Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Cell Culture
2.2.1. iCell
2.2.2. ChiPS22
2.2.3. iPSC C32
2.2.4. hESC SA121
2.3. Maturation Protocol
2.4. In Vitro Hypertrophy Cell Model
2.5. Characterization of hPSC-CMs
Bioenergetics Seahorse Analysis
2.6. Structural Analysis
2.6.1. Immunofluorescence Staining
2.6.2. Confocal Imaging and Image Analysis
2.6.3. Cell Morphology Analysis
2.6.4. Sarcomere Characterization
2.7. Ultrastructural Analysis
2.7.1. TEM Analysis
2.7.2. Mitochondria Content Quantification
2.7.3. Sarcomere Content Quantification
2.8. Functional Analyses
2.8.1. Calcium Imaging
2.8.2. Electrophysiological Analyses
2.8.3. Single-Cell Action Potential
2.9. Three-Dimensional Cardiac Microtissue Generation and Force Measurements
2.10. Cardiac Tissue Processing and Staining
2.11. RNA Sequencing Analysis
2.12. Identification and Analysis of Differentially Expressed Genes
2.13. Pathway Enrichment Analysis
2.14. Isolation of Total RNA from Cell Cultures and Quantitative Real-Time PCR (qPCR)
2.15. In Vitro-to-In Vivo Translatability of BCKDK Inhibition on BCAA Catabolism
2.16. Statistical Analysis
3. Results
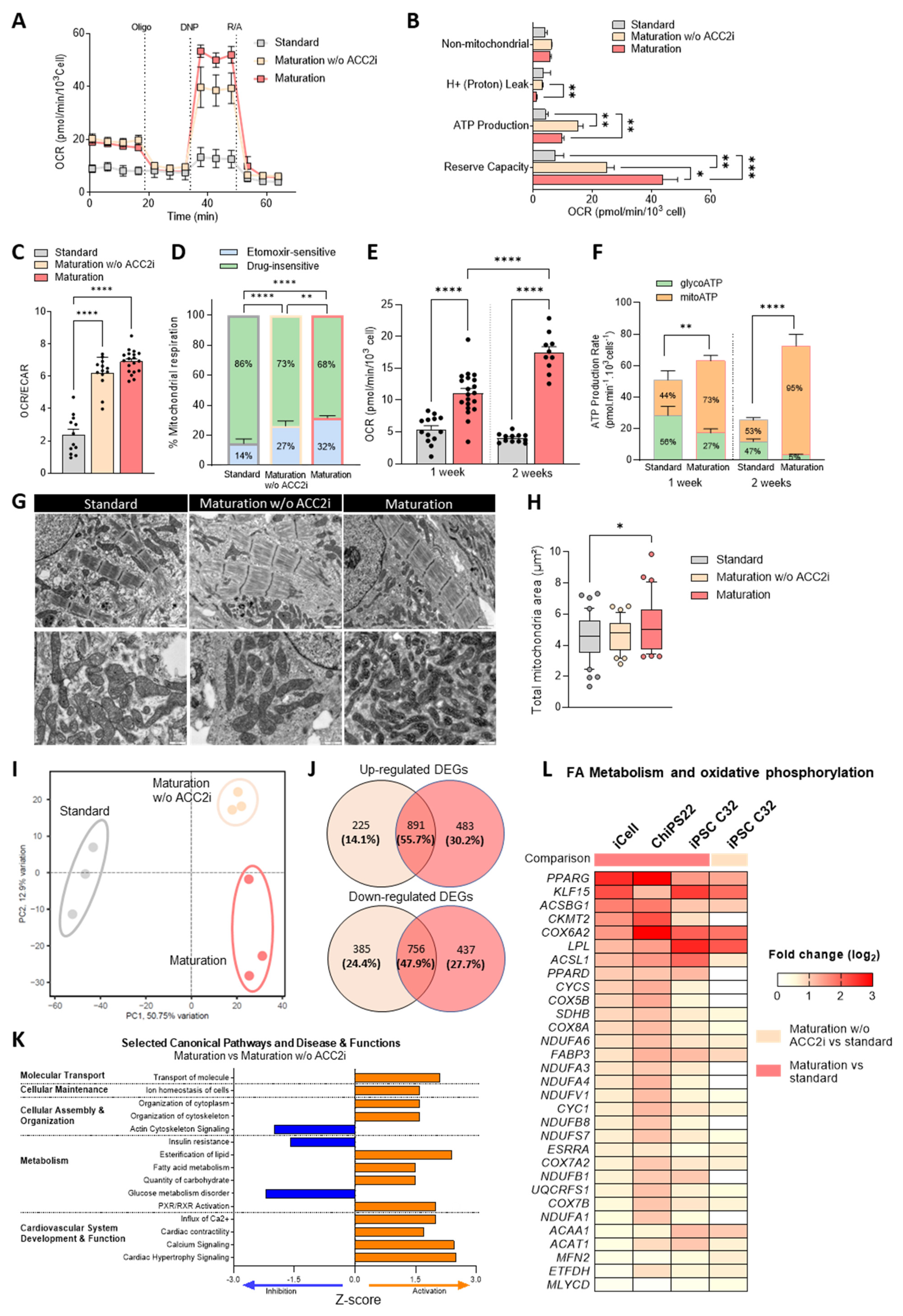
3.1. Enhancement of Mitochondrial Function, FAO, and Bioenergetics in hiPSC-CMs Cultured in Maturation Medium Containing an ACC2 Inhibitor
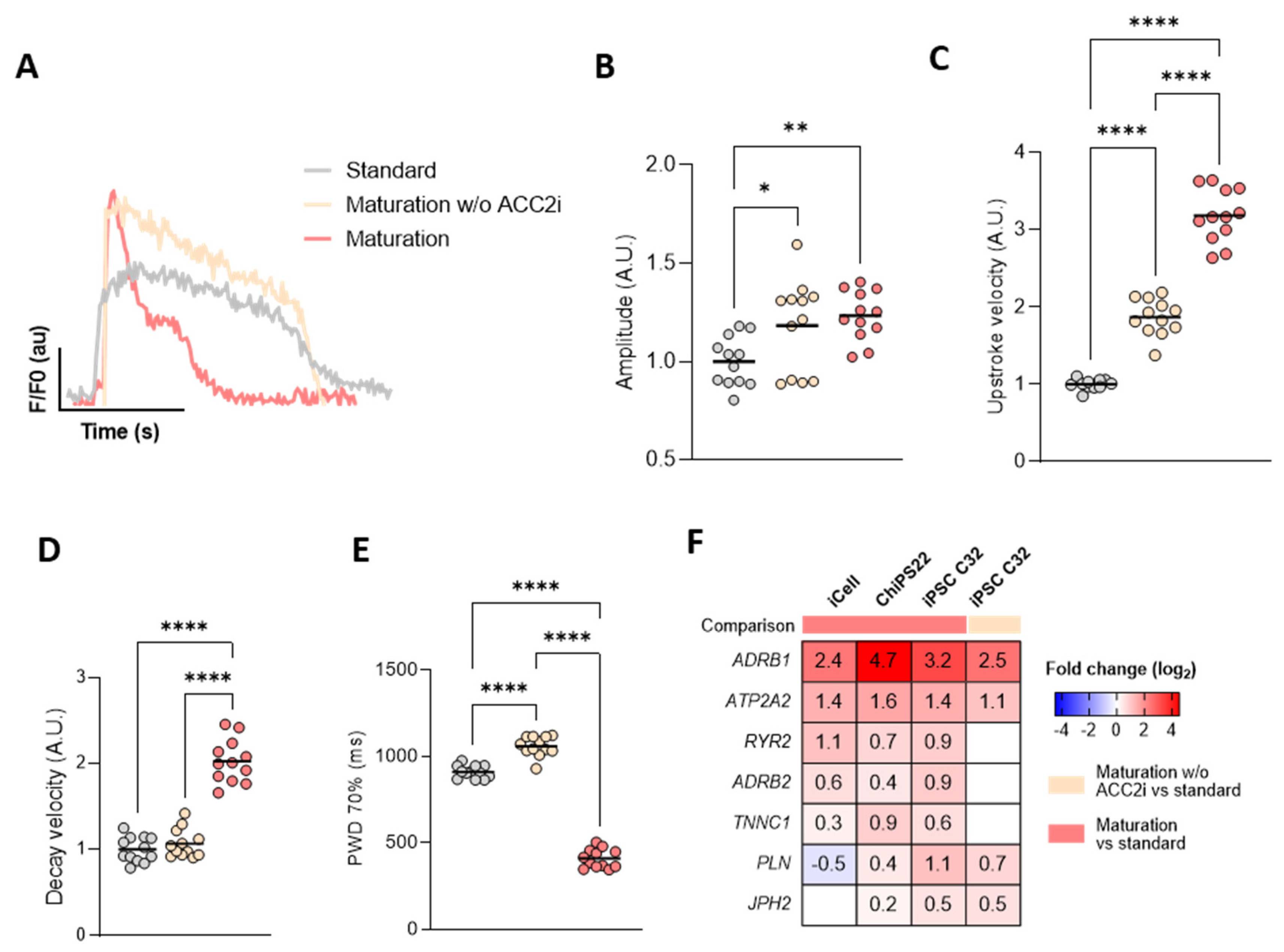
3.2. Maturation Medium Enhances Calcium Kinetics in hiPSC-CMs
3.3. Superior Electrophysiological Properties of hiPSC-CMs Cultured in Maturation Medium Compared to Standard Medium
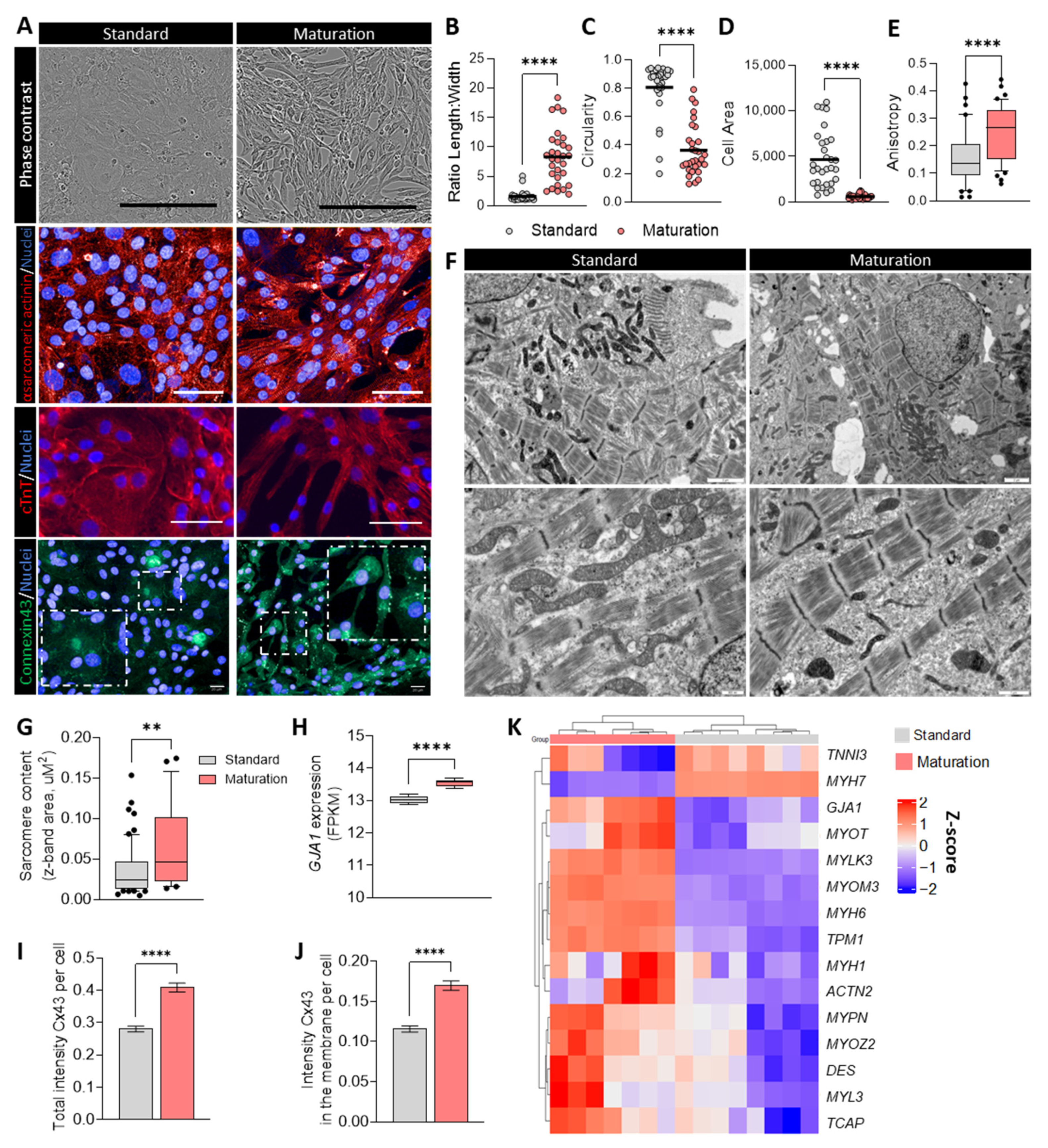
3.4. Improved Phenotypic Maturation of hiPSC-CMs Cultured in Maturation Medium
3.5. Maturation Medium Enhances Cell Structure, CM Marker Expression, and Ventricular Phenotype in hESC-CMs
3.6. Maturation Medium Improves Formation, Morphology, and Contractile Kinetics of hiPSC-CM-Derived 3D Microtissues
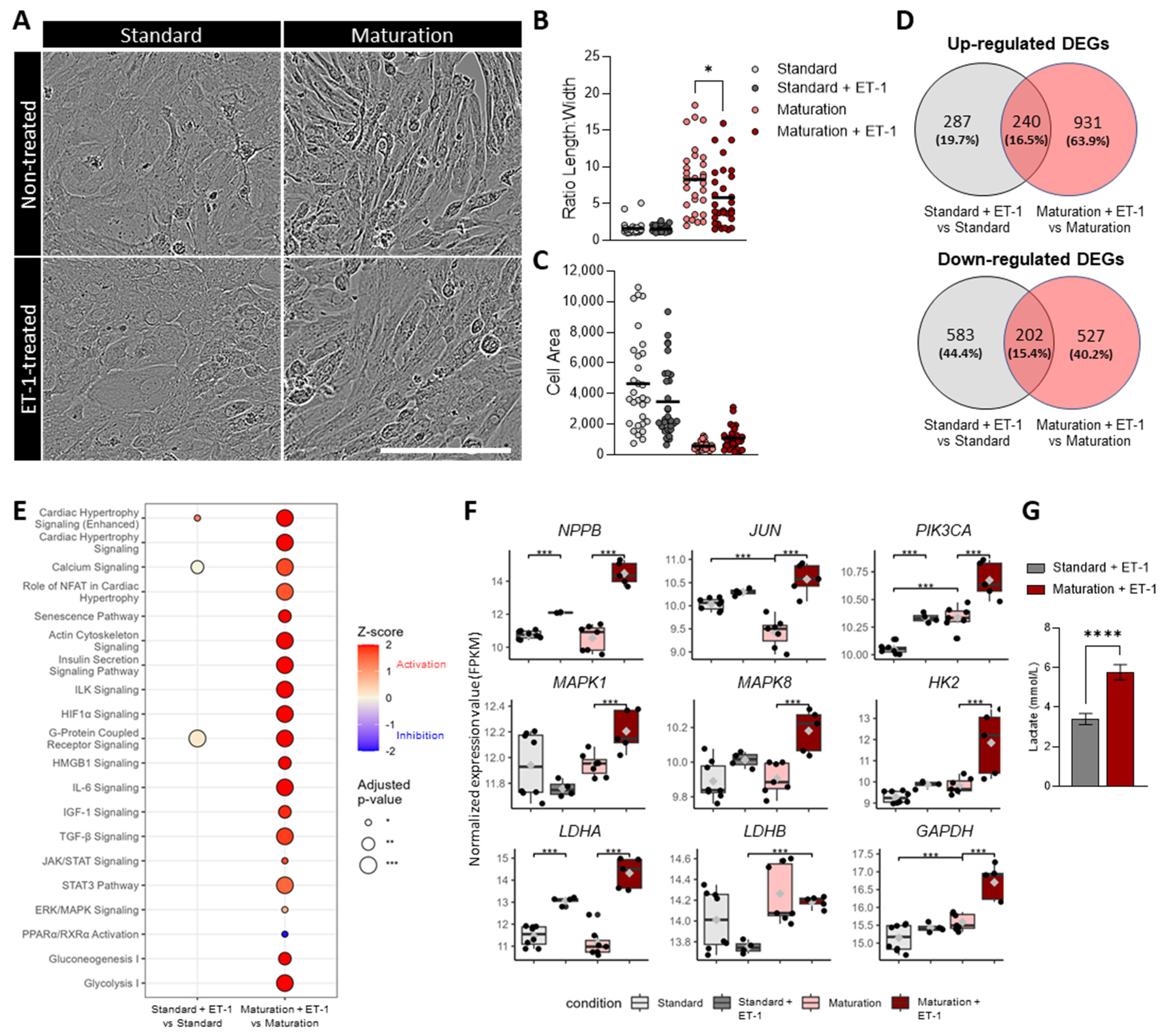
3.7. Improved Physiologically Relevant Response of hiPSC-CMs Cultured in Maturation Medium to Endothelin-1 Hypertrophic Stimulation
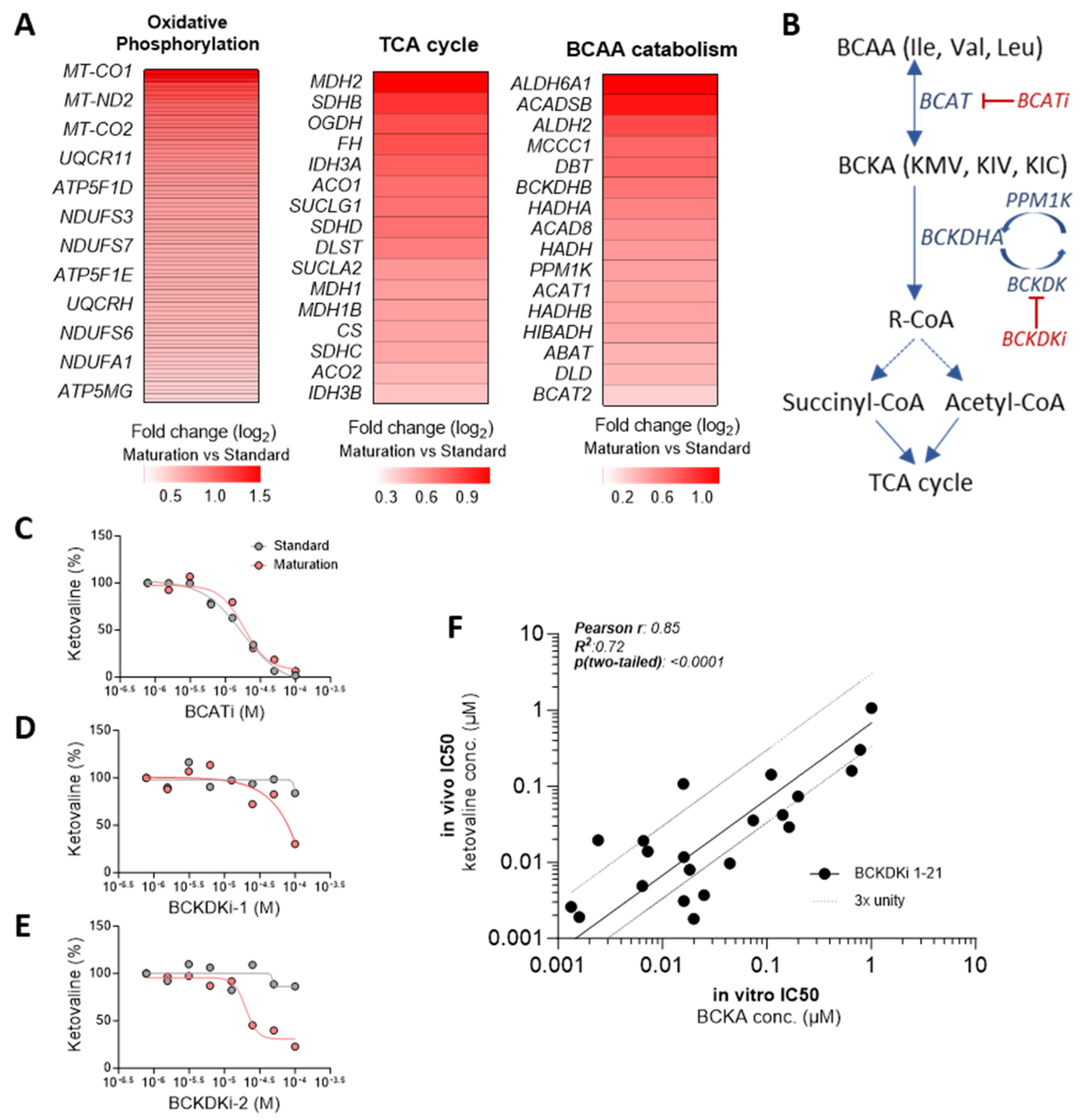
3.8. Enhanced In Vitro-to-In Vivo Correlation Achieved with hiPSC-CM Cultured in Maturation Medium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seibertz, F.; Sutanto, H.; Dülk, R.; Pronto, J.R.D.; Springer, R.; Rapedius, M.; Liutkute, A.; Ritter, M.; Jung, P.; Stelzer, L.; et al. Electrophysiological and Calcium-Handling Development during Long-Term Culture of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Basic. Res. Cardiol. 2023, 118, 14. [Google Scholar] [CrossRef]
- Lewandowski, J.; Rozwadowska, N.; Kolanowski, T.J.; Malcher, A.; Zimna, A.; Rugowska, A.; Fiedorowicz, K.; Łabędź, W.; Kubaszewski, Ł.; Chojnacka, K.; et al. The Impact of in Vitro Cell Culture Duration on the Maturation of Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells of Myogenic Origin. Cell Transpl. 2018, 27, 1047–1067. [Google Scholar] [CrossRef]
- Wang, L.; Wada, Y.; Ballan, N.; Schmeckpeper, J.; Huang, J.; Rau, C.D.; Wang, Y.; Gepstein, L.; Knollmann, B.C. Triiodothyronine and Dexamethasone Alter Potassium Channel Expression and Promote Electrophysiological Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell Cardiol. 2021, 161, 130–138. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.; Pabon, L.; Fischer, K.A.; Reinecke, H.; Regnier, M.; Sniadecki, N.J.; Ruohola-Baker, H.; Murry, C.E. Tri-Iodo-l-Thyronine Promotes the Maturation of Human Cardiomyocytes-Derived from Induced Pluripotent Stem Cells. J. Mol. Cell Cardiol. 2014, 72, 296–304. [Google Scholar] [CrossRef]
- Birket, M.J.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.P.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef]
- Chirico, N.; Kessler, E.L.; Maas, R.G.C.; Fang, J.; Qin, J.; Dokter, I.; Daniels, M.; Šarić, T.; Neef, K.; Buikema, J.W.; et al. Small Molecule-Mediated Rapid Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cell Res. Ther. 2022, 13, 531. [Google Scholar] [CrossRef]
- Murphy, S.A.; Miyamoto, M.; Kervadec, A.; Kannan, S.; Tampakakis, E.; Kambhampati, S.; Lin, B.L.; Paek, S.; Andersen, P.; Lee, D.I.; et al. PGC1/PPAR Drive Cardiomyocyte Maturation at Single Cell Level via YAP1 and SF3B2. Nat. Commun. 2021, 12, 1648. [Google Scholar] [CrossRef]
- Huang, C.Y.; Peres Moreno Maia-Joca, R.; Ong, C.S.; Wilson, I.; DiSilvestre, D.; Tomaselli, G.F.; Reich, D.H. Enhancement of Human IPSC-Derived Cardiomyocyte Maturation by Chemical Conditioning in a 3D Environment. J. Mol. Cell Cardiol. 2020, 138, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Carido, M.; Sebastião, M.J.; Gomes-Alves, P.; Elliott, D.A.; Domian, I.J.; Teixeira, A.P.; et al. 3D Aggregate Culture Improves Metabolic Maturation of Human Pluripotent Stem Cell Derived Cardiomyocytes. Biotechnol. Bioeng. 2018, 115, 630–644. [Google Scholar] [CrossRef]
- Lopez, C.A.; Al-Siddiqi, H.H.A.A.; Purnama, U.; Iftekhar, S.; Bruyneel, A.A.N.; Kerr, M.; Nazir, R.; da Luz Sousa Fialho, M.; Malandraki-Miller, S.; Alonaizan, R.; et al. Physiological and Pharmacological Stimulation for in Vitro Maturation of Substrate Metabolism in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Sci. Rep. 2021, 11, 7802. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.J.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Chen, J.H.; Lundy, D.J.; Liu, C.H.; Hwang, S.M.; Pabon, L.; Shieh, R.C.; Chen, C.C.; Wu, S.N.; Yan, Y.T.; et al. Defined MicroRNAs Induce Aspects of Maturation in Mouse and Human Embryonic-Stem-Cell-Derived Cardiomyocytes. Cell Rep. 2015, 12, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Campostrini, G.; Kosmidis, G.; Ward-Van Oostwaard, D.; Davis, R.P.; Yiangou, L.; Ottaviani, D.; Veerman, C.C.; Mei, H.; Orlova, V.V.; Wilde, A.A.M.; et al. Maturation of HiPSC-Derived Cardiomyocytes Promotes Adult Alternative Splicing of SCN5A and Reveals Changes in Sodium Current Associated with Cardiac Arrhythmia. Cardiovasc. Res. 2023, 119, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Teixeira, A.; Domian, I.; Serra, M.; Alves, P.M. Distinct Carbon Sources Affect Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Sci. Rep. 2017, 7, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Charrez, B.; Neiman, G.; Siemons, B.; Boggess, S.C.; Wall, S.; Charwat, V.; Jæger, K.H.; Cleres, D.; Telle, Å.; et al. Metabolically Driven Maturation of Human-Induced-Pluripotent-Stem-Cell-Derived Cardiac Microtissues on Microfluidic Chips. Nat. Biomed. Eng. 2022, 6, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human IPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef]
- Hu, D.; Linders, A.; Yamak, A.; Correia, C.; Kijlstra, J.D.; Garakani, A.; Xiao, L.; Milan, D.J.; Van Der Meer, P.; Serra, M.; et al. Metabolic Maturation of Human Pluripotent Stem Cellderived Cardiomyocytes by Inhibition of HIF1α and LDHA. Circ. Res. 2018, 123, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, N.M.; Sachs, D.; Shewale, B.; Gonzalez, D.M.; Dhanan-Krishnan, P.; Torre, D.; LaMarca, E.; Raimo, S.; Dariolli, R.; Serasinghe, M.N.; et al. PPARdelta Activation Induces Metabolic and Contractile Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Stem Cell 2022, 29, 559–576.e7. [Google Scholar] [CrossRef]
- Gentillon, C.; Li, D.; Duan, M.; Yu, W.M.; Preininger, M.K.; Jha, R.; Rampoldi, A.; Saraf, A.; Gibson, G.C.; Qu, C.K.; et al. Targeting HIF-1α in Combination with PPARα Activation and Postnatal Factors Promotes the Metabolic Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell Cardiol. 2019, 132, 120–135. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.L.; Leonard, A.; Sun, L.; Fischer, K.A.; Wang, Y.; Ritterhoff, J.; Zhao, L.; Kolwicz, S.C.; Pabon, L.; et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 13, 657–668. [Google Scholar] [CrossRef]
- Galow, A.M.; Brenmoehl, J.; Hoeflich, A. Synergistic Effects of Hormones on Structural and Functional Maturation of Cardiomyocytes and Implications for Heart Regeneration. Cell. Mol. Life Sci. 2023, 80, 240. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, D.; ter Huurne, M.; Elliott, D.A.; Bellin, M.; Mummery, C.L. Maturing Differentiated Human Pluripotent Stem Cells in Vitro: Methods and Challenges. Development 2023, 150, dev201103. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, K.A.; Blancard, M.; Lyra-Leite, D.M.; Vanoye, C.G.; Fonoudi, H.; Jouni, M.; DeKeyser, J.M.L.; Lenny, B.; Sapkota, Y.; George, A.L.; et al. Independent Compartmentalization of Functional, Metabolic, and Transcriptional Maturation of HiPSC-Derived Cardiomyocytes. Cell Rep. 2024, 43, 114160. [Google Scholar] [CrossRef] [PubMed]
- Piquereau, J.; Ventura-Clapier, R. Maturation of Cardiac Energy Metabolism during Perinatal Development. Front. Physiol. 2018, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Jaswal, J.S. Energy Metabolic Phenotype of the Cardiomyocyte During Development, Differentiation, and Postnatal Maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Glass, N.; Ovchinnikov, D.; Yang, S.K.; Zhang, X.; Mazzone, S.; Chen, C.; Wolvetang, E.; Cooper-White, J. Modelling Ischemia-Reperfusion Injury (IRI) in Vitro Using Metabolically Matured Induced Pluripotent Stem Cell-Derived Cardiomyocytes. APL Bioeng. 2018, 2, 026102. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef] [PubMed]
- Kolwicz, S.C.; Olson, D.P.; Marney, L.C.; Garcia-Menendez, L.; Synovec, R.E.; Tian, R. Cardiac-Specific Deletion of Acetyl CoA Carboxylase 2 Prevents Metabolic Remodeling during Pressure-Overload Hypertrophy. Circ. Res. 2012, 111, 728–738. [Google Scholar] [CrossRef]
- Ritterhoff, J.; McMillen, T.S.; Villet, O.; Young, S.; Kolwicz, S.C.; Senn, T.; Caudal, A.; Tian, R. Increasing Fatty Acid Oxidation Elicits a Sex-Dependent Response in Failing Mouse Hearts. J. Mol. Cell Cardiol. 2021, 158, 1–10. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Young, S.; Villet, O.; Shao, D.; Neto, F.C.; Bettcher, L.F.; Hsu, Y.W.A.; Kolwicz, S.C.; Raftery, D.; Tian, R. Metabolic Remodeling Promotes Cardiac Hypertrophy by Directing Glucose to Aspartate Biosynthesis. Circ. Res. 2020, 126, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Titmarsh, D.M.; Glass, N.R.; Mills, R.J.; Hidalgo, A.; Wolvetang, E.J.; Porrello, E.R.; Hudson, J.E.; Cooper-White, J.J. Induction of Human IPSC-Derived Cardiomyocyte Proliferation Revealed by Combinatorial Screening in High Density Microbioreactor Arrays. Sci. Rep. 2016, 6, 24637. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.S.; Lehtinen, M.L.; Leung, C.Y.; Lian, X.; Xu, J.; Keung, W.; Geng, L.; Kolstad, T.R.S.; Thams, S.; Wong, A.O.-T.; et al. Human ISL1+ Ventricular Progenitors Self-Assemble into an In Vivo Functional Heart Patch and Preserve Cardiac Function Post Infarction. Mol. Ther. 2018, 26, 1644–1659. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically Defined Generation of Human Cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Weiwad, W.K.K.; Linke, W.A.; Wussling, M.H.P. Sarcomere Length–Tension Relationship of Rat Cardiac Myocytes at Lengths Greater than Optimum. J. Mol. Cell Cardiol. 2000, 32, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like Honeycombs for Tissue Engineering of Cardiac Anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, K.; Chen, C.S.; Lejeune, E. Sarc-Graph: Automated Segmentation, Tracking, and Analysis of Sarcomeres in HiPSCderived Cardiomyocytes. PLoS Comput. Biol. 2021, 17, e1009443. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Karakikes, I.; Serrao, G.W.; Backeris, P.; Lee, J.-J.; Xie, C.; Senyei, G.; Gordon, R.E.; Li, R.A.; Akar, F.G.; et al. Advancing Functional Engineered Cardiac Tissues toward a Preclinical Model of Human Myocardium. FASEB J. 2014, 28, 644–654. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Voronova, V.; Sokolov, V.; Morias, Y.; Boezelman, M.J.; Wågberg, M.; Henricsson, M.; Hansson, K.; Goltsov, A.; Peskov, K.; Sundqvist, M. Evaluation of Therapeutic Strategies Targeting BCAA Catabolism Using a Systems Pharmacology Model. Front. Pharmacol. 2022, 13, 993422. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.C.; Chen, G.; Lynch, C.J. Quantification of Branched-Chain Keto Acids in Tissue by Ultra Fast Liquid Chromatography–Mass Spectrometry. Anal. Biochem. 2013, 439, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Harwood, H.J.; Petras, S.F.; Shelly, L.D.; Zaccaro, L.M.; Perry, D.A.; Makowski, M.R.; Hargrove, D.M.; Martin, K.A.; Tracey, W.R.; Chapman, J.G.; et al. Isozyme-Nonselective N-Substituted Bipiperidylcarboxamide Acetyl-CoA Carboxylase Inhibitors Reduce Tissue Malonyl-CoA Concentrations, Inhibit Fatty Acid Synthesis, and Increase Fatty Acid Oxidation in Cultured Cells and in Experimental Animals. J. Biol. Chem. 2003, 278, 37099–37111. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimo, D.A.; John, J.E.; Zhang, L.; Efraim, E.S.; Zhang, R.; Liao, X.; Jain, M.K. KLF15 and PPAR α Cooperate to Regulate Cardiomyocyte Lipid Gene Expression and Oxidation. PPAR Res. 2015, 2015, 201625. [Google Scholar] [CrossRef] [PubMed]
- De Waard, S.; Montnach, J.; Ribeiro, B.; Nicolas, S.; Forest, V.; Charpentier, F.; Mangoni, M.E.; Gaborit, N.; Ronjat, M.; Loussouarn, G.; et al. Functional Impact of Bekm-1, a High-Affinity Herg Blocker, on Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 7167. [Google Scholar] [CrossRef] [PubMed]
- Tso, S.C.; Gui, W.J.; Wu, C.Y.; Chuang, J.L.; Qi, X.; Skvorak, K.J.; Dorko, K.; Wallace, A.L.; Morlock, L.K.; Lee, B.H.; et al. Benzothiophene Carboxylate Derivatives as Novel Allosteric Inhibitors of Branched-Chain α-Ketoacid Dehydrogenase Kinase. J. Biol. Chem. 2014, 289, 20583–20593. [Google Scholar] [CrossRef]
- La Sala, G.; Pfleger, C.; Käck, H.; Wissler, L.; Nevin, P.; Böhm, K.; Janet, J.P.; Schimpl, M.; Stubbs, C.J.; De Vivo, M.; et al. Combining Structural and Coevolution Information to Unveil Allosteric Sites. Chem. Sci. 2023, 14, 7057–7067. [Google Scholar] [CrossRef] [PubMed]
- Rog-Zielinska, E.A.; Craig, M.A.; Manning, J.R.; Richardson, R.V.; Gowans, G.J.; Dunbar, D.R.; Gharbi, K.; Kenyon, C.J.; Holmes, M.C.; Hardie, D.G.; et al. Glucocorticoids Promote Structural and Functional Maturation of Foetal Cardiomyocytes: A Role for PGC-1α. Cell Death Differ. 2015, 22, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; He, S.; Mali, P. Systematic Discovery of Transcription Factors That Improve HPSC-Derived Cardiomyocyte Maturation via Temporal Analysis of Bioengineered Cardiac Tissues. APL Bioeng. 2023, 7, 026109. [Google Scholar] [CrossRef]
- Grubb, S.; Calloe, K.; Thomsen, M.B. Impact of KChiP2 on Cardiac Electrophysiology and the Progression of Heart Failure. Front. Physiol. 2012, 3, 24523. [Google Scholar] [CrossRef]
- Deschênes, I.; Armoundas, A.A.; Jones, S.P.; Tomaselli, G.F. Post-Transcriptional Gene Silencing of KChIP2 and Navβ1 in Neonatal Rat Cardiac Myocytes Reveals a Functional Association between Na and Ito Currents. J. Mol. Cell Cardiol. 2008, 45, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Balbuena, D.; Guerrero-Serna, G.; Valdivia, C.; Caballero, R.; Diez-Guerra, F.J.; Jiménez-Vázquez, E.; Ramírez, R.; Rocha, A.; Herron, T.; Campbell, K.; et al. Cardiac Kir2.1 and NaV1.5 Channels Traffic Together to the Sarcolemma to Control Excitability. Circ. Res. 2018, 122, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Goversen, B.; van der Heyden, M.A.G.; van Veen, T.A.B.; de Boer, T.P. The Immature Electrophysiological Phenotype of IPSC-CMs Still Hampers in Vitro Drug Screening: Special Focus on IK1. Pharmacol. Ther. 2018, 183, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Schram, G.; Pourrier, M.; Wang, Z.; White, M.; Nattel, S. Barium Block of Kir2 and Human Cardiac Inward Rectifier Currents: Evidence for Subunit-Heteromeric Contribution to Native Currents. Cardiovasc. Res. 2003, 59, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Markandeya, Y.S.; Kamp, T.J.; Makielski, J.C.; January, C.T.; Eckhardt, L.L. IK1-Enhanced Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes: An Improved Cardiomyocyte Model to Investigate Inherited Arrhythmia Syndromes. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Ackers-Johnson, M.; Li, P.Y.; Holmes, A.P.; O’Brien, S.-M.; Pavlovic, D.; Foo, R.S. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes from the Adult Mouse Heart. Circ. Res. 2016, 119, 909–920. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, C.; Christoffersson, J.; Tejedor, S.; El-Haou, S.; Matadamas-Guzman, M.; Nair, S.; Dönnes, P.; Musa, G.; Rohman, M.; Sundqvist, M.; et al. Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor. Cells 2024, 13, 1339. https://doi.org/10.3390/cells13161339
Correia C, Christoffersson J, Tejedor S, El-Haou S, Matadamas-Guzman M, Nair S, Dönnes P, Musa G, Rohman M, Sundqvist M, et al. Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor. Cells. 2024; 13(16):1339. https://doi.org/10.3390/cells13161339
Chicago/Turabian StyleCorreia, Cláudia, Jonas Christoffersson, Sandra Tejedor, Saïd El-Haou, Meztli Matadamas-Guzman, Syam Nair, Pierre Dönnes, Gentian Musa, Mattias Rohman, Monika Sundqvist, and et al. 2024. "Enhancing Maturation and Translatability of Human Pluripotent Stem Cell-Derived Cardiomyocytes through a Novel Medium Containing Acetyl-CoA Carboxylase 2 Inhibitor" Cells 13, no. 16: 1339. https://doi.org/10.3390/cells13161339







