The Untapped Biomarker Potential of MicroRNAs for Health Risk–Benefit Analysis of Vaping vs. Smoking
Abstract
1. Introduction
2. The Public Health Impact of Vaping
3. Overview of E-Cigs
4. MiRNAs in Health and Disease: Utility for Vaping Research
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wills, T.A.; Soneji, S.S.; Choi, K.; Jaspers, I.; Tam, E.K. E-cigarette use and respiratory disorders: An integrative review of converging evidence from epidemiological and laboratory studies. Eur. Respir. J. 2021, 57, 1901815. [Google Scholar] [CrossRef]
- Bozier, J.; Chivers, E.K.; Chapman, D.G.; Larcombe, A.N.; Bastian, N.A.; Masso-Silva, J.A.; Byun, M.K.; McDonald, C.F.; Crotty Alexander, L.E.; Ween, M.P. The Evolving Landscape of e-Cigarettes: A Systematic Review of Recent Evidence. Chest 2020, 157, 1362–1390. [Google Scholar] [CrossRef] [PubMed]
- Balfour, D.J.K.; Benowitz, N.L.; Colby, S.M.; Hatsukami, D.K.; Lando, H.A.; Leischow, S.J.; Lerman, C.; Mermelstein, R.J.; Niaura, R.; Perkins, K.A.; et al. Balancing Consideration of the Risks and Benefits of E-Cigarettes. Am. J. Public Health 2021, 111, 1661–1672. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2021, 4, CD010216. [Google Scholar]
- Besaratinia, A.; Tommasi, S. Vaping epidemic: Challenges and opportunities. Cancer Causes Control. CCC 2020, 31, 663–667. [Google Scholar] [CrossRef]
- Gotts, J.E.; Jordt, S.E.; McConnell, R.; Tarran, R. What are the respiratory effects of e-cigarettes? BMJ 2019, 366, l5275. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 301–322. [Google Scholar] [CrossRef]
- Ali, F.R.M.; Diaz, M.C.; Vallone, D.; Tynan, M.A.; Cordova, J.; Seaman, E.L.; Trivers, K.F.; Schillo, B.A.; Talley, B.; King, B.A. E-cigarette Unit Sales, by Product and Flavor Type—United States, 2014-2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.E.; Loretan, C.G.; Jamal, A.; Davis Lynn, B.C.; Mayer, M.; Alcantara, I.C.; Neff, L. Tobacco Product Use Among Adults—United States, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Gentzke, A.; Hu, S.S.; Cullen, K.A.; Apelberg, B.J.; Homa, D.M.; King, B.A. Tobacco Use Among Middle and High School Students—United States, 2011-2016. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kramarow, E.A.; Elgaddal, N. Current Electronic Cigarette Use among Adults Aged 18 and over: United States, 2021; National Center for Health Statistics: Hyattsville, MD, USA, 2023.
- Birdsey, J.; Cornelius, M.; Jamal, A.; Park-Lee, E.; Cooper, M.R.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; Neff, L. Tobacco Product Use Among U.S. Middle and High School Students—National Youth Tobacco Survey, 2023. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Crotty Alexander, L.E.; Christiani, D.C. Vaping and Lung Inflammation and Injury. Annu. Rev. Physiol. 2022, 84, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Tommasi, S. An opportune and unique research to evaluate the public health impact of electronic cigarettes. Cancer Causes Control. CCC 2017, 28, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Bhadriraju, S.; Glantz, S.A. E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am. J. Public Health 2021, 111, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.A. E-Cigarettes as Consumer Products. Am. J. Public Health 2022, 112, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Koldobskiy, M.A.; Gondor, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, S.; Caliri, A.W.; Caceres, A.; Moreno, D.E.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. Deregulation of Biologically Significant Genes and Associated Molecular Pathways in the Oral Epithelium of Electronic Cigarette Users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef]
- Andersen, A.; Reimer, R.; Dawes, K.; Becker, A.; Hutchens, N.; Miller, S.; Dogan, M.; Hundley, B.; James, A.M.; Jeffrey, D.L.; et al. DNA methylation differentiates smoking from vaping and non-combustible tobacco use. Epigenetics Off. J. DNA Methylation Soc. 2022, 17, 178–190. [Google Scholar] [CrossRef]
- Yan, R.; Chen, X.L.; Xu, Y.M.; Lau, A.T.Y. Epimutational effects of electronic cigarettes. Environ. Sci. Pollut. Res. Int. 2021, 28, 17044–17067. [Google Scholar] [CrossRef]
- Goodman, S.; Chappell, G.; Guyton, K.Z.; Pogribny, I.P.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: An update of a systematic literature review. Mutat. Res. Rev. Mutat. Res. 2022, 789, 108408. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Caceres, A.; Tommasi, S. DNA Hydroxymethylation in Smoking-Associated Cancers. Int. J. Mol. Sci. 2022, 23, 2657. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.M.; McElroy, J.P.; Weng, D.Y.; Chung, S.; Fadda, P.; Reisinger, S.A.; Ying, K.L.; Brasky, T.M.; Wewers, M.D.; Freudenheim, J.L.; et al. Lung mitochondrial DNA copy number, inflammatory biomarkers, gene transcription and gene methylation in vapers and smokers. EBioMedicine 2022, 85, 104301. [Google Scholar] [CrossRef] [PubMed]
- Song, M.A.; Mori, K.M.; McElroy, J.P.; Freudenheim, J.L.; Weng, D.Y.; Reisinger, S.A.; Brasky, T.M.; Wewers, M.D.; Shields, P.G. Accelerated epigenetic age, inflammation, and gene expression in lung: Comparisons of smokers and vapers with non-smokers. Clin. Epigenet. 2023, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Jones, A.; Evans, I.; Raut, J.R.; Zikan, M.; Cibula, D.; Wong, A.; Brenner, H.; Richmond, R.C.; Widschwendter, M. Cigarette Smoking and E-cigarette Use Induce Shared DNA Methylation Changes Linked to Carcinogenesis. Cancer Res. 2024, 84, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Caceres, A.; Tommasi, S.; Besaratinia, A. Hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation in vapers and smokers. Epigenetics Off. J. DNA Methylation Soc. 2020, 15, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Caliri, A.W.; Tommasi, S. The interplay of DNA damage and repair, gene expression, and mutagenesis in mammalian cells during oxidative stress. Carcinogenesis 2024. Online ahead of print. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Dong, H.; Lei, J.; Ding, L.; Wen, Y.; Ju, H.; Zhang, X. MicroRNA: Function, detection, and bioanalysis. Chem. Rev. 2013, 113, 6207–6233. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Tommasi, S. Epigenetics of human melanoma: Promises and challenges. J. Mol. Cell Biol. 2014, 6, 356–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, S.D.; Adcock, I.M.; Garssen, J.; Mortaz, E.; Varahram, M.; Mirsaeidi, M.; Velayati, A. The roles of miRNAs as potential biomarkers in lung diseases. Eur. J. Pharmacol. 2016, 791, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Diao, L.; Han, L. Small non-coding RNAs in human cancer: Function, clinical utility, and characterization. Oncogene 2021, 40, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ruiz-Manriquez, L.M.; Ambriz-Gonzalez, H.; Medina-Gomez, D.; Valenzuela-Coronado, E.; Moreno-Gomez, P.; Pathak, S.; Chakraborty, S.; Srivastava, A. Impact of smoking-induced dysregulated human miRNAs in chronic disease development and their potential use in prognostic and therapeutic purposes. J. Biochem. Mol. Toxicol. 2022, 36, e23134. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, K.; Bollati, V.; Nawrot, T.S. MicroRNAs as potential signatures of environmental exposure or effect: A systematic review. Environ. Health Perspect. 2015, 123, 399–411. [Google Scholar] [CrossRef]
- Dehmel, S.; Nathan, P.; Bartel, S.; El-Merhie, N.; Scherb, H.; Milger, K.; John-Schuster, G.; Yildirim, A.O.; Hylkema, M.; Irmler, M.; et al. Intrauterine smoke exposure deregulates lung function, pulmonary transcriptomes, and in particular insulin-like growth factor (IGF)-1 in a sex-specific manner. Sci. Rep. 2018, 8, 7547. [Google Scholar] [CrossRef]
- Li, M.; Huo, X.; Davuljigari, C.B.; Dai, Q.; Xu, X. MicroRNAs and their role in environmental chemical carcinogenesis. Environ. Geochem. Health 2019, 41, 225–247. [Google Scholar] [CrossRef]
- Malkani, S.; Chin, C.R.; Cekanaviciute, E.; Mortreux, M.; Okinula, H.; Tarbier, M.; Schreurs, A.S.; Shirazi-Fard, Y.; Tahimic, C.G.T.; Rodriguez, D.N.; et al. Circulating miRNA Spaceflight Signature Reveals Targets for Countermeasure Development. Cell Rep. 2020, 33, 108448. [Google Scholar] [CrossRef]
- Herrnreiter, C.J.; Li, X.; Luck, M.E.; Zilliox, M.J.; Choudhry, M.A. Integrated analysis of dysregulated microRNA and mRNA expression in intestinal epithelial cells following ethanol intoxication and burn injury. Sci. Rep. 2021, 11, 20213. [Google Scholar] [CrossRef] [PubMed]
- Moszyńska, A.; Jaśkiewicz, M.; Serocki, M.; Cabaj, A.; Crossman, D.K.; Bartoszewska, S.; Gebert, M.; Dąbrowski, M.; Collawn, J.F.; Bartoszewski, R. The hypoxia-induced changes in miRNA-mRNA in RNA-induced silencing complexes and HIF-2 induced miRNAs in human endothelial cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22412. [Google Scholar] [CrossRef] [PubMed]
- Kopa-Stojak, P.N.; Pawliczak, R. Comparison of effects of tobacco cigarettes, electronic nicotine delivery systems and tobacco heating products on miRNA-mediated gene expression. A systematic review. Toxicol. Mech. Methods 2023, 33, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, L.R.; Harris, A. The role of microRNAs in chronic respiratory disease: Recent insights. Biol. Chem. 2018, 399, 219–234. [Google Scholar] [CrossRef]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Sastre, B.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 608666. [Google Scholar]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Croce, C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009, 10, 704–714. [Google Scholar] [CrossRef]
- Besaratinia, A.; Tommasi, S. Vaping: A growing global health concern. eClinicalMedicine 2019, 17, 100208. [Google Scholar] [CrossRef]
- Glantz, S.A.; Nguyen, N.; Oliveira da Silva, A.L. Population-Based Disease Odds for E-Cigarettes and Dual Use versus Cigarettes. NEJM Evid. 2024, 3, EVIDoa2300229. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, M.; Newton, J.N. Tobacco Control Leaders Call for a Balanced Assessment of the Risks and Benefits of Nicotine Vaping. Am. J. Public Health 2021, 111, 1570–1571. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A. From Tobacco Cigarettes to Electronic Cigarettes: The Two Sides of a Nicotine Coin. Front. Oral Health 2021, 2, 790634. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Barrington-Trimis, J. E-Cigarettes and Harm Reduction: An Artificial Controversy Instead of Evidence and a Well-Framed Decision Context. Am. J. Public Health 2021, 111, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, C.P.; Hall, W.; Borland, R.; Wodak, A.; Beaglehole, R.; Benowitz, N.L.; Britton, J.; Bullen, C.; Etter, J.F.; McNeill, A.; et al. A critique of the Australian National Health and Medical Research Council CEO statement on electronic cigarettes. Addiction 2023, 118, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.K.; Stjepanović, D.; Lim, C.; Sun, T.; Shanmuga Anandan, A.; Connor, J.P.; Gartner, C.; Hall, W.D.; Leung, J. A systematic review of randomized controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addict. Behav. 2021, 119, 106912. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, I.; Oberndorfer, M.; Fischer, J.; Wiesinger, W.; Haider, S.; Dorner, T.E. Effectiveness of Electronic Cigarettes in Smoking Cessation: A Systematic Review and Meta-analysis. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2021, 23, 625–634.e4. [Google Scholar] [CrossRef] [PubMed]
- Hanewinkel, R.; Niederberger, K.; Pedersen, A.; Unger, J.B.; Galimov, A. E-cigarettes and nicotine abstinence: A meta-analysis of randomised controlled trials. Eur. Respir. Rev. 2022, 31, 210215. [Google Scholar] [CrossRef]
- Levett, J.Y.; Filion, K.B.; Reynier, P.; Prell, C.; Eisenberg, M.J. Efficacy and Safety of E-Cigarette Use for Smoking Cessation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Med. 2023, 136, 804–813. [Google Scholar] [CrossRef]
- Osibogun, O.; Bursac, Z.; Maziak, W. Longitudinal transition outcomes among adult dual users of e-cigarettes and cigarettes with the intention to quit in the United States: PATH Study (2013–2018). Prev. Med. Rep. 2022, 26, 101750. [Google Scholar] [CrossRef]
- Chen, R.; Pierce, J.P.; Leas, E.C.; Benmarhnia, T.; Strong, D.R.; White, M.M.; Stone, M.; Trinidad, D.R.; McMenamin, S.B.; Messer, K. Effectiveness of e-cigarettes as aids for smoking cessation: Evidence from the PATH Study cohort, 2017–2019. Tob. Control 2023, 32, e145–e152. [Google Scholar] [CrossRef]
- Banks, E.; Yazidjoglou, A.; Brown, S.; Nguyen, M.; Martin, M.; Beckwith, K.; Daluwatta, A.; Campbell, S.; Joshy, G. Electronic cigarettes and health outcomes: Umbrella and systematic review of the global evidence. Med. J. Aust. 2023, 218, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Hedman, L.; Galanti, M.R.; Ryk, L.; Gilljam, H.; Adermark, L. Electronic cigarette use and smoking cessation in cohort studies and randomized trials: A systematic review and meta-analysis. Tob. Prev. Cessat. 2021, 7, 62. [Google Scholar] [CrossRef]
- McNeill, A.; Brose, L.S.; Calder, R.; Bauld, L.; Robson, D. Evidence Review of E-Cigarettes and Heated Tobacco Products 2018; Public Health England: London, UK, 2018. [Google Scholar]
- Besaratinia, A. COVID-19: A pandemic converged with global tobacco epidemic and widespread vaping-state of the evidence. Carcinogenesis 2021, 42, 1009–1022. [Google Scholar] [CrossRef]
- Khouja, J.N.; Suddell, S.F.; Peters, S.E.; Taylor, A.E.; Munafò, M.R. Is e-cigarette use in non-smoking young adults associated with later smoking? A systematic review and meta-analysis. Tob. Control 2020, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gades, M.S.; Alcheva, A.; Riegelman, A.L.; Hatsukami, D.K. The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2022, 24, 1332–1343. [Google Scholar] [CrossRef]
- Soule, E.; Bansal-Travers, M.; Grana, R.; McIntosh, S.; Price, S.; Unger, J.B.; Walton, K. Electronic cigarette use intensity measurement challenges and regulatory implications. Tob. Control 2021, 32, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Snoderly, H.T.; Nurkiewicz, T.R.; Bowdridge, E.C.; Bennewitz, M.F. E-Cigarette Use: Device Market, Study Design, and Emerging Evidence of Biological Consequences. Int. J. Mol. Sci. 2021, 22, 12452. [Google Scholar] [CrossRef] [PubMed]
- Havermans, A.; Krüsemann, E.J.Z.; Pennings, J.; de Graaf, K.; Boesveldt, S.; Talhout, R. Nearly 20 000 e-liquids and 250 unique flavour descriptions: An overview of the Dutch market based on information from manufacturers. Tob. Control 2021, 30, 57–62. [Google Scholar] [CrossRef]
- Wei, B.; O’Connor, R.J.; Goniewicz, M.L.; Hyland, A. Emerging Chemicals of Health Concern in Electronic Nicotine Delivery Systems. Chem. Res. Toxicol. 2020, 33, 2637–2646. [Google Scholar] [CrossRef]
- Besaratinia, A.; Tommasi, S. Electronic cigarettes: The road ahead. Prev. Med. 2014, 66, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Schneller, L.M.; Bansal-Travers, M.; Goniewicz, M.L.; McIntosh, S.; Ossip, D.; O’Connor, R.J. Use of flavored electronic cigarette refill liquids among adults and youth in the US-Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015). PLoS ONE 2018, 13, e0202744. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Elias, R.J.; Silakov, A.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free. Radic. Biol. Med. 2018, 120, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Jabba, S.V.; DeWinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.E.; Zimmerman, J.B. Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2019, 21, 1248–1258. [Google Scholar] [CrossRef]
- Son, Y.; Mishin, V.; Laskin, J.D.; Mainelis, G.; Wackowski, O.A.; Delnevo, C.; Schwander, S.; Khlystov, A.; Samburova, V.; Meng, Q. Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem. Res. Toxicol. 2019, 32, 1087–1095. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. Enhancement of the mutagenicity of benzo(a)pyrene diol epoxide by a nonmutagenic dose of ultraviolet A radiation. Cancer Res. 2003, 63, 8708–8716. [Google Scholar]
- Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. [Google Scholar] [CrossRef]
- Tommasi, S.; Bates, S.E.; Behar, R.Z.; Talbot, P.; Besaratinia, A. Limited mutagenicity of electronic cigarettes in mouse or human cells in vitro. Lung Cancer 2017, 112, 41–46. [Google Scholar] [CrossRef]
- Correia-Alvarez, E.; Keating, J.E.; Glish, G.; Tarran, R.; Sassano, M.F. Reactive Oxygen Species, Mitochondrial Membrane Potential, and Cellular Membrane Potential Are Predictors of E-Liquid Induced Cellular Toxicity. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2020, 22 (Suppl. 1), S4–S13. [Google Scholar] [CrossRef]
- Barhdadi, S.; Mertens, B.; Van Bossuyt, M.; Van De Maele, J.; Anthonissen, R.; Canfyn, M.; Courselle, P.; Rogiers, V.; Deconinck, E.; Vanhaecke, T. Identification of flavouring substances of genotoxic concern present in e-cigarette refills. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 147, 111864. [Google Scholar] [CrossRef]
- Besaratinia, A.; Tommasi, S. The consequential impact of JUUL on youth vaping and the landscape of tobacco products: The state of play in the COVID-19 era. Prev. Med. Rep. 2021, 22, 101374. [Google Scholar] [CrossRef] [PubMed]
- McDonough, S.R.; Rahman, I.; Sundar, I.K. Recent updates on biomarkers of exposure and systemic toxicity in e-cigarette users and EVALI. Am. J. Physiology. Lung Cell. Mol. Physiol. 2021, 320, L661–L679. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.W.; Newmeyer, M.N.; Rule, A.M.; Prasse, C. Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography-High-Resolution Mass Spectrometry. Chem. Res. Toxicol. 2021, 34, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Khlystov, A.; Samburova, V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Desvignes, T.; Batzel, P.; Berezikov, E.; Eilbeck, K.; Eppig, J.T.; McAndrews, M.S.; Singer, A.; Postlethwait, J.H. miRNA Nomenclature: A View Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends Genet. TIG 2015, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- Du, T.; Zamore, P.D. Beginning to understand microRNA function. Cell Res. 2007, 17, 661–663. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Starkey Lewis, P.J.; Dear, J.; Platt, V.; Simpson, K.J.; Craig, D.G.; Antoine, D.J.; French, N.S.; Dhaun, N.; Webb, D.J.; Costello, E.M.; et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology 2011, 54, 1767–1776. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef]
- Shaik, S.; Martin, E.; Hayes, D.; Gimble, J.; Devireddy, R. microRNA Sequencing of CD34+ Sorted Adipose Stem Cells Undergoing Endotheliogenesis. Stem Cells Dev. 2021, 30, 265–288. [Google Scholar] [CrossRef]
- Jones Buie, J.N.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Fan, H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis 2016, 254, 271–281. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, X.; Yang, L.; Zhang, R.; Zhu, R.; Feng, R. Integrated analysis of mRNA and microRNA expression profiles reveals differential transcriptome signature in ischaemic and dilated cardiomyopathy induced heart failure. Epigenet. Off. J. DNA Methylation Soc. 2021, 16, 917–932. [Google Scholar] [CrossRef]
- Chu, M.; Wu, S.; Wang, W.; Mao, L.; Yu, Y.; Jiang, L.; Yuan, W.; Zhang, M.; Sang, L.; Huang, Q.; et al. miRNA sequencing reveals miRNA-4508 from peripheral blood lymphocytes as potential diagnostic biomarker for silica-related pulmonary fibrosis: A multistage study. Respirology 2020, 25, 511–517. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Zhang, N.; Zhao, X.; Li, R.; Wang, Y.; Chen, C.; Wang, D.; Zhang, X.; Chen, L.; et al. Comprehensive identification of RNA transcripts and construction of RNA network in chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 154. [Google Scholar] [CrossRef]
- Han, X.; Wang, H.; Li, Y.; Liu, L.; Gao, S. A 2 miRNAs-based signature for the diagnosis of atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 150. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Wang, X.; Li, Z.; Sun, H.; Wei, J.; Zeng, X.; Cao, X.; Wan, C. Integrated Analysis of miRNAs and Gene Expression Profiles Reveals Potential Biomarkers for Osteoarthritis. Front. Genet. 2022, 13, 814645. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Shimada, K.; Nakai, T.; Ohbayashi, C. MicroRNAs in Smoking-Related Carcinogenesis: Biomarkers, Functions, and Therapy. J. Clin. Med. 2018, 7, 98. [Google Scholar] [CrossRef]
- Dama, E.; Melocchi, V.; Mazzarelli, F.; Colangelo, T.; Cuttano, R.; Di Candia, L.; Ferretti, G.M.; Taurchini, M.; Graziano, P.; Bianchi, F. Non-Coding RNAs as Prognostic Biomarkers: A miRNA Signature Specific for Aggressive Early-Stage Lung Adenocarcinomas. Noncoding RNA 2020, 6, 48. [Google Scholar] [CrossRef]
- Agostini, M.; Ganini, C.; Candi, E.; Melino, G. The role of noncoding RNAs in epithelial cancer. Cell Death Discov. 2020, 6, 13. [Google Scholar] [CrossRef]
- Liu, X.; Liu, P.; Chernock, R.D.; Yang, Z.; Lang Kuhs, K.A.; Lewis, J.S.; Luo, J.; Li, H.; Gay, H.A.; Thorstad, W.L.; et al. A MicroRNA Expression Signature as Prognostic Marker for Oropharyngeal Squamous Cell Carcinoma. J. Natl. Cancer Inst. 2021, 113, 752–759. [Google Scholar] [CrossRef]
- Chen, D.; Yang, X.; Liu, M.; Zhang, Z.; Xing, E. Roles of miRNA dysregulation in the pathogenesis of multiple myeloma. Cancer Gene Ther. 2021, 28, 1256–1268. [Google Scholar] [CrossRef]
- Singh, K.P.; Maremanda, K.P.; Li, D.; Rahman, I. Exosomal microRNAs are novel circulating biomarkers in cigarette, waterpipe smokers, E-cigarette users and dual smokers. BMC Med. Genom. 2020, 13, 128. [Google Scholar] [CrossRef]
- Solleti, S.K.; Bhattacharya, S.; Ahmad, A.; Wang, Q.; Mereness, J.; Rangasamy, T.; Mariani, T.J. MicroRNA expression profiling defines the impact of electronic cigarettes on human airway epithelial cells. Sci. Rep. 2017, 7, 1081. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Bolognesi, C.; Kirsch-Volders, M.; Bonassi, S.; Zeiger, E.; Knasmueller, S.; Fenech, M. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat. Res. 2008, 659, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Holland, N.; Zeiger, E.; Chang, W.P.; Burgaz, S.; Thomas, P.; Bolognesi, C.; Knasmueller, S.; Kirsch-Volders, M.; Bonassi, S. The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells--past, present and future. Mutagenesis 2011, 26, 239–245. [Google Scholar] [CrossRef]
- Besaratinia, A.; Bates, S.E.; Pfeifer, G.P. Mutational signature of the proximate bladder carcinogen N-hydroxy-4-acetylaminobiphenyl: Inconsistency with the p53 mutational spectrum in bladder cancer. Cancer Res. 2002, 62, 4331–4338. [Google Scholar] [PubMed]
- Torres-Bugarin, O.; Zavala-Cerna, M.G.; Nava, A.; Flores-Garcia, A.; Ramos-Ibarra, M.L. Potential uses, limitations, and basic procedures of micronuclei and nuclear abnormalities in buccal cells. Dis. Markers 2014, 2014, 956835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Guo, J.; Carmella, S.G.; Lindgren, B.; Ikuemonisan, J.; Jensen, J.; Hatsukami, D.K.; Balbo, S.; Hecht, S.S. Increased Acrolein-DNA Adducts in Buccal Brushings of e-Cigarette Users. Carcinogenesis 2022, 43, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S. Oral Cancer, 5th ed.; BC Decker, Inc.: Hamilton, ON, Canada, 2003. [Google Scholar]
- Proia, N.K.; Paszkiewicz, G.M.; Nasca, M.A.; Franke, G.E.; Pauly, J.L. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer—A review. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J. Mutation selection and the natural history of cancer. Nature 1975, 255, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Rosin, M.P. The use of the micronucleus test on exfoliated cells to identify anti-clastogenic action in humans: A biological marker for the efficacy of chemopreventive agents. Mutat. Res. 1992, 267, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Foiles, P.G.; Miglietta, L.M.; Quart, A.M.; Quart, E.; Kabat, G.C.; Hecht, S.S. Evaluation of 32P-postlabeling analysis of DNA from exfoliated oral mucosa cells as a means of monitoring exposure of the oral cavity to genotoxic agents. Carcinogenesis 1989, 10, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.Y.; Hsu, T.; Santella, R.M. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis 2002, 23, 207–211. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Baker, V.; Larios, E.; Chung, F.L. Molecular and cellular effects of green tea on oral cells of smokers: A pilot study. Mol. Nutr. Food Res. 2005, 49, 43–51. [Google Scholar] [CrossRef]
- Slebos, R.J.; Li, M.; Vadivelu, S.; Burkey, B.B.; Netterville, J.L.; Sinard, R.; Gilbert, J.; Murphy, B.; Chung, C.H.; Shyr, Y.; et al. Microsatellite mutations in buccal cells are associated with aging and head and neck carcinoma. Br. J. Cancer 2008, 98, 619–626. [Google Scholar] [CrossRef][Green Version]
- Shani, T.; Onn, A.; Kabha, A.; Ben-Dov, I.; Adam, I.; Amariglio, N.; Yahalom, R.; Rechavi, G.; Trakhtenbrot, L.; Hirshberg, A. Chromosomal numerical aberrations in apparently normal oral mucosa of heavy smokers affected by lung cancer. Oral. Oncol. 2010, 46, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, S.; Blumenfeld, H.; Besaratinia, A. Vaping dose, device type, and e-liquid flavor are determinants of DNA damage in electronic cigarette users. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2023, 25, 1145–1154. [Google Scholar] [CrossRef]
- Bonassi, S.; Coskun, E.; Ceppi, M.; Lando, C.; Bolognesi, C.; Burgaz, S.; Holland, N.; Kirsh-Volders, M.; Knasmueller, S.; Zeiger, E.; et al. The HUman MicroNucleus project on eXfoLiated buccal cells (HUMN(XL)): The role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat. Res. 2011, 728, 88–97. [Google Scholar] [CrossRef]
- Greenspan, E.J.; Lee, H.; Dyba, M.; Pan, J.; Mekambi, K.; Johnson, T.; Blancato, J.; Mueller, S.; Berry, D.L.; Chung, F.L. High-throughput, quantitative analysis of acrolein-derived DNA adducts in human oral cells by immunohistochemistry. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 844–853. [Google Scholar] [CrossRef]
- Pan, J.; Awoyemi, B.; Xuan, Z.; Vohra, P.; Wang, H.T.; Dyba, M.; Greenspan, E.; Fu, Y.; Creswell, K.; Zhang, L.; et al. Detection of acrolein-derived cyclic DNA adducts in human cells by monoclonal antibodies. Chem. Res. Toxicol. 2012, 25, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, I.; Muzic, J.; Le, C.T.; Sebero, E.; Villalta, P.; Ma, B.; Jensen, J.; Hatsukami, D.; Hecht, S.S. Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol. 2013, 26, 37–45. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; Yang, Z.; Wong, A.; Pipinikas, C.P.; Jiao, Y.; Jones, A.; Anjum, S.; Hardy, R.; Salvesen, H.B.; Thirlwell, C.; et al. Correlation of Smoking-Associated DNA Methylation Changes in Buccal Cells With DNA Methylation Changes in Epithelial Cancer. JAMA Oncol. 2015, 1, 476–485. [Google Scholar] [CrossRef]
- De Oliveira, S.R.; Da Silva, I.C.; Mariz, B.A.; Pereira, A.M.; De Oliveira, N.F. DNA methylation analysis of cancer-related genes in oral epithelial cells of healthy smokers. Arch. Oral Biol. 2015, 60, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Pathak, A.K.; Fan, Y.H.; Liu, D.D.; Lee, J.J.; Tang, H.; Kurie, J.M.; Morice, R.C.; Kim, E.S.; Hong, W.K.; et al. Oral epithelium as a surrogate tissue for assessing smoking-induced molecular alterations in the lungs. Cancer Prev. Res. 2008, 1, 39–44. [Google Scholar] [CrossRef]
- Wangsri, S.; Subbalekha, K.; Kitkumthorn, N.; Mutirangura, A. Patterns and possible roles of LINE-1 methylation changes in smoke-exposed epithelia. PLoS ONE 2012, 7, e45292. [Google Scholar] [CrossRef]
- Shah, V.; Sridhar, S.; Beane, J.; Brody, J.S.; Spira, A. SIEGE: Smoking Induced Epithelial Gene Expression Database. Nucleic Acids Res. 2005, 33, D573–D579. [Google Scholar] [CrossRef] [PubMed]
- Spivack, S.D.; Hurteau, G.J.; Jain, R.; Kumar, S.V.; Aldous, K.M.; Gierthy, J.F.; Kaminsky, L.S. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Res. 2004, 64, 6805–6813. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.O.; Gumus, Z.H.; Kacker, A.; Choksi, V.L.; Bocker, J.M.; Zhou, X.K.; Yantiss, R.K.; Hughes, D.B.; Du, B.; Judson, B.L.; et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev. Res. 2010, 3, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Schembri, F.; Zeskind, J.; Shah, V.; Gustafson, A.M.; Steiling, K.; Liu, G.; Dumas, Y.M.; Zhang, X.; Brody, J.S.; et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genom. 2008, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.M.; White, V.L.; Jenkins, M.C.; Burian, D. Examining smoking-induced differential gene expression changes in buccal mucosa. BMC Med. Genom. 2010, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, M.; Egan, K.M.; Abruzzo, J.; Newcomb, P.A.; Titus-Ernstoff, L.; Franklin, T.; Bender, P.K.; Beck, J.C.; Le Marchand, L.; Lum, A.; et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2001, 10, 687–696. [Google Scholar]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef]
- Fritz, G.; Henninger, C. Rho GTPases: Novel Players in the Regulation of the DNA Damage Response? Biomolecules 2015, 5, 2417–2434. [Google Scholar] [CrossRef]
- Tommasi, S.; Pabustan, N.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. A novel role for vaping in mitochondrial gene dysregulation and inflammation fundamental to disease development. Sci. Rep. 2021, 11, 22773. [Google Scholar] [CrossRef]
- Besaratinia, A.; Maas, L.M.; Brouwer, E.M.; Kleinjans, J.C.; Van Schooten, F.J. Comparison between smoking-related DNA adduct analysis in induced sputum and peripheral blood lymphocytes. Carcinogenesis 2000, 21, 1335–1340. [Google Scholar] [PubMed]
- Zhong, J.; Agha, G.; Baccarelli, A.A. The Role of DNA Methylation in Cardiovascular Risk and Disease: Methodological Aspects, Study Design, and Data Analysis for Epidemiological Studies. Circ. Res. 2016, 118, 119–131. [Google Scholar] [CrossRef]
- Gesthalter, Y.B.; Vick, J.; Steiling, K.; Spira, A. Translating the transcriptome into tools for the early detection and prevention of lung cancer. Thorax 2015, 70, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Billatos, E.; Vick, J.L.; Lenburg, M.E.; Spira, A.E. The Airway Transcriptome as a Biomarker for Early Lung Cancer Detection. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2984–2992. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Li, T.; Ma, L.; Fan, F.; Jin, Y.; Shen, J. The whole transcriptome and proteome changes in the early stage of myocardial infarction. Cell Death Discov. 2019, 5, 73. [Google Scholar] [CrossRef]
- Shields, P.G.; Berman, M.; Brasky, T.M.; Freudenheim, J.L.; Mathe, E.; McElroy, J.P.; Song, M.A.; Wewers, M.D. A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Kleinjans, J.C.; Van Schooten, F.J. Biomonitoring of tobacco smoke carcinogenicity by dosimetry of DNA adducts and genotyping and phenotyping of biotransformational enzymes: A review on polycyclic aromatic hydrocarbons. Biomark. Biochem. Indic. Exposure Response Susceptibility Chem. 2002, 7, 209–229. [Google Scholar]
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef]
- Ali, S.A.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I.; Kapoor, M. The non-coding RNA interactome in joint health and disease. Nat. Rev. Rheumatol. 2021, 17, 692–705. [Google Scholar] [CrossRef]
- Bouchie, A. First microRNA mimic enters clinic. Nat. Biotechnol. 2013, 31, 577. [Google Scholar] [CrossRef]
- Scherr, M.; Venturini, L.; Battmer, K.; Schaller-Schoenitz, M.; Schaefer, D.; Dallmann, I.; Ganser, A.; Eder, M. Lentivirus-mediated antagomir expression for specific inhibition of miRNA function. Nucleic Acids Res. 2007, 35, e149. [Google Scholar] [CrossRef] [PubMed]
- Sidarovich, V.; Adami, V.; Quattrone, A. A cell-based high-throughput screen addressing 3′UTR-dependent regulation of the MYCN gene. Mol. Biotechnol. 2014, 56, 631–643. [Google Scholar] [CrossRef] [PubMed]

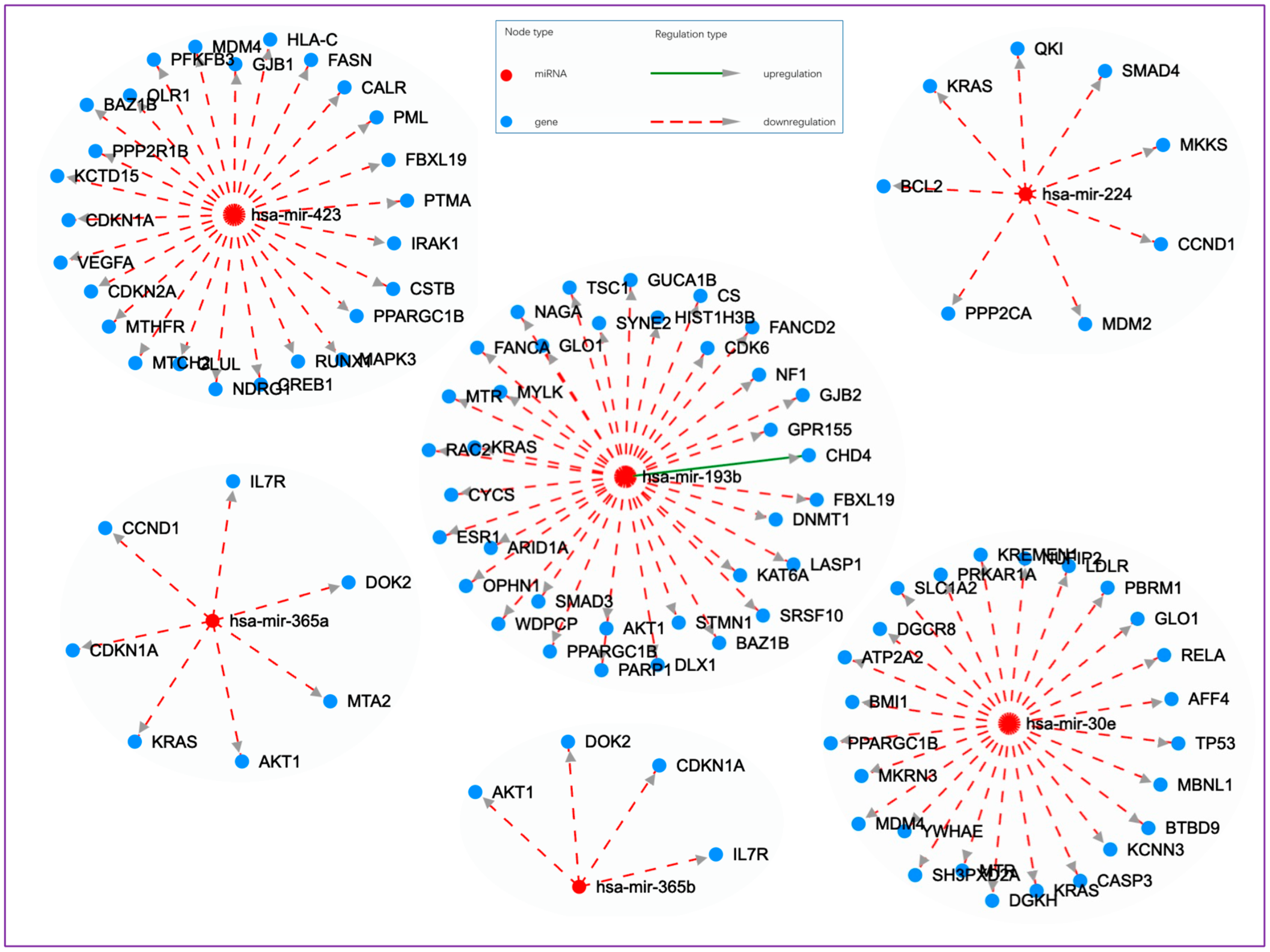
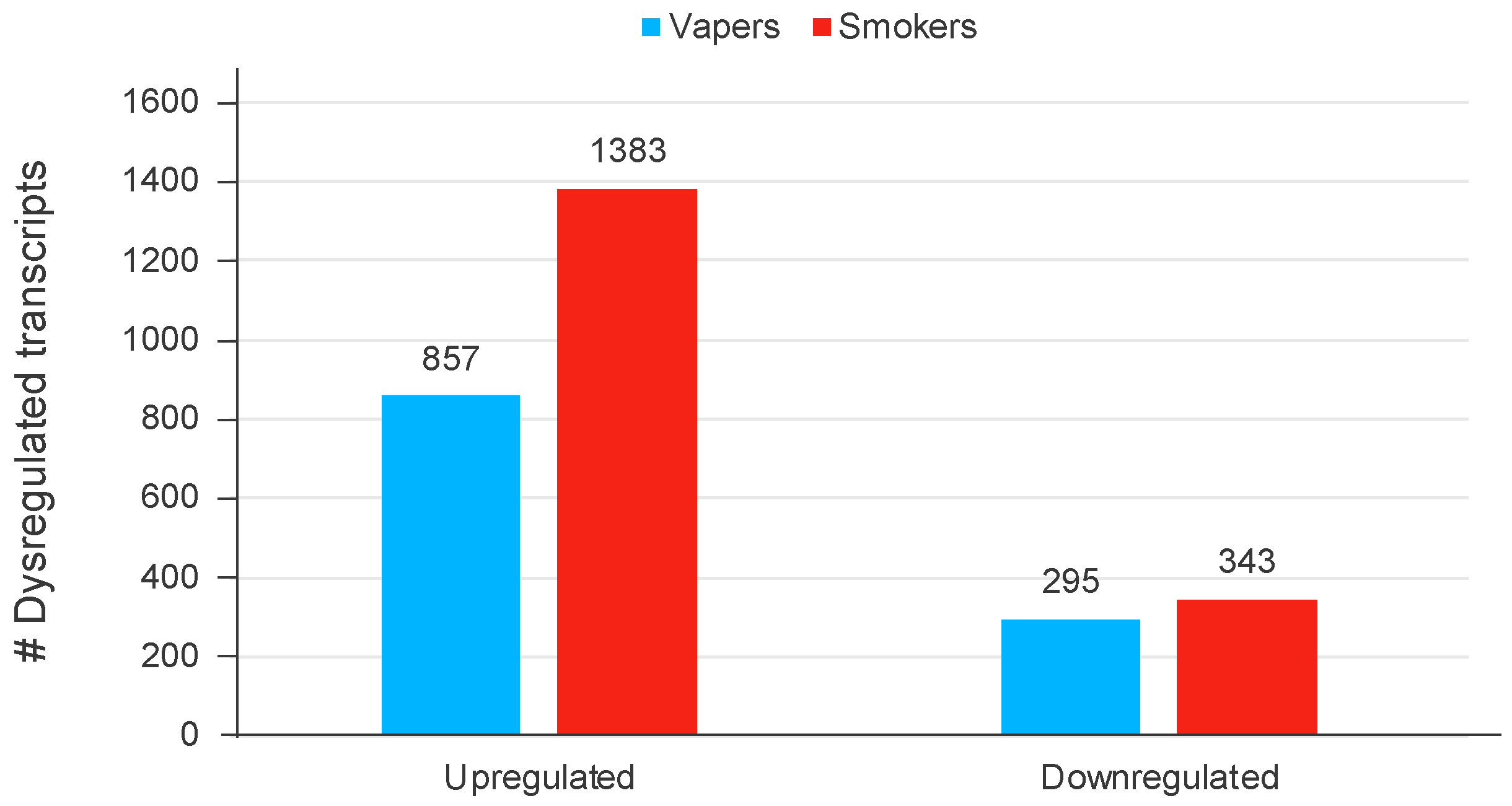
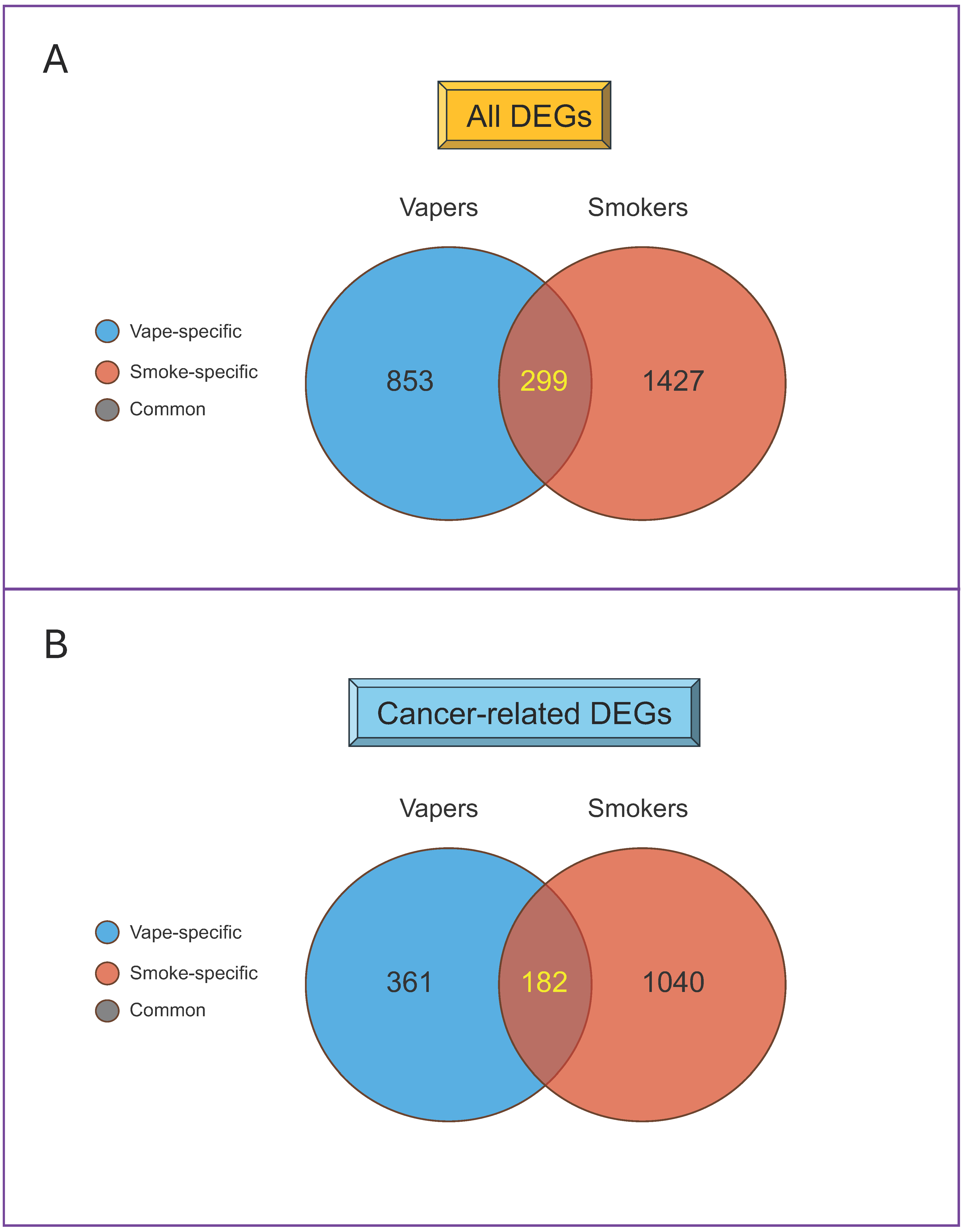
| MiRNA | Observed SAM Score (d) * | MiR-Target Network † |
|---|---|---|
| hsa-miR-421 ‡ | −8.84 | 831 predicted targets § |
| hsa-miR-23a-3p | −8.75 | TSC1, EN2, PPARGC1A, ADAM28, SMAD5, SOD2, LMAN2L, PTEN, SDHD, CHD4, PTPN11, TRPM7, ABCD1, ALDH5A1, SKI, PSAP, STAT3, STS, CXCL12, LDHA, CLDN16, NAV2, FAS, TNFAIP3, CCL8, IL6R, IRF1, GJA1, TSNAX, NUFIP2, RGS5, AMBRA1, KLF12, TGFB2, FKBP5, IKZF3, SLC1A5, MC2R, RFC2, CXCL8, ADK, LPAR1, MOG, C9orf3, NLGN4X, TBL2, SMAD3, MEF2C, PEX26 |
| hsa-miR-186-5p | −8.47 | SOD2, MATR3, RAF1, CNBP, CDKN1B, TOP2A, EEF2, DPP9, TNFSF15, KIF6, WNT5A, CPT1A, FGFR2, MYH9, ATM, ACVR1, HMGB1, OLR1, PTGIS, MAPK14, VEGFA, WDR11 |
| hsa-miR-140-5p | −7.4 | GDNF, SKI, ARID5B, SUZ12, FGF9, ADA, FGF2, CDK6, SHANK3, PRDM1, TPI1, NFYA, FPGS, SLC7A5, ITGA6, VEGFA |
| hsa-let-7f-5p | −6.61 | CCNG1, HDAC2, EPHA4, SMARCC1, ATXN2, EDN1, IKZF3, CYP19A1, BAZ1B, IL6, GLUL, CRX, IL13 |
| hsa-miR-28-5p | −6.56 | TPM4, TUFM, TP53 |
| hsa-miR-130a-3p | −6.55 | PPARGC1A, ADAM28, SOD2, PTPN14, SMAD4, ZBTB7A, CCND2, PTEN, MET, ABCG8, LDLR, COX10, TGFBR1, TGFBR2, IFNLR1, RNF125, ARSA, LIPA, MECP2, FKTN, IL23R, CTSA, SLC31A1, MAPK1, P2RY11, PPARA, PPARG, IER3IP1, QKI, APP, XIAP, MC2R, ESR1, SATB2, TPP1, CLCN3, PEX13, RUNX3 |
| hsa-miR-98-5p | −6.42 | ABCC4, PPARGC1A, FZD3, CD99, CCND2, CYP19A1, CHST3, PDLIM5, EDN1, IGF1, CREB1, IL6R, CHD7, SLC31A1, CDKN1A, THBS1, CAP1, LIFR, PLPP3, CDKN2B, TRAF1, VCL, REL, SIGMAR1, MPL, LGR4, KREMEN1, ICAM1, IGF2BP2, ZMPSTE24 |
| hsa-miR-151a-3p | −6.26 | TP53, ZBTB24, SLC6A1, MLH3, MPL, NOP10 |
| hsa-miR-182-5p | −6.11 | NDRG1, BDNF, CDKN1B, FGF9, FBXW7, PTEN, MITF, BARD1, PSD3, IGF1R, GSK3B, CREB1 |
| hsa-miR-1307-3p ‡ | 8.33 | VPS37C, CIC, TAGLN3, CCDC28B, AGAP1, ISM1, PRKCZ |
| hsa-miR-146b-3p | 8.34 | SQSTM1, KCTD15, CDKN1A, ERBB4, NUFIP2, NPAS4, REL, EGFR, SLC5A5, MYLK, KIT, IL6, TLR4, FAM107A |
| hsa-miR-25-5p | 8.57 | CDKN1C, NFIX, TP53, MDM4, ERBB2, NF2, SH2B3, 14-Sep, TNFSF10, FGF2, XPC, CDH1, MDM2, NOTCH2, RECK, DHFR, NRAS, TNFRSF10A |
| hsa-miR-148b-3p | 9.42 | PIK3CA, CYP1B1, CCL11, AHI1, PIK3CG, HMOX1, SRSF10, HLA-A, MCL1, MECP2, HLA-G, C3orf58, RUNX3 |
| hsa-miR-320a/b/c/d ‡ | 10.13 | (a) TSC1, TFAP2A, GAPDH, RPS6KA3, CDKN2A, MOCS1, PTEN, CCND2, PRDX3, MET, DIAPH1, PLS3, BRD4, CYBA, RNF125, KMT2D, TPI1, IGF2, CLN6, MMP9, FAS, KDM5C, YWHAE, GLUL, PPT1, HSD17B10, MDK, HNF1A, MLX, XBP1, CDK6, PKM, ATP7A, AR, TUBA1B, PACS1, NCOR1, RERE, SLC6A8, EZH2, VDAC1, VEGFA, RUNX2 (b) 1045 predicted targets § (c) 1045 predicted targets § (d) MDK |
| hsa-miR-342-5p | 12.51 | ACOX1, JUN, PFN1, FOSL2, NAA10, ZBTB7A, CCND2, COX10, DIAPH1, FAM107A, SKI, KCTD15, PLA2G4A, IDS, MMACHC, GGCX, MDM4, NDRG1, TFRC, IKBKG, CDK6, FXN, CLN8, ENG, ANTXR2, IKZF3, PPM1L, MAF, ACTG1, ABL1, PPM1D, IGF2R, ELN, UCP3, PTGIS, VAPB, IGF1R |
| hsa-miR-887-3p ‡ | 12.9 | CASK, CCPG1, PLD2, TCTN3, ATP2B2, CNPY2, C3orf80, FARP1, NPAT, CRLF3, MORC3, STARD13, POM121, TMEM139, TXNDC5, ST6GAL2, TMEM38B, PLEKHG2, MAP3K1, NCOR2, FADS6, CALM1, NT5C3A, FBXO34, RINL, PRH2, GSK3A, GAP43, HNRNPA3, GATD1, RRP36, GCNT2 |
| hsa-miR-7706 ‡ | 13.63 | TFAP2A, BLCAP, IQGAP1, CSNK2A1, ZNF468, ALAS2, ZBTB20, ANO8, ADO, SPNS2, FOSB, CTDSPL2, PWWP3B, RAB29, CELF4, TMEM65, DOCK4, RER1, CCDC103, GAS6, COMMD8, COL9A1, PCDHGA11, PCDHGA2, PCDHGC5, PCDHGA12, PCDHGB5, PCDHGA1, PCDHGA8, PCDHGA7, PCDHGB3, PCDHGB2, PCDHGA9, PCDHGB7, PCDHGA10, PCDHGC4, SELENOW, PCDHGC3, PCDHGB1, PCDHGA3, PCDHGB4, PCDHGA5, PCDHGA4, PCDHGB6, PCDHGA6, MZF1, KPNA1, DUSP18, PHTF1, KDM5A, PMF1-BGLAP, ZKSCAN3, ACTN2, INPP5A, GEMIN7, PPP1R9A, GNG12, DCP2, PMEPA1, CALHM3, FNBP1, GLG1, ATF6, CITED2, WNK1, DPYSL5, CCDC93, RETREG3, NFIX, SNX9, ATRX, MBOAT2, TGFBR3L, FCHSD1, ZNF385A, ZEB1, PAX6 |
| hsa-miR-130b-5p | 13.96 | PIK3CB, ARHGAP1, PTMA, DICER1, PPARGC1A, GRIN2A, PARP1, JAG2, SLC38A2, ADARB1, ZBTB7A, CCND2, PTEN, JARID2, MLXIPL, SLC30A3, RIMS3, LDLR, TCF7L2, TGFBR2, RB1, NR3C1, MMP2, LIPA, SH3PXD2A, WNK3, EDN1, HRH1, IGF1, RASSF1, RAPGEF6, SYNGR1, PLXNA3, SLC31A1, FMR1, MBNL1, TFRC, MAPK1, TES, PPARA, PLP1, PPARG, TCF4, SLC26A7, QKI, XIAP, LRP8, PSD3, EIF2S1, RFC2, CBLN2, CD86, SHOX, ACVR1, NRN1, ESR1, KMT2A, PLEKHA6, EPHA4, OXSR1, NOTCH2, CCNA2, EMX2, KREMEN1, CLCN3, CHEK2, ACSL4, RUNX3 |
| hsa-miR-941 ‡ | 14.01 | ZNF324, CROT, ATP8B2, FBXO42, PRDM16, PGP, ANKRD34B, CKS1B, NDUFAF7, SERTAD3, HINT3, MTMR9, CACNG8, RAI1, SPSB1, TMEM203, SAV1, CLCN3, KCMF1, POLH, SAR1A, RGS10, ORAI2, FOXN4, VPS53, ASAH2B, SLC35E4, SYNRG |
| MiRNA | Log2 Fold Change | t-Test p Value | FDR Adjusted p Value | MiR-Target Network * |
|---|---|---|---|---|
| hsa-miR-365a/b-3p | 24.32 ↑ | 2.49 × 10−34 | 1.18 × 10−31 | (a) CDKN1A, DOK2, AKT1, CCND1, KRAS, MTA2, IL7R (b) DOK2, AKT1, IL7R, CDKN1A |
| hsa-miR-362-5p | −44.56 ↓ | 9.82 × 10−23 | 2.32 × 10−20 | CASP8, FGF9 |
| hsa-miR-29b-3p | −24.34 ↓ | 2.75 × 10−17 | 4.33 × 10−15 | FBN1, REST, LOX, MDM2, TET2, CNBP, SMARCC1, LAMA2, COL5A1, BCL2, TNFAIP3, DNMT3A, TGFB2, VHL, IFNG, ESR1, BACE1, CCNA2, NOTCH2, HDAC4, AQP4 |
| hsa-let-7f-5p | 1.41 ↑ | 9.74 × 10−8 | 1.15 × 10−5 | CCNG1, HDAC2, EPHA4, SMARCC1, ATXN2, EDN1, IKZF3, CYP19A1, BAZ1B, IL6, GLUL, CRX, IL13 |
| hsa-miR-1299 † | 20.10 ↑ | 1.50 × 10−7 | 1.42 × 10−5 | 1119 predicted targets § |
| hsa-miR-21-5p | 1.30 ↑ | 7.13 × 10−7 | 5.29 × 10−5 | LATS1, DICER1, MIB1, PTPN14, REST, SLC17A5, RPS6KA3, GDF5, NR2C2, IGF1R, TGFBR2, CYCS, STAT3, RB1, COL4A1, PTPN3, OXTR, SOX11, CCL1, CADM1, LAMP2, DMD, CLCN5, BAZ1B, SLC9A6, GGCX, BCL2, TOP2A, KAT6A, KLF9, MDM4, PTGFR, SLC31A1, ZBTB20, FMR1, FUT2, SEMA5A, CCNG1, HS3ST3B1, PURA, KIF6, CCND1, PPARA, NBEA, CDK6, LIFR, TCF21, WNT5A, FKBP5, SOX5, RECK, PLAT, TRIM44, EIF2S1, TLR4, PPM1L, GTF2I, CEP152, AGAP1, NTF3, FOXO3, HPGD, CPM, HMGB1, EGFR, PIK3R1, GNE, RP2, NIPBL, TIMP3, SOX2, BMI1, MUC1, PREPL |
| hsa-let-7i-5p | 1.37 ↑ | 7.84 × 10−7 | 5.29 × 10−5 | MDM4, MYBPC3, SOD2, EPHA4, CCND1, EDN1, IKZF3, ACTA1, IGF1, IGF1R, MAP2K7, CRX, IL13 |
| hsa-let-7a-5p | 1.53 ↑ | 1.52 × 10−6 | 8.96 × 10−5 | MYC, ARG2, MDM2, SIK1, F2R, CRX, IFNLR1, CASP3, EDN1, IGF2, BCL2, AP1S1, MDM4, NPC1, THBS1, CDKN1A, DUSP6, CCNG1, TES, KRAS, CDK6, FXN, IKZF3, BTG1, EPHA4, VCL, MPL, ACTA1 |
| hsa-miR-30a-5p | 1.50 ↑ | 1.56 × 10−5 | 0.000735 | DGKH, DROSHA, TP53, CTNNB1, PPARD, SOD2, PRKAR1A, FBXO45, SLC38A2, ELOVL5, MAPK8, NPTN, MET, LDLR, OPHN1, HSPA5, ESR2, SLC1A2, PPARGC1B, FOXG1, BCL11A, CREM, CASP3, MPDU1, SH3PXD2A, GNAL, MECP2, MTR, PEX11B, SLC7A5, YWHAE, EEF2, ITGB3, CNP, THBS1, MAPK1, NUFIP2, NCAM1, PDCD10, KRAS, ATRX, CDK6, LIFR, WNT5A, ENTPD4, HDAC1, SCML2, PNPO, KCNN3, TGM2, KPNA1, KMT2A, SP4, EGFR, NDE1, PBRM1, KREMEN1, PPP3R1, IGF1R, RUNX2, PREPL |
| hsa-miR-193b-3p | 8.77 ↑ | 1.41 × 10−5 | 0.000735 | CS, TSC1, GUCA1B, PARP1, SYNE2, FANCD2, CHD4, DNMT1, OPHN1, CYCS, PPARGC1B, NAGA, DLX1, LASP1, AKT1, FANCA, GLO1, GJB2, MTR, GPR155, HIST1H3B, BAZ1B, STMN1, KAT6A, FBXL19, WDPCP, SRSF10, KRAS, CDK6, NF1, ARID1A, ESR1, SMAD3, MYLK, RAC2 |
| hsa-miR-100-5p | 1.25 ↑ | 8.37 × 10−5 | 0.003478 | AKT1, FKBP5, RB1, IGF1R |
| hsa-miR-423-3p | 1.48 ↑ | 8.84 × 10−5 | 0.003478 | PTMA, PML, FASN, CDKN2A, PPP2R1B, MAPK3, MTCH2, CSTB, KCTD15, PPARGC1B, RUNX1, BAZ1B, GLUL, FBXL19, CREB1, IRAK1, MDM4, NDRG1, CDKN1A, CALR, MTHFR, PFKFB3, GJB1, HLA-C, OLR1, VEGFA |
| hsa-miR-30c-5p | 1.50 ↑ | 0.000276283 | 0.010031 | TP53, SERPINE1, PPARGC1B, CTGF, NOTCH1, SUZ12, LIFR, MCL1, SLC7A5, LDLR |
| hsa-miR-451a † | −1.80 ↓ | 0.000781282 | 0.02634 | OSR1, CUX2, PSMB8, CXCL16, TARP, ST8SIA4, CDKN2D, MIF, FBLN5, CERK, SAMD4B, CAB39, VAPA, LETM2, MEX3C, USP46, CMTM6, TBC1D9B, PMM2, KIAA1217, MAU2, RNF217, MEGF6, S1PR2, EVL, FBXO33, ATF2, CDKN2B, UCK1, CAV1, C16orf72, DCAF5, RAB5A, CACHD1, LUZP2, EIF2AK3, AKTIP, FAM171A1, TTN, NEDD9 |
| hsa-miR-143-3p | 1.04 ↑ | 0.00091283 | 0.028724 | PAPPA, ADCY2, STAR, MDM2, NR2C2, MMP14, CNBP, TRAF3IP2, MMP2, AKT1, IDS, COL5A1, MMP9, THRA, GLUL, TNF, IL2RA, IRF1, MAPK1, DNMT3A, KRAS, IKZF3, XIAP, ITGB1, PTPN2, TEP1, PTGS2, PIK3R1, SMAD3, SMYD4, FHIT, LIMK1, IGF1R |
| hsa-miR-224-5p | 2.01 ↑ | 0.001192386 | 0.035175 | PPP2CA, CCND1, SMAD4, KRAS, MKKS, MDM2, QKI, BCL2 |
| hsa-miR-30e-5p | −1.30 ↓ | 0.001717024 | 0.047673 | DGKH, TP53, PRKAR1A, MKRN3, RELA, LDLR, SLC1A2, PPARGC1B, CASP3, SH3PXD2A, GLO1, MTR, YWHAE, MDM4, AFF4, MBNL1, NUFIP2, KRAS, BTBD9, KCNN3, ATP2A2, DGCR8, PBRM1, KREMEN1, BMI1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besaratinia, A.; Tommasi, S. The Untapped Biomarker Potential of MicroRNAs for Health Risk–Benefit Analysis of Vaping vs. Smoking. Cells 2024, 13, 1330. https://doi.org/10.3390/cells13161330
Besaratinia A, Tommasi S. The Untapped Biomarker Potential of MicroRNAs for Health Risk–Benefit Analysis of Vaping vs. Smoking. Cells. 2024; 13(16):1330. https://doi.org/10.3390/cells13161330
Chicago/Turabian StyleBesaratinia, Ahmad, and Stella Tommasi. 2024. "The Untapped Biomarker Potential of MicroRNAs for Health Risk–Benefit Analysis of Vaping vs. Smoking" Cells 13, no. 16: 1330. https://doi.org/10.3390/cells13161330
APA StyleBesaratinia, A., & Tommasi, S. (2024). The Untapped Biomarker Potential of MicroRNAs for Health Risk–Benefit Analysis of Vaping vs. Smoking. Cells, 13(16), 1330. https://doi.org/10.3390/cells13161330








