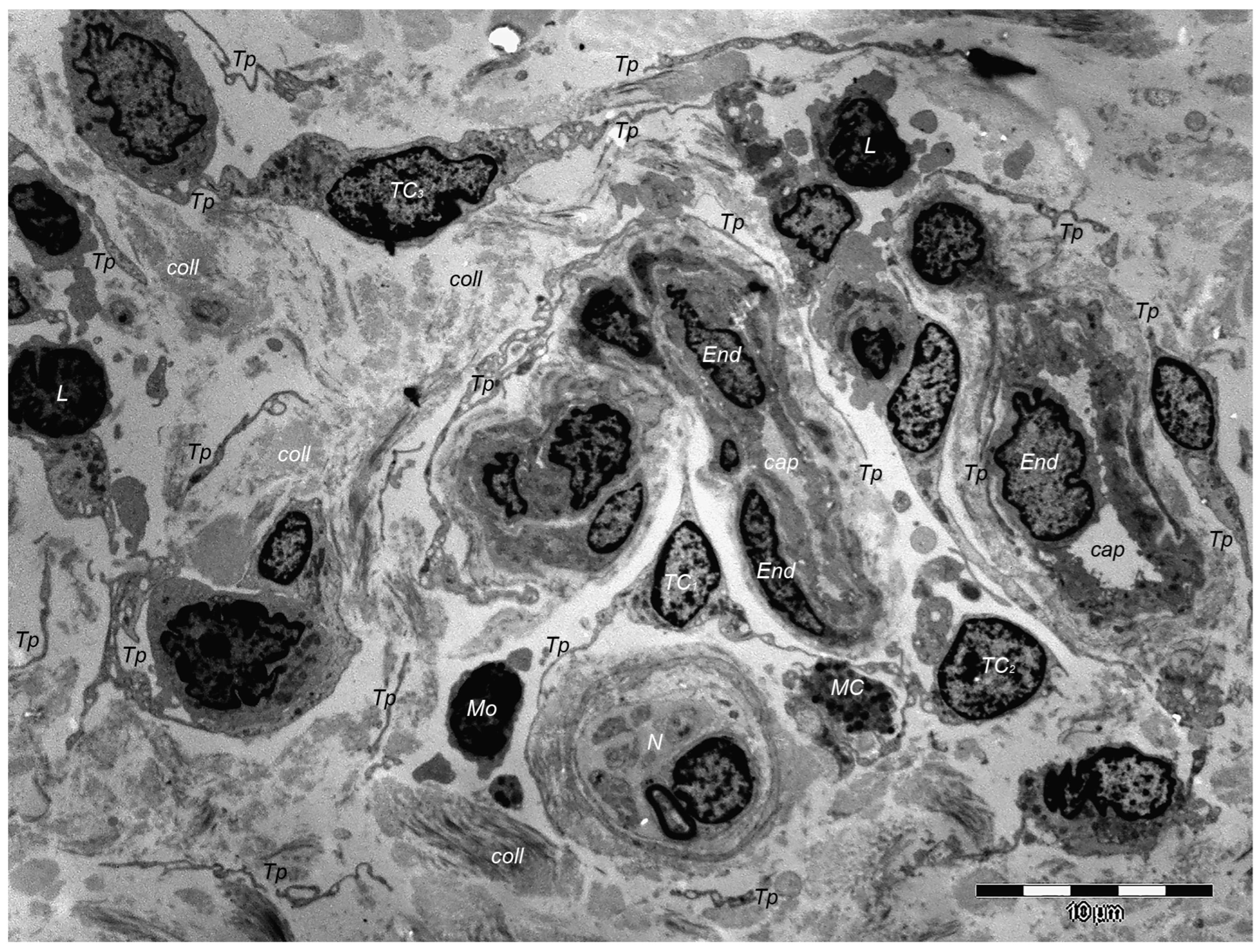
| GROWTH FACTORS | Platelet-derived growth factor (PDGF), AA-AB-BB |
| Transforming growth factor β (TGFβ) | |
| Vascular endothelial growth factor (VEGF) | |
| Epidermal growth factor (EGF) | |
| Fibroblast growth factor (FGF) | |
| Connective tissue growth factor (CTGF) | |
| Insulin-like growth factor (IGF-1) | |
| Hepatocyte growth factor (HGF) | |
| Keratinocyte growth factor/fibroblast growth factor-7 (KGF/FGF-7) | |
| Angiopoietin-1 (ANG-1) | |
| CHEMOKINES | Growth-regulated protein alpha (CXCL1/GRO α/KC/CINC-1) |
| Platelet factor 4/chemokine (C-X-C motif) ligand 4 (PF4/CXCL4) | |
| Epithelial-cell-derived neutrophil-activating peptide-78 (ENA-78)/C-X-C motif chemokine 5 (CXCL5) | |
| C-X-C motif chemokine 7 (CXCL7)/Neutrophil Activating Peptide 2 (NAP-2) | |
| C-X-C motif chemokine 8 (CXCL8)/interleukin-8 (IL-8) | |
| Stromal-cell-derived factor-1 α/C-X-C motif chemokine 12 (SDF-1α/CXCL12) | |
| Monocyte chemoattractant protein (MCP-1)/Chemokine C-C ligand—2 (CCL2) | |
| Macrophage inflammatory protein-1α (MIP-1α)/Chemokine C-C ligand—3 (CCL3) | |
| Chemokine C-C ligand—5 (CCL5)/regulated on activation, normal T-cell expressed and secreted (RANTES) | |
| ADHESION MOLECULES | Fibrinogen |
| Thrombospondin | |
| the von Willebrand factor (vWF) | |
| INTEGRAL MEMBRANE PROTEINS | Integrin αIIbβ3 |
| Glycoprotein (GP) Ib-IX-V complex | |
| Triggering receptor expressed on myeloid cells (TREM)-like transcript 1 (TLT-1) | |
| P-selectin | |
| COAGULANTS AND ANTICOAGULANTS | Platelet factor V |
| Factor IX (Christmas factor) | |
| Factor XIII | |
| Growth arrest specific-6 (Gas6) | |
| ANTIMICROBIAL AGENTS | |
| INFLAMMATORY/IMMUNE AGENTS |
| Nucleotides | Adenosine triphosphate (ATP) |
| Adenosine diphosphate (ADP) | |
| Cyclic adenosine monophosphate (cAMP) | |
| Uridine triphosphate (UTP) | |
| Guanosine-5′-triphosphate (GTP) | |
| Bioactive amines | Serotonin (5-hydroxytryptamine) |
| Histamine | |
| Phosphates (polyphosphate, pyrophosphate) | |
| Ions | Ca2+, Mg2+, K+, P+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manole, C.G.; Voiculescu, V.M.; Soare, C.; Ceafalan, L.C.; Gherghiceanu, M.; Hinescu, M.E. Skin Telocytes Could Fundament the Cellular Mechanisms of Wound Healing in Platelet-Rich Plasma Administration. Cells 2024, 13, 1321. https://doi.org/10.3390/cells13161321
Manole CG, Voiculescu VM, Soare C, Ceafalan LC, Gherghiceanu M, Hinescu ME. Skin Telocytes Could Fundament the Cellular Mechanisms of Wound Healing in Platelet-Rich Plasma Administration. Cells. 2024; 13(16):1321. https://doi.org/10.3390/cells13161321
Chicago/Turabian StyleManole, Catalin G., Vlad M. Voiculescu, Cristina Soare, Laura Cristina Ceafalan, Mihaela Gherghiceanu, and Mihail E. Hinescu. 2024. "Skin Telocytes Could Fundament the Cellular Mechanisms of Wound Healing in Platelet-Rich Plasma Administration" Cells 13, no. 16: 1321. https://doi.org/10.3390/cells13161321







