Experimental Study: The Development of a Novel Treatment for Chemotherapy-Resistant Tongue Cancer with the Inhibition of the Pathological Periostin Splicing Variant 1-2 with Exon 21
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cell Cultures
2.3. Anti-Human POSTN Antibody
2.4. In Situ Hybridization
2.5. MTS Cell Proliferation Assay
2.6. Western Blot Analysis
2.7. Xenograft Assay in Nude Mice
2.8. Statistical Analysis
3. Results
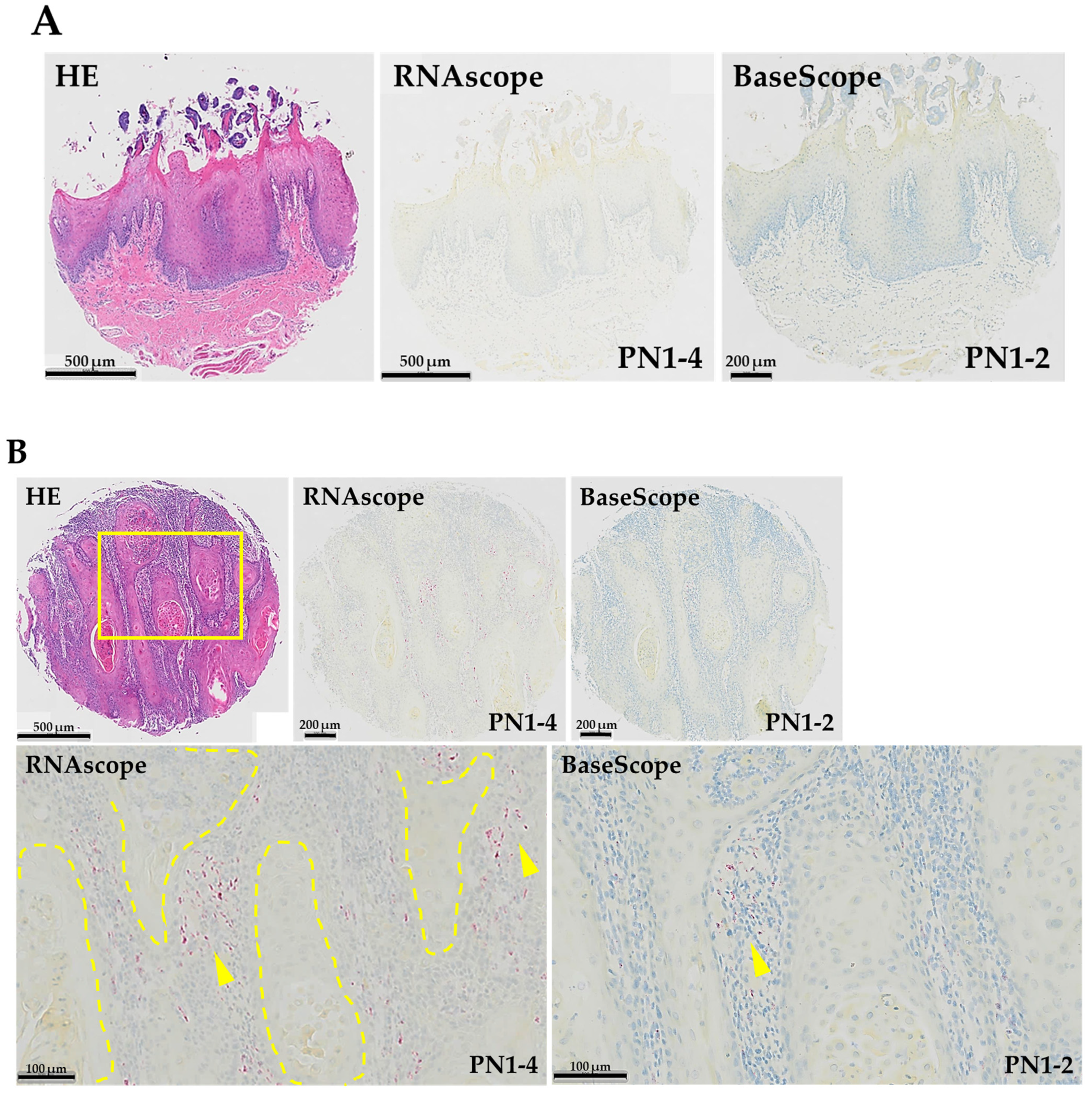
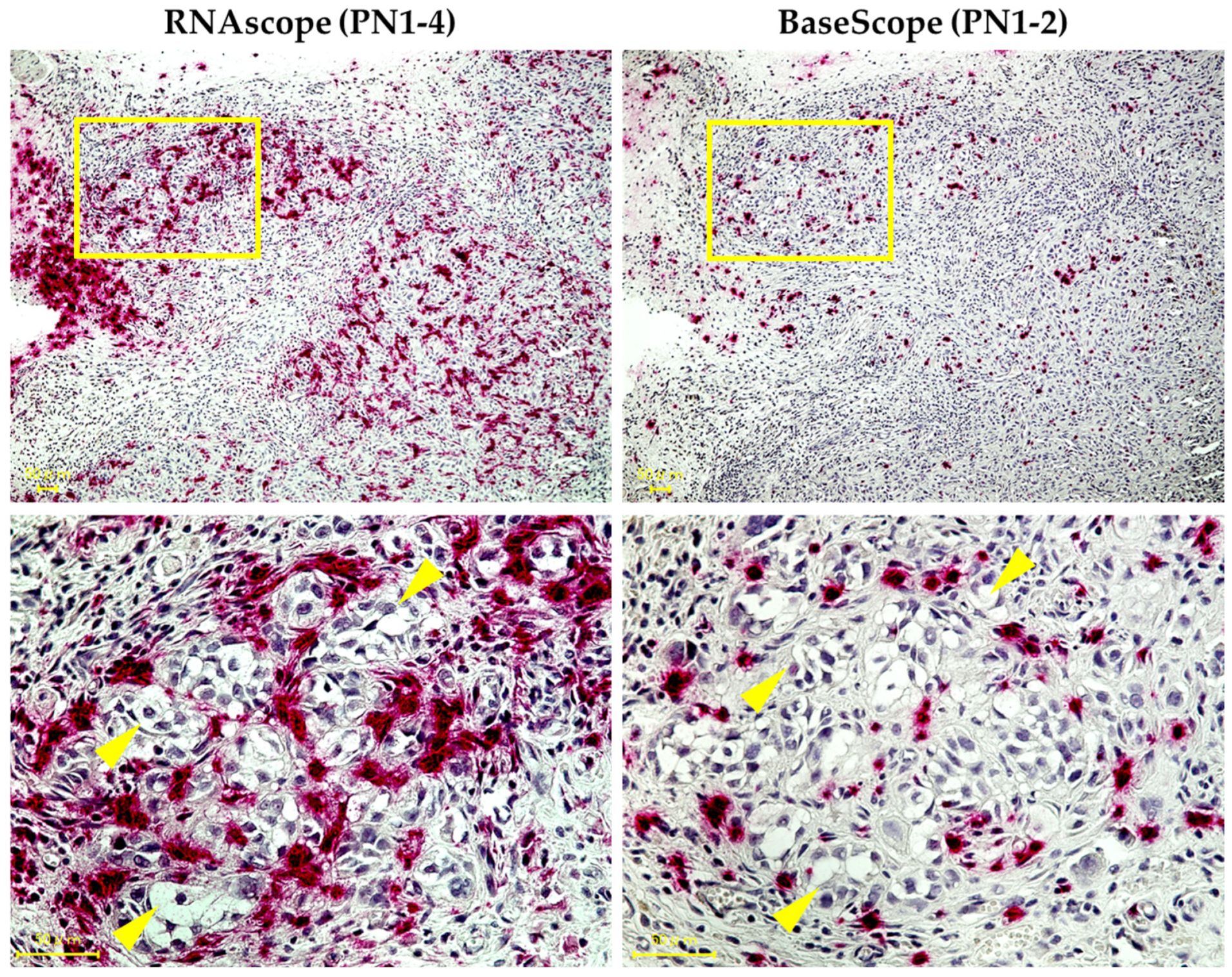
3.1. Expression Pattern of PN1-4 and PN1-2 in Human TSCC
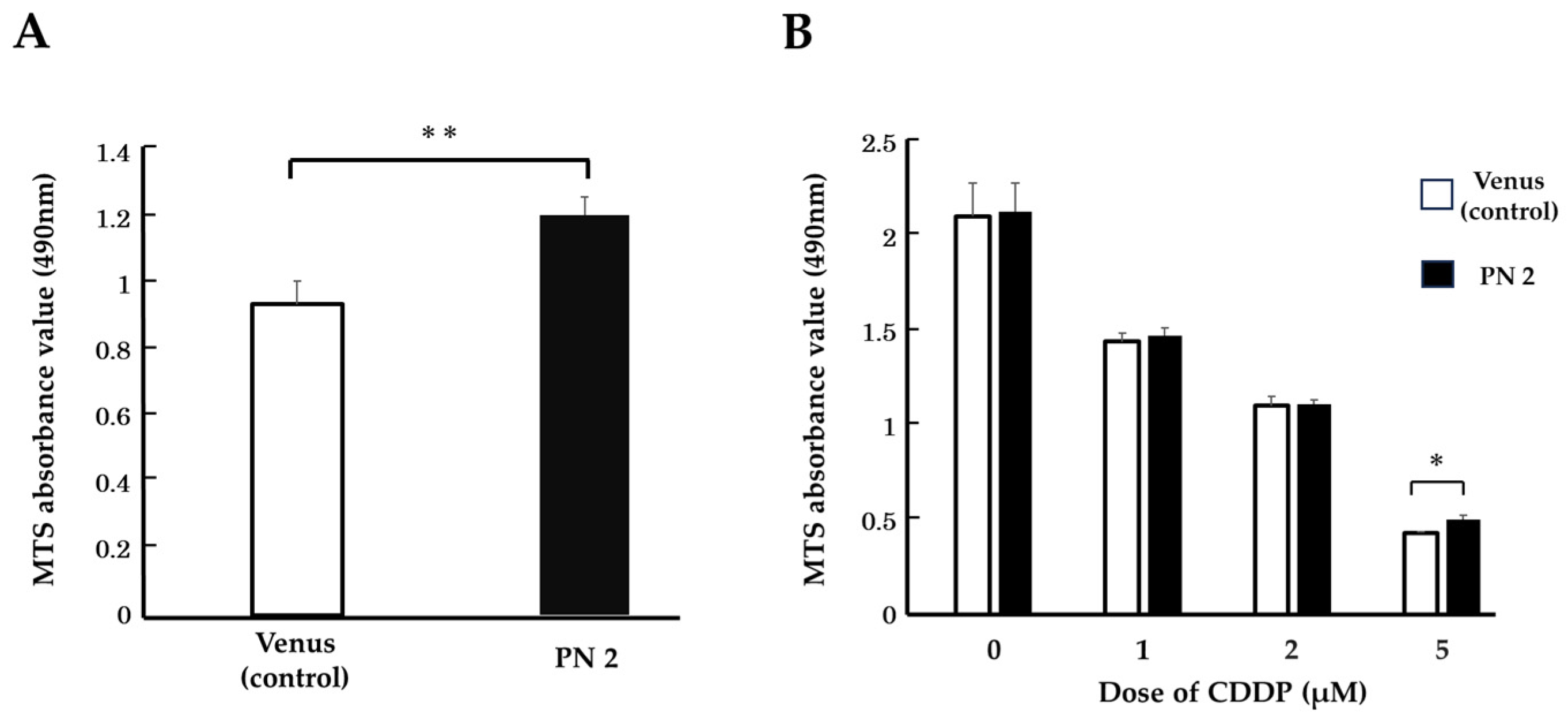
3.2. Effect of PN2 on Tongue Cancer Cell Viability
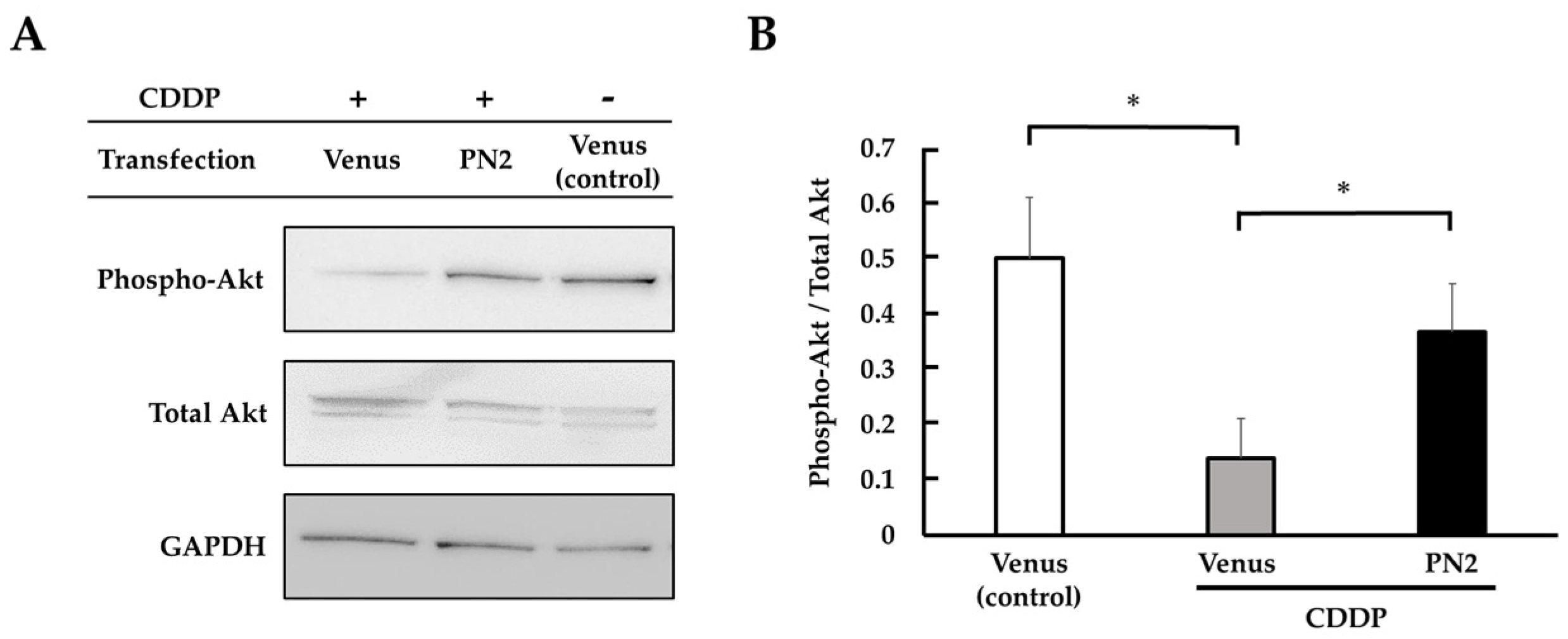
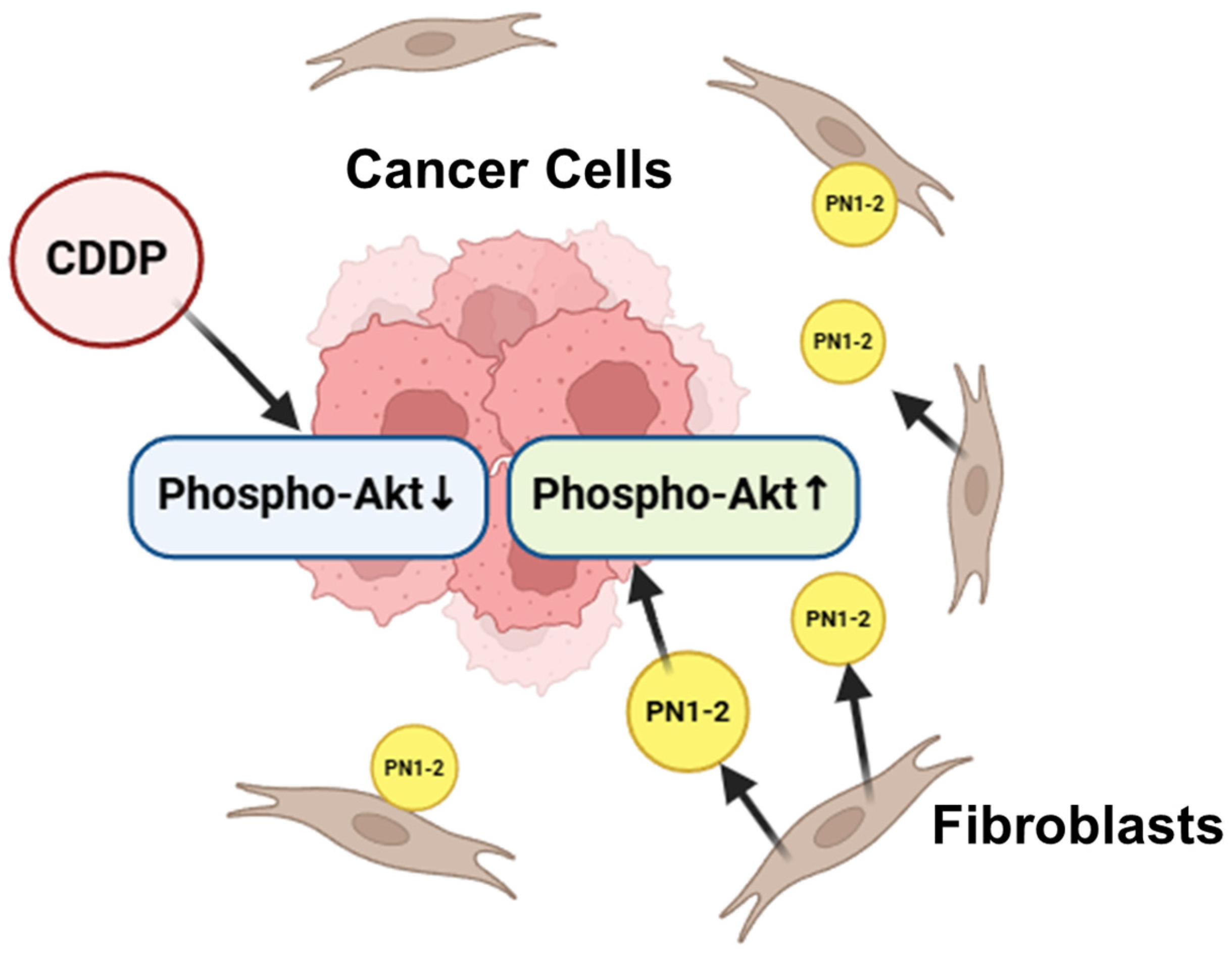
3.3. Activation of Akt by PN2 in Tongue Cancer Cells
3.4. Expression Pattern of PN1-4 and PN1-2 in an HSC-3 Xenograft Model
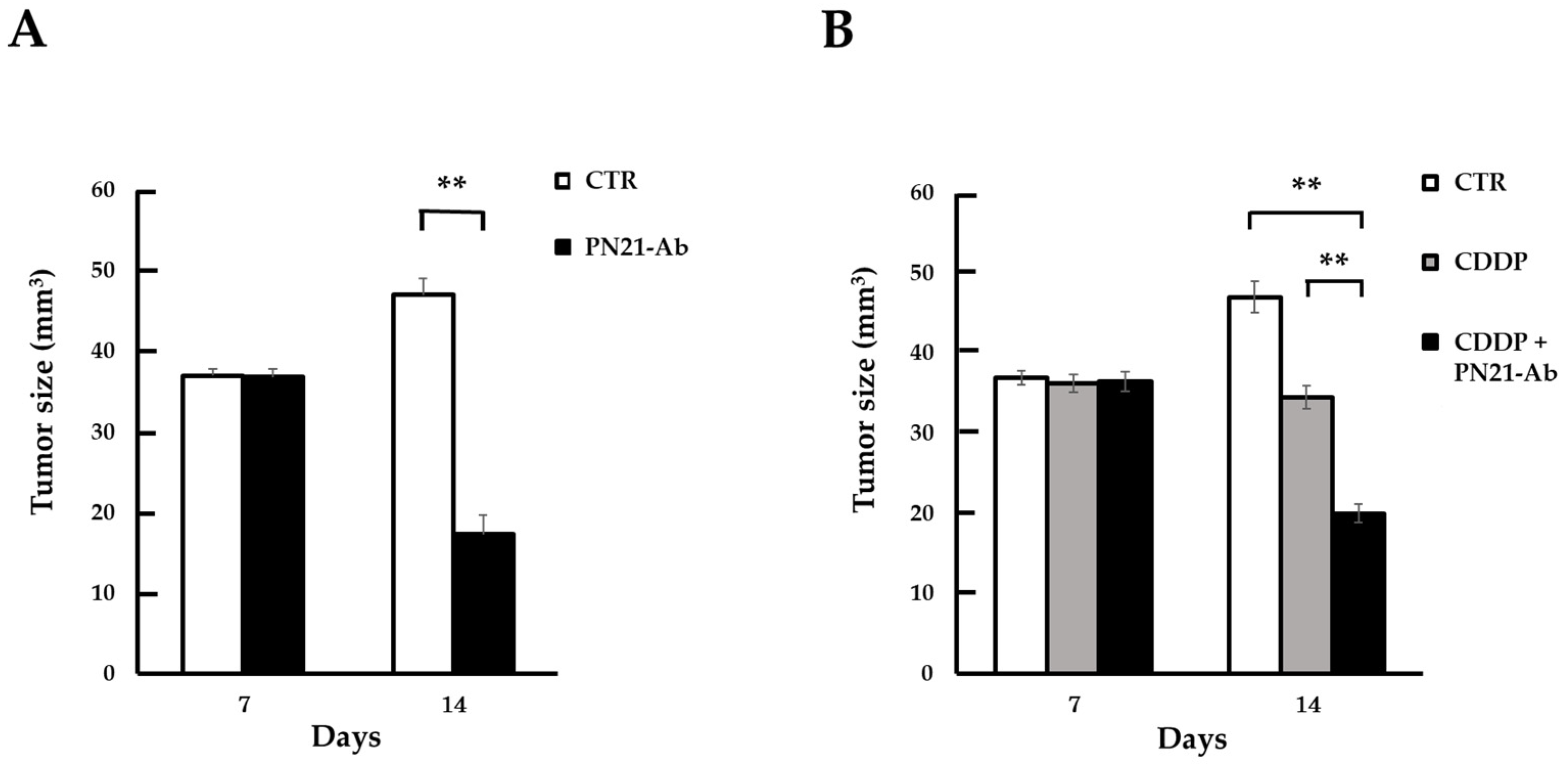
3.5. Effect of PN21-Ab
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| HNSCC | head and neck squamous cell carcinoma |
| TSCC | tongue squamous cell carcinoma |
| HSC-3 | human oral squamous carcinoma cell line-3 |
| 5-FU | 5-fluorouracil |
| POSTN | periostin |
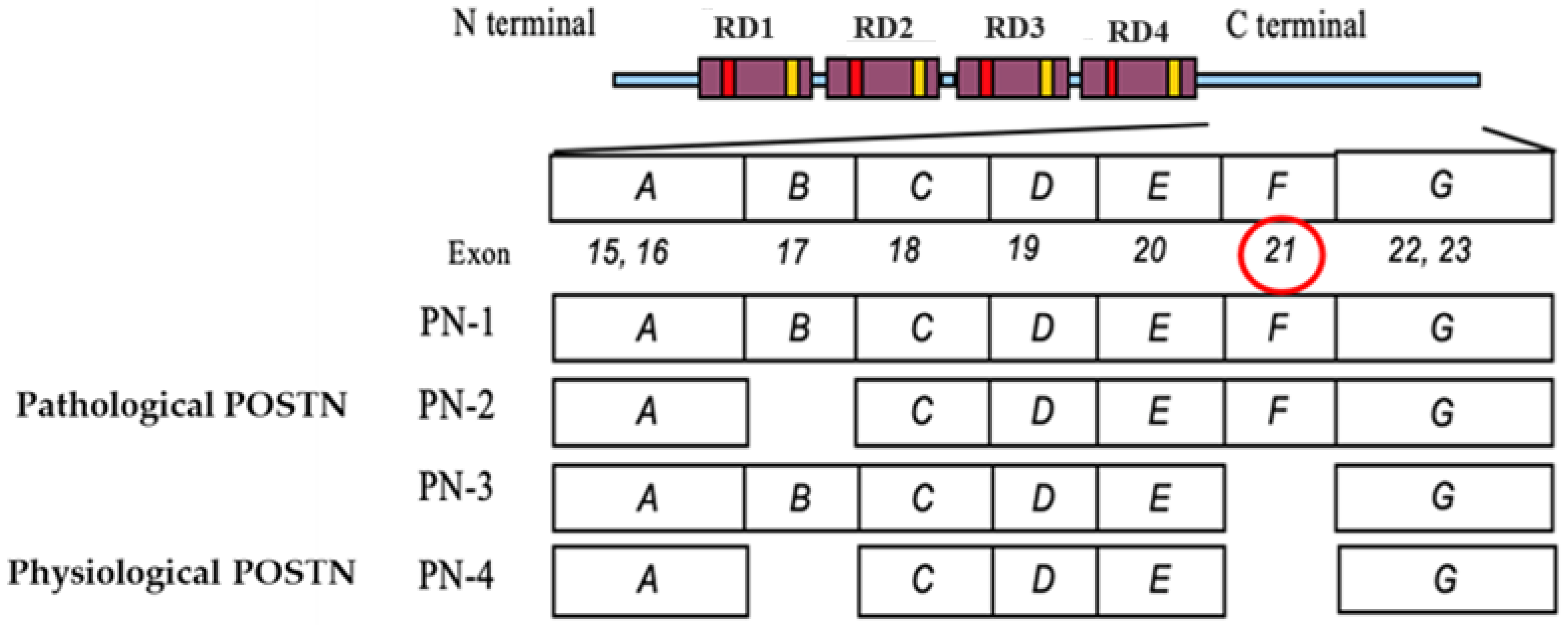
| PN1 | periostin 1, full-length periostin |
| PN2 | periostin 2, lack of periostin exon 17 |
| PN3 | periostin 3, lack of periostin exon 21 |
| PN4 | periostin 4, lack of periostin exon 17 and 21 |
| PN1-2 | periostin 1 and 2, periostin with exon 21 |
| PN1-4 | all periostin splicing variants, periostin 1, 2, 3 and 4 |
| Ab | antibody |
| PN21-Ab | periostin exon 21 specific neutralizing antibody |
| RPMI | Rosewell Park Memorial Institute |
| RIPA | radioimmunoprecipitation buffer |
| TBST | tris-buffered saline with tween 20 |
| vol | volume |
| FBS | fetal bovine serum |
| ISH | in situ hybridization |
| CDDP | cisplatin |
| Akt | PKB, protein kinase B |
| PVDF | poly vinyli dene fluoride |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| BALB/c | balb/c.PKAchu |
| HE | hematoxylin–eosin |
| FAK | focal adhesion kinase |
| PI3K | phosphoinositide 3-kinase |
References
- Mao, L.; Hong, W.K.; Papadimitrakopoulou, V.A. Focus on head and neck cancer. Cancer Cell 2004, 5, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zheng, Z.; Yuan, Y.; Pathak, J.L.; Yang, X.; Wang, L.; Ye, Z.; Cho, W.C.; Zeng, M.; Wu, L. The Emerging Role of Exosomes in Oral Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 628103. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.; Eu, D.; Loh, T.; Ahmed, Q.; Lim, C.M. Histopathologic prognostic indices in tongue squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.O.; Soini, Y.; Salo, T. Prognostic evaluation of oral tongue cancer: Means, markers and perspectives (I). Oral Oncol. 2010, 46, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.O.; Soini, Y.; Salo, T. Prognostic evaluation of oral tongue cancer: Means, markers and perspectives (II). Oral Oncol. 2010, 46, 636–643. [Google Scholar] [CrossRef]
- Omura, K. Current status of oral cancer treatment strategies: Surgical treatments for oral squamous cell carcinoma. Int. J. Clin. Oncol. 2014, 19, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xu, C.; Yu, D. Mechanisms correlated with chemotherapy resistance in tongue cancers. J. Cancer Res. Ther. 2018, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, H.; Qiu, N.; Huang, X.; Li, H. Periostin drives extracellular matrix degradation, stemness, and chemoresistance by activating the MAPK/ERK signaling pathway in triple–negative breast cancer cells. Lipids Health Dis. 2023, 22, 153. [Google Scholar] [CrossRef]
- Takatsu, F.; Suzawa, K.; Tomida, S.; Thu, Y.M.; Sakaguchi, M.; Toji, T.; Ohki, M.; Tsudaka, S.; Date, K.; Matsuda, N.; et al. Periostin secreted by cancer-associated fibroblasts promotes cancer progression and drug resistance in non-small cell lung cancer. J. Mol. Med. 2023, 101, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Gao, F.; Xing, L.; Qin, P.; Liang, X.; Zhang, J.; Qiao, X.; Lin, L.; Zhao, Q.; et al. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumor Biol. 2016, 37, 15283–15291. [Google Scholar] [CrossRef]
- Taniyama, Y.; Katsuragi, N.; Sanada, F.; Azuma, J.; Iekushi, K.; Koibuchi, N.; Okayama, K.; Ikeda-Iwabu, Y.; Muratsu, J.; Otsu, R.; et al. Selective Blockade of Periostin Exon 17 Preserves Cardiac Performance in Acute Myocardial Infarction. Hypertension 2016, 67, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Taniyama, Y.; Sanada, F.; Morishita, R.; Nakamori, S.; Morimoto, K.; Yeung, K.T.; Yang, J. Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci. Rep. 2018, 8, 4013. [Google Scholar] [CrossRef]
- Fujikawa, T.; Sanada, F.; Taniyama, Y.; Shibata, K.; Katsuragi, N.; Koibuchi, N.; Akazawa, K.; Kanemoto, Y.; Kuroyanagi, H.; Shimazu, K.; et al. Periostin exon-21 antibody neutralization of triple-negative breast cancer cell-derived periostin regulates tumor-associated macrophage polarization and angiogenesis. Cancers 2021, 13, 5072. [Google Scholar] [CrossRef]
- Nakama, T.; Yoshida, S.; Ishikawa, K.; Kobayashi, Y.; Abe, T.; Kiyonari, H.; Shioi, G.; Katsuragi, N.; Ishibashi, T.; Morishita, R.; et al. Different roles played by periostin splice variants in retinal neovascularization. Exp. Eye Res. 2016, 153, 133–140. [Google Scholar] [CrossRef]
- Kudo, Y.; Iizuka, S.; Yoshida, M.; Nguyen, P.T.; Siriwardena, S.B.S.M.; Tsunematsu, T.; Ohbayashi, M.; Ando, T.; Hatakeyama, D.; Shibata, T.; et al. Periostin Directly and Indirectly Promotes Tumor Lymphangiogenesis of Head and Neck Cancer. PLoS ONE 2012, 7, e0044488. [Google Scholar] [CrossRef]
- Wenhua, S.; Tsunematsu, T.; Umeda, M.; Tawara, H.; Fujiwara, N.; Mouri, Y.; Arakaki, R.; Ishimaru, N.; Kudo, Y. Cancer cell-derived novel periostin isoform promotes invasion in head and neck squamous cell carcinoma. Cancer Med. 2023, 12, 8510–8525. [Google Scholar] [CrossRef] [PubMed]
- Kyutoku, M.; Taniyama, Y.; Katsuragi, N.; Shimizu, H.; Kunugiza, Y.; Iekushi, K.; Koibuchi, N.; Sanada, F.; Oshita, Y.; Morishita, R. Role of periostin in cancer progression and metastasis: Inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int. J. Mol. Med. 2011, 28, 181–186. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Hu, M.; Dai, M.H.; Xiong, J.; Zhang, S.; Wu, H.J.; Zhang, S.S.; Gong, Z.J. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/beta-catenin signaling pathway. Mol. Cancer 2018, 17, 3. [Google Scholar] [CrossRef]
- Tang, Q.; Cheng, B.; Xie, M.; Chen, Y.; Zhao, J.; Zhou, X.; Chen, L. Circadian clock gene Bmal1 inhibits tumorigenesis and increases paclitaxel sensitivity in tongue squamous cell carcinoma. Cancer Res. 2017, 77, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Li, Q.L.; Pan, Q.; Zhang, H.Y.; Fu, X.Y.; Yao, F.; Wang, J.N.; Yang, A.K. Xenotropic and polytropic retrovirus receptor 1 (XPR1) promotes progression of tongue squamous cell carcinoma (TSCC) via activation of NF-κB signaling. J. Exp. Clin. Cancer Res. 2019, 38, 167. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.F.; Oner, M.; Abay, A.; Diyapoglu, A. MicroRNAs in human tongue squamous cell carcinoma: From pathogenesis to therapeutic implications. Oral Oncol. 2017, 67, 124–130. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, Q.; Liu, C.; Qiu, J. Plumbagin has an inhibitory effect on the growth of TSCC PDX model and it enhances the anticancer efficacy of cisplatin. Aging 2023, 15, 12225–12250. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.F.; Chen, X.J.; Liang, L.J.; Yu, L.; Wu, X.G.; Zhou, C.F.; Wang, Z.C.; Fan, L.S.; Hu, Z.; Liang, L.; et al. Periostin+cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 2021, 15, 210–227. [Google Scholar] [CrossRef]
- Bernardes, S.S.; Pinto, M.C.X.; Amorim, J.H.; Azevedo, V.A.D.C.; Resende, R.R.; Mintz, A.; Birbrair, A. Glioma Pericytes Promote Angiogenesis by Producing Periostin. Cell. Mol. Neurobiol. 2022, 42, 557–564. [Google Scholar] [CrossRef]
- Keklikoglou, I.; Kadioglu, E.; Bissinger, S.; Langlois, B.; Bellotti, A.; Orend, G.; Ries, C.H.; De Palma, M. Periostin Limits Tumor Response to VEGFA Inhibition. Cell Rep. 2018, 22, 2530–2540. [Google Scholar] [CrossRef]
- González-González, L.; Alonso, J. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Kudo, A. Periostin; Springer: Berlin/Heidelberg, Germany, 2019; p. 1132. [Google Scholar]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.-F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Yin, P.; Chen, J.; Wu, Y.; Gao, F.; Wen, J.; Zhang, W.; Su, Y.; Zhang, X. Chemoprevention of 4NQO-Induced Mouse Tongue Carcinogenesis by AKT Inhibitor through the MMP-9/RhoC Signaling Pathway and Autophagy. Anal. Cell. Pathol. 2022, 2022, 3770715. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Satoh, K.; Masamune, A.; Hirota, M.; Kimura, K.; Umino, J.; Hamada, S.; Satoh, A.; Egawa, S.; Motoi, F.; et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int. J. Cancer 2008, 122, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Ben, Q.W.; Zhao, Z.; Ge, S.F.; Zhou, J.; Yuan, F.; Yuan, Y.Z. Circulating levels of periostin may help identify patients with more aggressive Colorectal cancer. Int. J. Oncol. 2009, 34, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, C.; Li, D.; Xie, Y.; Shi, J.; Li, G.; Guan, Y.; Li, M.; Zhang, P.; Peng, F.; et al. Periostin, a stroma-associated protein, correlates with tumor invasiveness and progression in nasopharyngeal carcinoma. Clin. Exp. Metastasis 2012, 29, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak-Wielgomas, K.; Grzegrzolka, J.; Piotrowska, A.; Gomulkiewicz, A.; Witkiewicz, W.; Dziegiel, P. Periostin expression in cancer-associated fibroblasts of invasive ductal breast carcinoma. Oncol. Rep. 2016, 36, 2745–2754. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Kunita, A.; Iwata, C.; Komura, D.; Nishiyama, T.; Shimazu, K.; Takeshita, K.; Shibahara, J.; Kii, I.; Morishita, Y.; et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 2014, 184, 859–870. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kashima, T.G.; Nishiyama, T.; Shimazu, K.; Morishita, Y.; Shimazaki, M.; Kii, I.; Horie, H.; Nagai, H.; Kudo, A.; et al. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J. Histochem. Cytochem. 2018, 56, 753–764. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikebe, S.; Koibuchi, N.; Shibata, K.; Sanada, F.; Shimizu, H.; Takenobu, T.; Taniyama, Y. Experimental Study: The Development of a Novel Treatment for Chemotherapy-Resistant Tongue Cancer with the Inhibition of the Pathological Periostin Splicing Variant 1-2 with Exon 21. Cells 2024, 13, 1341. https://doi.org/10.3390/cells13161341
Ikebe S, Koibuchi N, Shibata K, Sanada F, Shimizu H, Takenobu T, Taniyama Y. Experimental Study: The Development of a Novel Treatment for Chemotherapy-Resistant Tongue Cancer with the Inhibition of the Pathological Periostin Splicing Variant 1-2 with Exon 21. Cells. 2024; 13(16):1341. https://doi.org/10.3390/cells13161341
Chicago/Turabian StyleIkebe, Shoji, Nobutaka Koibuchi, Kana Shibata, Fumihiro Sanada, Hideo Shimizu, Toshihiko Takenobu, and Yoshiaki Taniyama. 2024. "Experimental Study: The Development of a Novel Treatment for Chemotherapy-Resistant Tongue Cancer with the Inhibition of the Pathological Periostin Splicing Variant 1-2 with Exon 21" Cells 13, no. 16: 1341. https://doi.org/10.3390/cells13161341





