Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture of HCjEC
2.3. Fluorescence Calcium Imaging
2.4. Planar Patch-Clamp Recording
2.5. Statistical Data Analyses
3. Results
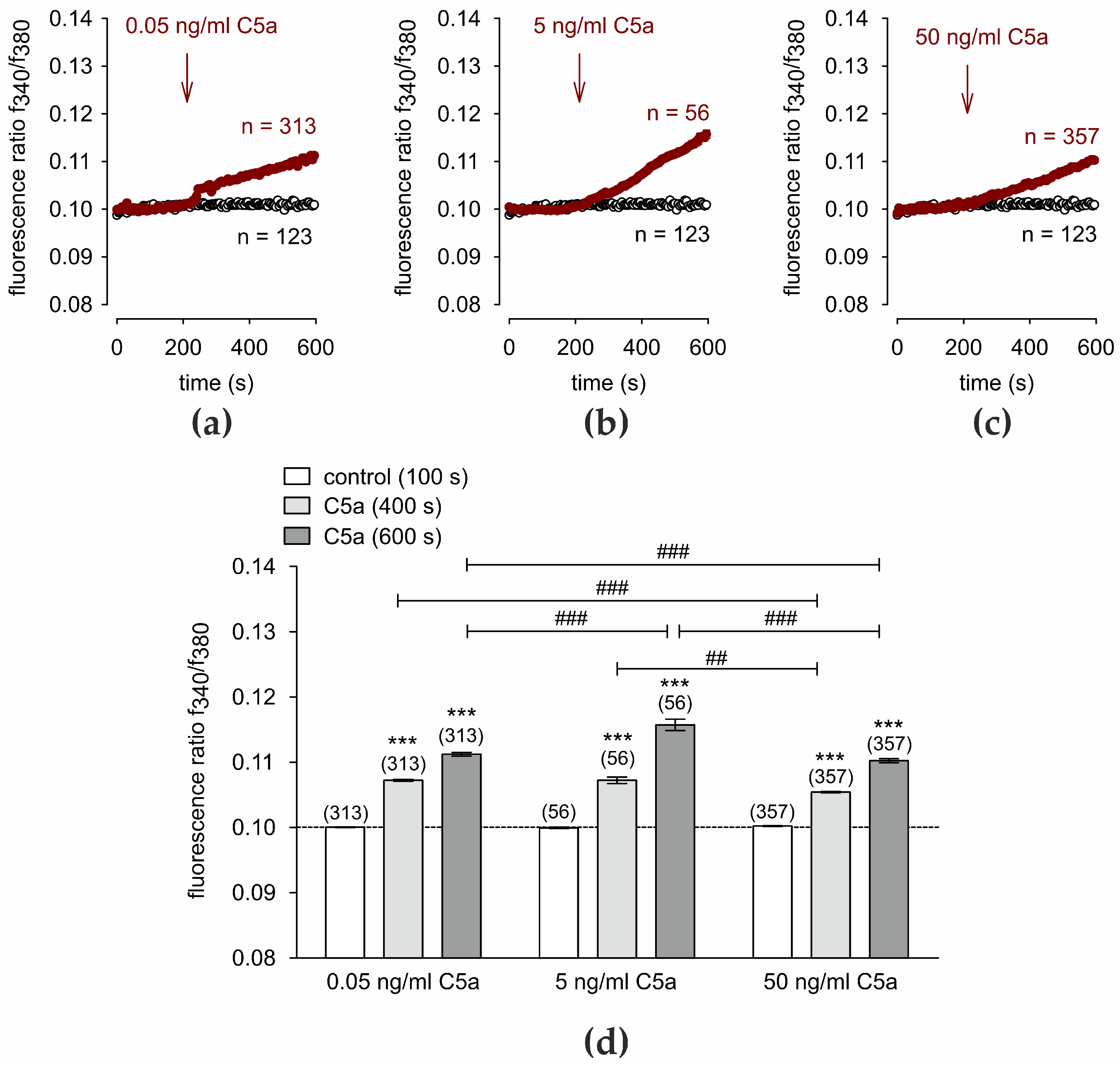
3.1. C5a Increases Intracellular Ca2+
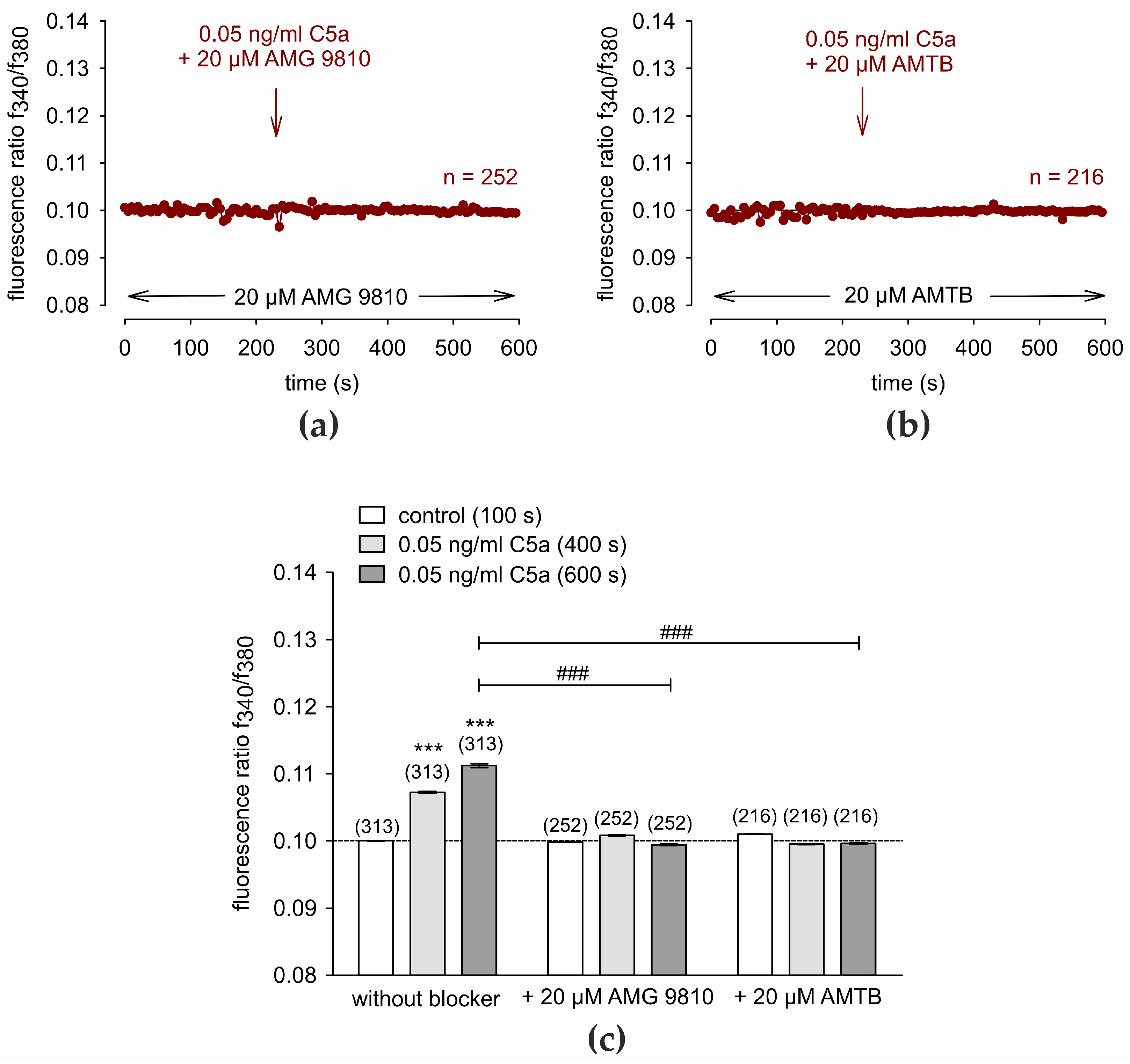
3.2. TRP Channel Blockers Abolish C5a-Induced Ca2+ Increase
3.3. C5L2p Suppresses the C5a-Induced Ca2+ Increase in a Dose-Dependent Matter
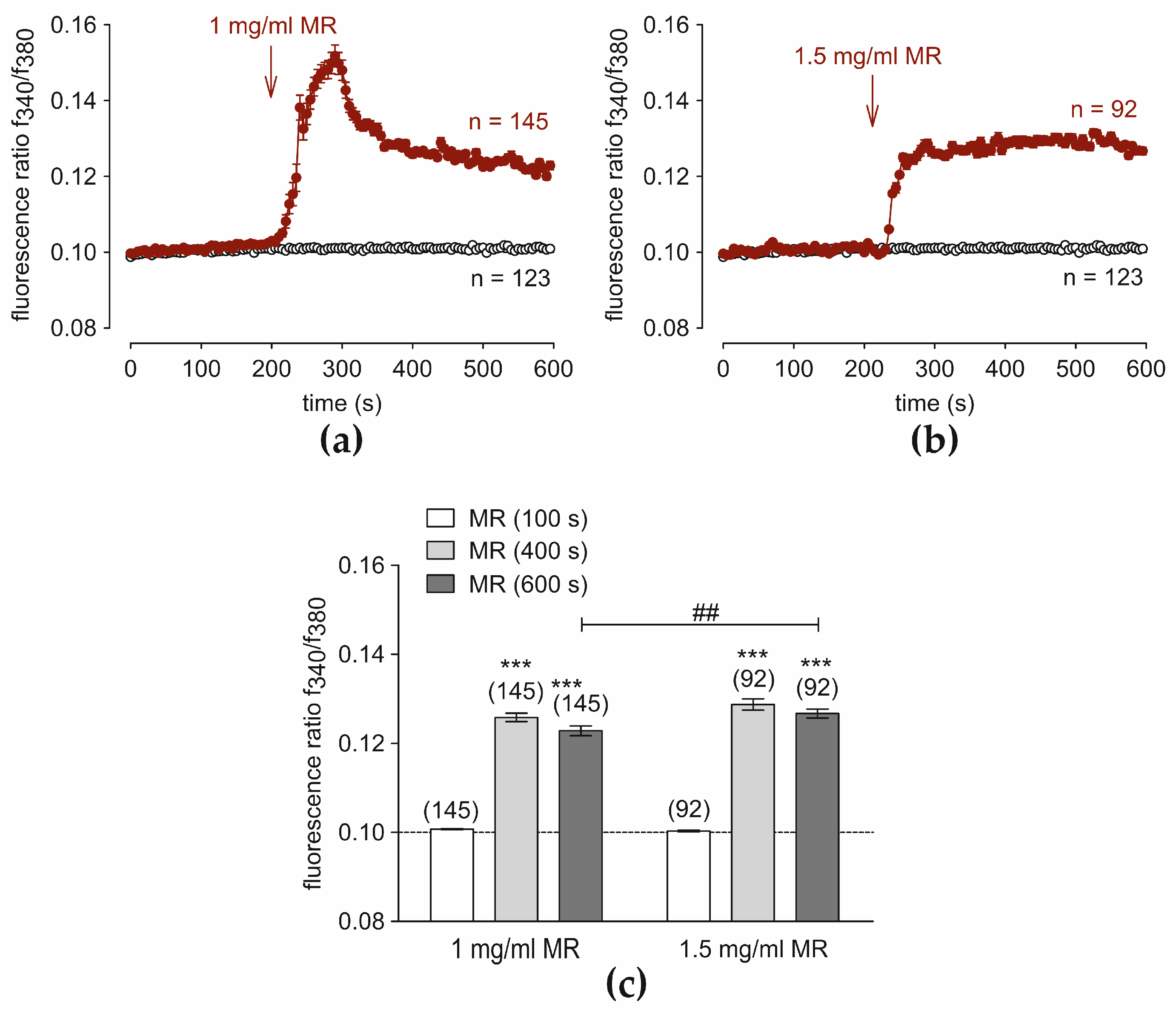
3.4. Fungal Pathogen MR Increases Intracellular Ca2+
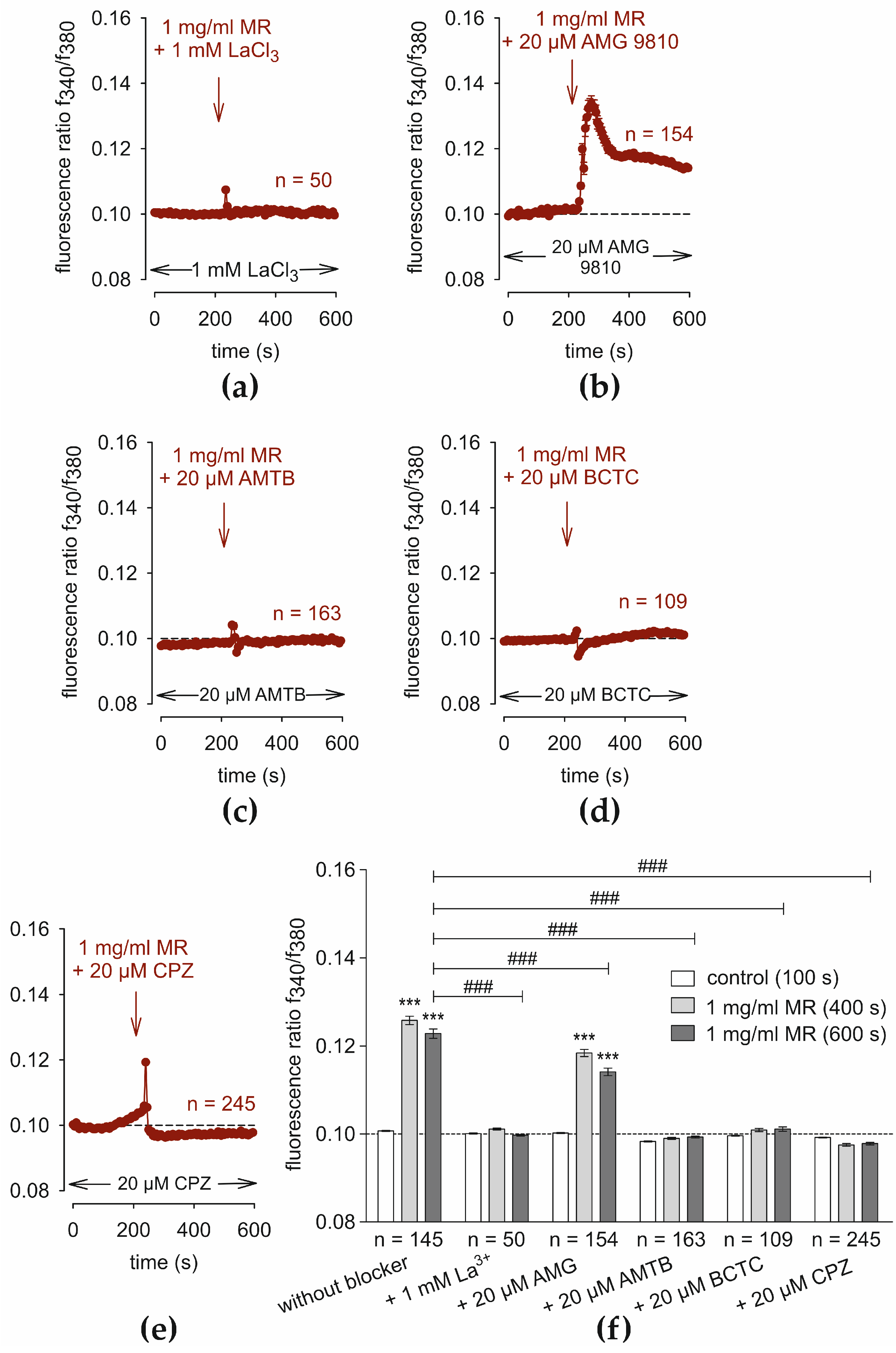
3.5. TRP Channel Blockers Suppress the MR-Induced Ca2+ Increase
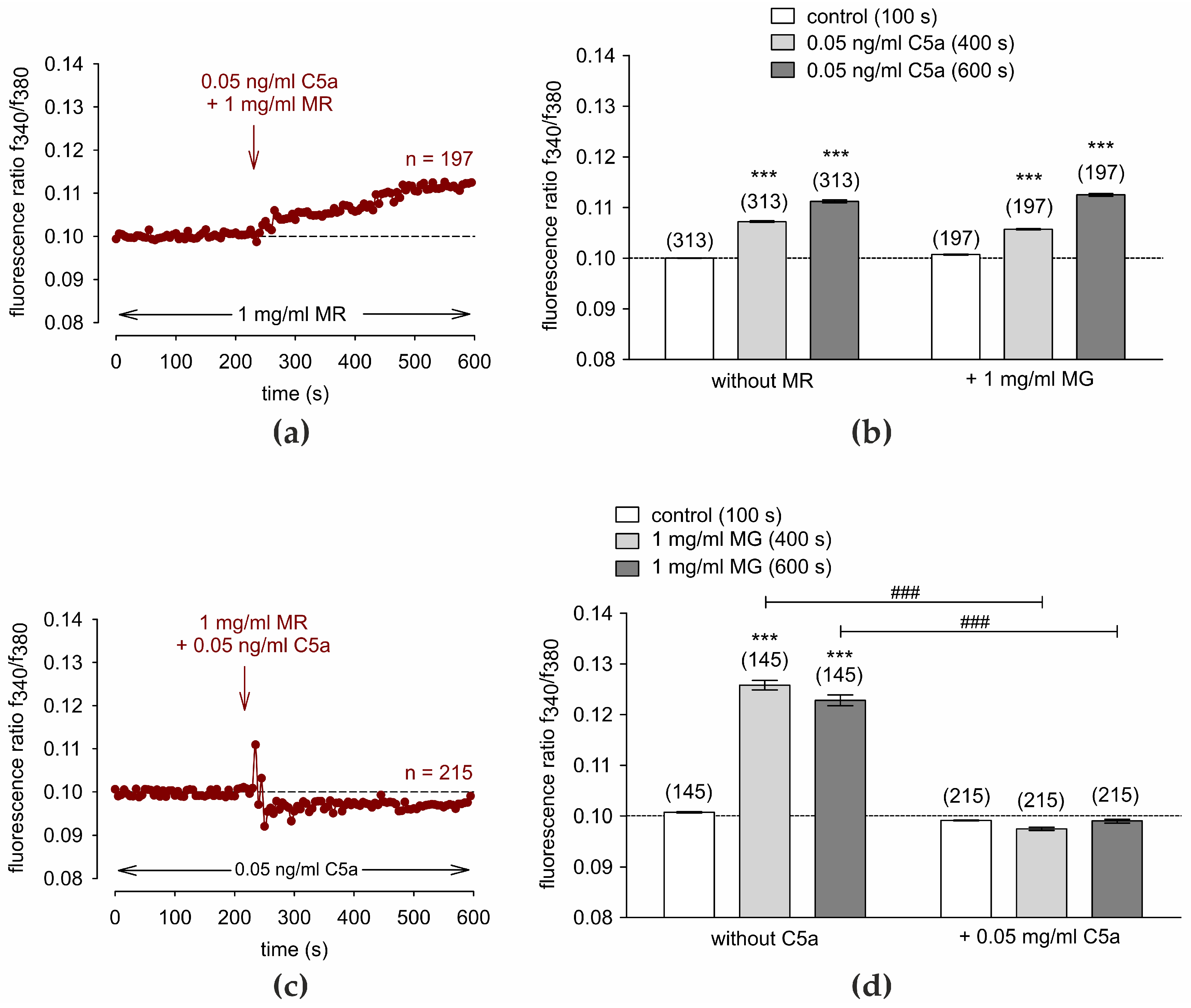
3.6. C5a Suppresses the MR-Induced Ca2+ Increase
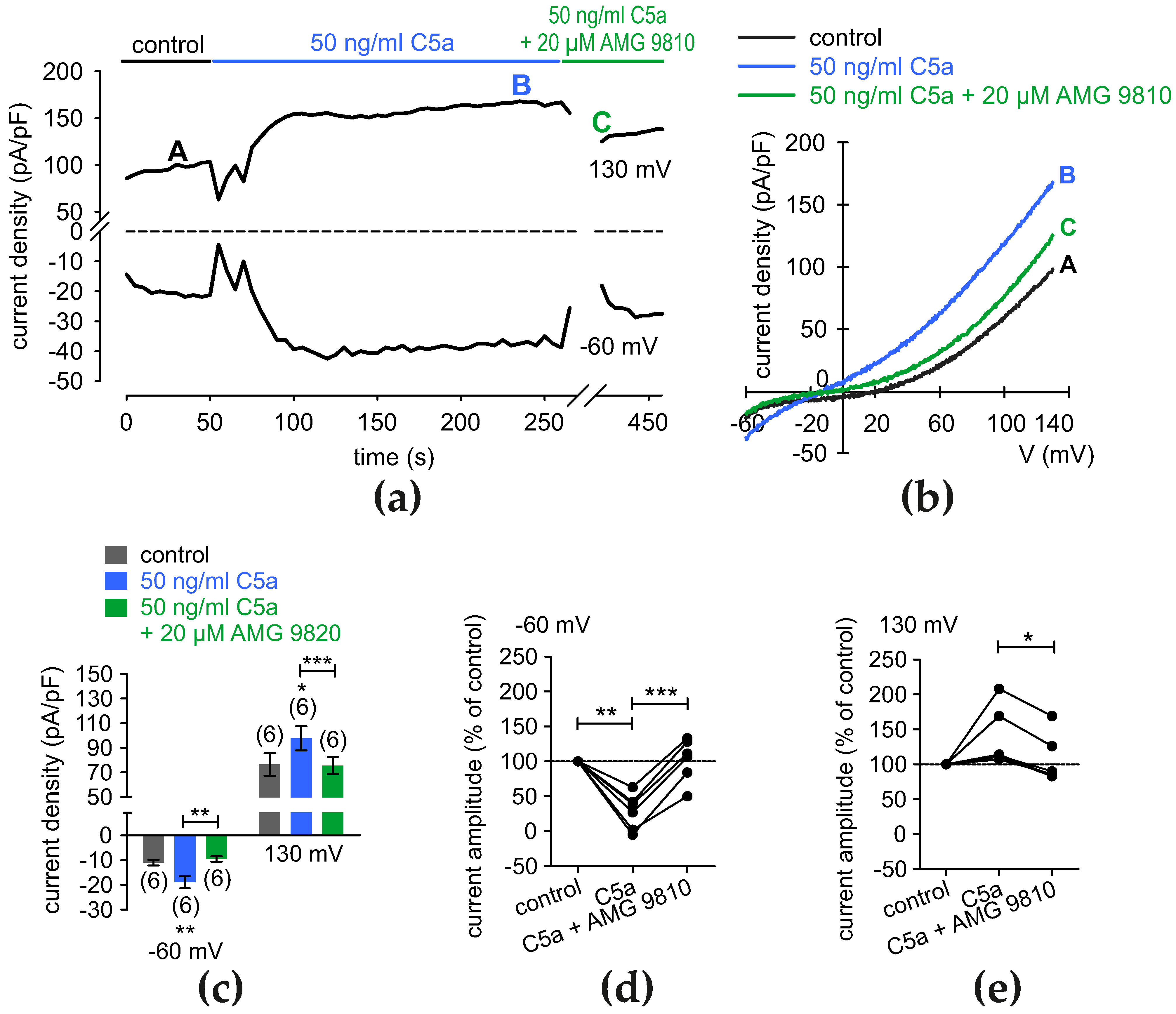
3.7. C5a-Increased Whole-Cell Currents Are Suppressed by TRP Channel Blockers
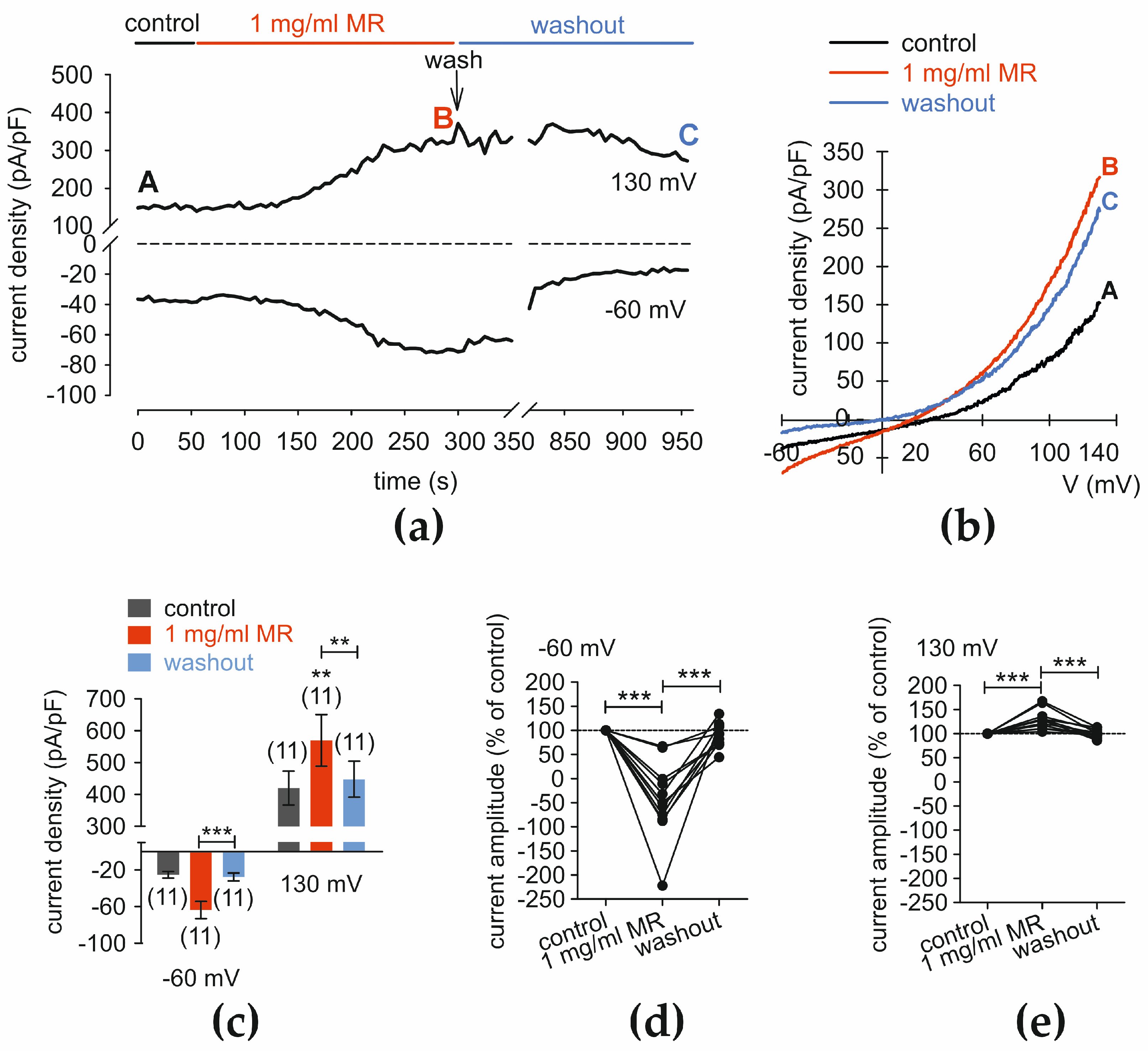
3.8. MR Increases Whole-Cell Currents
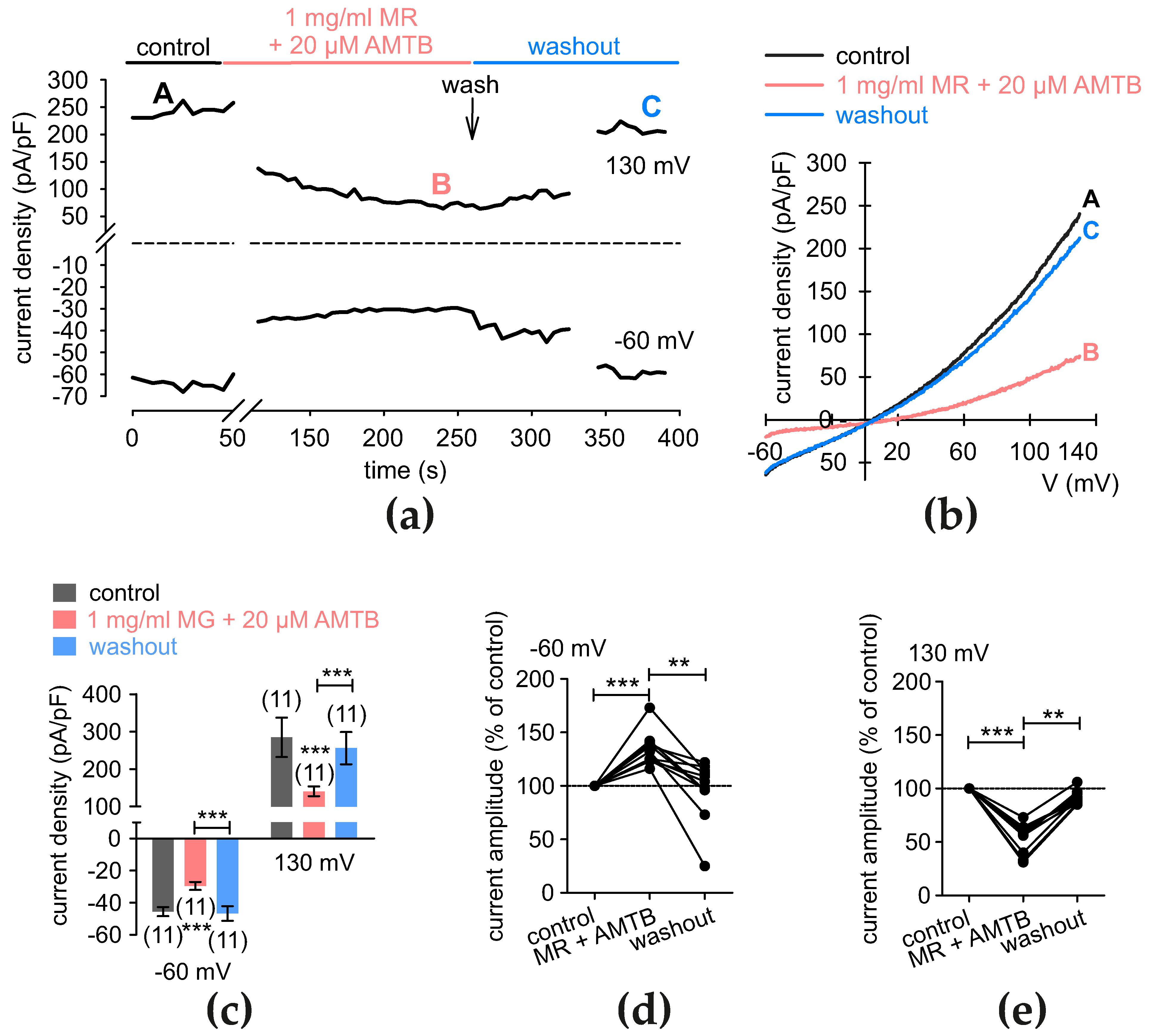
3.9. MR-Induces Rises in Whole-Cell Currents That TRP Channel Blockers Suppress
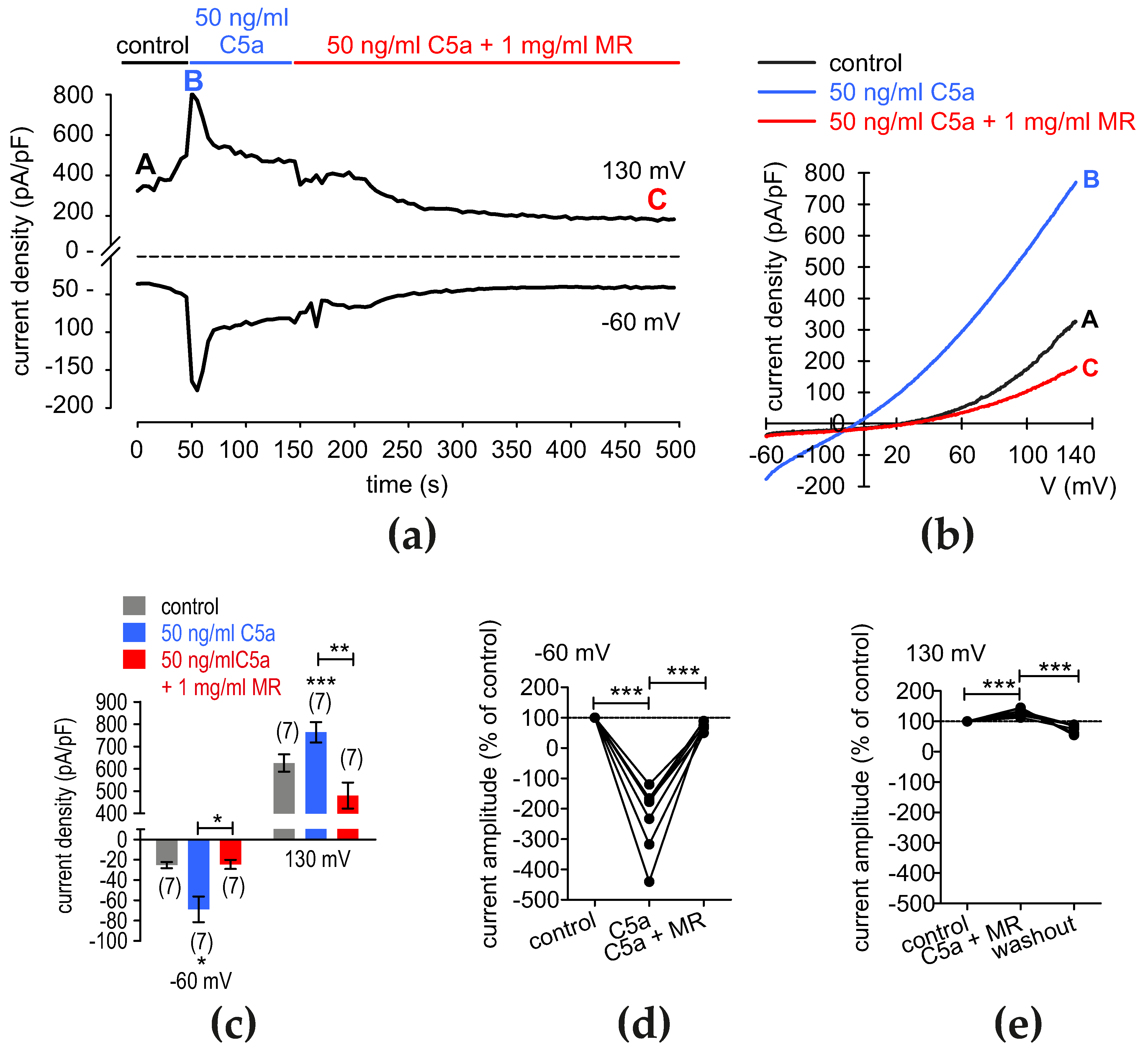
3.10. C5a Suppresses the Whole Cell Current Rises That MR Induces
4. Discussion
4.1. Role of C5a in Mediating Calcium Regulation
4.2. Mechanism of the C5a Ca2+ Effect with and without C5L2p
4.3. MR Affects Calcium Regulation
4.4. MR Is Associated with TRPs
4.5. Crosstalk between C5a and MR
4.6. C5a and MR Function on the Cell Membrane

4.7. Clinical Relevance
4.8. Limitations of This Study
4.9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şapte, E.; Costea, C.F.; Cărăuleanu, A.; Dancă, C.; Dumitrescu, G.F.; Dimitriu, G.; Chihaia, M.A.; Buzdugă, C.M.; Cucu, A.; Turliuc, M.D. Histological, immunohistochemical and clinical considerations on amniotic membrane transplant for ocular surface reconstruction. Rom. J. Morphol. Embryol. 2017, 58, 363–369. [Google Scholar] [PubMed]
- Knop, N.; Knop, E. Ultrastructural anatomy of CALT follicles in the rabbit reveals characteristics of M-cells, germinal centres and high endothelial venules. J. Anat. 2005, 207, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Kaercher, T.; Buchholz, P.; Kimmich, F. Treatment of patients with keratoconjunctivitis sicca with Optive: Results of a multicenter, open-label observational study in Germany. Clin. Ophthalmol. 2009, 3, 33–39. [Google Scholar] [PubMed]
- Borgia, P.; Sypherd, P.S. Control of beta-glucosidase synthesis in Mucor racemosus. J. Bacteriol. 1977, 130, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Sipsas, N.V.; Roilides, E.; Walsh, T.J. Rhino-orbital-cerebral mucormycosis. Curr. Infect. Dis. Rep. 2012, 14, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W. Allergen of the month–mucor. Ann. Allergy Asthma Immunol. 2015, 115, A15. [Google Scholar] [CrossRef] [PubMed]
- Kamradt, T. Komplementsystem. In Harrisons Innere Medizin; Harrison, T.R., Suttorp, N., Siegmund, B., Dietel, M., Eds.; ABW Wissenschaftsverlag GmbH: Berlin, Germany, 2020; Volume 3, p. 3042. [Google Scholar]
- Bartnicki-Garcia, S. Control of dimorphism in Mucor by hexoses: Inhibition of hyphal morphogenesis. J. Bacteriol. 1968, 96, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Benlamkaddem, S.; Zdaik, G.; Doughmi, D.; Bennis, A.; Chraibi, F.; Berdai, M.A.; Abdellaoui, M.; Benatiya Andaloussi, I.; Harandou, M. Rhino-Orbital Cerebral Mucormycosis: A Fatal Evolution. Cureus 2023, 15, e37837. [Google Scholar] [CrossRef]
- Fleckner, R.A.; Goldstein, J.H. Mucormycosis. Br. J. Ophthalmol. 1969, 53, 542–548. [Google Scholar] [CrossRef]
- Jutta Schröder-Braunstein, P.D.M.K. Das Komplementsystem: Von den Grundlagen zur klinischen Bedeutung. Transfusionsmedizin 2020, 2, 75–88. [Google Scholar] [CrossRef]
- de Vries, M.R.; Wezel, A.; Schepers, A.; van Santbrink, P.J.; Woodruff, T.M.; Niessen, H.W.; Hamming, J.F.; Kuiper, J.; Bot, I.; Quax, P.H. Complement factor C5a as mast cell activator mediates vascular remodelling in vein graft disease. Cardiovasc. Res. 2013, 97, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J. Modulation of human eosinophil polymorphonuclear leukocyte migration and function. Am. J. Pathol. 1976, 85, 419–436. [Google Scholar] [PubMed]
- Sogkas, G.; Vogtle, T.; Rau, E.; Gewecke, B.; Stegner, D.; Schmidt, R.E.; Nieswandt, B.; Gessner, J.E. Orai1 controls C5a-induced neutrophil recruitment in inflammation. Eur. J. Immunol. 2015, 45, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Bainton, D.F.; Borregaard, N.; Springer, T.A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Investig. 1987, 80, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, H.; Gong, X.; Shen, J.; Chen, X.; Wu, Z. The complement C3a-C3aR and C5a-C5aR pathways promote viability and inflammation of human retinal pigment epithelium cells by targeting NF-kappaB signaling. Exp. Ther. Med. 2022, 24, 493. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A. Functions of C5a receptors. J. Mol. Med. 2009, 87, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.A.; Shutov, L.P.; Shepherd, A.J.; Mohapatra, D.P.; Usachev, Y.M. Mechanisms underlying mechanical sensitization induced by complement C5a: The roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 2019, 160, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Quadros, A.U.; Cunha, T.M. C5a and pain development: An old molecule, a new target. Pharmacol. Res. 2016, 112, 58–67. [Google Scholar] [CrossRef]
- Mahajan, A.; Grüneboom, A.; Petru, L.; Podolska, M.J.; Kling, L.; Maueröder, C.; Dahms, F.; Christiansen, S.; Günter, L.; Krenn, V.; et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 2019, 105, 1087–1098. [Google Scholar] [CrossRef]
- Llorián-Salvador, M.; Byrne, E.M.; Szczepan, M.; Little, K.; Chen, M.; Xu, H. Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells. J. Neuroinflamm. 2022, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Cortright, D.N.; Meade, R.; Waters, S.M.; Chenard, B.L.; Krause, J.E. C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr. Eye Res. 2009, 34, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Hasikova, L.; Hampel, U.; Gruneboom, A.; Shan, X.; Herrmann, I.; Garreis, F.; Bock, F.; Knopf, J.; Singh, J.; et al. Aggregated neutrophil extracellular traps occlude Meibomian glands during ocular surface inflammation. Ocul. Surf. 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heppe, E.N.; Tofern, S.; Schulze, F.S.; Ishiko, A.; Shimizu, A.; Sina, C.; Zillikens, D.; Köhl, J.; Goletz, S.; Schmidt, E. Experimental Laminin 332 Mucous Membrane Pemphigoid Critically Involves C5aR1 and Reflects Clinical and Immunopathological Characteristics of the Human Disease. J. Investig. Dermatol. 2017, 137, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [CrossRef]
- Korbmacher, C.; Brenner, B.; Scholz, T. Calcium als Botenstoff. In Physiologie, 9th ed.; Pape, H.-C., Kurtz, A., Silbernagl, S., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2019. [Google Scholar]
- Verkhratsky, A.; Parpura, V. Calcium signalling and calcium channels: Evolution and general principles. Eur. J. Pharmacol. 2014, 739, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Korbmacher, C.; Brenner, B.; Scholz, T. Ionengradienten zwischen Extra- und Intrazellulärflüssigkeit. In Physiologie, 9th ed.; Pape, H.-C., Kurtz, A., Silbernagl, S., Eds.; Georg Thieme Verlag KG: Stuttgart, Germany, 2019. [Google Scholar]
- Mergler, S.; Pleyer, U. The human corneal endothelium: New insights into electrophysiology and ion channels. Prog. Retin. Eye Res. 2007, 26, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Elementary and global aspects of calcium signalling. J. Physiol. 1997, 499, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.G.; Oh, U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr. Pharm. Des. 2005, 11, 2687–2698. [Google Scholar] [CrossRef]
- Reinach, P.S.; Mergler, S.; Okada, Y.; Saika, S. Ocular transient receptor potential channel function in health and disease. BMC Ophthalmol. 2015, 15 (Suppl. S1), 153. [Google Scholar] [CrossRef]
- Mergler, S.; Pleyer, U. Importance of Transient Receptor Potential Channels of the Ocular Surface: Insights and Outlook—From Laboratory Research to Clinical Practice. Klin. Monbl Augenheilkd. 2020, 237, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Pingle, S.C.; Matta, J.A.; Ahern, G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 155–171. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 2005, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Garreis, F.; Sahlmuller, M.; Lyras, E.M.; Reinach, P.S.; Dwarakanath, A.; Paulsen, F.; Pleyer, U. Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem. Cell Biol. 2012, 137, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Budde, C.; Bohm, A.; Reinach, P.S.; Dhandapani, P.; Ljubojevic, N.; Schweiger, M.W.; von der Waydbrink, H.; Reimers, I.; Kohrle, J.; et al. TRPM8 Activation via 3-Iodothyronamine Blunts VEGF-Induced Transactivation of TRPV1 in Human Uveal Melanoma Cells. Front. Pharmacol. 2018, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- Ciura, S.; Liedtke, W.; Bourque, C.W. Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J. Neurosci. 2011, 31, 14669–14676. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflugers Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. Preface. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 223, pp. 5–6. [Google Scholar]
- Bereiter, D.A.; Rahman, M.; Thompson, R.; Stephenson, P.; Saito, H. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3739–3746. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef]
- Juarez-Contreras, R.; Mendez-Resendiz, K.A.; Rosenbaum, T.; Gonzalez-Ramirez, R.; Morales-Lazaro, S.L. TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 8882. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Sumioka, T.; Reinach, P.S.; Miyajima, M.; Saika, S. Roles of Epithelial and Mesenchymal TRP Channels in Mediating Inflammatory Fibrosis. Front. Immunol. 2021, 12, 731674. [Google Scholar] [CrossRef]
- İlhan, H.D.; Ünal, B.; Ayaz, Y.; Erin, N. Changes in TRPV1 Expression as Well as Substance P and Vasoactive Intestinal Peptide Levels Are Associated with Recurrence of Pterygium. Int. J. Mol. Sci. 2022, 23, 15692. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, T.; Chen, J.; Cai, X.; Fu, Y. Uncovering the role of transient receptor potential channels in pterygium: A machine learning approach. Inflamm. Res. 2023, 72, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Garreis, F.; Schroder, A.; Reinach, P.S.; Zoll, S.; Khajavi, N.; Dhandapani, P.; Lucius, A.; Pleyer, U.; Paulsen, F.; Mergler, S. Upregulation of Transient Receptor Potential Vanilloid Type-1 Channel Activity and Ca2+ Influx Dysfunction in Human Pterygial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2564–2577. [Google Scholar] [CrossRef]
- Kalangara, J.P.; Vanijcharoenkarn, K.; Chisolm, S.; Kuruvilla, M.E. Neuropathic pain and itch: Mechanisms in allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yang, W.; Guo, C.; Jiang, H.; Li, F.; Xiao, M.; Davidson, S.; Yu, G.; Duan, B.; Huang, T.; et al. Anatomical and functional dichotomy of ocular itch and pain. Nat. Med. 2018, 24, 1268–1276. [Google Scholar] [CrossRef]
- McKemy, D.D. TRPM8: The Cold and Menthol Receptor. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; Frontiers in Neuroscience; Taylor Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Henström, M.; Hadizadeh, F.; Beyder, A.; Bonfiglio, F.; Zheng, T.; Assadi, G.; Rafter, J.; Bujanda, L.; Agreus, L.; Andreasson, A.; et al. TRPM8 polymorphisms associated with increased risk of IBS-C and IBS-M. Gut 2017, 66, 1725–1727. [Google Scholar] [CrossRef]
- Iftinca, M.; Altier, C. The cool things to know about TRPM8! Channels 2020, 14, 413–420. [Google Scholar] [CrossRef]
- Gonzalez-Muniz, R.; Bonache, M.A.; Martin-Escura, C.; Gomez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef]
- Gavva, N.R.; Sandrock, R.; Arnold, G.E.; Davis, M.; Lamas, E.; Lindvay, C.; Li, C.M.; Smith, B.; Backonja, M.; Gabriel, K.; et al. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci. Rep. 2019, 9, 19655. [Google Scholar] [CrossRef]
- Keller, M.; Mergler, S.; Li, A.; Zahn, I.; Paulsen, F.; Garreis, F. Thermosensitive TRP Channels Are Functionally Expressed and Influence the Lipogenesis in Human Meibomian Gland Cells. Int. J. Mol. Sci. 2024, 25, 4043. [Google Scholar] [CrossRef]
- Brockmann, T.; Bertelmann, E.; Pleyer, U. Complement Anaphylatoxin Binders and Their Use in Treatment of a Subject Having an Ocular Wound and/or Fibrosis. Patent WO/2019/243555, 26 December 2019. [Google Scholar]
- Wiethoff, K.; Bader, G. Characterization and specification finding of Mucor racemosus preparation used as homeopathic starting material. Planta Med. 2013, 79, PK42. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M.; Enriquez de Salamanca, A.; Callejo, S.; Corrales, R.M.; Saez, V.; Siemasko, K.F.; Stern, M.E. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4263–4274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Chang, O.L.; Dolmetsch, R.E. Calcium imaging of cortical neurons using Fura-2 AM. J. Vis. Exp. 2009, 23, e1067. [Google Scholar] [CrossRef]
- Brooke, S.M.; Trafton, J.A.; Sapolsky, R.M. Autofluorescence as a confound in the determination of calcium levels in hippocampal slices using fura-2AM dye. Brain Res. 1996, 706, 283–288. [Google Scholar] [CrossRef]
- Mergler, S.; Mertens, C.; Valtink, M.; Reinach, P.S.; Szekely, V.C.; Slavi, N.; Garreis, F.; Abdelmessih, S.; Turker, E.; Fels, G.; et al. Functional significance of thermosensitive transient receptor potential melastatin channel 8 (TRPM8) expression in immortalized human corneal endothelial cells. Exp. Eye Res. 2013, 116, 337–349. [Google Scholar] [CrossRef]
- Ludwiczak, S.; Reinhard, J.; Reinach, P.S.; Li, A.; Oronowicz, J.; Yousf, A.; Kakkassery, V.; Mergler, S. Joint CB1 and NGF Receptor Activation Suppresses TRPM8 Activation in Etoposide-Resistant Retinoblastoma Cells. Int. J. Mol. Sci. 2024, 25, 1733. [Google Scholar] [CrossRef] [PubMed]
- Turker, E.; Garreis, F.; Khajavi, N.; Reinach, P.S.; Joshi, P.; Brockmann, T.; Lucius, A.; Ljubojevic, N.; Turan, E.; Cooper, D.; et al. Vascular Endothelial Growth Factor (VEGF) Induced Downstream Responses to Transient Receptor Potential Vanilloid 1 (TRPV1) and 3-Iodothyronamine (3-T(1)AM) in Human Corneal Keratocytes. Front. Endocrinol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Py, C.; Martina, M.; Diaz-Quijada, G.A.; Luk, C.C.; Martinez, D.; Denhoff, M.W.; Charrier, A.; Comas, T.; Monette, R.; Krantis, A.; et al. From understanding cellular function to novel drug discovery: The role of planar patch-clamp array chip technology. Front. Pharmacol. 2011, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Behrends, J.C.; Fertig, N. Planar Patch Clamping. In Patch-Clamp Analysis: Advanced Techniques; Walz, W., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 411–433. [Google Scholar]
- Bruggemann, A.; Farre, C.; Haarmann, C.; Haythornthwaite, A.; Kreir, M.; Stoelzle, S.; George, M.; Fertig, N. Planar patch clamp: Advances in electrophysiology. Methods Mol. Biol. 2008, 491, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods 1994, 51, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Leng, A.; Li, L.; Yang, B.; Shen, S.; Chen, H.; Zhu, E.; Xu, Q.; Ma, X.; Shi, P.; et al. AMTB, a TRPM8 antagonist, suppresses growth and metastasis of osteosarcoma through repressing the TGFbeta signaling pathway. Cell Death Dis. 2022, 13, 288. [Google Scholar] [CrossRef]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef]
- Pomonis, J.D.; Harrison, J.E.; Mark, L.; Bristol, D.R.; Valenzano, K.J.; Walker, K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 306, 387–393. [Google Scholar] [CrossRef]
- Liu, T.; Fang, Z.; Wang, G.; Shi, M.; Wang, X.; Jiang, K.; Yang, Z.; Cao, R.; Tao, H.; Wang, X.; et al. Anti-tumor activity of the TRPM8 inhibitor BCTC in prostate cancer DU145 cells. Oncol. Lett. 2016, 11, 182–188. [Google Scholar] [CrossRef]
- Halaszovich, C.R.; Zitt, C.; Jungling, E.; Luckhoff, A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J. Biol. Chem. 2000, 275, 37423–37428. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, S.; Strauss, O. Basal calcium entry in retinal pigment epithelial cells is mediated by TRPC channels. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5767–5772. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; McCloskey, M.A. Dual pathways for GTP-dependent regulation of chemoattractant-activated K+ conductance in murine J774 monocytes. J. Biol. Chem. 1994, 269, 31533–31543. [Google Scholar] [CrossRef] [PubMed]
- Lashinger, E.S.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Renal Physiol. 2008, 295, F803–F810. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhang, S.; Lu, L. A Transient Receptor Potential-like Calcium Ion Channel in the Filamentous Fungus Aspergillus nidulans. J. Fungi. 2021, 7, 920. [Google Scholar] [CrossRef]
- Li, R.; Coulthard, L.G.; Wu, M.C.; Taylor, S.M.; Woodruff, T.M. C5L2: A controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013, 27, 855–864. [Google Scholar] [CrossRef]
- Farkas, I.; Sarvari, M.; Aller, M.; Okada, N.; Okada, H.; Liko, I.; Liposits, Z. Estrogen receptor alpha and beta differentially mediate C5aR agonist evoked Ca2+-influx in neurons through L-type voltage-gated Ca2+ channels. Neurochem. Int. 2012, 60, 631–639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roach, T.I.; Rebres, R.A.; Fraser, I.D.; Decamp, D.L.; Lin, K.M.; Sternweis, P.C.; Simon, M.I.; Seaman, W.E. Signaling and cross-talk by C5a and UDP in macrophages selectively use PLCbeta3 to regulate intracellular free calcium. J. Biol. Chem. 2008, 283, 17351–17361. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Annamalai, B.; Abdusalamova, K.; Reichhart, N.; Huber, C.; Lin, Y.; Jo, E.A.H.; Zipfel, P.F.; Skerka, C.; Wildner, G.; et al. Anaphylatoxins Activate Ca(2+), Akt/PI3-Kinase, and FOXO1/FoxP3 in the Retinal Pigment Epithelium. Front. Immunol. 2017, 8, 703. [Google Scholar] [CrossRef]
- Shibasaki, K.; Suzuki, M.; Mizuno, A.; Tominaga, M. Effects of body temperature on neural activity in the hippocampus: Regulation of resting membrane potentials by transient receptor potential vanilloid 4. J. Neurosci. 2007, 27, 1566–1575. [Google Scholar] [CrossRef]
- Strauss, O.; Wienrich, M. Cultured retinal pigment epithelial cells from RCS rats express an increased calcium conductance compared with cells from non-dystrophic rats. Pflugers Arch. 1993, 425, 68–76. [Google Scholar] [CrossRef]
- Mergler, S.; Steinhausen, K.; Wiederholt, M.; Strauss, O. Altered regulation of L-type channels by protein kinase C and protein tyrosine kinases as a pathophysiologic effect in retinal degeneration. FASEB J. 1998, 12, 1125–1134. [Google Scholar] [CrossRef]
- Stein, M.A.; Mathers, D.A.; Yan, H.; Baimbridge, K.G.; Finlay, B.B. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect. Immun. 1996, 64, 4820–4825. [Google Scholar] [CrossRef]
- Jami, Z. The Influence of Complement Component C5a on Calcium Hemostasis in Human Corneal Keratocytes. Master’s Thesis, Charité Universitätsmedizin Berlin, Free University of Berlin, Berlin, Germany, 2023. Available online: https://refubium.fu-berlin.de/bitstream/handle/fub188/39079/diss_z.jami.pdf?sequence=1&isAllowed=y (accessed on 5 June 2024).
- Saul, S.; Stanisz, H.; Backes, C.S.; Schwarz, E.C.; Hoth, M. How ORAI and TRP channels interfere with each other: Interaction models and examples from the immune system and the skin. Eur. J. Pharmacol. 2014, 739, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lehen’kyi, V.; Flourakis, M.; Skryma, R.; Prevarskaya, N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene 2007, 26, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Bodding, M.; Fecher-Trost, C.; Flockerzi, V. Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J. Biol. Chem. 2003, 278, 50872–50879. [Google Scholar] [CrossRef]
- Gunn, L.; Ding, C.; Liu, M.; Ma, Y.; Qi, C.; Cai, Y.; Hu, X.; Aggarwal, D.; Zhang, H.G.; Yan, J. Opposing roles for complement component C5a in tumor progression and the tumor microenvironment. J. Immunol. 2012, 189, 2985–2994. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.A.; Monk, P.N. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74). J. Biol. Chem. 2002, 277, 7165–7169. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Poole, D.P.; Grace, M.; McIntyre, P.; Bunnett, N.W. The G protein-coupled receptor-transient receptor potential channel axis: Molecular insights for targeting disorders of sensation and inflammation. Pharmacol. Rev. 2015, 67, 36–73. [Google Scholar] [CrossRef]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Diamond, R.D. Fungal surfaces: Effects of interactions with phagocytic cells. Rev. Infect. Dis. 1988, 10 (Suppl. 2), S428–S431. [Google Scholar] [CrossRef]
- Kuzuya, F.; Naito, M.; Asai, K.; Shibata, K.; Iwata, Y. Effect of the calcium blocker, lanthanum, on bovine aortic smooth muscle cells in culture. Artery 1983, 12, 51–59. [Google Scholar] [PubMed]
- Heber, S.; Ciotu, C.I.; Hartner, G.; Gold-Binder, M.; Ninidze, N.; Gleiss, A.; Kress, H.G.; Fischer, M.J.M. TRPV1 antagonist BCTC inhibits pH 6.0-induced pain in human skin. Pain 2020, 161, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Walpole, C.S.; Bevan, S.; Bovermann, G.; Boelsterli, J.J.; Breckenridge, R.; Davies, J.W.; Hughes, G.A.; James, I.; Oberer, L.; Winter, J. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J.Med.Chem. 1994, 37, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Ravindran, J.; Yadav, V.R.; Aggarwal, B.B. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic. Biol. Med. 2012, 53, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, M.; Ling, J.; Tan, W.; Gu, J.G. TRPM8 mechanism of cold allodynia after chronic nerve injury. J. Neurosci. 2007, 27, 13680–13690. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Oriowo, M. Cooling-induced contraction of the rat gastric fundus: Mediation via transient receptor potential (TRP) cation channel TRPM8 receptor and Rho-kinase activation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, H.S.; Joo, C.K. TRPV1 Antagonist Suppresses Allergic Conjunctivitis in a Murine Model. Ocul. Immunol. Inflamm. 2018, 26, 440–448. [Google Scholar] [CrossRef]
- Lambrecht, B.N. An unexpected role for the anaphylatoxin C5a receptor in allergic sensitization. J. Clin. Investig. 2006, 116, 628–632. [Google Scholar] [CrossRef]
- Pandey, M.K. Molecular basis for downregulation of C5a-mediated inflammation by IgG1 immune complexes in allergy and asthma. Curr. Allergy Asthma Rep. 2013, 13, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Laumonnier, Y.; Korkmaz, R.; Nowacka, A.A.; Köhl, J. Complement-mediated immune mechanisms in allergy. Eur. J. Immunol. 2023, 53, e2249979. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.P.D.; Dossou, C.; Kashif, A.; Sharifinejad, N.; Azizi, G.; Hamedifar, H.; Sabzvari, A.; Zian, Z. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int. Immunopharmacol. 2022, 102, 108402. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3: As a Biomarker. In General Methods in Biomarker Research and their Applications; Preedy, V.R., Patel, V.B., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 223–249. [Google Scholar]
- Dybing, E.; Løvdal, T.; Hetland, R.B.; Løvik, M.; Schwarze, P.E. Respiratory allergy adjuvant and inflammatory effects of urban ambient particles. Toxicology 2004, 198, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.V.; Kumar, D.; Freiwald, T.; Chauss, D.; Johnson, M.D.; Abers, M.S.; Steinbrink, J.M.; Perfect, J.R.; Alexander, B.; Matzaraki, V.; et al. C5a-licensed phagocytes drive sterilizing immunity during systemic fungal infection. Cell 2023, 186, 2802–2822.e22. [Google Scholar] [CrossRef] [PubMed]
- Harpf, V.; Rambach, G.; Parth, N.; Neurauter, M.; Fleischer, V.; Lackner, M.; Lass-Flörl, C.; Würzner, R.; Speth, C. Complement, but Not Platelets, Plays a Pivotal Role in the Outcome of Mucormycosis In Vivo. J. Fungi 2023, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Hospenthal, D.R.; Petraitis, V.; Kontoyiannis, D.P. Necrotizing Mucormycosis of Wounds Following Combat Injuries, Natural Disasters, Burns, and Other Trauma. J. Fungi. 2019, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Granja, L.F.; Pinto, L.; Almeida, C.A.; Alviano, D.S.; Da Silva, M.H.; Ejzemberg, R.; Alviano, C.S. Spores of Mucor ramosissimus, Mucor plumbeus and Mucor circinelloides and their ability to activate human complement system in vitro. Med. Mycol. 2010, 48, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Maehara, H.; Norikawa, K.; Tanaka, K.; Kato, Y.; Kasai, A.; Omori, T.; Machida, T.; Sekine, H.; Sekiryu, T. Tear fluid and complement activation products in tears after ocular surgery. BMC Ophthalmol. 2023, 23, 329. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capo-Aponte, J.E.; Pleyer, U.; et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef]
- Milligan, C.J.; Li, J.; Sukumar, P.; Majeed, Y.; Dallas, M.L.; English, A.; Emery, P.; Porter, K.E.; Smith, A.M.; McFadzean, I.; et al. Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat. Protoc. 2009, 4, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Lucius, A.; Khajavi, N.; Reinach, P.S.; Kohrle, J.; Dhandapani, P.; Huimann, P.; Ljubojevic, N.; Grotzinger, C.; Mergler, S. 3-Iodothyronamine increases transient receptor potential melastatin channel 8 (TRPM8) activity in immortalized human corneal epithelial cells. Cell Signal 2016, 28, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, N.; Reinach, P.S.; Skrzypski, M.; Lude, A.; Mergler, S. L-carnitine reduces in human conjunctival epithelial cells hypertonic-induced shrinkage through interacting with TRPV1 channels. Cell Physiol. Biochem. 2014, 34, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, S.; Doerner, J.D.; Mueller-Buehl, A.M.; Koch, D.; Fuchshofer, R.; Dick, H.B.; Joachim, S.C. Cytokine and Complement Response in the Glaucomatous βB1-CTGF Mouse Model. Front. Cell Neurosci. 2021, 15, 718087. [Google Scholar] [CrossRef] [PubMed]
- Shutov, L.P.; Warwick, C.A.; Shi, X.; Gnanasekaran, A.; Shepherd, A.J.; Mohapatra, D.P.; Woodruff, T.M.; Clark, J.D.; Usachev, Y.M. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J. Neurosci. 2016, 36, 5055–5070. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.; Van Goor, F.; Luccioli, S.; Billings, E.M.; Vortmeyer, A.O.; Baranyi, L.; Szebeni, J.; Alving, C.R.; Carroll, M.C.; Berkower, I.; et al. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: A novel effector function of immunoglobulins. Nat. Med. 2003, 9, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.; Lu, M.; Fure, H.; Sun, W.; Sun, C.; Shi, N.Y.; Dou, Y.; Su, J.; Swanson, X.; Mollnes, T.E. Pre-neutralization of C5a-mediated effects by the monoclonal antibody 137-26 reacting with the C5a moiety of native C5 without preventing C5 cleavage. Clin. Exp. Immunol. 2003, 133, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.H.; Jin, X.; Li, L.M.; Li, S.Y.; Xie, H.P. A novel mutation in C5L2 gene was associated with hyperlipidemia and retinitis pigmentosa in a Chinese family. Lipids Health Dis. 2014, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Erdman, L.K.; Lu, Z.; Serghides, L.; Zhong, K.; Dhabangi, A.; Musoke, C.; Gerard, C.; Cserti-Gazdewich, C.; Liles, W.C.; et al. Functional roles for C5a and C5aR but not C5L2 in the pathogenesis of human and experimental cerebral malaria. Infect. Immun. 2014, 82, 371–379. [Google Scholar] [CrossRef]
- Brockmann, C.; Brockmann, T.; Dege, S.; Busch, C.; Kociok, N.; Vater, A.; Klussmann, S.; Strauss, O.; Joussen, A.M. Intravitreal inhibition of complement C5a reduces choroidal neovascularization in mice. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1695–1704. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Valtink, M.; Reinach, P.S.; Skupin, A.; Luo, H.; Brockmann, T.; Ba Salem, M.H.O.; Pleyer, U.; Mergler, S. L-carnitine suppresses transient receptor potential vanilloid type 1 activity and myofibroblast transdifferentiation in human corneal keratocytes. Lab. Investig. 2021, 101, 680–689. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rech, L.; Dietrich-Ntoukas, T.; Reinach, P.S.; Brockmann, T.; Pleyer, U.; Mergler, S. Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells. Cells 2024, 13, 1329. https://doi.org/10.3390/cells13161329
Rech L, Dietrich-Ntoukas T, Reinach PS, Brockmann T, Pleyer U, Mergler S. Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells. Cells. 2024; 13(16):1329. https://doi.org/10.3390/cells13161329
Chicago/Turabian StyleRech, Loreena, Tina Dietrich-Ntoukas, Peter S. Reinach, Tobias Brockmann, Uwe Pleyer, and Stefan Mergler. 2024. "Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells" Cells 13, no. 16: 1329. https://doi.org/10.3390/cells13161329
APA StyleRech, L., Dietrich-Ntoukas, T., Reinach, P. S., Brockmann, T., Pleyer, U., & Mergler, S. (2024). Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells. Cells, 13(16), 1329. https://doi.org/10.3390/cells13161329







