Abstract
Specific cancer therapy remains a problem to be solved. Breast and colorectal cancer are among the cancers with the highest prevalence and mortality rates. Although there are some therapeutic options, there are still few effective agents for those cancers, which constitutes a clinical problem that requires further research efforts. Lysosomes play an important role in cancer cells’ survival, and targeting lysosomes has gained increased interest. In recent years, our team has been synthetizing and testing novel benzo[a]phenoxazine derivatives, as they have been shown to possess potent pharmacological activities. Here, we investigated the anticancer activity of three of the most potent derivatives from our library, C9, A36, and A42, on colorectal- and breast-cancer-derived cell lines, and compared this with the effect on non-neoplastic cell lines. We observed that the three compounds were selective for the cancer cells, namely the RKO colorectal cancer cell line and the MCF7 breast cancer cell line. In both models, the compounds reduced cell proliferation, cell survival, and cell migration, accumulated on the lysosome, and induced cell death accompanied by lysosomal membrane permeabilization (LMP), increasing the intracellular pH and ROS accumulation. Our results demonstrated that these compounds specifically target lysosomes from cancer cells, making them promising candidates as LMP inducers for cancer therapy.
1. Introduction
Globally, the burden of cancer mortality and incidence is rising quickly, to the extent that cancer is now considered to be the leading cause of death and the most important barrier to increasing life expectancy around the world [1]. In the top three most commonly diagnosed cancers, breast cancer ranks first and colorectal cancer ranks third, with breast cancer being the leading cause of cancer-related death in women and colorectal cancer being the second leading cause of cancer-related death in both sexes [2].
Both colorectal and breast cancer are highly heterogeneous diseases with different risk factors, treatment outcomes, and prognoses depending on their molecular subtypes [3,4,5].
The current treatment options for these patients include surgery, radiation, chemotherapy, immunologic therapy, and some targeted therapies, but with limited results. Tumor resistance and severe side effects continue to be major therapeutic limitations, despite recent efforts to develop new therapies leading to improved survival rates. This highlights the need for the discovery of new, effective drugs [6,7,8,9].
In this field, one of the primary drivers in the discovery of drugs for therapeutical applications is the synthesis of novel organic compounds [10,11,12]. Among these, phenoxazine derivatives are of great interest, as they have been reported to possess a variety of pharmacological properties, such as antimicrobial, antifungal, antiviral, and antitumor activities [13,14,15,16,17,18,19]. Within the family of phenoxazine derivatives, benzo[a]phenoxazines have gained importance because, in addition to their fluorescent properties, they have shown potent antitumor activity [20,21,22,23]. However, information about their targets and mechanism of action is still very limited.
Our research team has been focused on synthesizing new benzo[a]phenoxazine derivatives, evaluating their pharmacological activity, and elucidating their mechanisms of action in order to assess their potential applications. To classify the most potent synthetized compounds, we applied a comparative approach involving an evaluation of their biological activity by determining their MIC (minimum inhibitory concentration), using a standard microdilution method for activity testing with the yeast S. cerevisiae as a eukaryotic cell model [17,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. We further evaluated one of our compounds, BaP1 (a lead compound displaying an MIC of 25 μM), against colorectal cancer cells, and discovered that it had promising anticancer activity. We discovered that this effect was directly related to its lysosomal accumulation and lysosomal membrane permeabilization [39].
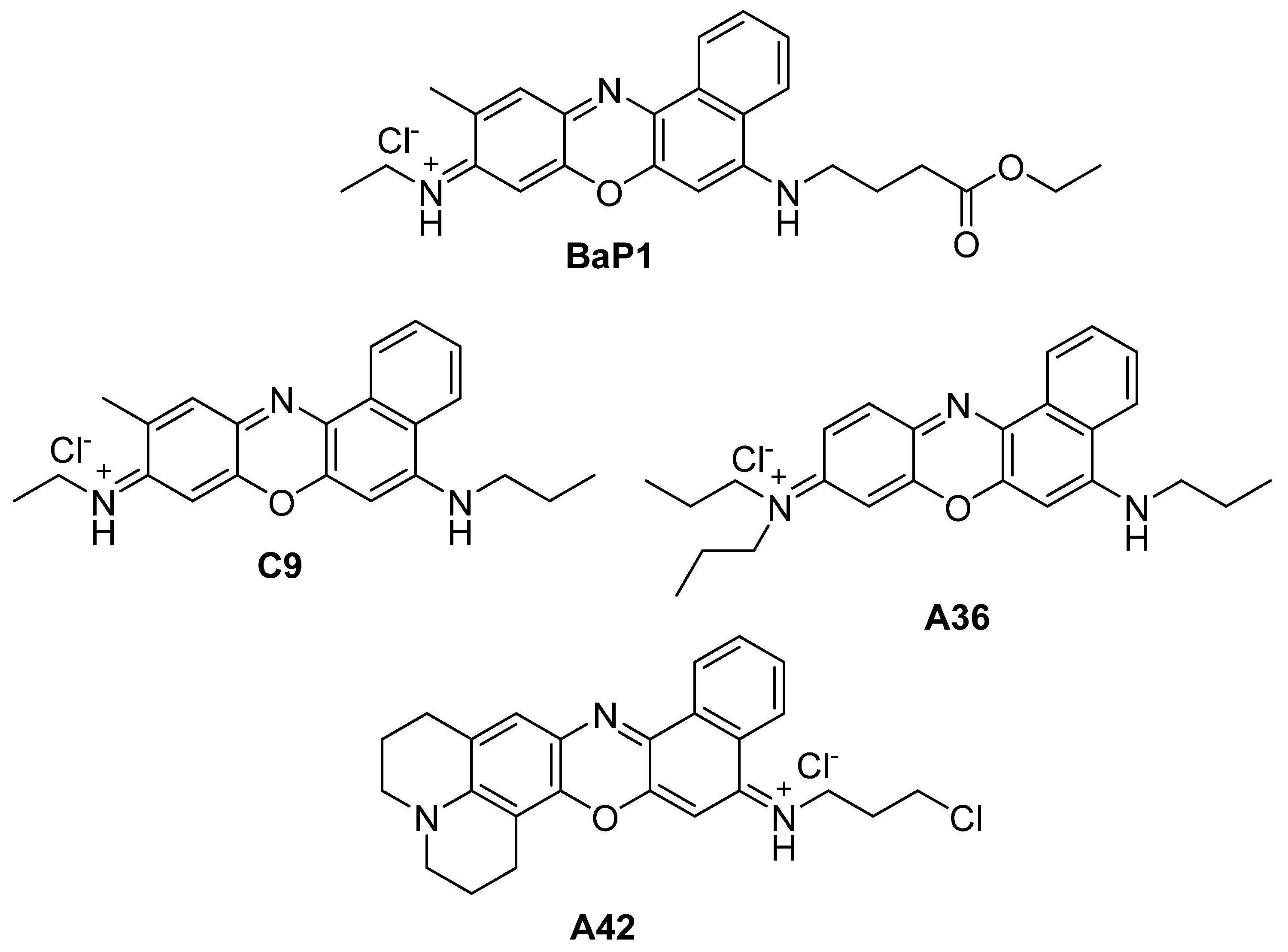
In the present work, we evaluate the anticancer potential of the most potent compounds from our library, C9, A36, and A42 with MICs of 6.25 μM, 1.56 μM, and 0.78 μM, respectively (Figure 1) [31,40,41,42,43]. Our aim is to uncover their anticancer activity not only against colorectal cancer, but also test their potential against breast cancer cells, as well as to evaluate their intracellular targets.
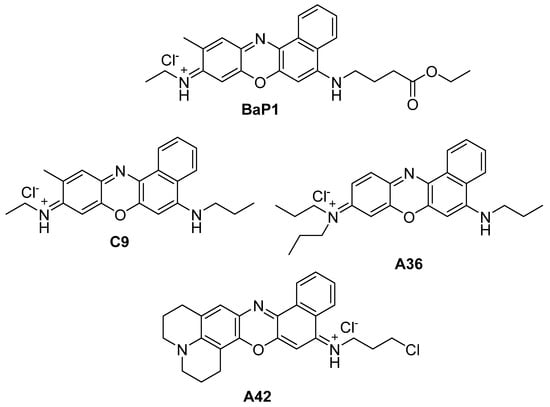
Figure 1.
Chemical structures of BaP1, C9, A36, and A42.
2. Materials and Methods
2.1. Compounds, Cell Lines, and Culture Conditions
The compounds C9, A36, and A42 were synthesized by our research team according to previously published experimental procedures [25,40,41,42,43]. 1H NMR spectra were used to confirm the structure and purity of each compound. It was verified that all compounds were >95% pure by 1H NMR.
We used two non-neoplastic cell lines, NCM460 cells derived from healthy mucosal epithelium and BJ-5ta fibroblast cells isolated from the foreskin of a male patient, obtained from InCell, San Antonio, TX, USA and ATCC (American Type Culture Collection), Manassas, VA, USA respectively. We also used three CRC-derived cell lines, RKO, SW480, and HCT116, and two breast-cancer-derived cell lines, MDA-MB-231 and MCF7, all obtained from ATCC.
The NCM460 and SW480 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 media containing stable glutamine (Biowest, Nuaillé, France), 1% penicillin–streptomycin (Biowest), and 10% heat-inactivated fetal bovine serum (FBS; Gibco, Invitrogen, Carlsbad, CA, USA). The HCT116 cells were grown in McCoy’s 5A Medium (Biowest) containing 1% penicillin–streptomycin and 10% heat-inactivated FBS (Gibco, Invitrogen). The RKO, MDA-MB-231, and MCF7 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) High Glucose (Biowest), which was supplemented with 1% penicillin–streptomycin (Biowest) and 10% heat-inactivated FBS. The BJ-5ta cells were grown in a 4:1 combination of DMEM (Biowest) and Medium 199 (Biowest), supplemented with 0.01 mg/mL of hygromycin (Sigma, Burlington, MA, USA) and 10% heat-inactivated FBS (Gibco, Invitrogen). The cell lines were plated onto 25 or 75 cm3 tissue culture flasks and maintained in a humidified incubator with 5% CO2 at 37 °C.
2.2. Sulforhodamine B Assay
To calculate their IC50 values, the various cell lines were plated into 24-well plates at a density sufficient to achieve 80% confluence after 48 h of growth under normal conditions (no treatment, in the respective culture medium) as follows: 4 × 104 cells/mL (RKO and MDA-MB-231), 5 × 104 cells/mL (HCT116), 6 × 104 cells/mL (MCF7), 8 × 104 cells/mL (BJ-5ta), 1.75 × 105 cells/mL (SW480), and 3 × 105 cells/mL (NCM460). The cells were allowed to adhere overnight, from 12 h to 18 h, and then treated with increasing concentrations of each compound, C9, A36, and A42 (0.125 μM, 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM), in their respective culture mediums for 48 h. In addition to a negative control that consisted only of cells and growth medium, a negative control containing the highest concentration of the solvent in which the compound was dissolved (DMSO 0.1%) was also used. The SRB assay was performed according to Marques et al. [44]. The IC50 values were obtained using GraphPad Prism version 8.2.1 through the application of a sigmoidal dose–response nonlinear regression, after logarithmic transformation. The selectivity index was determined by dividing the IC50 value of the non-neoplastic BJ-5ta cell line by the IC50 value of the cancer cell lines (IC50 BJ-5ta/IC50 cancer cell line); furthermore, for the CRC model, a second selectivity index was also determined using the non-neoplastic NCM460 cell line (IC50 NCM460/IC50 CRC cell line). According to the results of the selectivity index, the cell line with the highest selectivity value for each of the models, CRC and breast, was selected for subsequent characterization.
2.3. Cell Proliferation by CFSE
RKO and MCF7 cell proliferation was evaluated through flow cytometry using the Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) probe. Confluent cells were collected, rinsed with PBS 1×, and incubated with 5 μM of CFSE for 15 min at 37 °C. Then, the cells were rinsed, resuspended in growth medium, and seeded in 12-well plates at 1 × 105 cells/mL. After 24 h, adherent cells were treated with compounds C9, A36, and A42 at IC50 and 2 × IC50 concentrations or 0.1% DMSO (vehicle). Untreated cells were used as controls. The cells were harvested at different time points of 0 h, 24 h, 28 h, and 72 h. The cells were then analyzed in a flow cytometer and the CFSE median fluorescence intensity was quantified using the FITC-A channel. All median values were normalized to the time point 0 h.
2.4. Colony Formation Assay
A colony formation assay was conducted on the RKO and MCF7 cell lines. In 6-well plates, 600 cells/mL and 800 cells/mL RKO and MCF7 cells, respectively, were seeded. Adherent cells were treated with the compounds C9, A36, and A42 at IC50/2 and IC50 concentrations or 0.1% DMSO (vehicle). As a control, untreated cells were used. Following a 48 h incubation period, the cells were twice rinsed with PBS 1× before being supplemented with new medium. After that, the cells were given 10–14 days to grow (new medium was added every 3 days). Following a PBS 1× rinse, the colonies were fixed for 30 min using 0.5% crystal violet and 6% glutaraldehyde. The ImageJ software (V1.53) was used to calculate the number of colonies. The percentage of colonies was determined and normalized for the untreated control.
2.5. Wound Healing Assessment Assay
RKO and MCF7 cells were seeded in 12-well plates at densities of 5 × 105 cells/mL and 4.5 × 105 cells/mL, respectively. After 24 h, a wound was created by scraping the cell layer using a 1 mm pipette tip. The cells were treated with C9, A36, and A42 at IC50 and 2 × IC50 concentrations or 0.1% DMSO (vehicle). Untreated cells were used as controls. The wound regions were photographed at 0 and 12 h, and migration was assessed using the Image J software. The relative migration was calculated in relation to the untreated controls.
2.6. Annexin V/PI Assay
RKO and MCF7 cells were seeded in 12-well plates at a density of 1 × 105 cells/mL. After 24 h, the cells were treated with C9, A36, and A42 at 2 × IC50 and 4 × IC50 concentrations or 0.1% DMSO (vehicle). Untreated cells were used as controls. After 48 h, floating and adherent cells were collected. The cells were resuspended in 100 mL of binding buffer and treated for 15 min in the dark with 5 μL of Annexin V-FITC (Detection Kit-ab14085) and 5 μL of Propidium Iodide (50 μg/mL). The samples were subjected to flow cytometry analysis, PI fluorescence was detected using the ECD-A channel, and Annexin V fluorescence was detected using the FITC-A channel.
2.7. Evaluation of ROS Levels
The RKO and MCF7 ROS levels were evaluated through flow cytometry using the dihydroethidium (DHE) probe. RKO and MCF7 cells were seeded in 12-well plates at a density of 1 × 105 cells/mL. After 24 h, the cells were treated with the compounds C9, A36, and A42 at 2 × IC50 and 4 × IC50 concentrations or 0.1% DMSO (vehicle). As a control, untreated cells were used. The cells were collected after 48 h of treatment, washed with PBS 1×, and stained for 30 min at 37 °C in the dark using 0.5 μM DHE. The DHE mean fluorescence intensity was analyzed by flow cytometry using the PE-A channel.
2.8. Fluorescence Staining Evaluation
For fluorescence staining observations, RKO and MCF7 cells were co-stained with the compounds and with LysoSensor Green DND-189 and DAPI. The RKO and MCF7 cells were plated on microscopy slides with a density of 1.5 × 105 cells/mL and allowed to adhere for 24 h. On the next day, the cells were exposed to C9, A36, and A42 IC50 for 3 h. The cells were washed and resuspended in PBS 1× and then stained for 30 min at 37 °C with 2 μM of LysoSensor Green. The cells stained with LysoSensor Green were co-stained with DAPI at a final concentration of 10 μg/mL. The Olympus BX6F2 microscope (40× and 60× oil immersion objectives) (Tokyo, Japan) was utilized to analyze the samples. It was fitted with the appropriate filter cubes, which included U-FDICT (differential interference contrast), TLV-U-FF-FITC (green), U-FYW (far-red), and U-FUNA (blue).
2.9. Lysosomal Membrane Permeabilization Assessment
RKO and MCF7 lysosomal membrane permeabilization (LMP) was evaluated through flow cytometry using the Acridine Orange (A.O) probe. RKO and MCF7 cells were seeded in 12-well plates at a density of 1 × 105 cells/mL. After 24 h, the cells were treated with the compounds C9, A36, and A42 at 2 × IC50 and 4 × IC50 concentrations or 0.1% DMSO (vehicle). As a control, untreated cells were employed. Following a 48 h period, the adherent and floating cells were collected and subjected to a 15-min dark incubation period at 37 °C with 1 μM A.O. After that, the samples were examined by flow cytometry using the PC5.5 channel.
2.10. Intracellular pH Evaluation
Evaluations of the intracellular pHs (pHi) of the RKO and MCF7 cells were performed through a flow cytometry optimized protocol using the pH-sensitive probe BCECF-AM. RKO and MCF7 cells were seeded in 12-well plates at a density of 1 × 105 cells/mL. After 24 h, the cells were treated with the compounds C9, A36, and A42 at 2 × IC50 and 4 × IC50 concentrations or 0.1% DMSO (vehicle). As a control, untreated cells were used. Attached and floating cells were collected, washed, and then resuspended in Hank’s balanced salt solution (HBSS) after a 48 h treatment period. After that, the cells were stained with 1 μM of BCECF-AM for 30 min at 37 °C. The samples were analyzed by flow cytometry. The FITC-A and PE-A channels were used to detect the fluorescence mean of BCECF-AM. The percentage of cells exhibiting intracellular acidification was estimated from the percentage of cells displaying an FITC-A/PE-A ratio lower than the control cells.
2.11. Fluorescence Microscopy and Flow Cytometry General Considerations
An Olympus BX6F2 microscope equipped with filter cubes U-FDICT (differential interference contrast), U-FYW (far-red), U-FGNA (red), and U-FUNA (blue) together with a 40× and 60× oil immersion objective was used for examining the microscopy samples. The Olympus ImageLS software (V2.2) was used to process the photos.
A Beckman Coulter Cytoflex System B4-R2-V0 flow cytometer fitted with a 488 nm solid-state laser (50 mW), FS, SS, FITC (525/40 BP), PE (585/42), ECD (610/20 BP), and PC5.5 (690/50 BP) channels was used to evaluate the flow cytometry samples. Each sample was examined, and twenty thousand cells, at a low flow rate, were considered. The flow cytometric analysis was carried out using the CytExpert Data software (V2.6).
2.12. Data Analysis
Results from at least three independent experiments are presented as means ± SD. Dunnett’s post-test was used in conjunction with one-way or two-way ANOVA to assess the data. p-values < 0.05 were considered to be statistically significant. Statistical analyses were carried out on macOS using GraphPad Prism 8.2.1.
3. Results and Discussion
3.1. Biological Activity of the Benzo[a]phenoxazine Compounds C9, A36, and A42
3.1.1. Effect of C9, A36, and A42 on Cell Viability
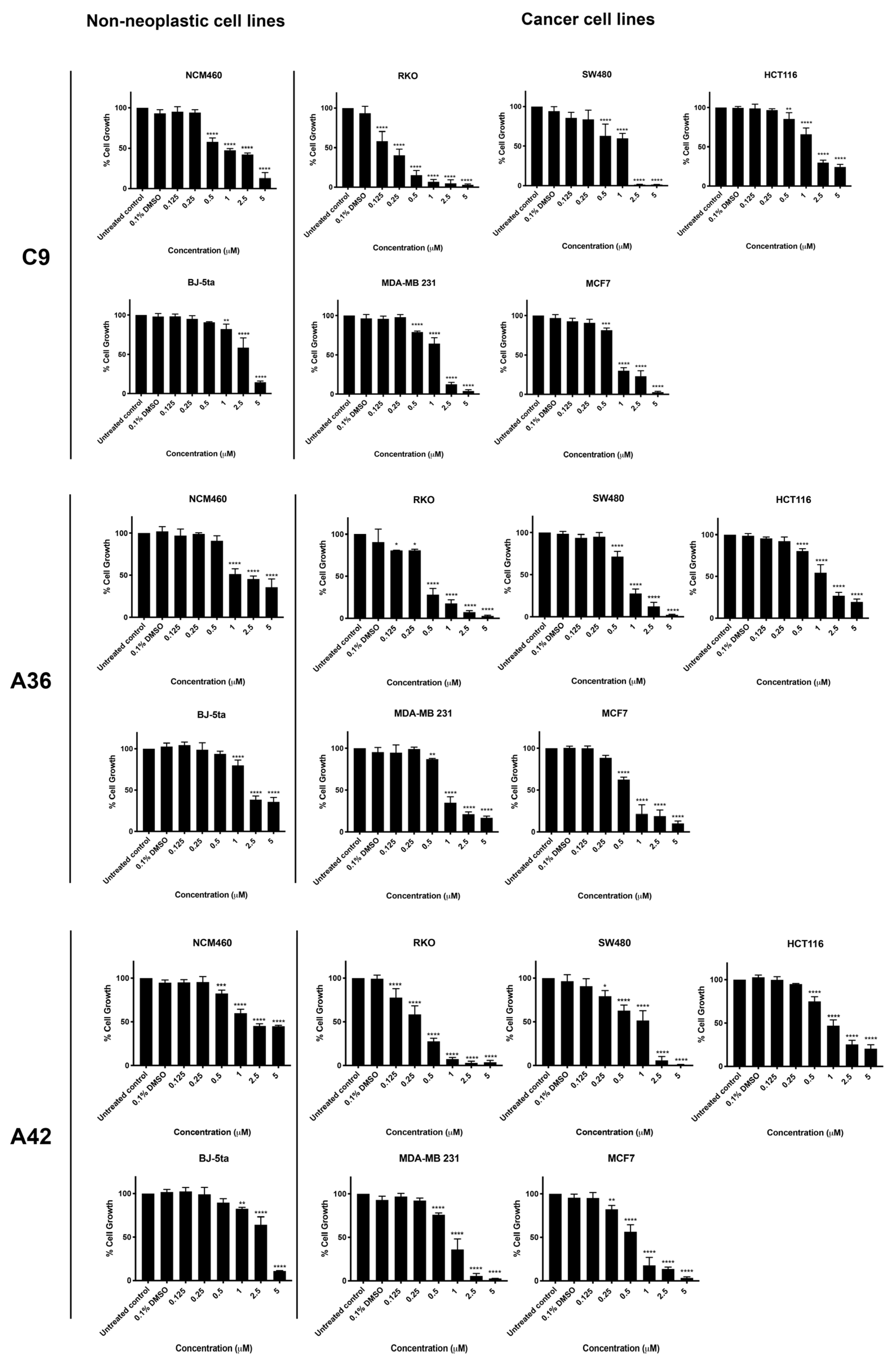
Three CRC-derived cell lines, SW480 (KRASG12V), HCT116 (KRASG13D), and RKO (BRAFV600E), harboring somatic mutations on KRAS or BRAF, and two breast-cancer-derived cell lines, MDA-MB-231 (basal like) and MCF7 (luminal A subtype), together with two non-neoplastic cell lines, NCM460 (normal colon mucosal epithelial cell line) and BJ-5ta (normal foreskin fibroblast cell line), were used to study the activity and selectivity of the three new benzo[a]phenoxazine compounds, C9, A36, and A42. All cell lines were treated with a range of drug concentrations (0.125 μM, 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM) for 48 h, and the effect on cell viability was assessed using a sulforhodamine B assay (SRB) (Figure 2). The results were used to determine the IC50 value of each compound for all the cell lines (Table 1). In addition, the selectivity index was determined using the BJ-5ta cell line as a reference, as well as the NCM460 cell line for the CRC model (Table 1).
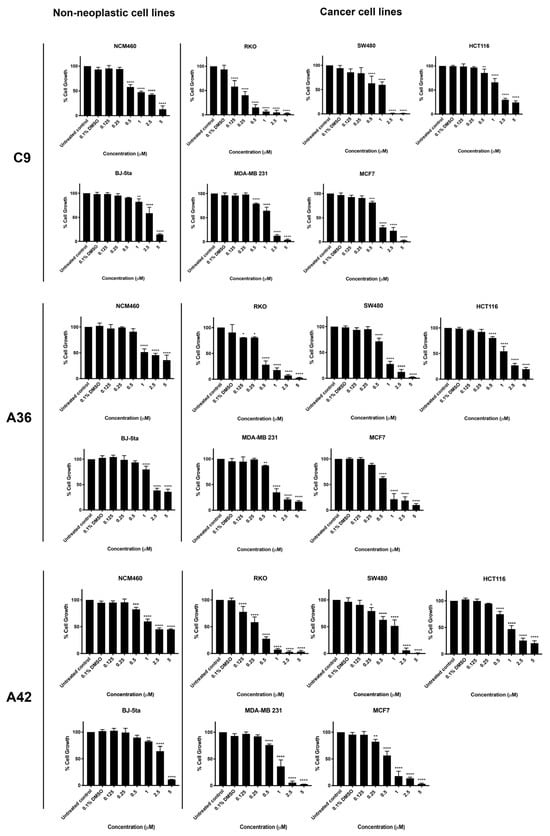
Figure 2.
Effects of C9, A36, and A42 on cell viability of BJ-5ta, NCM460, SW480, HCT116, RKO, MCF7, and MDA-MB-231 cell lines. Cell lines were subjected to increasing concentrations of compounds (0.125 μM, 0.25 μM, 0.5 μM, 1 μM, 2.5 μM, and 5 μM) or DMSO (0.1%) for 48 h. After incubation, cell viability was determined using the sulforhodamine B assay. The values are means with SD (n ≥ 3). The statistical analysis was conducted using two-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.

Table 1.
IC50 (μM) ± SD for compounds BaP1 (lead compound), C9, A36, and A42 in BJ-5ta, NCM460, SW480, HCT116, RKO, MCF7, and MDA-MB-231 cell lines.
The two non-neoplastic cell lines, BJ-5ta and NCM460, were the most resistant to the compounds and had the highest IC50 values (Figure 2 and Table 1). All CRC cell lines were quite sensitive to all compounds. Treatment significantly reduced cell viability, resulting in low IC50 values. In particular, the RKO cell line was the most sensitive for the CRC model, showing very low IC50 values and very high selectivity indices for all compounds (Figure 2 and Table 1). This is consistent with our previous results, where we tested the anticancer potential of a related benzo[a]phenoxazine compound (BaP1–lead compound) and observed that RKO cells were highly sensitive to the drug (Table 1) [39]. This is particularly important, as most colorectal cancer patients displaying the BRAF (V600E) mutational activation present in the RKO cell line display resistance to chemotherapy and targeted therapies such as EGFR inhibitors [45,46,47,48].
In the breast cancer model, all three compounds significantly decreased the viability of MCF7 and MDA-MB-231 cells (Figure 2). The compounds C9, A36, and A42 showed lower IC50 values and a higher selectivity index for the MCF7 cells compared to the MDA-MB-231 cells, indicating a greater effectiveness against the luminal A subtype of breast cancer. Although patients with this subtype may respond to targeted endocrine therapy, such as selective small molecules of estrogen biosynthesis and estrogen receptor modulators, long-term endocrine therapy has been linked to systemic toxicity, tumor resistance, and the emergence of drug-resistant cancer stem cells that interfere with therapy and promote disease progression, emphasizing the need for new chemotherapeutic agents [49,50,51,52].
Overall, all the new tested compounds revealed a high anticancer potential and exhibited lower IC50 values and higher selectivity indices than our lead compound BaP1. In particular, the CRC-derived cell line RKO and the breast-cancer-derived cell line MCF7 were found to be the most sensitive cell lines to the three compounds. To better assess the phenotypic alterations associated with the anticancer potential of the compounds and their mechanisms of action, we selected these cell lines and performed a more detailed characterization of the biological activity of the three compounds.
3.1.2. C9, A36, and A42 Decrease RKO and MCF7 Cell Proliferation
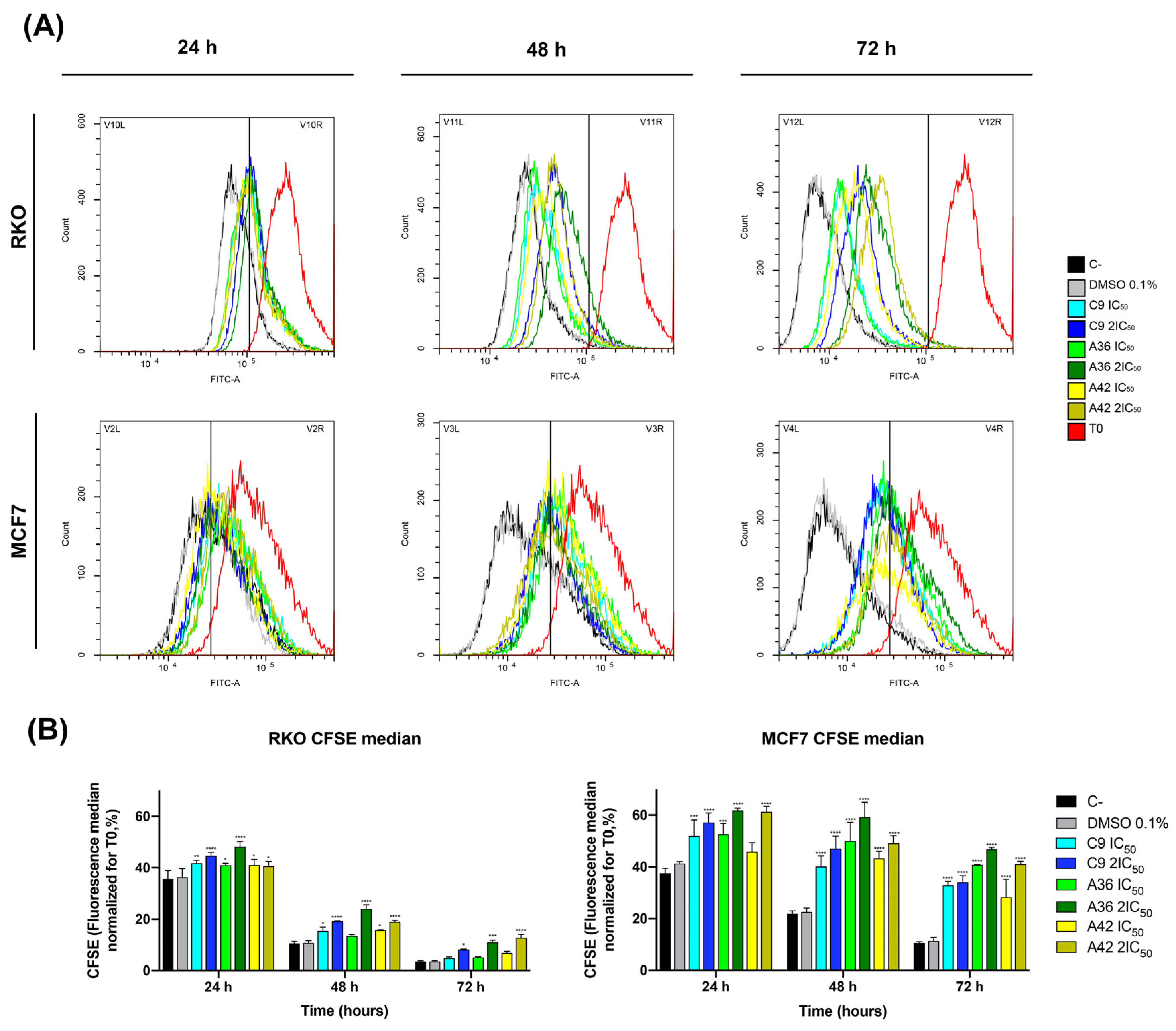
The benzo[a]phenoxazines C9, A36, and A42 were tested for their capacity to decrease cell growth using an optimized flow cytometry Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) labeling procedure. This procedure enables the differentiation of successive rounds of cell division, since the CFSE probe is uniformly distributed among daughter cells during cell division. As a result, a decrease in green CFSE fluorescence was employed as an indicator of cell proliferation.
RKO and MCF7 cells were treated with IC50 and 2 × IC50 concentrations of the compounds, and the effect on cell proliferation was analyzed after 24 h, 48 h, and 72 h of treatment by examining the decrease in green CFSE fluoresce in comparison with the untreated controls. The three compounds showed a significant effect, as a reduction in the proliferation of the treated cells was observed for the two cell lines, with the highest effect occurring with the compound A36 (Figure 3A,B). However, this inhibition proved to be much stronger in the breast cancer line MCF7. With the compound A36, it was possible to observe that the CFSE cell fluorescence decreased much less in the treated cells than in the untreated controls. We observed that the CFSE median fluorescence of the untreated cells decreased to 37% at 24 h, 21% at 48 h, and 10% at 72 h, in contrast to the 53% IC50, 62% 2 × IC50 at 24 h, 50% IC50, 59% 2 × IC50 at 48 h, and 41% IC50, 47% 2 × IC50 at 72 h (Figure 3B), reaching statistical significance. These results led us to conclude that these compounds are potent proliferation inhibitors, what is an important feature in anticancer drugs.
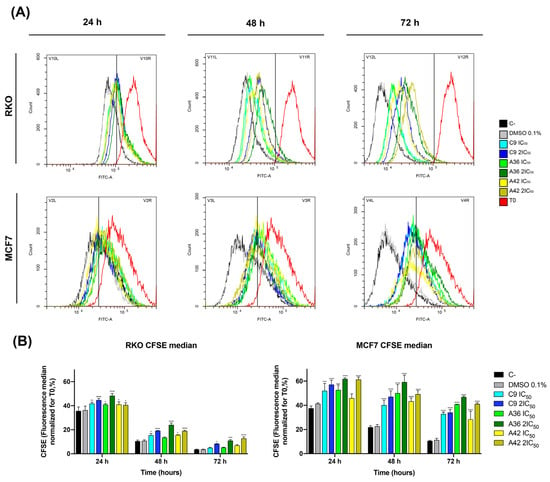
Figure 3.
Effects of C9, A36, and A42 on cell proliferation of RKO and MCF7 cell lines. (A) Representative histograms of RKO and MCF7 cell proliferation assays using carboxyfluorescein diacetate succinimidyl ester (CFSE). Cell lines were exposed to compounds C9, A36, and A42 at IC50 and 2 × IC50 or DMSO (0.1%) for 0 h, 24 h, 48 h, and 72 h. (B) Quantification of the CFSE fluorescence median, values normalized to T0 after 24 h, 48 h, and 72 h of exposure. The values are means with SD (n ≥ 3). The statistical analysis was conducted using two-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3.1.3. C9, A36, and A42 Reduce Cell Migration of RKO and MCF7 Cells
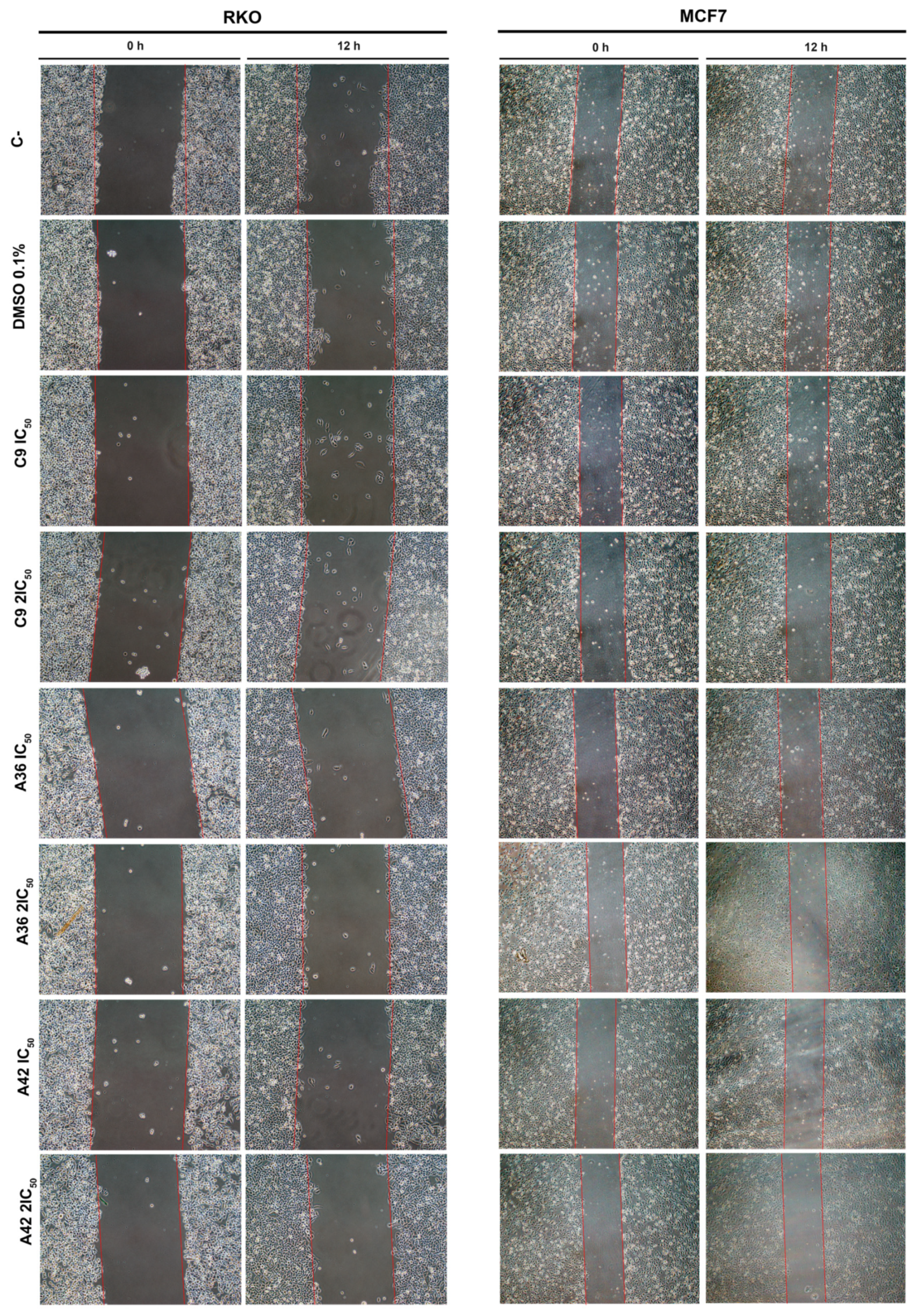
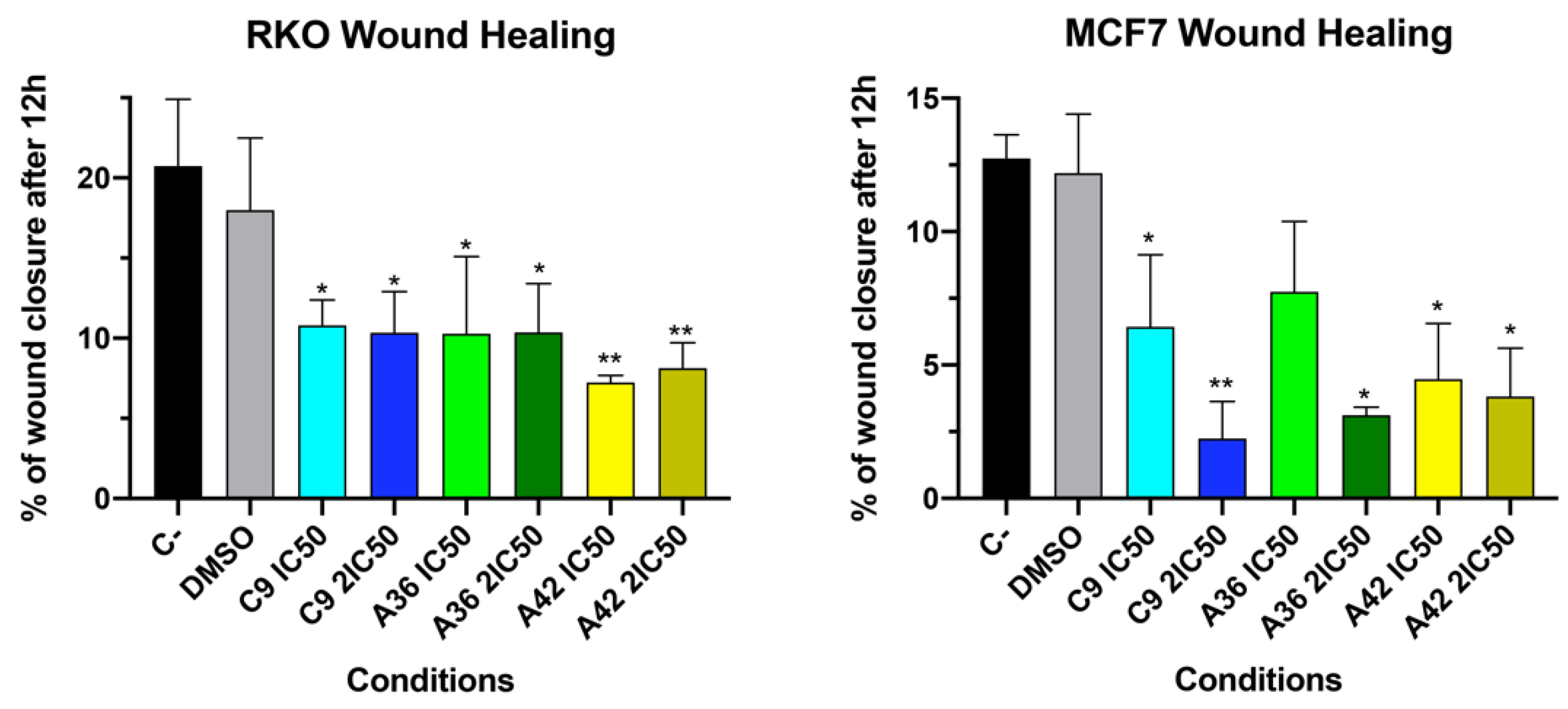
The development of metastases is one of the most lethal aspects of cancer. Cell migration is a central point in the pathological and physiological development of this process [53]. The wound healing assay is a simple in vitro prediction approach that can be used to assess the inhibitory potency of anticancer drugs against cell migration [54]. To understand whether some of the benzo[a]phenoxazine compounds have the ability to inhibit cell migration, we performed a wound healing assay by exposing RKO and MCF7 cells to the IC50 and 2 × IC50 of the compounds for 12 h. After incubation, wound closure was assessed and the wounds treated with the compounds were compared with the untreated controls (Figure 4). Wound closure in the controls was approximately 20% and 12.5% for the RKO and MCF7 cells, respectively. In the RKO cell line, all compounds had a similar effect at both IC50 and 2 × IC50, decreasing cell migration by half to values around 10%. A similar decrease was observed in the MCF7 cell line, but 2 × IC50 in general, and the compound C9 in particular, had a more pronounced effect (Figure 4). These results clearly show that all compounds inhibited cell motility, suggesting that they may be able to prevent metastatic dissemination.
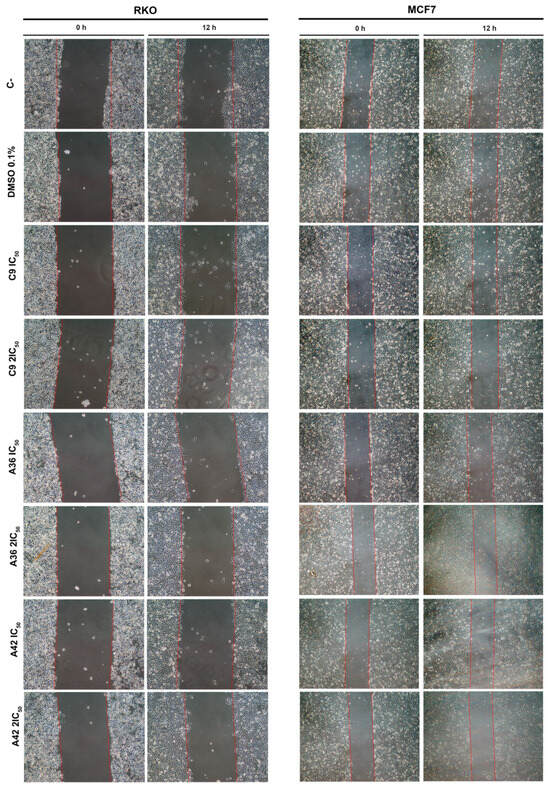
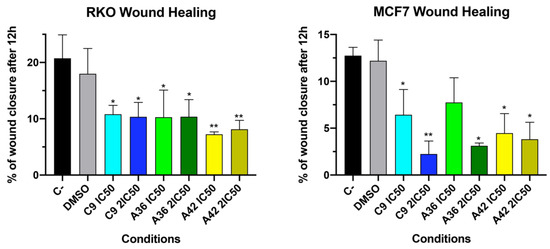
Figure 4.
Effects of C9, A36, and A42 on cell migration of RKO and MCF7 cell lines. Representative images (10× magnification) of wound healing assay after exposure of RKO and MCF7 cells to C9, A36, and A42 at IC50 and 2 × IC50 for 0 h and 12 h. Untreated cells (C– and DMSO (0.1%)-exposed cells were used as negative controls. Quantification of the % of wound closure after 12 h. The values are means with SD (n ≥ 3). The statistical analysis was conducted using one-way ANOVA. * p < 0.05 and ** p < 0.01.
3.1.4. C9, A36, and A42 Reduce RKO and MCF7 Cell Survival
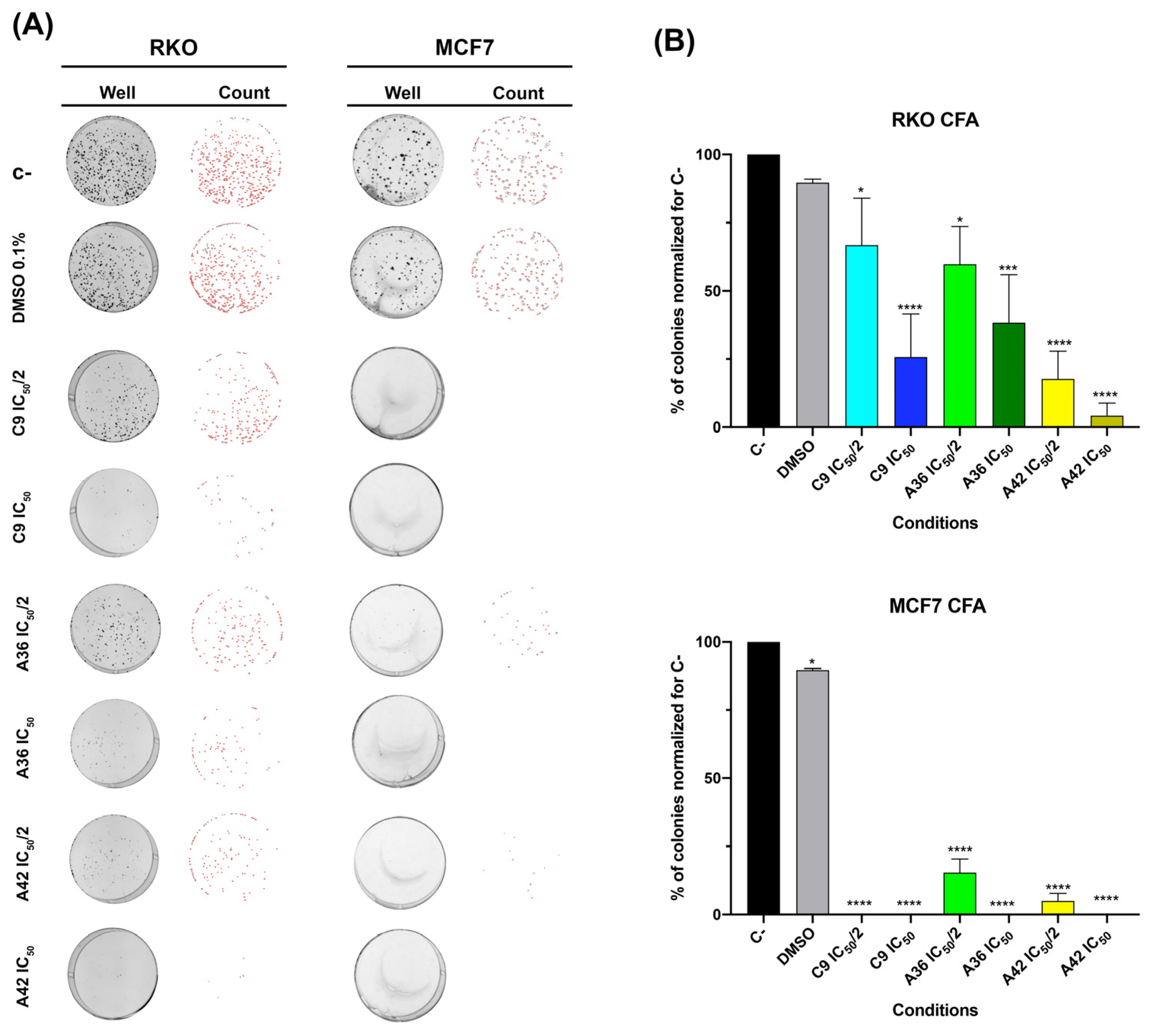
One of the biggest problems found in chemotherapy regimens is that, even after several cycles, some cancer cells have the ability to resist and relapse, maintaining their malignant behavior [55,56]. Thus, the perfect scenario is to use agents that are effective and prevent/reduce this relapse rate after chemotherapy cycles. With this in mind, we examined the effects of the agents on the survival of the RKO and MCF7 cells by evaluating their effects on the clonogenic ability of the cells (the ability of a single cell to survive and form a large colony), performing a colony formation assay. This assay can be used to determine a cell’s ability to survive exposure to an exogenous drug and form colonies when the substance is removed, replicating in vitro what happens during chemotherapy rounds.
As such, we assessed the effects of C9, A36, and A42 at IC50/2 and IC50 after 48 h of treatment. We found that, in both cell lines, all compounds were able to significantly reduce clonogenic ability (Figure 5). In the RKO cell line, the compound A42 was the most effective, with reductions of more than 75% and 90% of the colonies formed for IC50/2 and IC50, respectively. In the breast cancer cell line, these effects were even more pronounced. In fact, we only detected a reduced number of small colonies in the A36 and A42 IC50/2 conditions (Figure 5A,B).
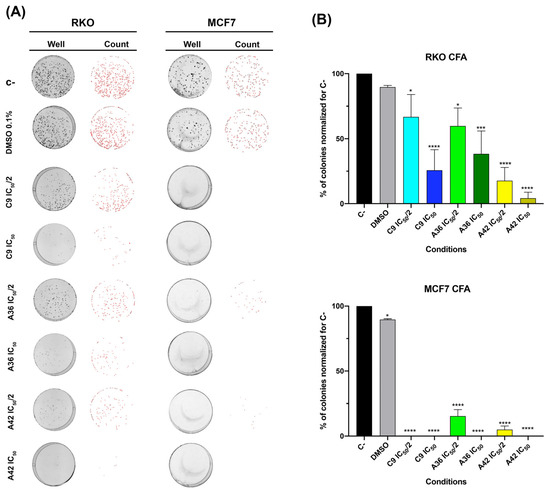
Figure 5.
Effects of C9, A36, and A42 on clonogenic ability of RKO and MCF7 cell lines. (A) Representative images (acquired with 5× magnification) of colony formation assay after RKO and MCF7 cells exposure to C9, A36, and A42 at IC50/2 and IC50 for 48 h. Untreated cells (C–) and DMSO (0.1%)-exposed cells were used as negative controls. (B) Quantification of the number of colonies, values normalized to C–. The values are means with SD (n ≥ 3). The statistical analysis was conducted using one-way ANOVA. * p < 0.05, *** p < 0.001, and **** p < 0.0001.
These results show the efficiency of these compounds in inducing a loss of cellular viability and, thus, reducing colony formation even at low doses, demonstrating their inhibitory effect on cell survival. Cell survival is a balance between proliferation and death, so we next studied the effects of these compounds on cell death.
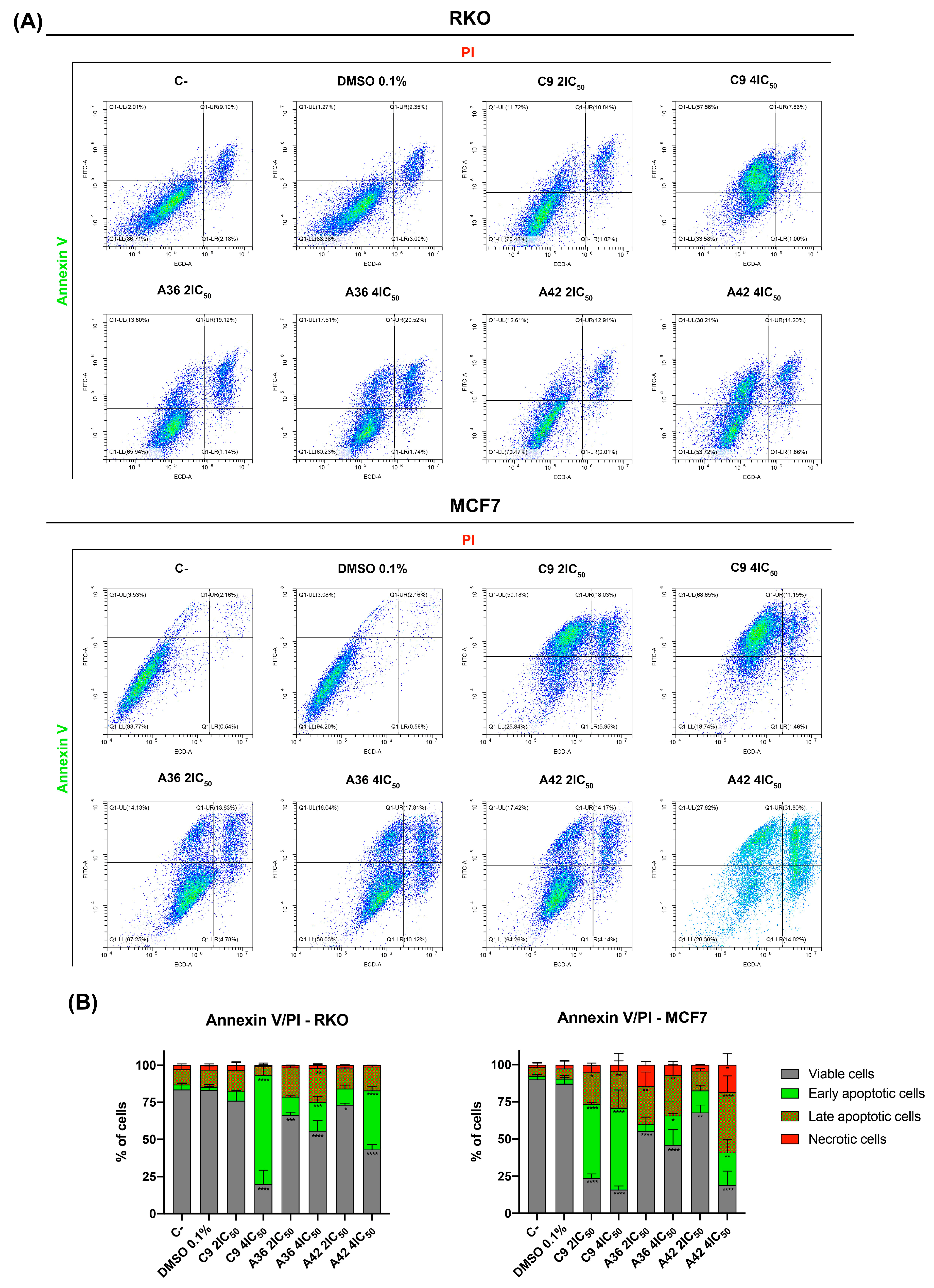
3.1.5. C9, A36, and A42 Induce RKO and MCF7 Cell Death
One of the main goals of clinical oncology is the development of compounds and treatments capable of selectively eliminating cancer cells. In this sense, we next evaluated the capability of the compounds to induce cell death in both cell lines. For this, we performed a flow cytometry analysis using annexin V-FITC and propidium iodide PI double staining. The conjugation of these two probes allowed for the discrimination of viable (negative for both), early apoptotic (annexin + and PI−), late apoptotic (annexin+ and PI+), and necrotic (annexin− and PI+) cells. RKO and MCF7 cells were exposed to 2 × IC50 and 4 × IC50 of the compounds for 48 h. Both flouting and attached cells were harvested and double stained. The results are summarized in Figure 6. The analysis showed that, globally, all the compounds induced cell death in the RKO and MCF7 cells and mainly increased the number of apoptotic cells (early and late) (Figure 6B). C9 and A42 were revealed to be the most powerful compounds, and the MCF7 cell line was the most sensitive cell line.
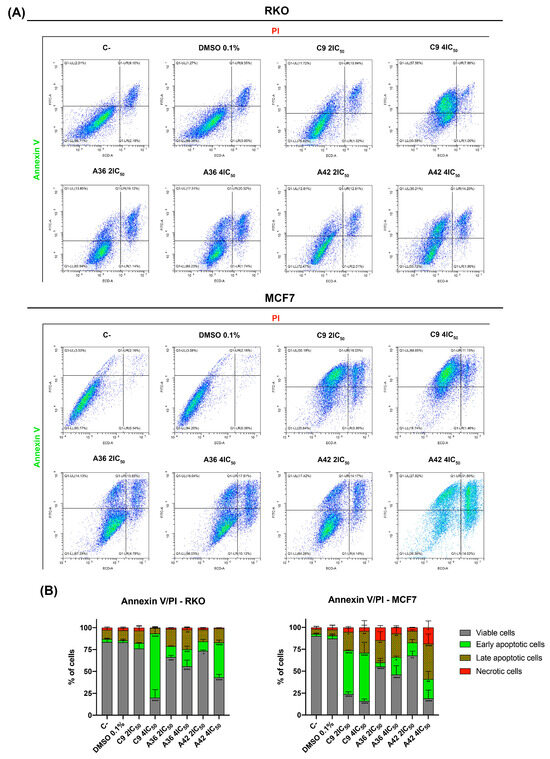
Figure 6.
Effects of C9, A36, and A42 on RKO and MCF7 cell death. (A) Representative bi-parametric dot plots of RKO and MCF7 Annexin V/PI analysis with Annexin V and propidium iodide (PI). Cell lines were exposed to compounds C9, A36, and A42 at 2 × IC50 and 4 × IC50 or DMSO (0.1%) for 48 h. (B) Quantification of the cells in the different stages (viable, early apoptotic, late apoptotic, and necrotic). The values are means with SD (n ≥ 3). The statistical analysis was conducted using two-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Overall, these results show that, at concentrations above IC50, these compounds were capable of inducing apoptotic cell death, similar to what was previously observed for our lead compound (BaP1) [39]. This is particularly important, since both RKO and MCF7 cells are known to be intrinsically resistant to apoptotic induction [57,58]. Indeed, our results are consistent with other reports in the literature, where it was shown that other phenoxazine compounds are capable of inducing apoptotic cell death in various cell types [59,60,61,62].
3.2. Intracellular Target Evaluation of the Benzo[a]phenoxazine Compounds C9, A36, and A42
3.2.1. C9, A36, and A42 Accumulate and Emit Fluorescence on RKO and MCF7 Cell Lysosomes
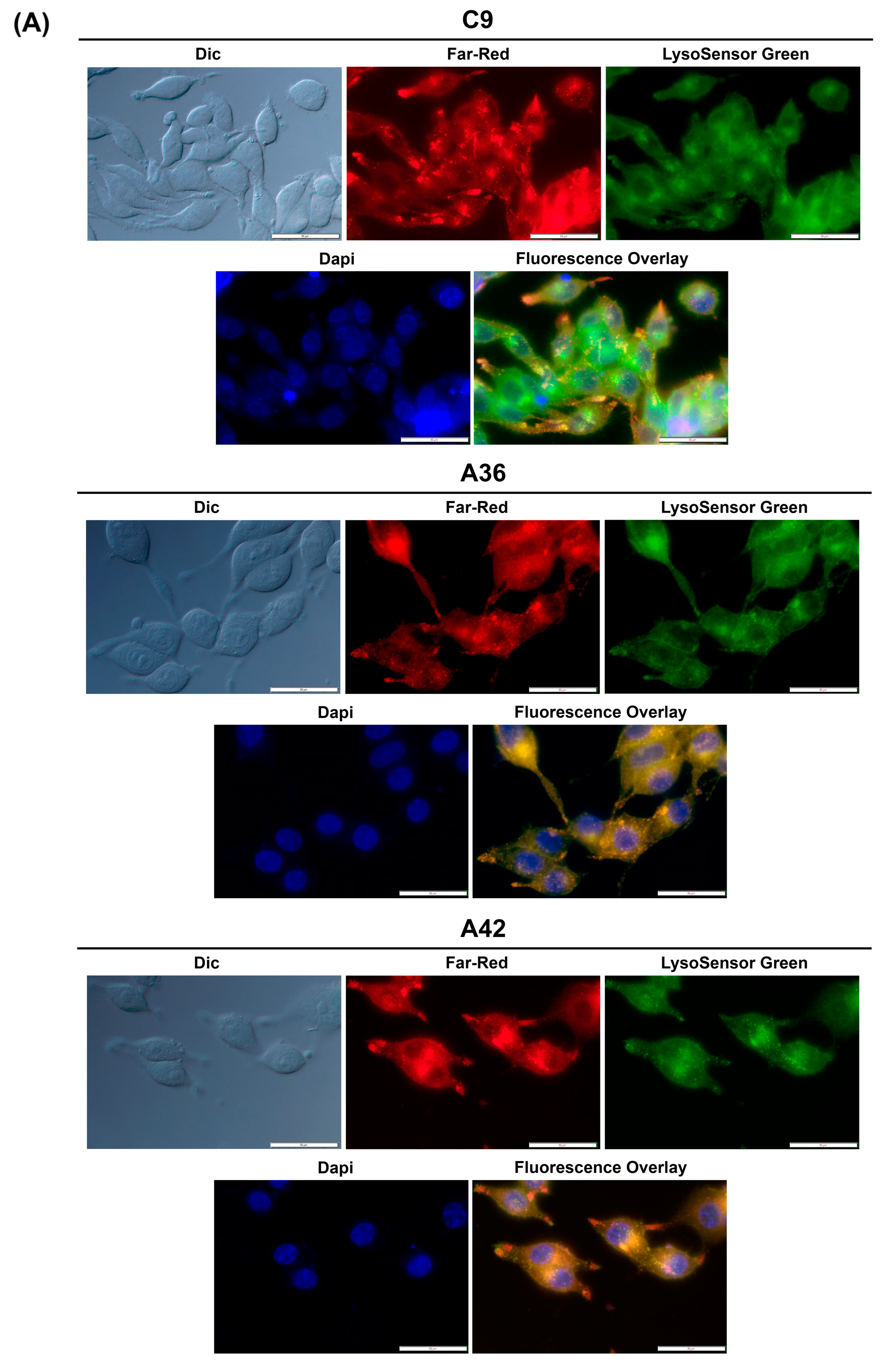
As mentioned above, our recent work has focused on the synthesis and characterization of new benzo[a]phenoxazine compounds. We attempt to preserve the central structures of the molecules and introduce various functional groups to functionalize the compounds. However, we have observed that all these compounds tend to accumulate and emit fluorescence at the vacuolar membrane (yeast) and the lysosomes (human CRC cells) [25,26,39,63].
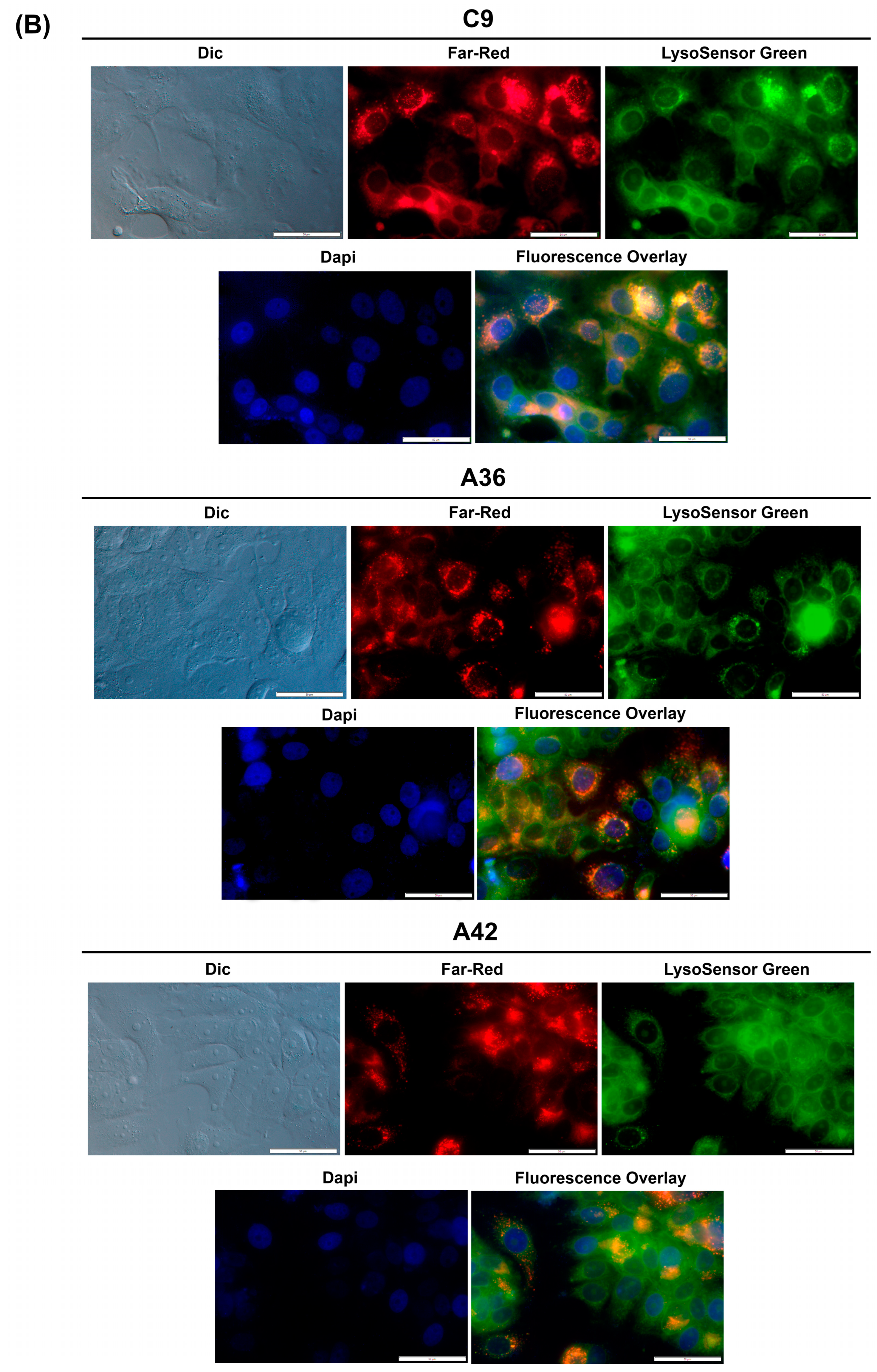
To determine whether this accumulation and emission also occurs for C9, A36, and A42, we took advantage of their intrinsic far-red fluorescence and co-stained RKO and MCF7 cells with the compounds, LysoSensor Green DND-189 (a probe that accumulates and stains lysosomes), and DAPI (stains the nuclei). Using the IC50 concentrations of the compounds, we observed that in both the RKO and MCF7 cells (Figure 7A,B), C9, A36, and A42 far-red fluorescence appeared in punctuated structures that co-localized with the LysoSensor Green lysosome staining, proving that there was an accumulation of these compounds in the lysosomes.
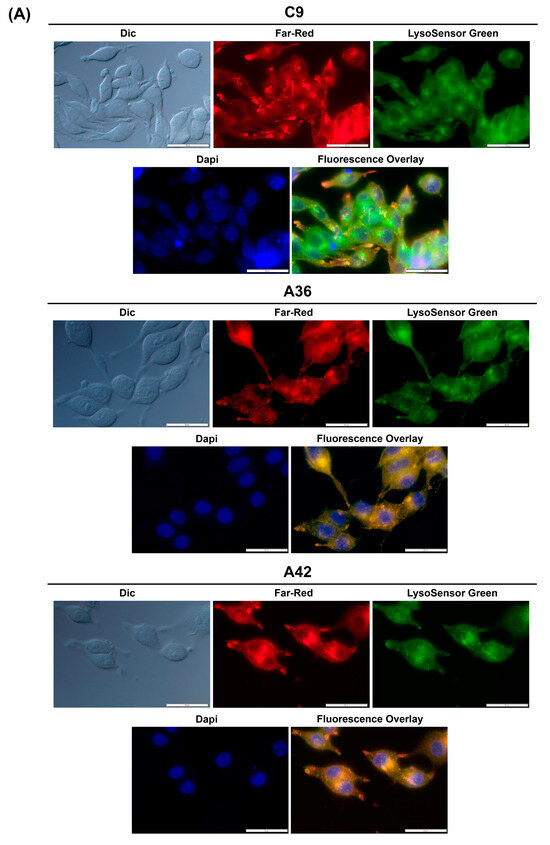
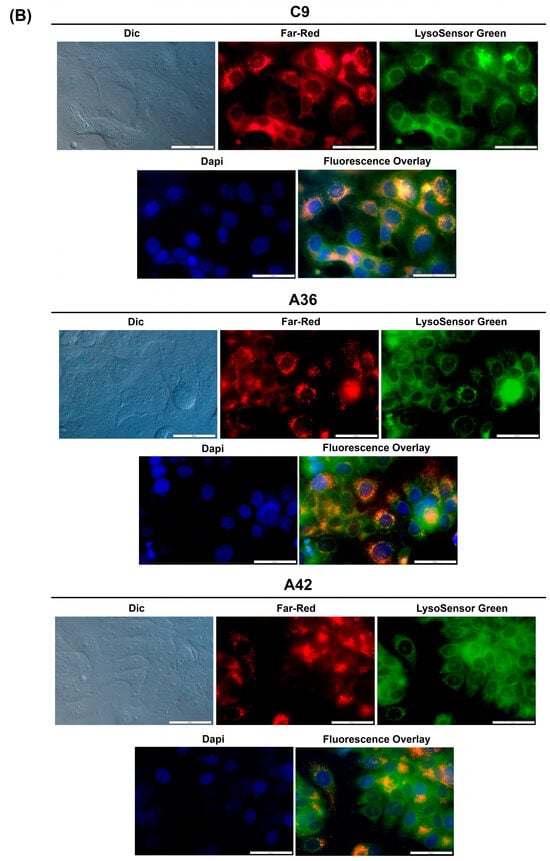
Figure 7.
C9, A36, and A42 lysosome accumulation. (A) Fluorescence microscopy images of RKO cells after incubation with C9, A36, and A42 at IC50, cells were co-stained with 2 μM of LysoSensor Green and co-stained with 10 μg/mL of DAPI. (B) Fluorescence microscopy images of MCF7 cells after incubation with C9, A36, and A42 at IC50, cells were co-stained 2 μM of LysoSensor Green and co-stained with 10 μg/mL of DAPI. Scale bar 50 μm.
These observations are consistent with those previously made for this family of compounds, including for the lead compound (BaP1), and with other reports from the literature, showing that compounds of this family accumulate and emit fluorescence in acidic lysosomes [64,65,66].
3.2.2. C9, A36, and A42 Lysosomal Membrane Permeabilization, Cytosolic Acidification, and ROS Generation
In our previous observations using BaP1, we found that its accumulation and fluorescence emission at the lysosome (CRC model) or vacuolar membrane (yeast model) were not irrelevant. Indeed, using yeast and colorectal cancer cell lines as complementary models, we observed that these organelles were direct targets of the drug, as we found permeabilization of their membranes and the involvement of vacuolar/lysosomal proteins in BaP1’s toxic effect [26,39].
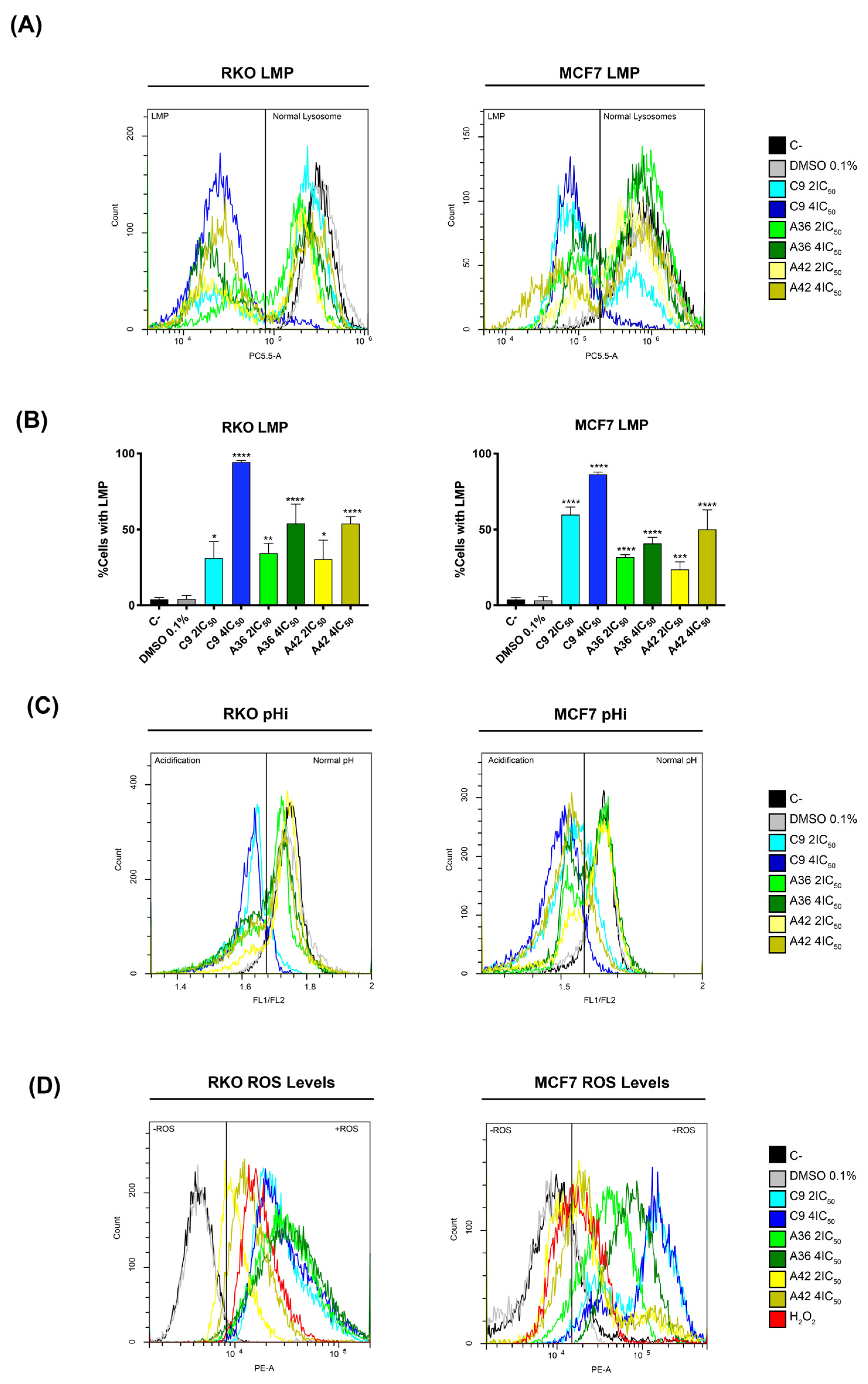
Following the information obtained with the lead compound and considering the microscopic observations made with these new compounds, we next investigated whether their toxicity was also associated with lysosome targeting. To this end, we evaluated the induction of lysosomal membrane permeabilization (LMP) by flow cytometry, using acridine orange (AO) as probe. This probe allows for an assessment of permeabilization, as its fluorescence decreases upon the release of lysosomal protons. RKO and MCF7 cells were treated with 2 × IC50 and 4 × IC50 (cell-death-inducing concentrations) of the compounds for 48 h, and were then incubated with AO and evaluated in the flow cytometer. The cells that presented decreased AO fluorescence were considered to have permeabilized lysosomes. As observed in the histograms in Figure 8A, treatment with the compounds resulted in a decrease in AO fluorescence, representing the existence of cell populations with permeabilized lysosomes.
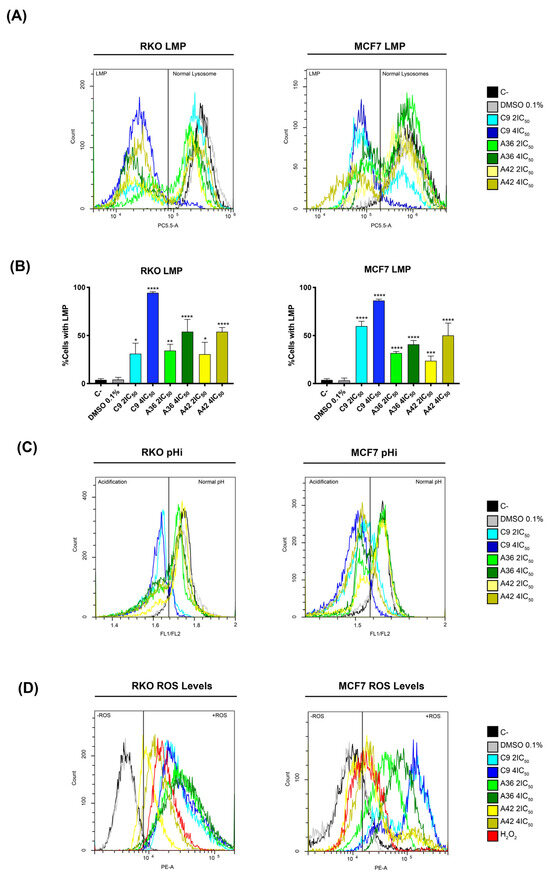
Figure 8.
Effects of C9, A36, and A42 on LMP, pHi, and ROS levels. Cell lines were exposed to compounds C9, A36, and A42 at 2 × IC50 and 4 × IC50 or DMSO (0.1%) for 48 h. (A) Representative histograms of RKO and MCF7 LMP analysis with acridine orange (AO). (B) Quantification of the cells in LMP induction. The values are means with SD (n ≥ 3). The statistical analysis was conducted using two-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. (C) Representative histograms of RKO and MCF7 intracellular acidification with BCECF-AM, indicated by the fluorescence ratio between BCECF-AM green (FITC-FL1) and red fluorescence (PE-FL2) (FL1/FL2). (D) Representative histograms of RKO and MCF7 ROS levels with DHE.
After quantifying the percentage of cells with LMP (Figure 8B), we found that compound C9 had the greatest effect on both cell lines, with 4 × IC50 leading to a very high percentage of cells with permeabilized lysosomes, more than 80% of the total population. These levels are consistent with what we observed for the cell death induction, as the compound C9 also proved to be the compound with the greatest ability to induce cell death in both cell lines. Moreover, in the case of the compounds A36 and A42, the levels of inviable cells and the levels of cells with LMP were also identical. These correlations suggest that the induction of LMP by these compounds is implicated in their mechanisms of cell death induction.
To further support LMP’s role in cell death induction, we investigated the effects of the compounds on intracellular pH (pHi), as LMP induction is associated with an increase in cytosolic proton levels and, as a result, a reduction in intracellular pH [67,68,69]. We used the dual-emission ratiometric pH-sensitive probe BCECF-AM, which emits fluorescence based on pHi. The result of acidification was shown by a decrease in the fluorescence ratio between BCECF-AM green (FITC-FL1) and red fluorescence (PE-FL2) (FL1/FL2). The cells with lower pHi values could be identified by a lower FL1/FL2 ratio compared to the control cells (C–). This is defined by the gates in the histograms in Figure 8B. Indeed, our results are consistent with LMP induction, as we observed the appearance of populations with lower FL1/FL2 ratios, representing cells with lower pHi values, after 48 h of treatment with the compounds for both cell lines (Figure 8C). Moreover, the compound C9 showed the most pronounced effect, as expected, demonstrating a direct association between LMP and intracellular acidification.
Since ROS generation is reported to precede LMP and cell death [70], and in view of reports showing that some compounds of this family increase intracellular ROS levels under certain circumstances [71,72], we also investigated whether C9, A36, and A42 had any effects in this regard. Indeed, by flow cytometry using dihydroethidium (DHE), a probe that allows for the direct detection of superoxide and hydrogen peroxide, we observed that all compounds, including C9 with a higher intensity, led to a very significant increase in the ROS levels in the two cell lines, as shown by an increase in the fluorescence mean of DHE (Figure 8D). In fact, these high levels of ROS seem to suggest that there may be a link with LMP, as they may be related to lysosomal membrane damage and consequent lysosomal permeabilization, as occurs in several cases of LMP-associated cell death [73,74,75].
There are several pharmacological studies in the literature that have explored the applications and endogenous targets of phenoxazine derivatives in general [13,14,15,16,17,18,19]. However, as far as benzo[a]phenoxazines are concerned, although there are studies demonstrating their suitability as anticancer drugs [20,21,22,23], there is not much information on their mechanisms of action. Our results are relevant, as they contribute to the elucidation of the mechanisms of action of these compounds and show that, at least in the specific case of these compounds, the lysosome arises as their target, which is consistent with what has been previously reported for BaP1 [39].
4. Conclusions
Here, we investigated the anticancer activity of the three of the most potent benzo[a]phenoxazine derivatives from our library of compounds, C9, A36, and A42, on colorectal and breast cancer cell lines. The three compounds proved to be selective for the cancer cells, improving selectivity compared with our lead compound BaP1. They showed IC50 values in the low micromolar range and exhibited greater selectivity indices for the cancer cells, especially for the RKO colorectal cancer cells harboring BRAFV600E mutations and the MCF7 breast cancer cells of the luminal subtype A. We performed a detailed characterization of the biological activity of these compounds and found that they were able to inhibit cell proliferation, cell migration, and cell survival in both cancer models.
We found that they accumulated in the lysosomes of the RKO and MCF7 cells, and this might be the reason why they were able to induce apoptotic cell death. At cell-death-inducing concentrations, we observed the permeabilization of lysosomal membranes accompanied by intracellular acidification and an increase in intracellular ROS accumulation. These results are consistent with what we observed previously for our lead benzo[a]phenoxazine compound BaP1, suggesting that the lysosome is the target of these compounds, but now we extend these results to more active compounds and also to breast-cancer-derived cell lines. Lysosomes are very attractive from a therapeutic point of view, as cancer cells have more numerous, larger, and less stable lysosomes and alterations in their lysosomal structure and function that make them more susceptible to lysosome destabilization. This could be the reason behind the selectivity and higher toxicity of C9, A36, and A42 towards cancer cells.
Our work is relevant, as it contributes to the characterization of the mechanisms of action of this family of compounds. Moreover, we show that the anticancer activity and mechanisms of action of these compounds are conserved in different cell tissues, making C9, A36, and A42 promising candidates as LMP inducers for cancer therapy.
Author Contributions
J.C.C.F. (conception and design, data acquisition, analysis, and interpretation; original draft preparation; writing review and editing); M.S.T.G., A.P. and M.J.S. (conception and design, review of the manuscript, and supervision). All authors have read and agreed to the published version of the manuscript.
Funding
Doctoral Grant J. Canossa Ferreira SFRH/BD/133207/2017 and COVID/BD/151978/2021 acknowledged to Fundação para a Ciência e Tecnologia. This work was supported by the strategic programmes UID/BIA/04050/2020 and UID/QUI/0686/2020 funded by national funds through the FCT I.P. Thanks are due to FCT for financial support to the Portuguese NMR network (PTNMR, Bruker Avance III 400-Univ. Minho). This work was also funded by FCT within the scope of project PTDC/QUIQIN/28662/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Linnekamp, J.F.; Wang, X.; Medema, J.P.; Vermeulen, L. Colorectal cancer heterogeneity and targeted therapy: A case for molecular disease subtypes. Cancer Res. 2015, 75, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Sanz-Pamplona, R.; Nadal, E.; Grasselli, J.; Pernas, S.; Dienstmann, R.; Moreno, V.; Tabernero, J.; Salazar, R. Intrinsic cancer subtypes-next steps into personalized medicine. Cells Oncol. 2015, 38, 3–16. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor heterogeneity in breast cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef]
- Peart, O. Breast intervention and breast cancer treatment options. Radiol. Technol. 2015, 86, 535M–558M. [Google Scholar]
- Yu, J.X.; Hubbard-Lucey, V.M.; Tang, J. The global pipeline of cell therapies for cancer. Nat. Rev. Drug Discov. 2019, 18, 821–822. [Google Scholar]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of novel heterocyclic compounds based on dialdehyde cellulose: Characterization, antimicrobial, antitumor activity, molecular dynamics simulation and target identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Volochnyuk, D.M.; Ryabukhin, S.V.; Judd, D.B. The Symbiotic Relationship Between Drug Discovery and Organic Chemistry. Chem. A Eur. J. 2020, 26, 1196–1237. [Google Scholar] [CrossRef]
- Ezeokonkwo, M.A.; Okafor, S.N.; Ogbonna, O.N.; Onoabedje, E.A.; Ibeanu, F.N.; Godwin-Nwakwasi, E.U.; Ezema, B.E. New antimalarial agents derived from nonlinear phenoxazine ring system. Med. Chem. Res. 2020, 29, 63–74. [Google Scholar] [CrossRef]
- Patil, V.S.; Padalkar, V.S.; Phatangare, K.R.; Umape, P.G.; Borase, B.N.; Sekar, N. Synthesis, Characterization, and Antibacterial Activity of Novel (1H-Benzo[d]imidazole-2-yl)-6-(diethylamino)-3H-one-xanthene, Phenoxazine, and Oxazine. J. Heterocycl. Chem. 2015, 52, 124–129. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Jegan, A.; Premnath, D.; Periasamy, V.S.; Vasanthkumar, S. Design, synthesis, molecular docking as histone deacetylase (HDAC8) inhibitors, cytotoxicity and antibacterial evaluation of novel 6-(4-(4-aminophenylsulfonyl)phenylamino)-5H-benzo[a]phenoxazin-5-one derivatives. Med. Chem. Res. 2015, 24, 197–208. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Andrei, G.; Orlov, A.A.; Khvatov, E.V.; Koruchekov, A.A.; Belyaev, E.S.; Nikolaev, E.N.; Korshun, V.A.; Snoeck, R.; Osolodkin, D.I.; et al. Antiviral activity spectrum of phenoxazine nucleoside derivatives. Antiviral Res. 2019, 163, 117–124. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Ferreira, J.C.C.; Sousa, M.J.; Gonçalves, M.S.T. N-(5-Amino-9H-benzo[a]phenoxazin-9-ylidene)propan-1-aminium chlorides as antifungal agents and NIR fluorescent probes. New J. Chem. 2021, 45, 7808–7815. [Google Scholar] [CrossRef]
- Koshibu-Koizumi, J.; Akazawa, M.; Iwamoto, T.; Takasaki, M.; Mizuno, F.; Kobayashi, R.; Abe, A.; Tomoda, A.; Hamatake, M.; Ishida, R. Antitumor activity of a phenoxazine compound, 2-amino-4, 4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one against human B cell and T cell lymphoblastoid cell lines: Induction of mixed types of cell death, apoptosis, and necrosis. J. Cancer Res. Clin. Oncol. 2002, 128, 363–368. [Google Scholar]
- Kozlovskaya, L.I.; Volok, V.P.; Shtro, A.A.; Nikolaeva, Y.V.; Chistov, A.A.; Matyugina, E.S.; Belyaev, E.S.; Jegorov, A.V.; Snoeck, R.; Korshun, V.A.; et al. Phenoxazine nucleoside derivatives with a multiple activity against RNA and DNA viruses. Eur. J. Med. Chem. 2021, 220, 113467. [Google Scholar] [CrossRef]
- Wesolowska, O.; Molnar, J.; Westman, G.; Samuelsson, K.; Kawase, M.; Ocsovszki, I.; Motohashi, N.; Michalak, K. Benzo[a]phenoxazines: A new group of potent P-glycoprotein inhibitors. In Vivo 2006, 20, 109–114. [Google Scholar] [PubMed]
- Pal, S.; Konkimalla, V.B.; Kathawate, L.; Rao, S.S.; Gejji, S.P.; Puranik, V.G.; Weyhermüller, T.; Salunke-Gawali, S. Targeting a chemorefractory COLO205 (BRAF V600E) cell line using substituted benzo[a]phenoxazines. RSC Adv. 2015, 5, 82549–82563. [Google Scholar] [CrossRef]
- Sen, K.; Sen, K.; Shirley, D.A. Potential Carcinostatic Derivatives of Benzo[a]- and Benzo[b]phenoxazine. J. Org. Chem. 1961, 26, 3861–3863. [Google Scholar] [CrossRef]
- Thorne, S.H.; Barak, Y.; Liang, W.; Bachmann, M.H.; Rao, J.; Contag, C.H.; Matin, A. CNOB/ChrR6, a new prodrug enzyme cancer chemotherapy. Mol. Cancer Ther. 2009, 8, 333–341. [Google Scholar] [CrossRef]
- Lopes, M.; Alves, C.T.; Rama Raju, B.; Gonçalves, M.S.T.; Coutinho, P.J.G.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B Biol. 2014, 141, 93–99. [Google Scholar] [CrossRef]
- Raju, B.R.; Garcia, A.M.F.; Costa, A.L.S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of new benzo[a]phenoxazinium probes possessing carboxylic ester, hydroxyl and amino functional groups: Photophysical studies in dry ethanol and conjugation with CdTe quantum dots. Dye. Pigment. 2014, 110, 203–213. [Google Scholar] [CrossRef]
- Raju, B.R.; Carvalho, M.M.T.; Leitão, M.I.P.S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis, photophysical characterisation and photostability studies of NIR probes with aliphatic, aromatic and chlorinated terminals in 5- and 9-amino positions of benzo[a]phenoxazines. Dye. Pigment. 2016, 132, 204–212. [Google Scholar] [CrossRef][Green Version]
- Raju, B.R.; Sampaio, D.M.F.; Silva, M.M.; Coutinho, P.J.G.; Gonçalves, M.S.T. Ultrasound promoted synthesis of Nile Blue derivatives. Ultrason. Sonochem. 2014, 21, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.H.J.; Coutinho, P.J.G.; Moura, J.C.V.P.; Gonçalves, M.S.T. Functionalised benzo[a]phenoxazine dyes as long-wavelength fluorescent probes for amino acids. Tetrahedron 2007, 63, 1654–1663. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Coutinho, P.J.G.; Gonçalves, M.S.T. Synthesis of short and long-wavelength functionalised probes: Amino acids’ labelling and photophysical studies. Tetrahedron 2007, 63, 12405–12418. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Sousa, M.J.; Moura, J.C.V.P.; Gonçalves, M.S.T. Synthesis, characterisation and antimicrobial activity of new benzo[a]phenoxazine based fluorophores. Tetrahedron Lett. 2007, 48, 8347–8352. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Rama Raju, B.; Cerqueira, N.M.F.S.A.; Sousa, M.J.; Gonçalves, M.S.T. Benzo[a]phenoxazinium chlorides: Synthesis, antifungal activity, in silico studies and evaluation as fluorescent probes. Bioorg. Chem. 2020, 98, 103730. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.C.; Lopes, C.; Preto, A.; Gonçalves, M.S.T.; Sousa, M.J. Novel Nile Blue Analogue Stains Yeast Vacuolar Membrane, Endoplasmic Reticulum, and Lipid Droplets, Inducing Cell Death through Vacuole Membrane Permeabilization. J. Fungi 2021, 7, 971. [Google Scholar] [CrossRef]
- Rama Raju, B.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel Nile Blue derivatives as fluorescent probes for DNA. Dye. Pigment. 2013, 99, 220–227. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Gonçalves, M.S.T.; Moura, J.C.V.P. Synthesis of fluorescent water-soluble functionalised benzo[a]phenoxazinium salts. Tetrahedron Lett. 2006, 47, 8567–8570. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Barros, S.A.; Moura, J.C.V.P.; Gonçalves, M.S.T. Fluorescence derivatisation of amino acids in short and long-wavelengths. Tetrahedron Lett. 2007, 48, 3403–3407. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Gonçalves, M.S.T.; Moura, J.C.V.P. Synthesis and fluorescence properties of side-chain carboxylated 5,9-diaminobenzo[a]phenoxazinium salts. Tetrahedron Lett. 2005, 46, 4949–4952. [Google Scholar] [CrossRef]
- Firmino, A.D.G.; Gonçalves, M.S.T. Bifunctionalised long-wavelength fluorescent probes for biological applications. Tetrahedron Lett. 2012, 53, 4946–4950. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel DNA fluorescence probes based on N-[5-(11-functionalised-undecylamino)-9H-benzo[a]phenoxazin-9-ylidene]propan-1-aminium chlorides: Synthesis and photophysical studies. Tetrahedron Lett. 2011, 52, 112–116. [Google Scholar] [CrossRef]
- Ferreira, J.C.C.; Granja, S.; Almeida, A.F.; Baltazar, F.; Gonçalves, M.S.T.; Preto, A.; Sousa, M.J. Targeting Lysosomes in Colorectal Cancer: Exploring the Anticancer Activity of a New Benzo[a]phenoxazine Derivative. Int. J. Mol. Sci. 2023, 24, 614. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. New long alkyl side-chain benzo[a]phenoxazines as micellisation probes. Tetrahedron Lett. 2009, 50, 4470–4474. [Google Scholar] [CrossRef]
- Alves, C.M.A.; Naik, S.; Coutinho, P.J.G.; Gonçalves, M.S.T. Novel long alkyl side chain benzo[a]phenoxazinium chlorides: Synthesis, photophysical behaviour and DNA interaction. Tetrahedron 2009, 65, 10441–10452. [Google Scholar] [CrossRef]
- Leitão, M.I.P.S.; Raju, B.R.; Naik, S.; Coutinho, P.J.G.; Sousa, M.J.; Gonçalves, M.S.T. Synthesis and photophysical studies of new benzo[a]phenoxazinium chlorides as potential antifungal agents. Tetrahedron Lett. 2016, 57, 3936–3941. [Google Scholar] [CrossRef]
- Raju, B.R.; Leitão, M.I.P.S.; Sousa, M.J.; Coutinho, P.J.G.; Gonçalves, M.S.T. New NIR dyes based on quinolizino[1,9-hi]phenoxazin-6-iminium chlorides: Synthesis, photophysics and antifungal activity. Dye. Pigment. 2020, 173, 107870. [Google Scholar] [CrossRef]
- Marques, C.; Oliveira, C.S.F.; Alves, S.; Chaves, S.R.; Coutinho, O.P.; Corte-Real, M.; Preto, A. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013, 4, e507. [Google Scholar] [CrossRef]
- Samowitz, W.S.; Sweeney, C.; Herrick, J.; Albertsen, H.; Levin, T.R.; Murtaugh, M.A.; Wolff, R.K.; Slattery, M.L. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005, 65, 6063–6069. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Dienstmann, R.; Tabernero, J. BRAF as a Target for Cancer Therapy. Anticancer Agents Med. Chem. 2012, 11, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Lapeyre-Prost, A.; Laurent Puig, P.; Zaanan, A. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br. J. Cancer 2019, 121, 434–442. [Google Scholar] [CrossRef]
- Munzone, E.; Colleoni, M. Optimal management of luminal breast cancer: How much endocrine therapy is long enough? Ther. Adv. Med. Oncol. 2018, 10, 175883591877743. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef]
- Telang, N.T.; Li, G.; Katdare, M.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y.C. The nutritional herb epimedium grandiflorum inhibits the growth in a model for the luminal a molecular subtype of breast cancer. Oncol. Lett. 2017, 13, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 6024. [Google Scholar] [CrossRef]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Nooter, K.; Stoter, G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol. Res. Pract. 1996, 192, 768–780. [Google Scholar] [CrossRef]
- Simstein, R.; Burow, M.; Parker, A.; Weldon, C.; Beckman, B. Apoptosis, chemoresistance, and breast cancer: Insights from the MCF-7 cell model system. Exp. Biol. Med. 2003, 228, 995–1003. [Google Scholar] [CrossRef]
- Kawakami, H.; Huang, S.; Pal, K.; Dutta, S.K.; Mukhopadhyay, D.; Sinicrope, F.A. Mutant BRAF Upregulates MCL-1 to Confer Apoptosis Resistance that Is Reversed by MCL-1 Antagonism and Cobimetinib in Colorectal Cancer. Mol. Cancer Ther. 2016, 15, 3015–3027. [Google Scholar] [CrossRef]
- Shirato, K.; Imaizumi, K.; Abe, A.; Tomoda, A. Phenoxazine derivatives 2-amino-4,4alpha-dihydro-4alpha-phenoxazine-3-one and 2-aminophenoxazine-3-one-induced apoptosis through a caspase-independent mechanism in human neuroblastoma cell line NB-1 cells. Biol. Pharm. Bull. 2007, 30, 331–336. [Google Scholar] [CrossRef][Green Version]
- Shirato, K.; Imaizumi, K.; Abe, A.; Tomoda, A. Phenoxazine derivatives induce caspase-independent cell death in human glioblastoma cell lines, A-172 and U-251 MG. Oncol. Rep. 2007, 17, 201–208. [Google Scholar] [CrossRef][Green Version]
- Kato, S.; Shirato, K.; Imaizumi, K.; Toyota, H.; Mizuguchi, J.; Odawara, M.; Che, X.F.; Akiyama, S.; Abe, A.; Tomoda, A. Anticancer effects of phenoxazine derivatives combined with tumor necrosis factor-related apoptosis-inducing ligand on pancreatic cancer cell lines, KLM-1 and MIA-PaCa-2. Oncol. Rep. 2006, 15, 843–848. [Google Scholar] [CrossRef]
- Chadar, D.; Rao, S.S.; Khan, A.; Gejji, S.P.; Bhat, K.S.; Weyhermüller, T.; Salunke-Gawali, S. Benzo[α]phenoxazines and benzo[a]phenothiazine from vitamin K3: Synthesis, molecular structures, DFT studies and cytotoxic activity. RSC Adv. 2015, 5, 57917–57929. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Ferreira, J.C.C.; Sousa, M.J.M.F.; Gonçalves, M.S.T. New Nile Blue derivatives as NIR fluorescent probes and antifungal agents. Proceedings 2019, 9, 65. [Google Scholar]
- Zhan, Y.H.; Li, X.J.; Sun, R.; Xu, Y.J.; Ge, J.F. Distinguishing normal cells from cancer cells via lysosome-targetable pH biomarkers with benzo[a]phenoxazine skeleton. Anal. Chim. Acta 2016, 10, 5796–5803. [Google Scholar] [CrossRef]
- Sun, R.; Liu, W.; Xu, Y.J.; Lu, J.M.; Ge, J.F.; Ihara, M. A cyanobenzo[a]phenoxazine-based near infrared lysosome-tracker for in cellulo imaging. Chem. Commun. 2013, 49, 10709–10711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Niu, J.Y.; Sun, R.; Xu, Y.J.; Ge, J.F. The improvement of lysosome targetability with oligoethyleneoxy chains linked benzo[a]phenoxazine. Bioorganic Med. Chem. Lett. 2018, 28, 2953–2956. [Google Scholar] [CrossRef]
- Brunk, U.T.; Svensson, I. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 1999, 4, 3–11. [Google Scholar] [CrossRef]
- Zhao, M.; Eaton, J.W.; Brunk, U.T. Protection against oxidant-mediated lysosomal rupture: A new anti-apoptotic activity of Bcl-2? FEBS Lett. 2000, 485, 104–108. [Google Scholar] [CrossRef]
- Nilsson, C.; Johansson, U.; Johansson, A.C.; Kågedal, K.; Öllinger, K. Cytosolic acidification and lysosomal alkalinization during TNF-α induced apoptosis in U937 cells. Apoptosis 2006, 11, 1149–1159. [Google Scholar] [CrossRef]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; De Sousa, W.T.; Da Silva, V.C.M.; Rodrigues, M.C.; Morais, J.A.V.; Song, J.L.; Cheng, Z.Q.; Longo, J.P.F.; Azevedo, R.B.; Jiang, C.S.; et al. Synthesis and evaluation of new potential benzo[a]phenoxazinium photosensitizers for anticancer photodynamic therapy. Molecules 2018, 23, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-L.; Che, X.-F.; Akiyama, S.-I.; Miyazawa, K.; Tomoda, A. 2-Aminophenoxazine-3-one induces cellular apoptosis by causing rapid intracellular acidification and generating reactive oxygen species in human lung adenocarcinoma cells. Int. J. Oncol. 2010, 36, 641–650. [Google Scholar]
- Cai, X.; Liu, Y.; Hu, Y.; Liu, X.; Jiang, H.; Yang, S.; Shao, Z.; Xia, Y.; Xiong, L. ROS-mediated lysosomal membrane permeabilization is involved in bupivacaine-induced death of rabbit intervertebral disc cells. Redox Biol. 2018, 18, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, N.D.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.Q.; Shen, H.M. (-)-Epigallocatechin-3-Gallate Induces Non-Apoptotic Cell Death in Human Cancer Cells via ROS-Mediated Lysosomal Membrane Permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Kwon, T.K. Induction of lysosomal membrane permeabilization is a major event of FTY720-mediated non-apoptotic cell death in human glioma cells. Cancers 2020, 12, 3388. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).