Centriole Translational Planar Polarity in Monociliated Epithelia
Abstract
:1. Introduction
2. Role of Polarity Proteins in Centriole Translational Polarization
2.1. Asymmetric Enrichment of Core PCP Proteins Controls Centriole Planar Polarization
2.2. Apico-Basal Polarity Proteins Modulate PCP Proteins’ Distribution
2.3. Role of Apico-Basal Polarity Proteins as Downstream Effectors of PCP
3. “Ciliary” Proteins Modulate Translational Planar Polarity
3.1. Role of BBS Proteins in Translational PCP
3.2. Role of IFT Proteins in Translational PCP
3.3. Role of Rpgrip1l in Translational PCP
4. Upstream Polarity Cues for PCP Orientation
4.1. Wnt Ligands
4.2. Fat/Dchs Protocadherins
4.3. Mechanical Forces
5. Control of Basal Body Movements Downstream of Polarity and Ciliary Proteins
5.1. Role of Actin and Myosin
5.2. Role of Microtubules
5.2.1. The Role of Microtubules in the Localization of PCP Proteins
5.2.2. Direct Role of Microtubules in Basal Body Movement
5.2.3. Role of Daple and Par3 in Microtubule-Mediated Basal Body Polarization
5.3. Links between Basal Body Appendages and the Cytoskeletal Network during Polarization
6. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Wallingford, J.B. Planar Cell Polarity Signaling, Cilia and Polarized Ciliary Beating. Curr. Opin. Cell Biol. 2010, 22, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T.; Stearns, T. Centriole Age Underlies Asynchronous Primary Cilium Growth in Mammalian Cells. Curr. Biol. 2009, 19, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Al Jord, A.; Lemaître, A.-I.; Delgehyr, N.; Faucourt, M.; Spassky, N.; Meunier, A. Centriole Amplification by Mother and Daughter Centrioles Differs in Multiciliated Cells. Nature 2014, 516, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Reiter, J. How the Centriole Builds Its Cilium: Of Mothers, Daughters, and the Acquisition of Appendages. Curr. Opin. Struct. Biol. 2021, 66, 41–48. [Google Scholar] [CrossRef]
- Sandoz, D.; Chailley, B.; Boisvieux-Ulrich, E.; Lemullois, M.; Laine, M.C.; Bautista-Harris, G. Organization and Functions of Cytoskeleton in Metazoan Ciliated Cells. Biol. Cell 1988, 63, 183–193. [Google Scholar] [CrossRef]
- Kunimoto, K.; Yamazaki, Y.; Nishida, T.; Shinohara, K.; Ishikawa, H.; Hasegawa, T.; Okanoue, T.; Hamada, H.; Noda, T.; Tamura, A.; et al. Coordinated Ciliary Beating Requires Odf2-Mediated Polarization of Basal Bodies via Basal Feet. Cell 2012, 148, 189–200. [Google Scholar] [CrossRef]
- Anstrom, J.A. Organization of the Ciliary Basal Apparatus in Embryonic Cells of the Sea Urchin, Lytechinus Pictus. Cell Tissue Res. 1992, 269, 305–313. [Google Scholar] [CrossRef]
- Mahen, R. The Structure and Function of Centriolar Rootlets. J. Cell Sci. 2021, 134, jcs258544. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Han, Y.-G.; Soriano-Navarro, M.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cilia Organize Ependymal Planar Polarity. J. Neurosci. 2010, 30, 2600–2610. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Rachel, R.A.; Lanford, P.J.; Copeland, N.G.; Jenkins, N.A.; Kelley, M.W. Identification of Vangl2 and Scrb1 as Planar Polarity Genes in Mammals. Nature 2003, 423, 173–177. [Google Scholar] [CrossRef]
- Hashimoto, M.; Shinohara, K.; Wang, J.; Ikeuchi, S.; Yoshiba, S.; Meno, C.; Nonaka, S.; Takada, S.; Hatta, K.; Wynshaw-Boris, A.; et al. Planar Polarization of Node Cells Determines the Rotational Axis of Node Cilia. Nat. Cell Biol. 2010, 12, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.; Labedan, P.; Dimidschstein, J.; Richard, F.; Cremer, H.; André, P.; Yang, Y.; Montcouquiol, M.; Goffinet, A.M.; Tissir, F. A Dual Role for Planar Cell Polarity Genes in Ciliated Cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3129–E3138. [Google Scholar] [CrossRef] [PubMed]
- Borovina, A.; Superina, S.; Voskas, D.; Ciruna, B. Vangl2 Directs the Posterior Tilting and Asymmetric Localization of Motile Primary Cilia. Nat. Cell Biol. 2010, 12, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, K.; Sai, X.; Hamada, H. Role of Wnt Signaling and Planar Cell Polarity in Left-Right Asymmetry. Curr. Top Dev. Biol. 2023, 153, 181–193. [Google Scholar] [CrossRef]
- Schenkelaars, Q.; Fierro-Constain, L.; Renard, E.; Borchiellini, C. Retracing the Path of Planar Cell Polarity. BMC Evol. Biol. 2016, 16, 69. [Google Scholar] [CrossRef]
- Hale, R.; Strutt, D. Conservation of Planar Polarity Pathway Function Across the Animal Kingdom. Annu. Rev. Genet. 2015, 49, 529–551. [Google Scholar] [CrossRef]
- Carvajal-Gonzalez, J.M.; Mulero-Navarro, S.; Mlodzik, M. Centriole Positioning in Epithelial Cells and Its Intimate Relationship with Planar Cell Polarity. Bioessays 2016, 38, 1234–1245. [Google Scholar] [CrossRef]
- Donati, A.; Anselme, I.; Schneider-Maunoury, S.; Vesque, C. Planar Polarization of Cilia in the Zebrafish Floor-Plate Involves Par3-Mediated Posterior Localization of Highly Motile Basal Bodies. Development 2021, 148, dev196386. [Google Scholar] [CrossRef]
- Lepelletier, L.; de Monvel, J.B.; Buisson, J.; Desdouets, C.; Petit, C. Auditory Hair Cell Centrioles Undergo Confined Brownian Motion throughout the Developmental Migration of the Kinocilium. Biophys. J. 2013, 105, 48–58. [Google Scholar] [CrossRef]
- Carvajal-Gonzalez, J.M.; Roman, A.-C.; Mlodzik, M. Positioning of Centrioles Is a Conserved Readout of Frizzled Planar Cell Polarity Signalling. Nat. Commun. 2016, 7, 11135. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Sans, N.; Huss, D.; Kach, J.; Dickman, J.D.; Forge, A.; Rachel, R.A.; Copeland, N.G.; Jenkins, N.A.; Bogani, D.; et al. Asymmetric Localization of Vangl2 and Fz3 Indicate Novel Mechanisms for Planar Cell Polarity in Mammals. J. Neurosci. 2006, 26, 5265–5275. [Google Scholar] [CrossRef]
- Wang, J.; Mark, S.; Zhang, X.; Qian, D.; Yoo, S.-J.; Radde-Gallwitz, K.; Zhang, Y.; Lin, X.; Collazo, A.; Wynshaw-Boris, A.; et al. Regulation of Polarized Extension and Planar Cell Polarity in the Cochlea by the Vertebrate PCP Pathway. Nat. Genet. 2005, 37, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Antic, D.; Stubbs, J.L.; Suyama, K.; Kintner, C.; Scott, M.P.; Axelrod, J.D. Planar Cell Polarity Enables Posterior Localization of Nodal Cilia and Left-Right Axis Determination during Mouse and Xenopus Embryogenesis. PLoS ONE 2010, 5, e8999. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, J.; Chen, W.; Elliott, G.; Andre, P.; Gao, B.; Yang, Y. Planar Cell Polarity Breaks Bilateral Symmetry by Controlling Ciliary Positioning. Nature 2010, 466, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sai, X.; Ikawa, Y.; Nishimura, H.; Mizuno, K.; Kajikawa, E.; Katoh, T.A.; Kimura, T.; Shiratori, H.; Takaoka, K.; Hamada, H.; et al. Planar Cell Polarity-Dependent Asymmetric Organization of Microtubules for Polarized Positioning of the Basal Body in Node Cells. Development 2022, 149, dev200315. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.F.; Mathewson, A.W.; Moens, C.B. PCP Signaling between Migrating Neurons and Their Planar-Polarized Neuroepithelial Environment Controls Filopodial Dynamics and Directional Migration. PLoS Genet. 2016, 12, e1005934. [Google Scholar] [CrossRef]
- Jussila, M.; Boswell, C.W.; Griffiths, N.W.; Pumputis, P.G.; Ciruna, B. Live Imaging and Conditional Disruption of Native PCP Activity Using Endogenously Tagged Zebrafish sfGFP-Vangl2. Nat. Commun. 2022, 13, 5598. [Google Scholar] [CrossRef]
- Wu, J.; Mlodzik, M. The Frizzled Extracellular Domain Is a Ligand for Van Gogh/Stbm during Nonautonomous Planar Cell Polarity Signaling. Dev. Cell 2008, 15, 462–469. [Google Scholar] [CrossRef]
- Mathewson, A.W.; Berman, D.G.; Moens, C.B. Microtubules Are Required for the Maintenance of Planar Cell Polarity in Monociliated Floorplate Cells. Dev. Biol. 2019, 452, 21–33. [Google Scholar] [CrossRef]
- Mitchell, B.; Stubbs, J.L.; Huisman, F.; Taborek, P.; Yu, C.; Kintner, C. The PCP Pathway Instructs the Planar Orientation of Ciliated Cells in the Xenopus Larval Skin. Curr. Biol. 2009, 19, 924–929. [Google Scholar] [CrossRef]
- Sokol, S.Y. Analysis of Dishevelled Signalling Pathways during Xenopus Development. Curr. Biol. 1996, 6, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Mitchell, B.J.; Abitua, P.B.; Kintner, C.; Wallingford, J.B. Dishevelled Controls Apical Docking and Planar Polarization of Basal Bodies in Ciliated Epithelial Cells. Nat. Genet. 2008, 40, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Meunier, A.; Huang, S.; Shimozawa, T.; Yamada, O.; Kida, Y.S.; Inoue, M.; Ito, T.; Kato, H.; Sakaguchi, M.; et al. Planar Polarity of Multiciliated Ependymal Cells Involves the Anterior Migration of Basal Bodies Regulated by Non-Muscle Myosin II. Development 2010, 137, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Guirao, B.; Meunier, A.; Mortaud, S.; Aguilar, A.; Corsi, J.-M.; Strehl, L.; Hirota, Y.; Desoeuvre, A.; Boutin, C.; Han, Y.-G.; et al. Coupling between Hydrodynamic Forces and Planar Cell Polarity Orients Mammalian Motile Cilia. Nat. Cell Biol. 2010, 12, 341–350. [Google Scholar] [CrossRef]
- Tissir, F.; Qu, Y.; Montcouquiol, M.; Zhou, L.; Komatsu, K.; Shi, D.; Fujimori, T.; Labeau, J.; Tyteca, D.; Courtoy, P.; et al. Lack of Cadherins Celsr2 and Celsr3 Impairs Ependymal Ciliogenesis, Leading to Fatal Hydrocephalus. Nat. Neurosci. 2010, 13, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Momose, T.; Kraus, Y.; Houliston, E. A Conserved Function for Strabismus in Establishing Planar Cell Polarity in the Ciliated Ectoderm during Cnidarian Larval Development. Development 2012, 139, 4374–4382. [Google Scholar] [CrossRef]
- Djiane, A.; Yogev, S.; Mlodzik, M. The Apical Determinants aPKC and dPatj Regulate Frizzled-Dependent Planar Cell Polarity in the Drosophila Eye. Cell 2005, 121, 621–631. [Google Scholar] [CrossRef]
- Wasserscheid, I.; Thomas, U.; Knust, E. Isoform-Specific Interaction of Flamingo/Starry Night with Excess Bazooka Affects Planar Cell Polarity in the Drosophila Wing. Dev. Dyn. 2007, 236, 1064–1071. [Google Scholar] [CrossRef]
- Kharfallah, F.; Guyot, M.C.; El Hassan, A.R.; Allache, R.; Merello, E.; De Marco, P.; Di Cristo, G.; Capra, V.; Kibar, Z. Scribble1 Plays an Important Role in the Pathogenesis of Neural Tube Defects through Its Mediating Effect of Par-3 and Vangl1/2 Localization. Hum. Mol. Genet. 2017, 26, 2307–2320. [Google Scholar] [CrossRef]
- Besson, C.; Bernard, F.; Corson, F.; Rouault, H.; Reynaud, E.; Keder, A.; Mazouni, K.; Schweisguth, F. Planar Cell Polarity Breaks the Symmetry of PAR Protein Distribution Prior to Mitosis in Drosophila Sensory Organ Precursor Cells. Curr. Biol. 2015, 25, 1104–1110. [Google Scholar] [CrossRef]
- Banerjee, J.J.; Aerne, B.L.; Holder, M.V.; Hauri, S.; Gstaiger, M.; Tapon, N. Meru Couples Planar Cell Polarity with Apical-Basal Polarity during Asymmetric Cell Division. eLife 2017, 6, e25014. [Google Scholar] [CrossRef] [PubMed]
- Bellaïche, Y.; Beaudoin-Massiani, O.; Stuttem, I.; Schweisguth, F. The Planar Cell Polarity Protein Strabismus Promotes Pins Anterior Localization during Asymmetric Division of Sensory Organ Precursor Cells in Drosophila. Development 2004, 131, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Bellaïche, Y.; Radovic, A.; Woods, D.F.; Hough, C.D.; Parmentier, M.L.; O’Kane, C.J.; Bryant, P.J.; Schweisguth, F. The Partner of Inscuteable/Discs-Large Complex Is Required to Establish Planar Polarity during Asymmetric Cell Division in Drosophila. Cell 2001, 106, 355–366. [Google Scholar] [CrossRef]
- Bellaïche, Y.; Gho, M.; Kaltschmidt, J.A.; Brand, A.H.; Schweisguth, F. Frizzled Regulates Localization of Cell-Fate Determinants and Mitotic Spindle Rotation during Asymmetric Cell Division. Nat. Cell Biol. 2001, 3, 50–57. [Google Scholar] [CrossRef]
- Aigouy, B.; Le Bivic, A. The PCP Pathway Regulates Baz Planar Distribution in Epithelial Cells. Sci. Rep. 2016, 6, 33420. [Google Scholar] [CrossRef] [PubMed]
- Chuykin, I.; Ossipova, O.; Sokol, S.Y. Par3 Interacts with Prickle3 to Generate Apical PCP Complexes in the Vertebrate Neural Plate. eLife 2018, 7, e37881. [Google Scholar] [CrossRef]
- Landin Malt, A.; Dailey, Z.; Holbrook-Rasmussen, J.; Zheng, Y.; Hogan, A.; Du, Q.; Lu, X. Par3 Is Essential for the Establishment of Planar Cell Polarity of Inner Ear Hair Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 4999–5008. [Google Scholar] [CrossRef]
- Siletti, K.; Tarchini, B.; Hudspeth, A.J. Daple Coordinates Organ-Wide and Cell-Intrinsic Polarity to Pattern Inner-Ear Hair Bundles. Proc. Natl. Acad. Sci. USA 2017, 114, E11170–E11179. [Google Scholar] [CrossRef]
- Hua, K.; Ferland, R.J. Primary Cilia Proteins: Ciliary and Extraciliary Sites and Functions. Cell Mol. Life Sci. 2018, 75, 1521–1540. [Google Scholar] [CrossRef]
- Nachury, M.V.; Mick, D.U. Establishing and Regulating the Composition of Cilia for Signal Transduction. Nat. Rev. Mol. Cell Biol. 2019, 20, 389–405. [Google Scholar] [CrossRef]
- Ross, A.J.; May-Simera, H.; Eichers, E.R.; Kai, M.; Hill, J.; Jagger, D.J.; Leitch, C.C.; Chapple, J.P.; Munro, P.M.; Fisher, S.; et al. Disruption of Bardet-Biedl Syndrome Ciliary Proteins Perturbs Planar Cell Polarity in Vertebrates. Nat. Genet. 2005, 37, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Badano, J.L.; Sibold, S.; Esmail, M.A.; Hill, J.; Hoskins, B.E.; Leitch, C.C.; Venner, K.; Ansley, S.J.; Ross, A.J.; et al. The Bardet-Biedl Protein BBS4 Targets Cargo to the Pericentriolar Region and Is Required for Microtubule Anchoring and Cell Cycle Progression. Nat. Genet. 2004, 36, 462–470. [Google Scholar] [CrossRef]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The Intraflagellar Transport Protein IFT20 Is Associated with the Golgi Complex and Is Required for Cilia Assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- May-Simera, H.L.; Petralia, R.S.; Montcouquiol, M.; Wang, Y.-X.; Szarama, K.B.; Liu, Y.; Lin, W.; Deans, M.R.; Pazour, G.J.; Kelley, M.W. Ciliary Proteins Bbs8 and Ift20 Promote Planar Cell Polarity in the Cochlea. Development 2015, 142, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.D.; Coyaud, É.; Gonçalves, J.; Mojarad, B.A.; Liu, Y.; Wu, Q.; Gheiratmand, L.; Comartin, D.; Tkach, J.M.; Cheung, S.W.T.; et al. A Dynamic Protein Interaction Landscape of the Human Centrosome-Cilium Interface. Cell 2015, 163, 1484–1499. [Google Scholar] [CrossRef]
- Jones, C.; Roper, V.C.; Foucher, I.; Qian, D.; Banizs, B.; Petit, C.; Yoder, B.K.; Chen, P. Ciliary Proteins Link Basal Body Polarization to Planar Cell Polarity Regulation. Nat. Genet. 2008, 40, 69–77. [Google Scholar] [CrossRef]
- Sipe, C.W.; Lu, X. Kif3a Regulates Planar Polarization of Auditory Hair Cells through Both Ciliary and Non-Ciliary Mechanisms. Development 2011, 138, 3441–3449. [Google Scholar] [CrossRef]
- Delaval, B.; Bright, A.; Lawson, N.D.; Doxsey, S. The Cilia Protein IFT88 Is Required for Spindle Orientation in Mitosis. Nat. Cell Biol. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Borovina, A.; Ciruna, B. IFT88 Plays a Cilia- and PCP-Independent Role in Controlling Oriented Cell Divisions during Vertebrate Embryonic Development. Cell Rep. 2013, 5, 37–43. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Malicki, J. Intraflagellar Transport Genes Are Essential for Differentiation and Survival of Vertebrate Sensory Neurons. Neuron 2004, 42, 703–716. [Google Scholar] [CrossRef]
- Kodani, A.; Salomé Sirerol-Piquer, M.; Seol, A.; Garcia-Verdugo, J.M.; Reiter, J.F. Kif3a Interacts with Dynactin Subunit P150 Glued to Organize Centriole Subdistal Appendages. EMBO J. 2013, 32, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Mahuzier, A.; Gaudé, H.-M.; Grampa, V.; Anselme, I.; Silbermann, F.; Leroux-Berger, M.; Delacour, D.; Ezan, J.; Montcouquiol, M.; Saunier, S.; et al. Dishevelled Stabilization by the Ciliopathy Protein Rpgrip1l Is Essential for Planar Cell Polarity. J. Cell Biol. 2012, 198, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Ohata, S.; Herranz-Pérez, V.; Nakatani, J.; Boletta, A.; García-Verdugo, J.M.; Álvarez-Buylla, A. Mechanosensory Genes Pkd1 and Pkd2 Contribute to the Planar Polarization of Brain Ventricular Epithelium. J. Neurosci. 2015, 35, 11153–11168. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.M.; van Amerongen, R.; Nusse, R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev. Cell 2016, 38, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.J.; Chen, B.-C.; Tsai, F.-C.; Anastassiadis, K.; Meyer, T.; Betzig, E.; Nusse, R. A Localized Wnt Signal Orients Asymmetric Stem Cell Division in Vitro. Science 2013, 339, 1445–1448. [Google Scholar] [CrossRef]
- Wu, J.; Roman, A.-C.; Carvajal-Gonzalez, J.M.; Mlodzik, M. Wg and Wnt4 Provide Long-Range Directional Input to Planar Cell Polarity Orientation in Drosophila. Nat. Cell Biol. 2013, 15, 1045–1055. [Google Scholar] [CrossRef]
- Ewen-Campen, B.; Comyn, T.; Vogt, E.; Perrimon, N. No Evidence That Wnt Ligands Are Required for Planar Cell Polarity in Drosophila. Cell Rep. 2020, 32, 108121. [Google Scholar] [CrossRef]
- Yu, J.J.S.; Maugarny-Calès, A.; Pelletier, S.; Alexandre, C.; Bellaiche, Y.; Vincent, J.-P.; McGough, I.J. Frizzled-Dependent Planar Cell Polarity without Secreted Wnt Ligands. Dev. Cell 2020, 54, 583–592. [Google Scholar] [CrossRef]
- Gubb, D.; García-Bellido, A. A Genetic Analysis of the Determination of Cuticular Polarity during Development in Drosophila Melanogaster. J. Embryol. Exp. Morphol. 1982, 68, 37–57. [Google Scholar]
- Qian, D.; Jones, C.; Rzadzinska, A.; Mark, S.; Zhang, X.; Steel, K.P.; Dai, X.; Chen, P. Wnt5a Functions in Planar Cell Polarity Regulation in Mice. Dev. Biol. 2007, 306, 121–133. [Google Scholar] [CrossRef]
- Landin Malt, A.; Hogan, A.K.; Smith, C.D.; Madani, M.S.; Lu, X. Wnts Regulate Planar Cell Polarity via Heterotrimeric G Protein and PI3K Signaling. J. Cell Biol. 2020, 219, e201912071. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, K.; Hashimoto, M.; Ajima, R.; Takaoka, K.; Shinohara, K.; Ikawa, Y.; Nishimura, H.; McMahon, A.P.; Willert, K.; Okada, Y.; et al. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Dev. Cell 2017, 40, 439–452. [Google Scholar] [CrossRef]
- Willecke, M.; Hamaratoglu, F.; Sansores-Garcia, L.; Tao, C.; Halder, G. Boundaries of Dachsous Cadherin Activity Modulate the Hippo Signaling Pathway to Induce Cell Proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 14897–14902. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.; Brittle, A.L.; Fisher, K.H.; Monk, N.A.M.; Strutt, D. Cellular Interpretation of the Long-Range Gradient of Four-Jointed Activity in the Drosophila Wing. eLife 2015, 4, e05789. [Google Scholar] [CrossRef] [PubMed]
- Brittle, A.; Thomas, C.; Strutt, D. Planar Polarity Specification through Asymmetric Subcellular Localization of Fat and Dachsous. Curr. Biol. 2012, 22, 907–914. [Google Scholar] [CrossRef]
- Shimada, Y.; Yonemura, S.; Ohkura, H.; Strutt, D.; Uemura, T. Polarized Transport of Frizzled along the Planar Microtubule Arrays in Drosophila Wing Epithelium. Dev. Cell 2006, 10, 209–222. [Google Scholar] [CrossRef]
- Garrido-Jimenez, S.; Roman, A.-C.; Carvajal-Gonzalez, J.M. Diminished Expression of Fat and Dachsous PCP Proteins Impaired Centriole Planar Polarization in Drosophila. Front. Genet. 2019, 10, 328. [Google Scholar] [CrossRef]
- Mao, Y.; Kuta, A.; Crespo-Enriquez, I.; Whiting, D.; Martin, T.; Mulvaney, J.; Irvine, K.D.; Francis-West, P. Dchs1-Fat4 Regulation of Polarized Cell Behaviours during Skeletal Morphogenesis. Nat. Commun. 2016, 7, 11469. [Google Scholar] [CrossRef]
- Saburi, S.; Hester, I.; Fischer, E.; Pontoglio, M.; Eremina, V.; Gessler, M.; Quaggin, S.E.; Harrison, R.; Mount, R.; McNeill, H. Loss of Fat4 Disrupts PCP Signaling and Oriented Cell Division and Leads to Cystic Kidney Disease. Nat. Genet. 2008, 40, 1010–1015. [Google Scholar] [CrossRef]
- Mitchell, B.; Jacobs, R.; Li, J.; Chien, S.; Kintner, C. A Positive Feedback Mechanism Governs the Polarity and Motion of Motile Cilia. Nature 2007, 447, 97–101. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Srinivasan, S.; Keller, R.; Kintner, C. Mechanical Strain Determines Cilia Length, Motility, and Planar Position in the Left-Right Organizer. Dev. Cell 2018, 45, 316–330. [Google Scholar] [CrossRef]
- Garrido-Jimenez, S.; Roman, A.-C.; Alvarez-Barrientos, A.; Carvajal-Gonzalez, J.M. Centriole Planar Polarity Assessment in Drosophila Wings. Development 2018, 145, dev169326. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, I.; Stylianou, P.; Skourides, P.A. Making the Connection: Ciliary Adhesion Complexes Anchor Basal Bodies to the Actin Cytoskeleton. Dev. Cell 2014, 28, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Bornens, M. The Centrosome in Cells and Organisms. Science 2012, 335, 422–426. [Google Scholar] [CrossRef]
- Vladar, E.K.; Bayly, R.D.; Sangoram, A.M.; Scott, M.P.; Axelrod, J.D. Microtubules Enable the Planar Cell Polarity of Airway Cilia. Curr. Biol. 2012, 22, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Wepf, R.; Simons, K. Roles for Rac1 and Cdc42 in Planar Polarization and Hair Outgrowth in the Wing of Drosophila. J. Cell Biol. 1996, 135, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Hannus, M.; Feiguin, F.; Heisenberg, C.-P.; Eaton, S. Planar Cell Polarization Requires Widerborst, a B’ Regulatory Subunit of Protein Phosphatase 2A. Development 2002, 129, 3493–3503. [Google Scholar] [CrossRef]
- Olofsson, J.; Sharp, K.A.; Matis, M.; Cho, B.; Axelrod, J.D. Prickle/Spiny-Legs Isoforms Control the Polarity of the Apical Microtubule Network in Planar Cell Polarity. Development 2014, 141, 2866–2874. [Google Scholar] [CrossRef]
- Matis, M.; Russler-Germain, D.A.; Hu, Q.; Tomlin, C.J.; Axelrod, J.D. Microtubules Provide Directional Information for Core PCP Function. eLife 2014, 3, e02893. [Google Scholar] [CrossRef]
- Sepich, D.S.; Usmani, M.; Pawlicki, S.; Solnica-Krezel, L. Wnt/PCP Signaling Controls Intracellular Position of MTOCs during Gastrulation Convergence and Extension Movements. Development 2011, 138, 543–552. [Google Scholar] [CrossRef]
- Shi, D.; Usami, F.; Komatsu, K.; Oka, S.; Abe, T.; Uemura, T.; Fujimori, T. Dynamics of Planar Cell Polarity Protein Vangl2 in the Mouse Oviduct Epithelium. Mech. Dev. 2016, 141, 78–89. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Keller, R.; Kintner, C.; Shook, D.R. Mechanical Strain Determines the Axis of Planar Polarity in Ciliated Epithelia. Curr. Biol. 2015, 25, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.E.; Ren, X.; Ward, L.C.; Girdler, G.C.; Araya, C.; Green, M.J.; Clark, B.S.; Link, B.A.; Clarke, J.D.W. Mirror-Symmetric Microtubule Assembly and Cell Interactions Drive Lumen Formation in the Zebrafish Neural Rod. EMBO J. 2013, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Ezan, J.; Lasvaux, L.; Gezer, A.; Novakovic, A.; May-Simera, H.; Belotti, E.; Lhoumeau, A.-C.; Birnbaumer, L.; Beer-Hammer, S.; Borg, J.-P.; et al. Primary Cilium Migration Depends on G-Protein Signalling Control of Subapical Cytoskeleton. Nat. Cell Biol. 2013, 15, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Tarchini, B.; Lu, X. New Insights into Regulation and Function of Planar Polarity in the Inner Ear. Neurosci. Lett. 2019, 709, 134373. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Miyazaki, N.; Murata, K.; Yasuo, H.; Ueno, N. Physical Association between a Novel Plasma-Membrane Structure and Centrosome Orients Cell Division. eLife 2016, 5, e16550. [Google Scholar] [CrossRef]
- Yi, J.; Wu, X.; Chung, A.H.; Chen, J.K.; Kapoor, T.M.; Hammer, J.A. Centrosome Repositioning in T Cells Is Biphasic and Driven by Microtubule End-on Capture-Shrinkage. J. Cell Biol. 2013, 202, 779–792. [Google Scholar] [CrossRef]
- Takagishi, M.; Esaki, N.; Takahashi, K.; Takahashi, M. Cytoplasmic Dynein Functions in Planar Polarization of Basal Bodies within Ciliated Cells. iScience 2020, 23, 101213. [Google Scholar] [CrossRef]
- Nakayama, S.; Yano, T.; Namba, T.; Konishi, S.; Takagishi, M.; Herawati, E.; Nishida, T.; Imoto, Y.; Ishihara, S.; Takahashi, M.; et al. Planar Cell Polarity Induces Local Microtubule Bundling for Coordinated Ciliary Beating. J. Cell Biol. 2021, 220, e202010034. [Google Scholar] [CrossRef]
- Schmoranzer, J.; Fawcett, J.P.; Segura, M.; Tan, S.; Vallee, R.B.; Pawson, T.; Gundersen, G.G. Par3 and Dynein Associate to Regulate Local Microtubule Dynamics and Centrosome Orientation during Migration. Curr. Biol. 2009, 19, 1065–1074. [Google Scholar] [CrossRef]
- Schober, M.; Schaefer, M.; Knoblich, J.A. Bazooka Recruits Inscuteable to Orient Asymmetric Cell Divisions in Drosophila Neuroblasts. Nature 1999, 402, 548–551. [Google Scholar] [CrossRef]
- Moore, R.; Theveneau, E.; Pozzi, S.; Alexandre, P.; Richardson, J.; Merks, A.; Parsons, M.; Kashef, J.; Linker, C.; Mayor, R. Par3 Controls Neural Crest Migration by Promoting Microtubule Catastrophe during Contact Inhibition of Locomotion. Development 2013, 140, 4763–4775. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Aoki, T.; Ohwada, N.; Fujimoto, T. Identification of a 195 kDa Protein in the Striated Rootlet: Its Expression in Ciliated and Ciliogenic Cells. Cell Motil. Cytoskelet. 2000, 45, 200–210. [Google Scholar] [CrossRef]
- Clare, D.K.; Magescas, J.; Piolot, T.; Dumoux, M.; Vesque, C.; Pichard, E.; Dang, T.; Duvauchelle, B.; Poirier, F.; Delacour, D. Basal Foot MTOC Organizes Pillar MTs Required for Coordination of Beating Cilia. Nat. Commun. 2014, 5, 4888. [Google Scholar] [CrossRef]
- Werner, M.E.; Hwang, P.; Huisman, F.; Taborek, P.; Yu, C.C.; Mitchell, B.J. Actin and Microtubules Drive Differential Aspects of Planar Cell Polarity in Multiciliated Cells. J. Cell Biol. 2011, 195, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Herawati, E.; Taniguchi, D.; Kanoh, H.; Tateishi, K.; Ishihara, S.; Tsukita, S. Multiciliated cell basal bodies align in stereo-typical patterns coordinated by the apical cytoskeleton. J. Cell Biol. 2016, 214, 571–586. [Google Scholar] [CrossRef]
- Basquin, C.; Ershov, D.; Gaudin, N.; Vu, H.T.-K.; Louis, B.; Papon, J.-F.; Orfila, A.-M.; Mansour, S.; Rink, J.C.; Azimzadeh, J. Emergence of a Bilaterally Symmetric Pattern from Chiral Components in the Planarian Epidermis. Dev. Cell 2019, 51, 516–525. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Yue, G.; Adamian, M.; Bulgakov, O.; Li, T. Rootletin, a Novel Coiled-Coil Protein, Is a Structural Component of the Ciliary Rootlet. J. Cell Biol. 2002, 159, 431–440. [Google Scholar] [CrossRef]
- Chong, W.M.; Wang, W.-J.; Lo, C.-H.; Chiu, T.-Y.; Chang, T.-J.; Liu, Y.-P.; Tanos, B.; Mazo, G.; Tsou, M.-F.B.; Jane, W.-N.; et al. Super-Resolution Microscopy Reveals Coupling between Mammalian Centriole Subdistal Appendages and Distal Appendages. eLife 2020, 9, e53580. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Balashova, E.E.; Smirnov, V.N.; Bystrevskaya, V.B. Detection of the Centriole Tyr- or Acet-Tubulin Changes in Endothelial Cells Treated with Thrombin Using Microscopic Immunocytochemistry. Cell Motil. Cytoskelet. 2005, 62, 1–12. [Google Scholar] [CrossRef]
- Gaudin, N.; Martin Gil, P.; Boumendjel, M.; Ershov, D.; Pioche-Durieu, C.; Bouix, M.; Delobelle, Q.; Maniscalco, L.; Phan, T.B.N.; Heyer, V.; et al. Evolutionary Conservation of Centriole Rotational Asymmetry in the Human Centrosome. eLife 2022, 11, e72382. [Google Scholar] [CrossRef]
- Buckley, C.E.; Moore, R.E.; Reade, A.; Goldberg, A.R.; Weiner, O.D.; Clarke, J.D.W. Reversible Optogenetic Control of Subcellular Protein Localization in a Live Vertebrate Embryo. Dev. Cell 2016, 36, 117–126. [Google Scholar] [CrossRef]
- Pitaval, A.; Senger, F.; Letort, G.; Gidrol, X.; Guyon, L.; Sillibourne, J.; Théry, M. Microtubule Stabilization Drives 3D Centrosome Migration to Initiate Primary Ciliogenesis. J. Cell Biol. 2017, 216, 3713–3728. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Jayachandran, P.; Brewster, R. The Polarity Protein Pard3 Is Required for Centrosome Positioning during Neurulation. Dev. Biol. 2010, 341, 335–345. [Google Scholar] [CrossRef]
- Inaba, M.; Venkei, Z.G.; Yamashita, Y.M. The Polarity Protein Baz Forms a Platform for the Centrosome Orientation during Asymmetric Stem Cell Division in the Drosophila Male Germline. eLife 2015, 4, e04960. [Google Scholar] [CrossRef] [PubMed]
- Humphries, A.C.; Narang, S.; Mlodzik, M. Mutations Associated with Human Neural Tube Defects Display Disrupted Planar Cell Polarity in Drosophila. eLife 2020, 9, e53532. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; An, Y.; Gao, Y.; Guo, L.; Rui, L.; Xie, H.; Sun, M.; Lam Hung, S.; Sheng, X.; Zou, J.; et al. Rare Deleterious PARD3 Variants in the aPKC-Binding Region Are Implicated in the Pathogenesis of Human Cranial Neural Tube Defects Via Disrupting Apical Tight Junction Formation. Hum. Mutat. 2017, 38, 378–389. [Google Scholar] [CrossRef]
- Marguet, F.; Vezain, M.; Marcorelles, P.; Audebert-Bellanger, S.; Cassinari, K.; Drouot, N.; Chambon, P.; Gonzalez, B.J.; Horowitz, A.; Laquerriere, A.; et al. Neuropathological Hallmarks of Fetal Hydrocephalus Linked to CCDC88C Pathogenic Variants. Acta. Neuropathol. Commun. 2021, 9, 104. [Google Scholar] [CrossRef]
- Wallmeier, J.; Frank, D.; Shoemark, A.; Nöthe-Menchen, T.; Cindric, S.; Olbrich, H.; Loges, N.T.; Aprea, I.; Dougherty, G.W.; Pennekamp, P.; et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am. J. Hum. Genet. 2019, 105, 1030–1039. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Valente, E.M. Motile and Non-Motile Cilia in Human Pathology: From Function to Phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef]
- Rose, C.D.; Pompili, D.; Henke, K.; Van Gennip, J.L.M.; Meyer-Miner, A.; Rana, R.; Gobron, S.; Harris, M.P.; Nitz, M.; Ciruna, B. SCO-Spondin Defects and Neuroinflammation Are Conserved Mechanisms Driving Spinal Deformity across Genetic Models of Idiopathic Scoliosis. Curr. Biol. 2020, 30, 2363–2373.e6. [Google Scholar] [CrossRef] [PubMed]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Curr. Biol. 2020, 30, 2353–2362.e3. [Google Scholar] [CrossRef] [PubMed]
- Blasky, A.J.; Pan, L.; Moens, C.B.; Appel, B. Pard3 Regulates Contact between Neural Crest Cells and the Timing of Schwann Cell Differentiation but Is Not Essential for Neural Crest Migration or Myelination. Dev. Dyn. 2014, 243, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Jokura, K.; Shibata, D.; Yamaguchi, K.; Shiba, K.; Makino, Y.; Shigenobu, S.; Inaba, K. CTENO64 Is Required for Coordinated Paddling of Ciliary Comb Plate in Ctenophores. Curr. Biol. 2019, 29, 3510–3516.e4. [Google Scholar] [CrossRef]
- Jokura, K.; Sato, Y.; Shiba, K.; Inaba, K. Two Distinct Compartments of a Ctenophore Comb Plate Provide Structural and Functional Integrity for the Motility of Giant Multicilia. Curr. Biol. 2022, 32, 5144–5152.e6. [Google Scholar] [CrossRef]
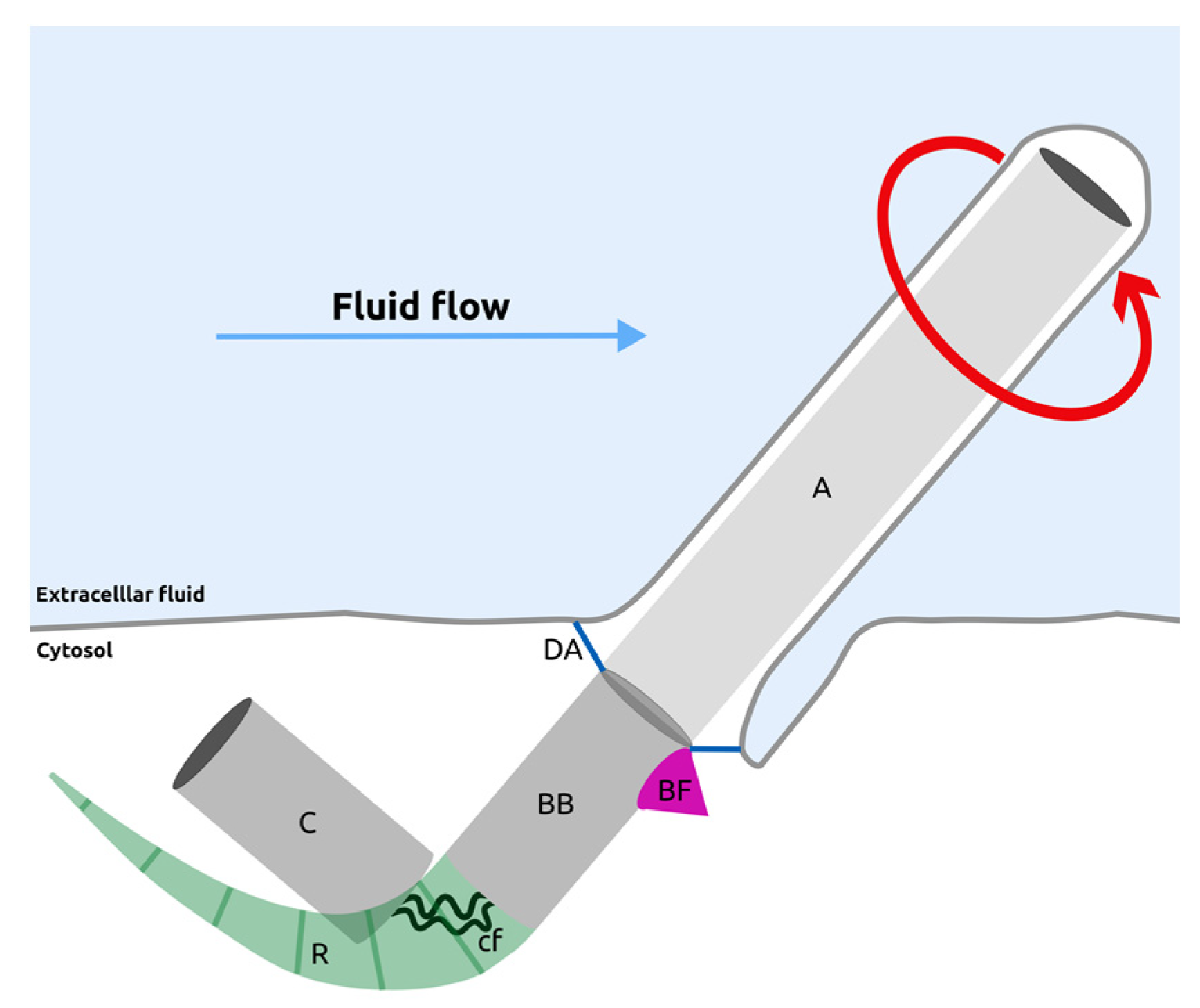

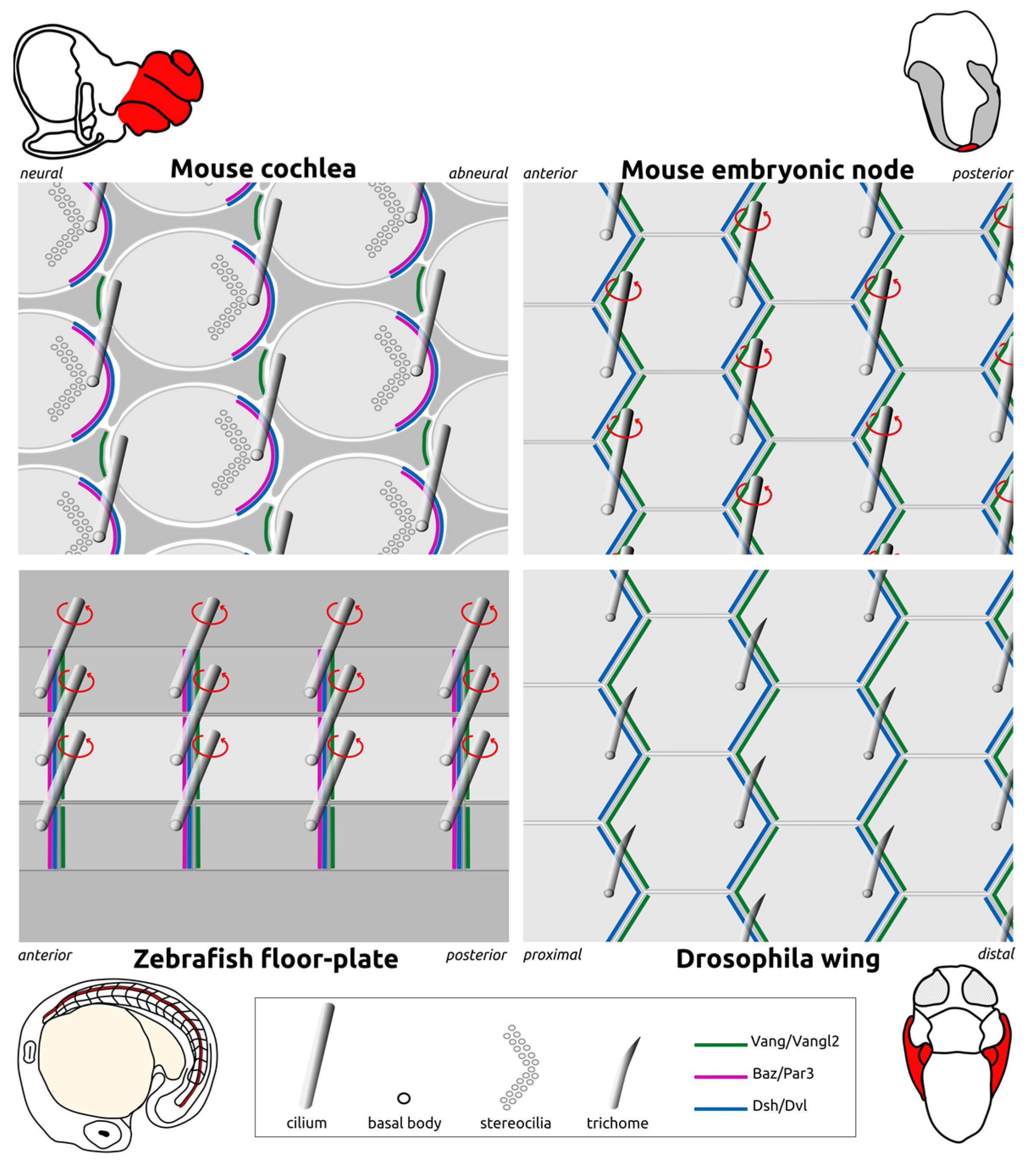
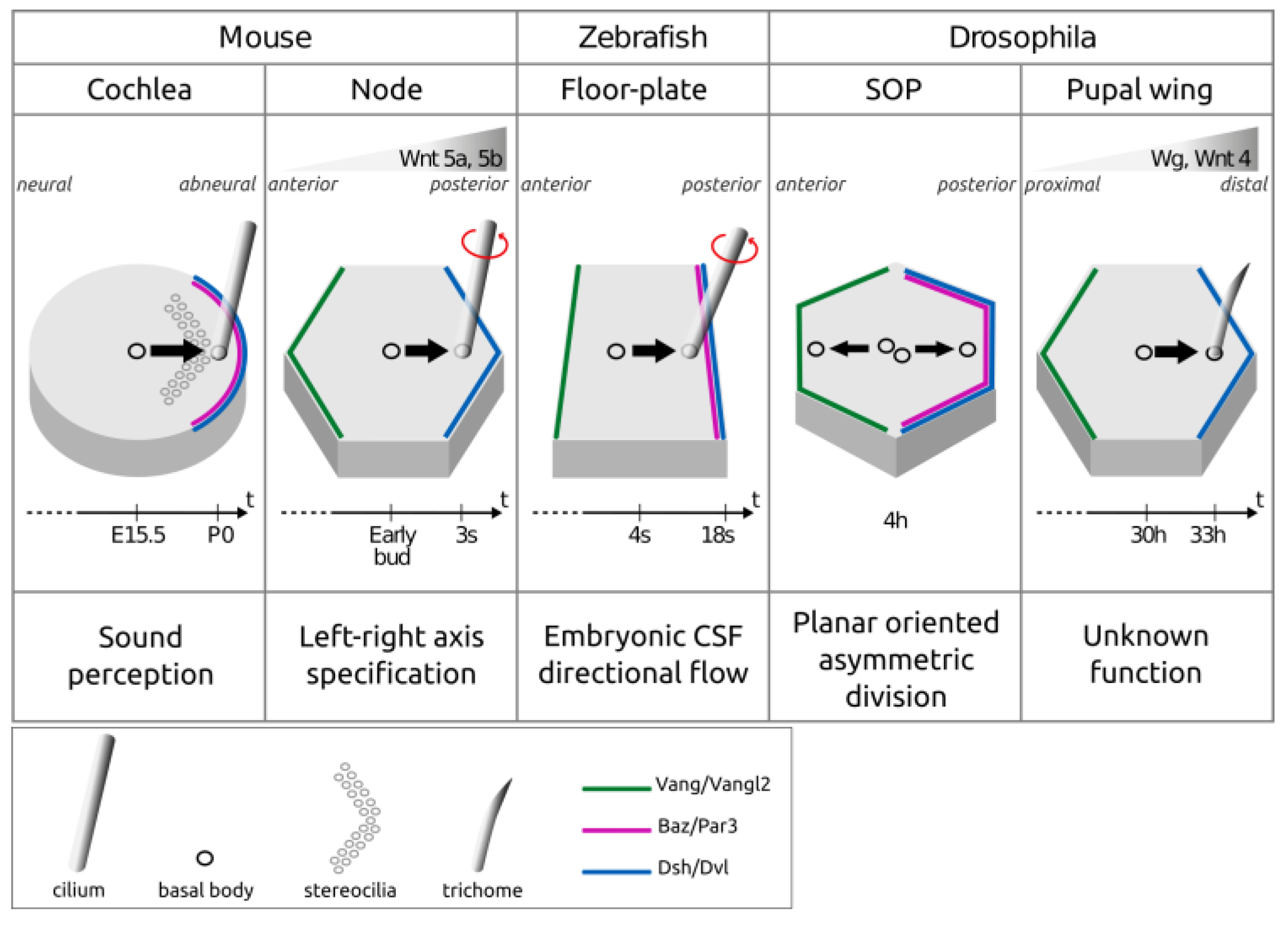

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, A.; Schneider-Maunoury, S.; Vesque, C. Centriole Translational Planar Polarity in Monociliated Epithelia. Cells 2024, 13, 1403. https://doi.org/10.3390/cells13171403
Donati A, Schneider-Maunoury S, Vesque C. Centriole Translational Planar Polarity in Monociliated Epithelia. Cells. 2024; 13(17):1403. https://doi.org/10.3390/cells13171403
Chicago/Turabian StyleDonati, Antoine, Sylvie Schneider-Maunoury, and Christine Vesque. 2024. "Centriole Translational Planar Polarity in Monociliated Epithelia" Cells 13, no. 17: 1403. https://doi.org/10.3390/cells13171403
APA StyleDonati, A., Schneider-Maunoury, S., & Vesque, C. (2024). Centriole Translational Planar Polarity in Monociliated Epithelia. Cells, 13(17), 1403. https://doi.org/10.3390/cells13171403





