Analysis of the Effector Functions of Vδ2 γδ T Cells and NK Cells against Cholangiocarcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Derivation of Vδ2 γδ T Cells
2.2. Flow Cytometric Analysis
2.3. Microscopic Analysis
2.4. Maintenance of Human Cholangiocarcinoma Cell Lines
2.5. Time-Resolved Fluorescence-Based Short-Term Cellular Cytotoxicity Assay
2.6. Luciferase-Based Long-Term Cellular Cytotoxicity Assay
2.7. Enzyme-Linked Immunosorbent Assay for IFN-γ
2.8. Derivation of NK Cells
2.9. Statistical Analysis
3. Results
3.1. Vδ2 γδ T Cells Exhibit Cytotoxic Activity against CCA Cells
3.2. Vδ2 γδ T Cells Require an Extended Timeframe to Exhibit Cytotoxicity against CCA Cells
3.3. TCR Signaling Facilitates the Effector Functions of Vδ2 γδ T Cells against CCA
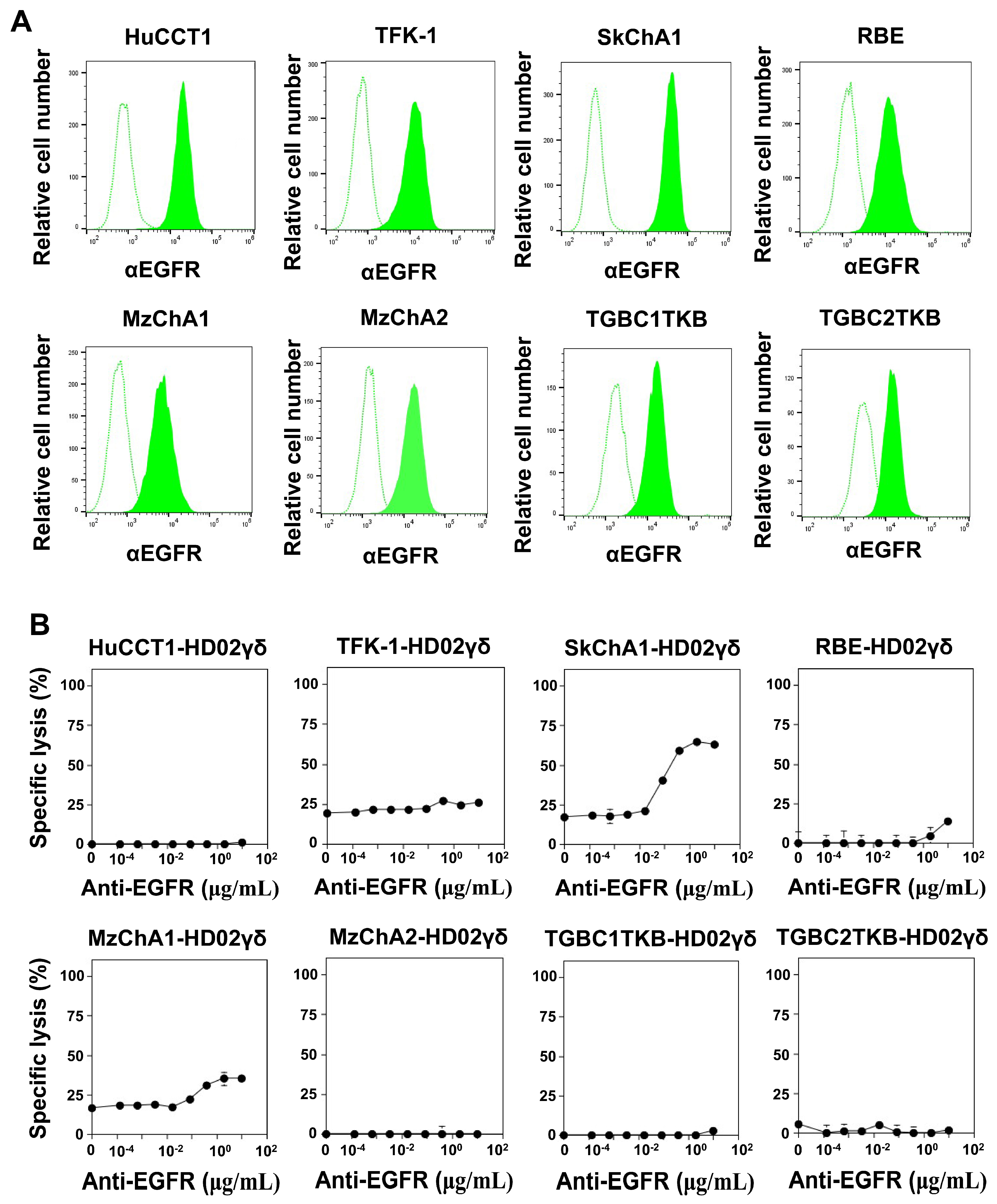
3.4. CD16-Mediated Signaling Facilitates the Effector Functions of Vδ2 γδ T Cells against CCA
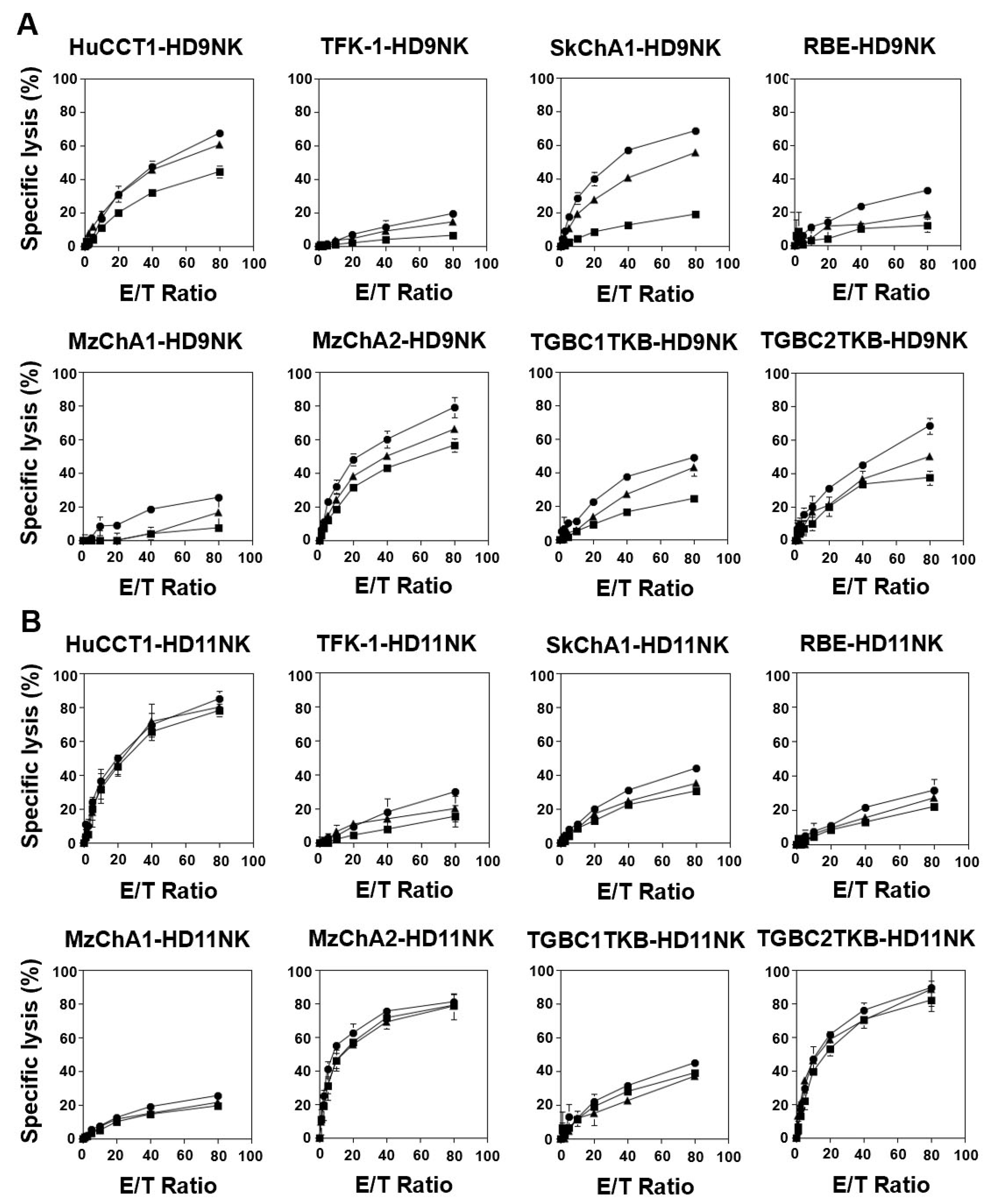
3.5. IL2/IL-18-Induced NK Cells Exhibited Potent Cellular Cytotoxicity against CCA Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wattanawongdon, W.; Hahnvajanawong, C.; Namwat, N.; Kanchanawat, S.; Boonmars, T.; Jearanaikoon, P.; Leelayuwat, C.; Techasen, A.; Seubwai, W. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int. J. Oncol. 2015, 47, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Tepsiri, N.; Chaturat, L.; Sripa, B.; Namwat, W.; Wongkham, S.; Bhudhisawasdi, V.; Tassaneeyakul, W. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J. Gastroenterol. 2005, 11, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Vatanasapt, V.; Martin, N.; Sriplung, H.; Chindavijak, K.; Sontipong, S.; Sriamporn, H.; Parkin, D.M.; Ferlay, J. Cancer incidence in Thailand, 1988–1991. Cancer Epidemiol. Biomarkers Prev. 1995, 4, 475–483. [Google Scholar] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 1, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kamsa-ard, S.; Luvira, V.; Suwanrungruang, K.; Vatanasapt, P.; Wiangnon, S. Risk factors for cholangiocarcinoma in Thailand: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2018, 19, 605–614. [Google Scholar] [CrossRef]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef]
- Flavell, D.J.; Flavell, S.U. Opisthorchis viverrini: Pathogenesis of infection in immunodeprived hamsters. Parasite Immunol. 1986, 8, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Haswell-Elkins, M.R.; Sithithaworn, P.; Mairiang, E.; Elkins, D.B.; Wongratanacheewin, S.; Kaewkes, S.; Mairiang, P. Immune responsiveness and parasite-specific antibody levels in human hepatobiliary disease associated with Opisthorchis viverrini infection. Clin. Exp. Immunol. 1991, 84, 213–218. [Google Scholar] [CrossRef]
- Valverde, A.; Bonhomme, N.; Farges, O.; Sauvanet, A.; Flejou, J.F.; Belghiti, J. Resection of intrahepatic cholangiocarcinoma: A Western experience. J. Hepatobiliary Pancreat. Surg. 1999, 6, 122–127. [Google Scholar] [CrossRef]
- Doherty, B.; Nambudiri, V.E.; Palmer, W.C. Update on the diagnosis and treatment of cholangiocarcinoma. Curr. Gastroenterol. Rep. 2017, 19, 2. [Google Scholar] [CrossRef]
- Malaguarnera, G.; Paladina, I.; Giordano, M.; Malaguarnera, M.; Bertino, G.; Berretta, M. Serum markers of intrahepatic cholangiocarcinoma. Dis. Markers 2013, 34, 219–228. [Google Scholar] [CrossRef]
- Skipworth, J.R.; Olde Damink, S.W.; Imber, C.; Bridgewater, J.; Pereira, S.P.; Malagó, M. Review article: Surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment. Pharmacol. Ther. 2011, 34, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Li, G.X.; Hu, Z.N.; Zhu, P.; Zhang, B.X.; Ding, Z.Y. Significant response to anti-PD-1 based immunotherapy plus lenvatinib for recurrent intrahepatic cholangiocarcinoma with bone metastasis: A case report and literature review. Medicine 2019, 98, e17832. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W.; Pan, M.; Scully, E.; Girardi, M.; Augenlicht, L.H.; Craft, J.; Yin, Z. γδ T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003, 198, 433–442. [Google Scholar] [CrossRef]
- Hintz, M.; Reichenberg, A.; Altincicek, B.; Bahr, U.; Gschwind, R.M.; Kollas, A.K.; Beck, E.; Wiesner, J.; Eberl, M.; Jomaa, H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001, 509, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W. Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections. Cell. Mol. Immunol. 2013, 10, 58–64. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef]
- Sandstrom, A.; Peigné, C.M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E. Molecular targets of the nitrogen containing bisphosphonates: The molecular pharmacology of prenyl synthase inhibition. Curr. Pharm. Des. 2010, 16, 2961–2969. [Google Scholar] [CrossRef] [PubMed]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.M.; Roberti, M.P.; Mordoh, J. Natural killer cells in human cancer: From biological functions to clinical applications. J. Biomed. Biotechnol. 2011, 2011, 676198. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The broad spectrum of human natural killer cell diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Fattori, S.; Gorvel, L.; Granjeaud, S.; Rochigneux, P.; Rouvière, M.S.; Ben Amara, A.; Boucherit, N.; Paul, M.; Dauplat, M.M.; Thomassin-Piana, J.; et al. Quantification of immune variables from liquid biopsy in breast cancer patients links Vδ2. Cancers 2021, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Cazzetta, V.; Bruni, E.; Terzoli, S.; Carenza, C.; Franzese, S.; Piazza, R.; Marzano, P.; Donadon, M.; Torzilli, G.; Cimino, M.; et al. NKG2A expression identifies a subset of human Vδ2 T cells exerting the highest antitumor effector functions. Cell Rep. 2021, 37, 109871. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Tanaka, Y.; Kobayashi, H.; Murata-Hirai, K.; Miyabe, H.; Sugie, T.; Toi, M.; Minato, N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur. J. Immunol. 2011, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.R.; Yang, C.L.; Li, T.; Du, T.; Zhang, P.; Li, X.L.; Dou, Y.C.; Duan, R.S. Circulating CXCR5+ natural killer cells are expanded in patients with myasthenia gravis. Clin. Transl. Immunol. 2023, 12, e1450. [Google Scholar] [CrossRef]
- Kaulfuss, M.; Mietz, J.; Fabri, A.; Vom Berg, J.; Münz, C.; Chijioke, O. The NK cell checkpoint NKG2A maintains expansion capacity of human NK cells. Sci. Rep. 2023, 13, 10555. [Google Scholar] [CrossRef]
- Mariotti, F.R.; Ingegnere, T.; Landolina, N.; Vacca, P.; Munari, E.; Moretta, L. Analysis of the mechanisms regulating soluble PD-1 production and function in human NK cells. Front. Immunol. 2023, 14, 1229341. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, Q.; Li, Y.; Lu, L.; Xiang, Z.; Yin, Z.; Kabelitz, D.; Wu, Y. γδ T cells: Origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Della Chiesa, M.; Carlomagno, S.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Mariotti, F.R.; Mingari, M.C.; Pende, D.; et al. Inhibitory receptors and checkpoints in human NK cells, Implications for the immunotherapy of cancer. Front. Immunol. 2020, 11, 2156. [Google Scholar] [CrossRef] [PubMed]
- Senju, H.; Kumagai, A.; Nakamura, Y.; Yamaguchi, H.; Nakatomi, K.; Fukami, S.; Shiraishi, K.; Harada, Y.; Nakamura, M.; Okamura, H.; et al. Effect of IL-18 on the expansion and phenotype of human natural killer cells: Application to cancer immunotherapy. Int. J. Biol. Sci. 2018, 14, 331–340. [Google Scholar] [CrossRef]
- Knuth, A.; Gabbert, H.; Dippold, W.; Klein, O.; Sachsse, W.; Bitter-Suermann, D.; Prellwitz, W.; Meyer zum Büschenfelde, K.H. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J. Hepatol. 1985, 1, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lv, X.; Zhang, Z.; Huang, Z.; Zhang, E. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma. Front. Immunol. 2023, 14, 1142690. [Google Scholar] [CrossRef]
- Kabelitz, D.; Wesch, D.; Pitters, E.; Zöller, M. Potential of human γδ T lymphocytes for immunotherapy of cancer. Int. J. Cancer 2004, 112, 727–732. [Google Scholar] [CrossRef]
- Hayday, A.C. γδ cells: A right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef]
- Costa, G.P.; Mensurado, S.; Silva-Santos, B. Therapeutic avenues for γδ T cells in cancer. J. Immunother. Cancer 2023, 11, e007955. [Google Scholar] [CrossRef]
- Alnaggar, M.; Xu, Y.; Li, J.; He, J.; Chen, J.; Li, M.; Wu, Q.; Lin, L.; Liang, Y.; Wang, X.; et al. Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: A case study for cholangiocarcinoma. J. Immunother. Cancer 2019, 7, 36. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, Z.; Alnaggar, M.; Kouakanou, L.; Li, J.; He, J.; Yang, J.; Hu, Y.; Chen, Y.; Lin, L.; et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell. Mol. Immunol. 2021, 18, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Tanaka, Y.; Okamura, H.; Kato, T.; Imaizumi, Y.; Nagai, K.; Miyazaki, Y.; Murota, H. Development of Innate-Immune-Cell-Based Immunotherapy for Adult T-Cell Leukemia-Lymphoma. Cells 2024, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Umeyama, Y.; Taniguchi, H.; Gyotoku, H.; Senju, H.; Tomono, H.; Takemoto, S.; Yamaguchi, H.; Tagod, M.S.O.; Iwasaki, M.; Tanaka, Y.; et al. Three distinct mechanisms underlying human γδ T cell-mediated cytotoxicity against malignant pleural mesothelioma. Front. Immunol. 2023, 14, 1058838. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Recognition and destruction of virus-infected cells by human gamma delta CTL. J. Immunol. 1994, 153, 5133–5140. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Zaidi, I.; Loizon, S.; Mercereau-Puijalon, O.; Déchanet-Merville, J.; Mamani-Matsuda, M. Human Vγ9Vδ2 T Lymphocytes in the Immune Response to. Front. Immunol. 2018, 9, 2760. [Google Scholar] [CrossRef] [PubMed]
- Farrington, L.A.; Jagannathan, P.; McIntyre, T.I.; Vance, H.M.; Bowen, K.; Boyle, M.J.; Nankya, F.; Wamala, S.; Auma, A.; Nalubega, M.; et al. Frequent Malaria Drives Progressive Vδ2 T-Cell Loss, Dysfunction, and CD16 Up-regulation During Early Childhood. J. Infect. Dis. 2016, 213, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Tongtawe, P.; Kriangkum, J.; Wimonwattrawatee, T.; Pattanapanyasat, K.; Bryant, L.; Shafiq, J.; Suntharsamai, P.; Looareesuwan, S.; Webster, H.K.; et al. Polyclonal expansion of peripheral γδ T cells in human Plasmodium falciparum malaria. Infect. Immun. 1994, 62, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, P.; Lutwama, F.; Boyle, M.J.; Nankya, F.; Farrington, L.A.; McIntyre, T.I.; Bowen, K.; Naluwu, K.; Nalubega, M.; Musinguzi, K.; et al. Vδ2+ T cell response to malaria correlates with protection from infection but is attenuated with repeated exposure. Sci. Rep. 2017, 7, 11487. [Google Scholar] [CrossRef]
- Shen, L.; Huang, D.; Qaqish, A.; Frencher, J.; Yang, R.; Shen, H.; Chen, Z.W. Fast-acting γδ T-cell subpopulation and protective immunity against infections. Immunol. Rev. 2020, 298, 254–263. [Google Scholar] [CrossRef]
- Rasi, V.; Wood, D.C.; Eickhoff, C.S.; Xia, M.; Pozzi, N.; Edwards, R.L.; Walch, M.; Bovenschen, N.; Hoft, D.F. Granzyme A Produced by γ9δ2 T Cells Activates ER Stress Responses and ATP Production, and Protects Against Intracellular Mycobacterial Replication Independent of Enzymatic Activity. Front. Immunol. 2021, 12, 712678. [Google Scholar] [CrossRef]
- Barnes, P.F.; Grisso, C.L.; Abrams, J.S.; Band, H.; Rea, T.H.; Modlin, R.L. γδ T lymphocytes in human tuberculosis. J. Infect. Dis. 1992, 165, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Dieli, F.; Troye-Blomberg, M.; Ivanyi, J.; Fournié, J.J.; Krensky, A.M.; Bonneville, M.; Peyrat, M.A.; Caccamo, N.; Sireci, G.; Salerno, A. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9/Vδ2 T lymphocytes. J. Infect. Dis. 2001, 184, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, N.; Zhao, H.; Jin, H.; Wang, G.; Niu, C.; Terunuma, H.; He, H.; Li, W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int. J. Cancer 2014, 134, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Han, J.; Baek, J.S.; Tak, E.; Song, G.W.; Lee, S.G.; Jung, D.H.; Park, G.C.; Ahn, C.S.; Kim, N. Cytotoxicity of human hepatic intrasinusoidal CD56bright natural killer cells against hepatocellular carcinoma cells. Int. J. Mol. Sci. 2019, 20, 1564. [Google Scholar] [CrossRef]
- Kang, Y.; Han, M.; Kim, M.; Hwang, H.J.; Ahn, B.C.; Tak, E.; Song, G.W.; Hwang, S.; Koh, K.N.; Jung, D.H.; et al. Cytotoxicity of human hepatic intrasinusoidal γδ T cells depends on phospho-antigen and NK receptor signaling. Anticancer Res. 2023, 43, 63–73. [Google Scholar] [CrossRef]
- Tanaka, Y.; Murata-Hirai, K.; Iwasaki, M.; Matsumoto, K.; Hayashi, K.; Kumagai, A.; Nada, M.H.; Wang, H.; Kobayashi, H.; Kamitakahara, H.; et al. Expansion of human γδ T cells for adoptive immunotherapy using a bisphosphonate prodrug. Cancer Sci. 2018, 109, 587–599. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulma, I.; Na-Bangchang, K.; Carvallo Herrera, A.; Ndubuisi, I.T.; Iwasaki, M.; Tomono, H.; Morita, C.T.; Okamura, H.; Mukae, H.; Tanaka, Y. Analysis of the Effector Functions of Vδ2 γδ T Cells and NK Cells against Cholangiocarcinoma Cells. Cells 2024, 13, 1322. https://doi.org/10.3390/cells13161322
Kulma I, Na-Bangchang K, Carvallo Herrera A, Ndubuisi IT, Iwasaki M, Tomono H, Morita CT, Okamura H, Mukae H, Tanaka Y. Analysis of the Effector Functions of Vδ2 γδ T Cells and NK Cells against Cholangiocarcinoma Cells. Cells. 2024; 13(16):1322. https://doi.org/10.3390/cells13161322
Chicago/Turabian StyleKulma, Inthuon, Kesara Na-Bangchang, Andrea Carvallo Herrera, Ifeanyi Theodora Ndubuisi, Masashi Iwasaki, Hiromi Tomono, Craig T. Morita, Haruki Okamura, Hiroshi Mukae, and Yoshimasa Tanaka. 2024. "Analysis of the Effector Functions of Vδ2 γδ T Cells and NK Cells against Cholangiocarcinoma Cells" Cells 13, no. 16: 1322. https://doi.org/10.3390/cells13161322





