Effect of Cyclin-Dependent Kinase 4/6 Inhibitors on Circulating Cells in Patients with Metastatic Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Peripheral Blood Mononuclear Cell Isolation and Immune Cell Characterization
2.3. Serum IFN-γ Levels
2.4. CTC Isolation and Characterization
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Baseline Characteristics of the Study Population
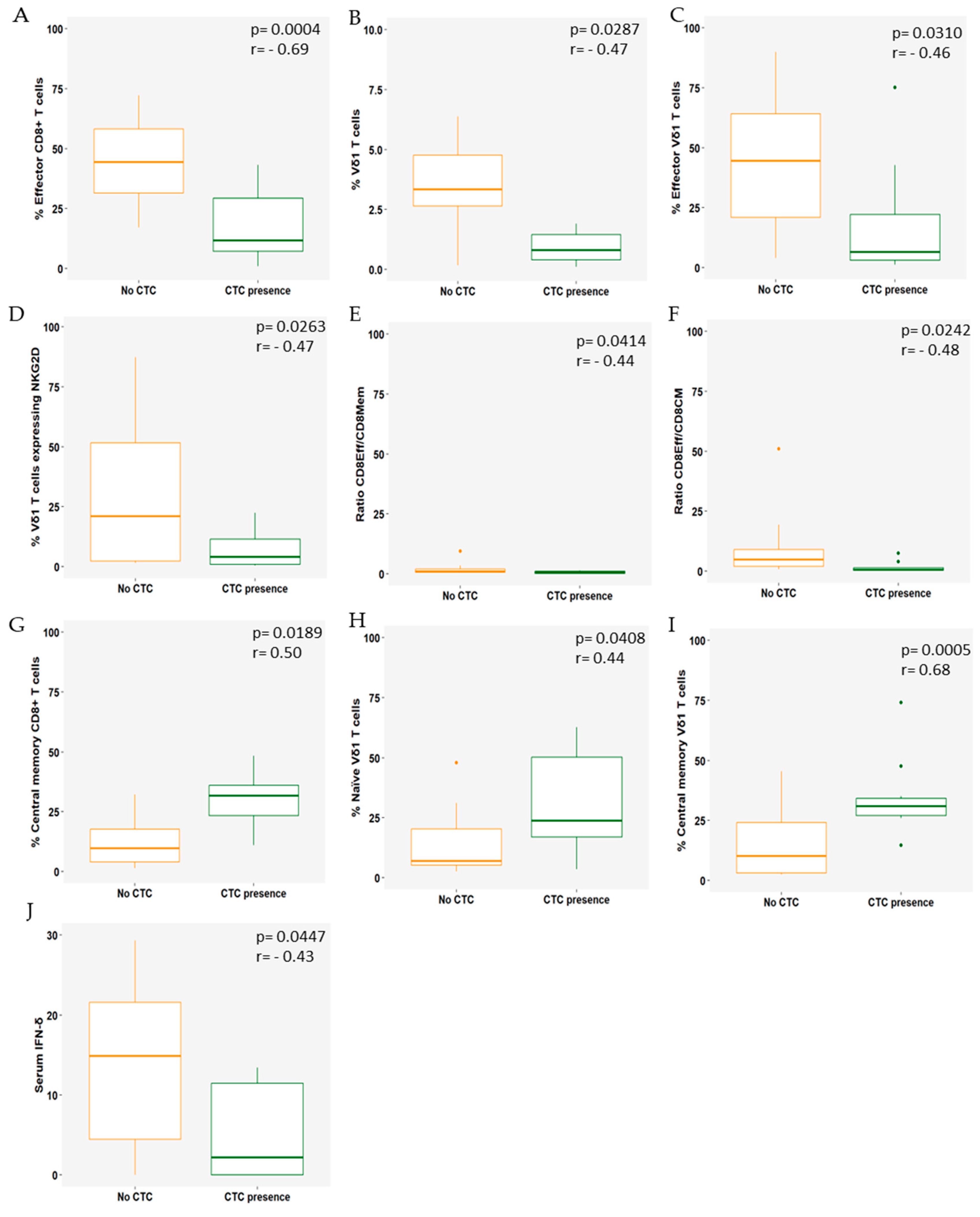
3.2. Immune Cell Populations Correlate with the Presence of CTCs
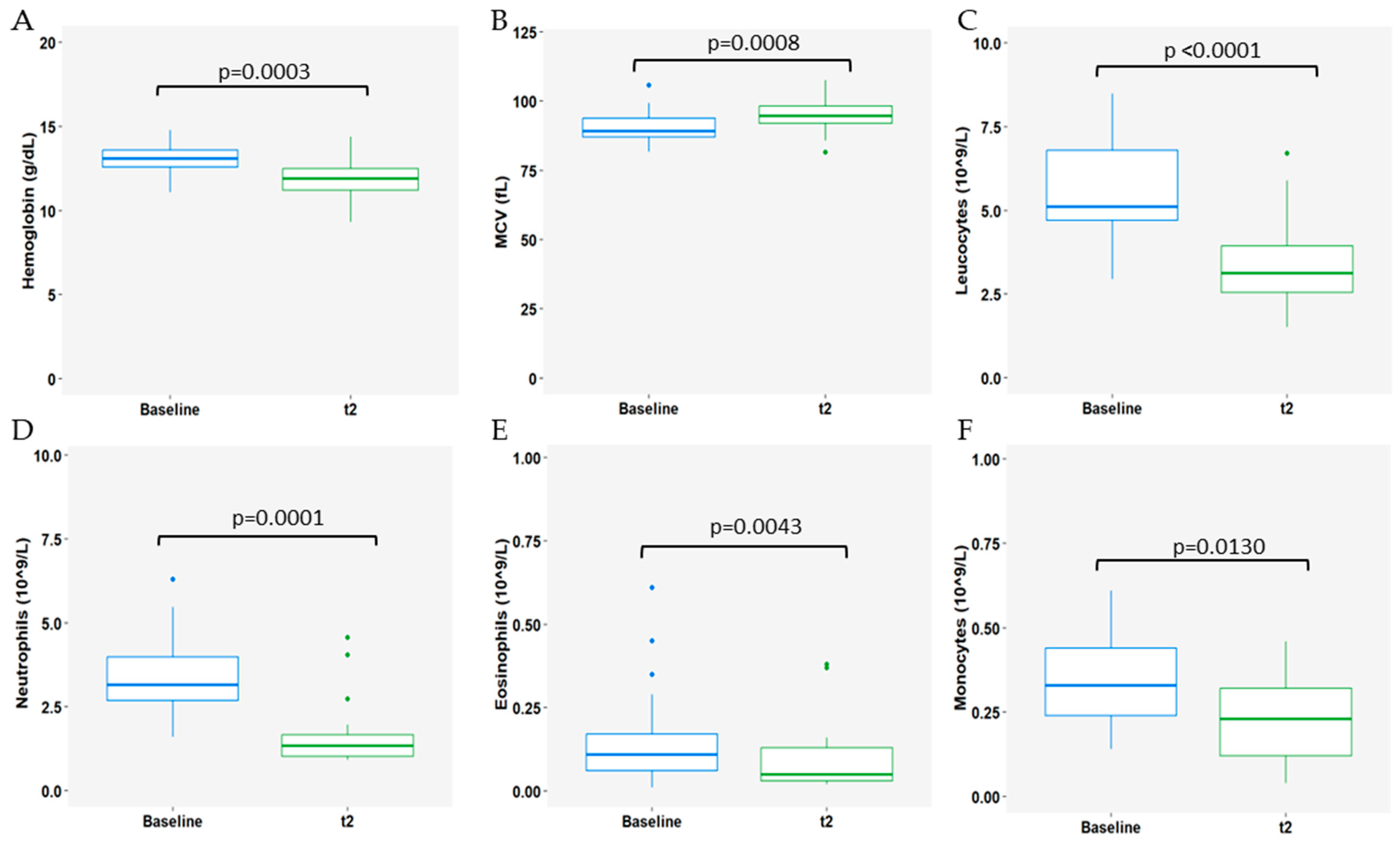
3.3. Impact of CDK4/6i Treatment Plus ET on Immune Populations
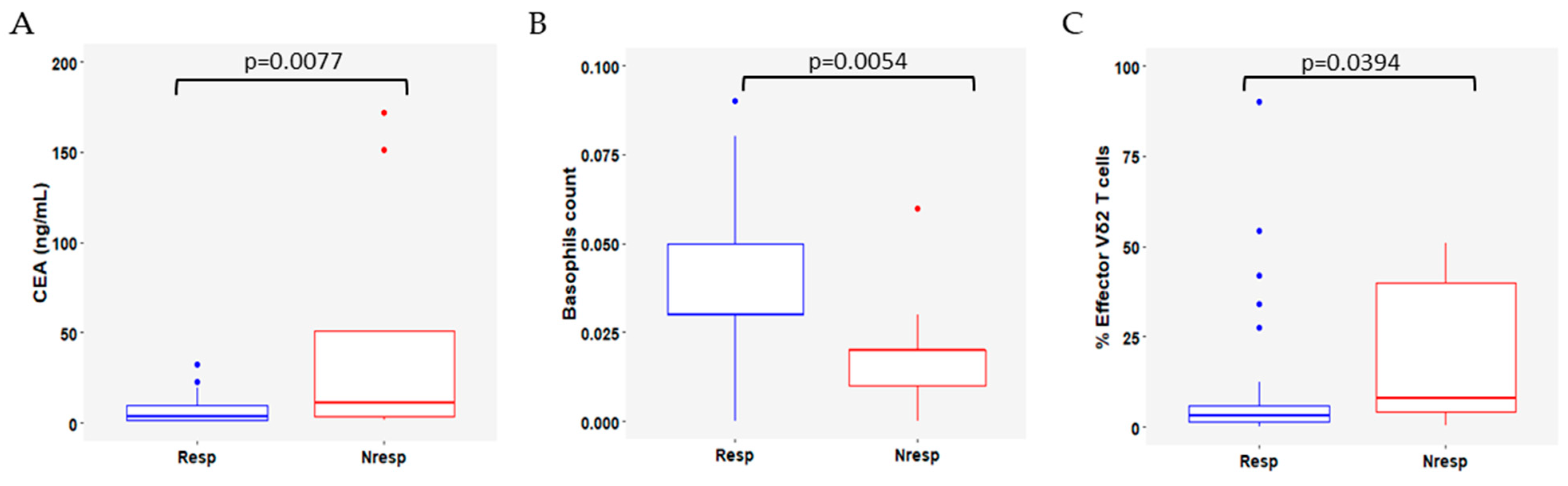
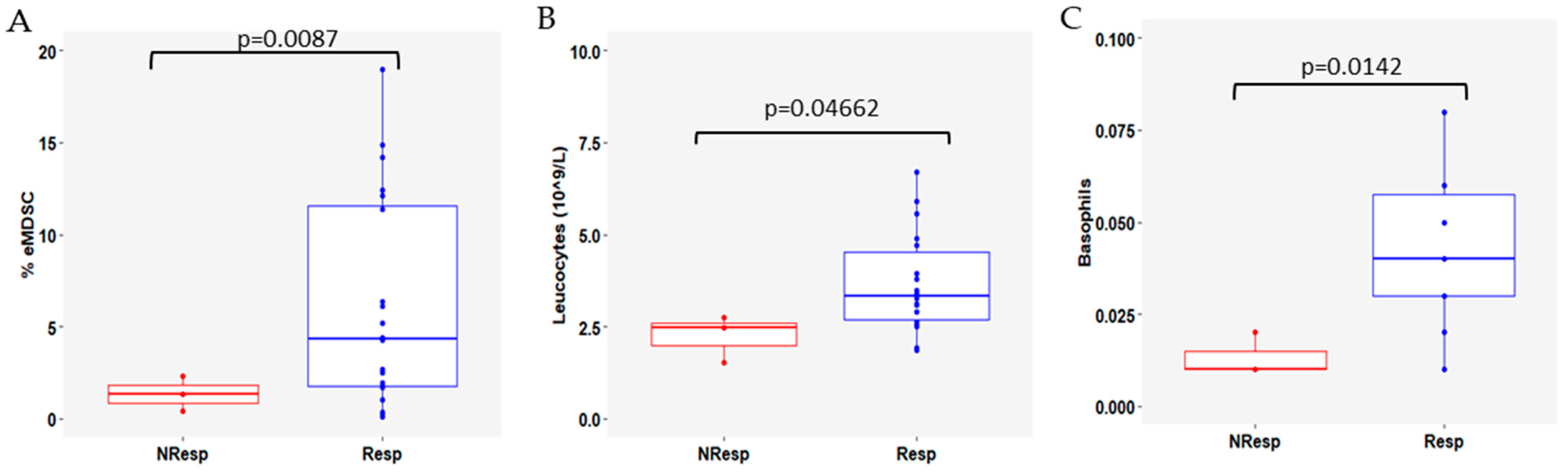
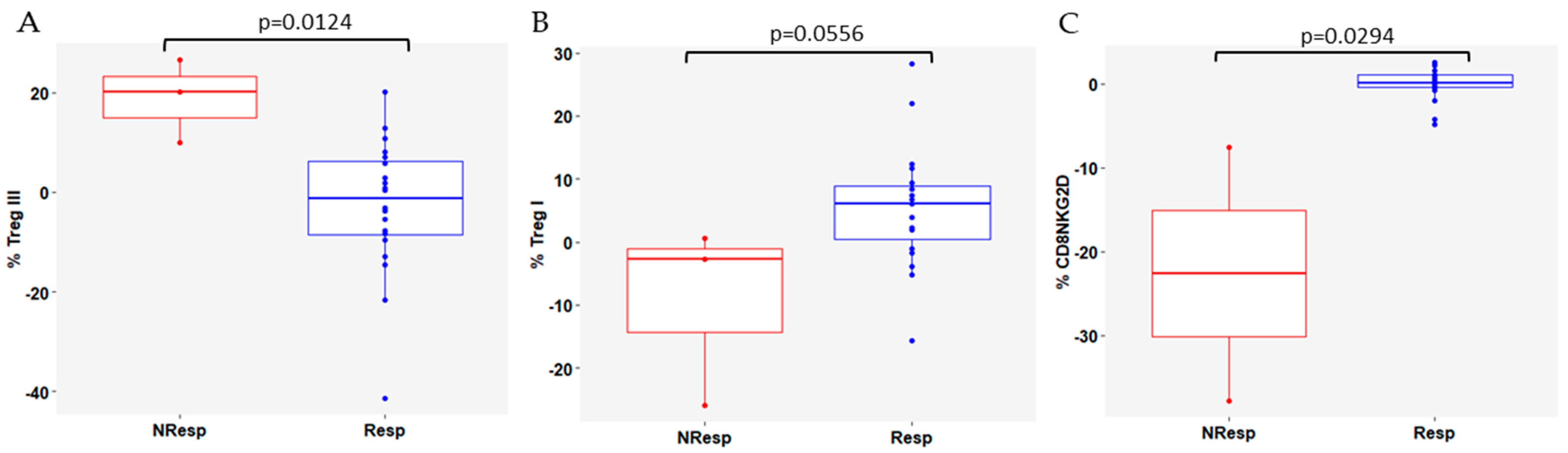
3.4. Variation in Immune Cell Subsets According to Response to CDK4/6i Plus ET
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Rugo, H.S.; Dieras, V.C.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Walshe, J.M.; Martin, M.; Chavez Mac Gregor, M.; Bananis, E.; et al. Overall Survival (OS) with First-Line Palbociclib plus Letrozole (PAL+LET) versus Placebo plus Letrozole (PBO+LET) in Women with Estrogen Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol. 2022, 40, LBA1003. [Google Scholar] [CrossRef]
- Klein, M.E.; Kovatcheva, M.; Davis, L.E.; Tap, W.D.; Koff, A. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell 2018, 34, 9–20. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4 + and CD8 + T Cells Have Opposing Roles in Breast Cancer Progression and Outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef]
- Muntasell, A.; Rojo, F.; Servitja, S.; Rubio-Perez, C.; Cabo, M.; Tamborero, D.; Costa-García, M.; Martínez-Garcia, M.; Menéndez, S.; Vazquez, I.; et al. NK Cell Infiltrates and HLA Class I Expression in Primary HER2+ Breast Cancer Predict and Uncouple Pathological Response and Disease-Free Survival. Clin. Cancer Res. 2019, 25, 1535–1545. [Google Scholar] [CrossRef]
- Shou, D.; Wen, L.; Song, Z.; Yin, J.; Sun, Q.; Gong, W. Suppressive Role of Myeloid-Derived Suppressor Cells (MDSCs) in the Microenvironment of Breast Cancer and Targeted Immunotherapies. Oncotarget 2016, 7, 64505–64511. [Google Scholar] [CrossRef] [PubMed]
- Kovacsovics-Bankowski, M.; Chisholm, L.; Vercellini, J.; Tucker, C.G.; Montler, R.; Haley, D.; Newell, P.; Ma, J.; Tseng, P.; Wolf, R.; et al. Detailed Characterization of Tumor Infiltrating Lymphocytes in Two Distinct Human Solid Malignancies Show Phenotypic Similarities. J. Immunother. Cancer 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the Role of CD4+ T Cells in Cancer Immunotherapy—New Insights into Old Paradigms. Cancer Gene Ther 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of Circulating T-, NK-, and NKT Cell Subsets in Patients with Colorectal Cancer: The Peripheral Blood Immune Cell Profile. Cancer Immunol. Immunother. 2019, 68, 1011–1024. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.-K.; Du, W.-X.; Li, R.-G.; Yang, J.; Li, J.; Li, F.; Tan, H.-B. Immune Cells within the Tumor Microenvi-ronment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Willemsen, A.E.C.A.B.; He, X.; van Cranenbroek, B.; de Jong, P.C.; de Boer, M.; Joosten, I.; Koenen, H.J.P.M.; van Herpen, C.M.L.; Gerritsen, W.R. Baseline Effector Cells Predict Response and NKT Cells Predict Pulmonary Toxicity in Advanced Breast Cancer Patients Treated with Everolimus and Exemestane. Int. Immunopharmacol. 2021, 93, 107404. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Func-tional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Scirocchi, F.; Scagnoli, S.; Botticelli, A.; Di Filippo, A.; Napoletano, C.; Zizzari, I.G.; Strigari, L.; Tomao, S.; Cortesi, E.; Rughetti, A.; et al. Immune Effects of CDK4/6 Inhibitors in Patients with HR+/HER2− Metastatic Breast Cancer: Relief from Immunosuppression Is Associated with Clinical Response. EBioMedicine 2022, 79, 104010. [Google Scholar] [CrossRef]
- Vella, M.; Coniglio, D.; Abrate, A.; Scalici Gesolfo, C.; Lo Presti, E.; Meraviglia, S.; Serretta, V.; Simonato, A. Characteriza-tion of Human Infiltrating and Circulating Gamma-Delta T Cells in Prostate Cancer. Investig. Clin. Urol. 2019, 60, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, A.C.; Rouvière, M.S.; Salem, N.; Ben Amara, A.; Orlanducci, F.; Vey, N.; Gorvel, L.; Chretien, A.-S.; Olive, D. Prognostic Immune Effector Signature in Adult Acute Lymphoblastic Leukemia Patients Is Domi-nated by γδ T Cells. Cells 2023, 12, 1693. [Google Scholar] [CrossRef]
- Mariani, S.; Muraro, M.; Pantaleoni, F.; Fiore, F.; Nuschak, B.; Peola, S.; Foglietta, M.; Palumbo, A.; Coscia, M.; Castella, B.; et al. Effector Gammadelta T Cells and Tumor Cells as Immune Targets of Zoledronic Acid in Multiple Myeloma. Leukemia 2005, 19, 664–670. [Google Scholar] [CrossRef]
- Gordino, G.; Costa-Pereira, S.; Corredeira, P.; Alves, P.; Costa, L.; Gomes, A.Q.; Silva-Santos, B.; Ribot, J.C. MicroRNA-181a Restricts Human Γδ T Cell Differentiation by Targeting Map3k2 and Notch2. EMBO Rep. 2022, 23, e52234. [Google Scholar] [CrossRef] [PubMed]
- Cimini, E.; Piacentini, P.; Sacchi, A.; Gioia, C.; Leone, S.; Lauro, G.M.; Martini, F.; Agrati, C. Zoledronic Acid Enhances Vδ2 T-Lymphocyte Antitumor Response to Human Glioma Cell Lines. Int. J. Immunopathol. Pharmacol. 2011, 24, 139–148. [Google Scholar] [CrossRef]
- Raverdeau, M.; Cunningham, S.P.; Harmon, C.; Lynch, L. Γδ T Cells in Cancer: A Small Population of Lymphocytes with Big Implications. Clin. Transl. Immunol. 2019, 8, e01080. [Google Scholar] [CrossRef]
- Lee, D.; Rosenthal, C.J.; Penn, N.E.; Dunn, Z.S.; Zhou, Y.; Yang, L. Human Γδ T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers 2022, 14, 3005. [Google Scholar] [CrossRef]
- Katoh, H.; Watanabe, M. Myeloid-Derived Suppressor Cells and Therapeutic Strategies in Cancer. Mediat. Inflamm. 2015, 2015, e159269. [Google Scholar] [CrossRef]
- Bhat, D.K.; Olkhanud, P.B.; Gangaplara, A.; Seifuddin, F.; Pirooznia, M.; Biancotto, A.; Fantoni, G.; Pittman, C.; Francis, B.; Dagur, P.K.; et al. Early Myeloid Derived Suppressor Cells (eMDSCs) Are Associated With High Donor Myeloid Chimer-ism Following Haploidentical HSCT for Sickle Cell Disease. Front. Immunol. 2021, 12, 757279. [Google Scholar] [CrossRef]
- Wherry, E.J.; Teichgräber, V.; Becker, T.C.; Masopust, D.; Kaech, S.M.; Antia, R.; von Andrian, U.H.; Ahmed, R. Lineage Relationship and Protective Immunity of Memory CD8 T Cell Subsets. Nat. Immunol. 2003, 4, 225–234. [Google Scholar] [CrossRef]
- Han, J.; Khatwani, N.; Searles, T.G.; Turk, M.J.; Angeles, C.V. Memory CD8+ T Cell Responses to Cancer. Semin. Immunol. 2020, 49, 101435. [Google Scholar] [CrossRef]
- Sarkar, I.; Pati, S.; Dutta, A.; Basak, U.; Sa, G. T-Memory Cells against Cancer: Remembering the Enemy. Cell Immunol. 2019, 338, 27–31. [Google Scholar] [CrossRef]
- Ring, A.; Nguyen-Sträuli, B.D.; Wicki, A.; Aceto, N. Biology, Vulnerabilities and Clinical Applications of Circulating Tu-mour Cells. Nat. Rev. Cancer 2023, 23, 95–111. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; Vries, E.d.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Ribeiro-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and Efficient Microfluidic Cell Filter for Isolation of Circulating Tumor Cells from Unpro-cessed Whole Blood of Colorectal Cancer Patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef]
- Lopes, C.; Piairo, P.; Chícharo, A.; Abalde-Cela, S.; Pires, L.R.; Corredeira, P.; Alves, P.; Muinelo-Romay, L.; Costa, L.; Dié-guez, L. HER2 Expression in Circulating Tumour Cells Isolated from Metastatic Breast Cancer Patients Using a Size-Based Microfluidic Device. Cancers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Leitão, T.P.; Corredeira, P.; Kucharczak, S.; Rodrigues, M.; Piairo, P.; Rodrigues, C.; Alves, P.; Cavaco, A.M.; Miranda, M.; Antunes, M.; et al. Clinical Validation of a Size-Based Microfluidic Device for Circulating Tumor Cell Isolation and Analy-sis in Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 8404. [Google Scholar] [CrossRef] [PubMed]
- Point Biserial Correlation—Kornbrot—2005—Major Reference Works—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/0470013192.bsa485 (accessed on 6 June 2024).
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Rugo, H.S.; Im, S.-A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2− ABC: Updated Exploratory Analyses of PALOMA-3, a Double-Blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Giudici, F.; Chapman, R.; Darlow, J.; Kilili, H.; Sobhani, N.; Cinelli, M.; Cappelletti, M.R.; Strina, C.; Milani, M.; et al. Clinico-Immunological Effects of a Single-Agent CDK4/6 Inhibitor in Advanced HR+/HER2− Breast Cancer Based on a Window of Opportunity Study. Curr. Issues Mol. Biol. 2022, 44, 4255–4267. [Google Scholar] [CrossRef]
- Rugo, H.S.; Cristofanilli, M.; Loibl, S.; Harbeck, N.; DeMichele, A.; Iwata, H.; Park, Y.H.; Brufsky, A.; Theall, K.P.; Huang, X.; et al. Prognostic Factors for Overall Survival in Patients with Hormone Receptor-Positive Advanced Breast Cancer: Anal-yses From PALOMA-3. Oncologist 2021, 26, e1339–e1346. [Google Scholar] [CrossRef]
- Cuyún Carter, G.; Mohanty, M.; Stenger, K.; Morato Guimaraes, C.; Singuru, S.; Basa, P.; Singh, S.; Tongbram, V.; Kuemmel, S.; Guarneri, V.; et al. Prognostic Factors in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative (HR+/HER2−) Advanced Breast Cancer: A Systematic Literature Review. Cancer Manag. Res. 2021, 13, 6537–6566. [Google Scholar] [CrossRef] [PubMed]
- Belghali, M.Y.; El Moumou, L.; Hazime, R.; Brahimi, M.; El Marrakchi, M.; Belaid, H.A.; Benali, S.A.; Khouchani, M.; Ba-M’hamed, S.; Admou, B. Phenotypic Characterization of Human Peripheral γδT-Cell Subsets in Glioblastoma. Microbiol. Immunol. 2022, 66, 465–476. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Palazón-Carrión, N.; Jiménez-Cortegana, C.; Sánchez-León, M.L.; Henao-Carrasco, F.; Nogales-Fernández, E.; Chiesa, M.; Caballero, R.; Rojo, F.; Nieto-García, M.-A.; Sánchez-Margalet, V.; et al. Circulating Immune Biomarkers in Peripheral Blood Correlate with Clinical Outcomes in Advanced Breast Cancer. Sci. Rep. 2021, 11, 14426. [Google Scholar] [CrossRef]
- Peuker, C.A.; Yaghobramzi, S.; Grunert, C.; Keilholz, L.; Gjerga, E.; Hennig, S.; Schaper, S.; Na, I.-K.; Keller, U.; Brucker, S.; et al. Treatment with Ribociclib Shows Favourable Immunomodulatory Effects in Patients with Hormone Recep-tor-Positive Breast Cancer—Findings from the RIBECCA Trial. Eur. J. Cancer 2022, 162, 45–55. [Google Scholar] [CrossRef] [PubMed]
- de Kruijf, E.M.; Sajet, A.; van Nes, J.G.; Putter, H.; Smit, V.T.; Eagle, R.A.; Jafferji, I.; Trowsdale, J.; Jan Liefers, G.; van de Velde, C.J.H.; et al. NKG2D Ligand Tumor Expression and Association with Clinical Outcome in Early Breast Cancer Patients: An Observa-tional Study. BMC Cancer 2012, 12, 24. [Google Scholar]
- Cristofanilli, M.; Pierga, J.-Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The Clinical Use of Circulating Tumor Cells (CTCs) Enumeration for Staging of Metastatic Breast Cancer (MBC): International Expert Consensus Paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, E.; Dieli, F.; Meraviglia, S. Tumor-Infiltrating Γδ T Lymphocytes: Pathogenic Role, Clinical Significance, and Differential Programing in the Tumor Microenvironment. Front. Immunol. 2014, 5, 607. [Google Scholar] [CrossRef]
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and Disseminated Tumour Cells—Mechanisms of Immune Surveillance and Escape. Nat. Rev. Clin. Oncol. 2017, 14, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, X.; Qin, F.; Yang, J.; Ai, L.; Wang, Q.; Zhai, Y. The Clinical Significance of Circulating Tumor Cells and T Lymphocyte Subtypes in Pancreatic Cancer Patients. Bioengineered 2022, 13, 2128–2136. [Google Scholar] [CrossRef]

| Signature | Role | |
|---|---|---|
| T cell subtype | ||
| CD4 T cells [12] | CD3+ CD4+ | T-helper cells
|
| CD8 T cells [13] | CD3+ CD8+ | Cytotoxic T cells
|
| Treg cells [12,14,15,16,17] | CD3+ CD4+ FoxP3+ | Regulatory T cells
|
| γδ T-cells [18,19,20,21,22,23,24] | A subset of T cells that express a T-cell receptor composed of a γ and a δ chain.
| |
| Vδ1 | CD3+ Vδ1+ | Mostly found in mucosal and epithelial tissues. |
| Vδ2 | CD3+ Vδ2+ | Mostly found in circulation. |
| eMDSCs [14,25,26] | CD3− CD16− CD14− CD11b+ | Early-stage myeloid-derived suppressor cells
|
| Functional state | ||
| Naïve [27] | CD45RA+ CD27+ | Naïve cells
|
| Eff [27] | CD45RA+ CD27− | Effector cells
|
| EM [27,28,29] | CD45RA− CD27− | Effector memory cells
|
| CM [28,29] | CD45RA− CD27+ | Central memory cells
|
| Responders | Non-Responders | Total | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 34 (77.3%) | 10 (22.7%) | 44 (100.0%) | ||||||
| Female, n (%) | 34 (100.0) | 10 (100.0) | 44 (100.0) | - | ||||
| Histology, n (%) | ||||||||
| NST | 25 (73.5) | 7 (70.0) | 32 (72.7) | 0.850 | ||||
| Lobular | 6 (17.6) | 2 (20.0) | 8 (18.2) | |||||
| MD | 3 (8.8) | 1 (10.0) | 4 (9.1) | |||||
| Grade, n (%) | ||||||||
| 1 | 1 (2.9) | 1 (10.0) | 2 (4.5) | 0.686 | ||||
| 2 | 17 (50.0) | 6 (60.0) | 23 (52.3) | |||||
| 3 | 11 (32.4) | 3 (30.0) | 14 (31.8) | |||||
| MD | 5 (14.7) | 0 (0.0) | 5 (11.4) | |||||
| ER | ||||||||
| Median (range) | 100.0 (25.0–100.0) | 90.0 (5.0–100.0) | 95.0 (5.0–100.0) | 0.174 | ||||
| MD, n (%) | 10 (29.4) | 2 (20.0) | 12 (27.3) | |||||
| PR | ||||||||
| Median (range) | 40.0 (0.0–100.0) | 15.0 (1.0–100.0) | 30.0 (0.0–100.0) | 0.970 | ||||
| MD, n (%) | 8 (23.5) | 2 (20.0) | 10 (22.7) | |||||
| Ki67 | ||||||||
| Median | 30.0 (5.0–75.0) | 20.0 (5–40.0) | 27.5 (5–75) | 0.189 | ||||
| <20, n (%) | 5 (14.7) | 3 (30.0) | 8 (18.2) | 0.287 | ||||
| ≥20, n (%) | 21 (61.8) | 5 (50.0) | 26 (59.1) | |||||
| MD, n (%) | 8 (23.5) | 2 (20.0) | 10 (22.7) | |||||
| HER2, n (%) | ||||||||
| 0 | 23 (67.6) | 6 (60.0) | 29 (65.9) | 0.848 | ||||
| 1+ or 2+ ISH non-amplified | 6 (17.6) | 4 (40.0) | 10 (22.7) | |||||
| HER2-negative (unclassified) † | 5 (14.7) | 0 (0.0) | 5 (11.4) | |||||
| DFS | ||||||||
| De novo | 7 (20.6) | 1 (10.0) | 8 (18.2) | 0.747 | ||||
| ≤24 mo | 3 (8.8) | 1 (10.0) | 4 (9.1) | |||||
| >24 mo | 24 (70.6) | 8 (80.0) | 32 (72.7) | |||||
| Median (range), mo * | 85.6 (8.0–245.3) | 80.69 (15.1–131.3) | 84.4 (8.0–245.3) | 0.064 | ||||
| Symptomatic at metastatic disease diagnosis, n (%) | ||||||||
| Yes | 15 (44.1) | 2 (20.0) | 17 (38.6) | 0.759 | ||||
| Previous CT, n (%) | ||||||||
| (Neo)Adjuvant setting | 17 (50.0) | 7 (70.0) | 24 (54.5) | 0.533 | ||||
| Metastatic setting | 2 (5.9) | 1 (10.0) | 3 (6.8) | 0.650 | ||||
| Clinicopathological characteristics at baseline | ||||||||
| Age at baseline, years | ||||||||
| Median (range) | 57 (27–78) | 50.5 (36–71) | 56.5 (27–88) | 0.305 | ||||
| Menopausal status at baseline, n (%) | ||||||||
| Pre- or peri-menopausal | 12 (35.3) | 4 (40.0) | 16 (36.4) | 0.786 | ||||
| Post-menopausal | 22 (64.7) | 6 (60.0) | 28 (63.6) | |||||
| Number of metastatic sites at baseline | ||||||||
| Median (range) | 1.5 (1.0–3.0) | 1.0 (1.0–4.0) | 1.0 (1.0–4.0) | 0.226 | ||||
| 1 metastatic site | 17 (50.0) | 9 (90.0) | 26 (59.1) | 0.024 | ||||
| ≥2 metastatic sites | 17 (50.0) | 1 (10.0) | 18 (40.9) | |||||
| Metastatic sites at baseline, n (%) | ||||||||
| Bone only | 11 (32.4) | 6 (60.0) | 17 (38.6) | 0.114 | ||||
| Bone | 22 (64.7) | 7 (70–0) | 29 (65.9) | 0.756 | ||||
| Lung | 8 (23.5) | 1 (10.0) | 9 (20.5) | 0.351 | ||||
| Liver | 11 (32.4) | 4 (40.0) | 15 (34.1) | 0.654 | ||||
| CNS | 0 (0.0) | 1 (10.0) | 1 (2.3) | 0.062 | ||||
| CDK4/6i therapy line, n (%) | ||||||||
| First | 26 (76.5) | 7 (70.0) | 33 (75.0) | 0.678 | ||||
| Second | 8 (23.5) | 3 (30.0) | 11 (25.0) | |||||
| ET used in combination with CDK4/6i, n (%) | ||||||||
| AI | 23 (67.6) | 5 (50.0) | 28 (63.6) | 0.308 | ||||
| Fulvestrant | 11 (32.4) | 5 (50.0) | 16 (36.4) | |||||
| OFS ¥ | 8 (23.5) | 2 (20.0) | 10 (22.7) | 0.827 | ||||
| CDK4/6i, n (%) | ||||||||
| Ribociclib | 13 (38.2) | 3 (30.0) | 16 (36.4) | 0.605 | ||||
| Palbociclib | 18 (52.9) | 5 (50.0) | 23 (52.3) | |||||
| Abemaciclib | 3 (8.8) | 2 (20.0) | 5 (11.4) | |||||
| BTA, n (%) | 16 (47.1) | 6 (60.0) | 22 (50.0) | 0.295 | ||||
| ECOG-PS at baseline, n (%) | ||||||||
| 0 | 22 (64.7) | 7 (70.0) | 29 (65.9) | 0.640 | ||||
| ≥1 | 7 (20.6) | 1 (10.0) | 8 (18.2) | |||||
| MD | 5 (14.7) | 2 (20.0) | 6 (15.9) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo-Martins, S.; Corredeira, P.; Cavaco, A.; Rodrigues, C.; Piairo, P.; Lopes, C.; Fraga, J.; Silva, M.; Alves, P.; Wachholz Szeneszi, L.; et al. Effect of Cyclin-Dependent Kinase 4/6 Inhibitors on Circulating Cells in Patients with Metastatic Breast Cancer. Cells 2024, 13, 1391. https://doi.org/10.3390/cells13161391
Lobo-Martins S, Corredeira P, Cavaco A, Rodrigues C, Piairo P, Lopes C, Fraga J, Silva M, Alves P, Wachholz Szeneszi L, et al. Effect of Cyclin-Dependent Kinase 4/6 Inhibitors on Circulating Cells in Patients with Metastatic Breast Cancer. Cells. 2024; 13(16):1391. https://doi.org/10.3390/cells13161391
Chicago/Turabian StyleLobo-Martins, Soraia, Patrícia Corredeira, Ana Cavaco, Carolina Rodrigues, Paulina Piairo, Cláudia Lopes, Joana Fraga, Madalena Silva, Patrícia Alves, Lisiana Wachholz Szeneszi, and et al. 2024. "Effect of Cyclin-Dependent Kinase 4/6 Inhibitors on Circulating Cells in Patients with Metastatic Breast Cancer" Cells 13, no. 16: 1391. https://doi.org/10.3390/cells13161391
APA StyleLobo-Martins, S., Corredeira, P., Cavaco, A., Rodrigues, C., Piairo, P., Lopes, C., Fraga, J., Silva, M., Alves, P., Wachholz Szeneszi, L., Barradas, A., Castro Duran, C., Antunes, M., Nogueira-Costa, G., Sousa, R., Pinto, C., Ribeiro, L., Abreu, C., Torres, S., ... Costa, L. (2024). Effect of Cyclin-Dependent Kinase 4/6 Inhibitors on Circulating Cells in Patients with Metastatic Breast Cancer. Cells, 13(16), 1391. https://doi.org/10.3390/cells13161391







