Effects of Antipsychotics on the Hypothalamus–Pituitary–Adrenal Axis in a Phencyclidine Animal Model of Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treatment Groups
- (1)
- NaCl group (control): these animals received perinatal NaCl treatment (n = 7); starting on PN day 35, acetic acid (at a final concentration of 1mM) was introduced into the drinking water, matching the concentration used for antipsychotic dissolution.
- (2)
- PCP group: these animals received perinatal PCP treatment (n = 7); starting on PN day 35, acetic acid was added to drinking water as in group 1.
- (3)
- NaCl–H group: these animals received perinatal NaCl treatment (n = 7); starting on PN day 35, haloperidol (H) treatment (Krka, Slovenia) was initiated at a dosage of 1 mg/kg/day.
- (4)
- PCP–H group: these animals received perinatal PCP treatment (n = 7); starting on PN day 35, this group received haloperidol therapy like group 3.
- (5)
- NaCl–C group: these animals received perinatal NaCl treatment (n = 6); starting on PN day 35, clozapine (C) treatment (Sandoz, Barleben, Germany) was initiated at a dosage of 20 mg/kg/day.
- (6)
- PCP–C group: animals received perinatal PCP treatment (n = 6); starting on PN35 received clozapine therapy like group 5.
2.3. Dissection of Rats
2.4. Determination of Corticosterone Concentration
2.5. Quantitative Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Effects of Perinatal PCP Administration and Antipsychotic Treatment on Corticosterone Concentration in the Serum of Rats
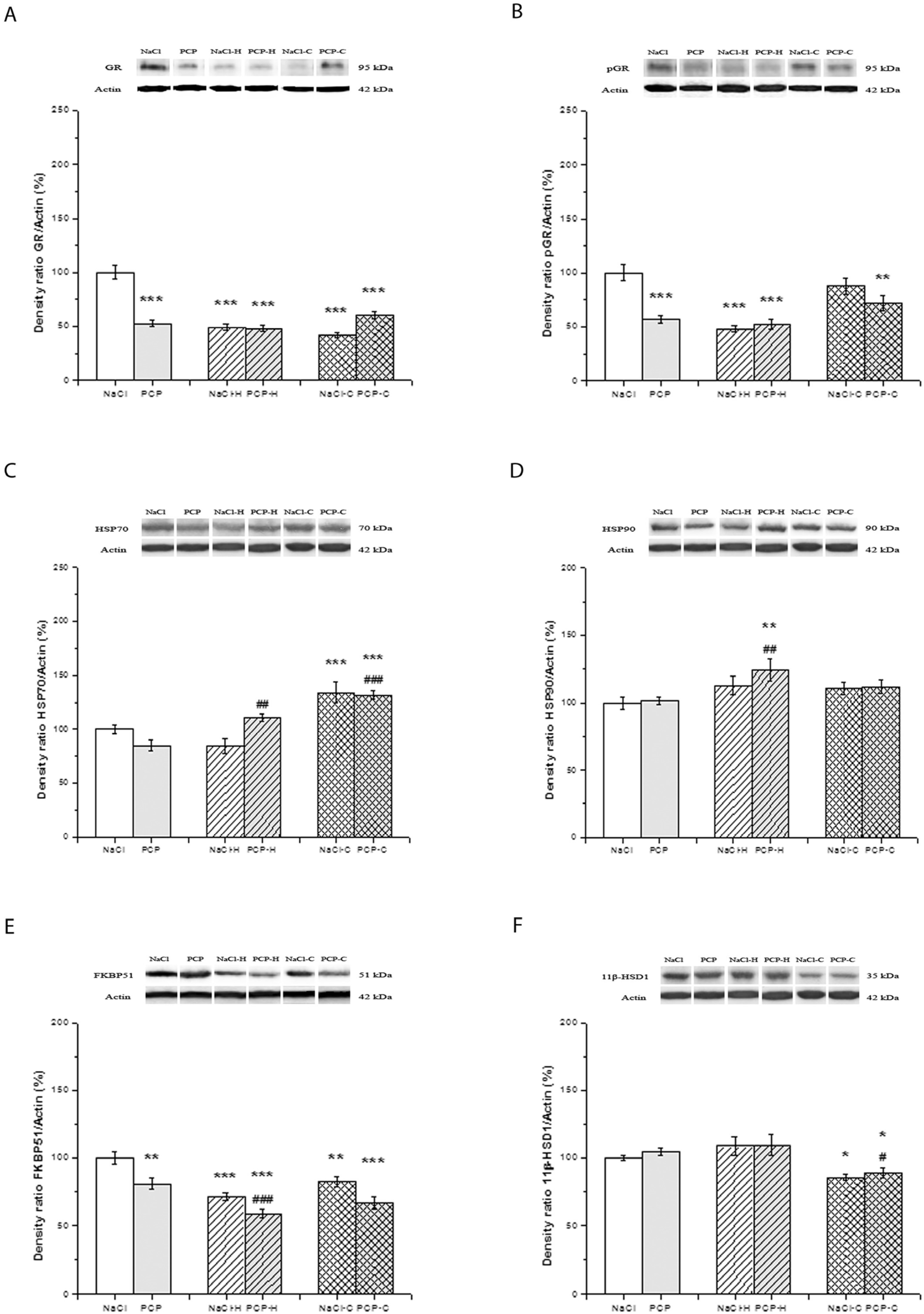
3.2. Effects of Perinatal PCP Administration and Antipsychotic Treatment on the Activity of GR Receptor and 11β-HSD1 Expression in the Cortex
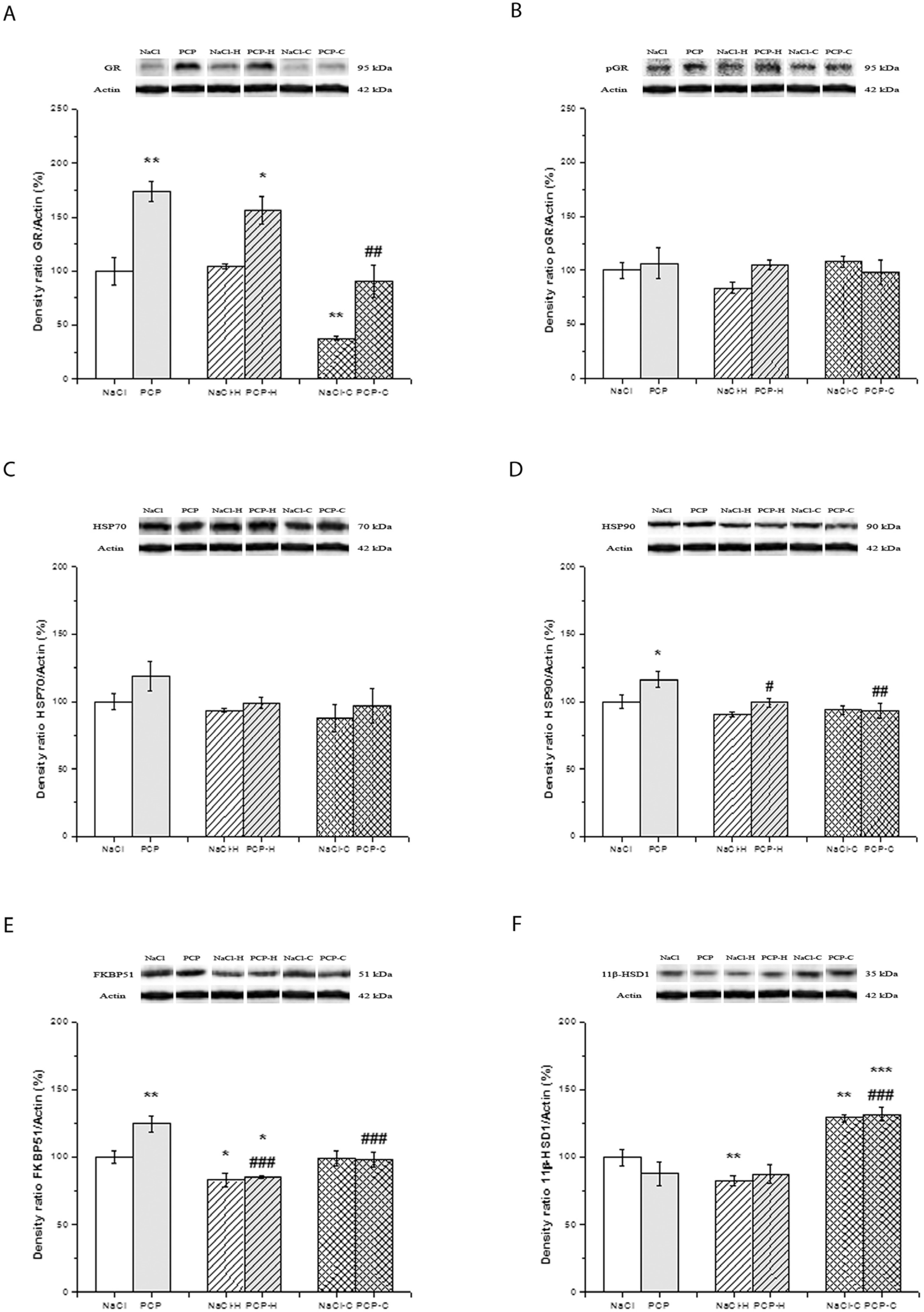
3.3. Effects of Perinatal PCP Administration and Antipsychotic Treatment on the Activity of GR Receptor and 11β-HSD1 Expression in the Hippocampus
3.4. Effects of Perinatal PCP Administration and Antipsychotic Treatment on the Activity of GR Receptor and 11β-HSD1 Expression in the Thalamus
3.5. Effects of Perinatal PCP Administration and Antipsychotic Treatment on the Activity of GR Receptor and 11β-HSD1 Expression in the Caudate Nucleus
4. Discussion
4.1. Long-Term Effects of Perinatal PCP Treatment on the HPA Axis
4.2. Effects of Antipsychotic Treatment on the HPA Axis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, W.; Liu, Z.; An, Q.; Hu, X. Cognitive impairment in psychiatric diseases: Biomarkers of diagnosis, treatment, and prevention. Front. Cell. Neurosci. 2022, 16, 1046692. [Google Scholar] [CrossRef] [PubMed]
- Chaumette, B.; Kebir, O.; Mam-Lam-Fook, C.; Morvan, Y.; Bourgin, J.; Godsil, B.P.; Plaze, M.; Gaillard, R.; Jay, T.M.; Krebs, M.O. Salivary cortisol in early psychosis: New findings and meta-analysis. Psychoneuroendocrinology 2016, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.S. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Konings, M.; Stefanis, N.; Kuepper, R.; de Graaf, R.; ten Have, M.; van Os, J.; Bakoula, C.; Henquet, C. Replication in two independent population-based samples that childhood maltreatment and cannabis use synergistically impact on psychosis risk. Psychol. Med. 2012, 42, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, R.; Addington, J.; Rusjan, P.M.; Suridjan, I.; Ng, A.; Boileau, I.; Pruessner, J.C.; Remington, G.; Houle, S.; Wilson, A.A. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry 2012, 71, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, S.; Da Silva, T.; Gerritsen, C.; Kiang, M.; Bagby, R.M.; Prce, I.; Wilson, A.A.; Houle, S.; Rusjan, P.M.; Mizrahi, R. Imaging Microglial Activation in Individuals at Clinical High Risk for Psychosis: An In Vivo PET Study with [18F]FEPPA. Neuropsychopharmacology 2017, 42, 2474–2481. [Google Scholar] [CrossRef]
- Schifani, C.; Hafizi, S.; Tseng, H.H.; Gerritsen, C.; Kenk, M.; Wilson, A.A.; Houle, S.; Rusjan, P.M.; Mizrahi, R. Preliminary data indicating a connection between stress-induced prefrontal dopamine release and hippocampal TSPO expression in the psychosis spectrum. Schizophr. Res. 2019, 213, 80–86. [Google Scholar] [CrossRef]
- Walker, E.; Mittal, V.; Tessner, K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008, 4, 189–216. [Google Scholar] [CrossRef]
- Szymańska, M.; Budziszewska, B.; Jaworska-Feil, L.; Basta-Kaim, A.; Kubera, M.; Leśkiewicz, M.; Regulska, M.; Lasoń, W. The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology 2009, 34, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, M.; Suska, A.; Budziszewska, B.; Jaworska-Feil, L.; Basta-Kaim, A.; Leśkiewicz, M.; Kubera, M.; Gergont, A.; Kroczka, S.; Kaciński, M.; et al. Prenatal stress decreases glycogen synthase kinase-3 phosphorylation in the rat frontal cortex. Pharmacol. Rep. 2009, 61, 612–620. [Google Scholar] [CrossRef]
- Bradley, A.J.; Dinan, T.G. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: Implications for mortality. J. Psychopharmacol. 2010, 24 (Suppl. S4), 91–118. [Google Scholar] [CrossRef] [PubMed]
- Rivier, C.; Rivest, S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: Peripheral and central mechanisms. Biol. Reprod. 1991, 45, 523–532. [Google Scholar] [CrossRef]
- Matuszewska, A.; Kowalski, K.; Jawień, P.; Tomkalski, T.; Gaweł-Dąbrowska, D.; Merwid-Ląd, A.; Szeląg, E.; Błaszczak, K.; Wiatrak, B.; Danielewski, M.; et al. The Hypothalamic-Pituitary-Gonadal Axis in Men with Schizophrenia. Int. J. Mol. Sci. 2023, 24, 6492. [Google Scholar] [CrossRef]
- Brzezinski-Sinai, N.A.; Brzezinski, A. Schizophrenia and Sex Hormones: What Is the Link? Front. Psychiatry 2020, 11, 693. [Google Scholar] [CrossRef]
- Markham, J.A. Sex steroids and schizophrenia. Rev. Endocr. Metab. Disord. 2012, 13, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.O.; Sagar, R.; Ammini, A.C.; Khurana, M.L.; Alias, A.G. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann. N. Y. Acad. Sci. 2004, 1032, 291–294. [Google Scholar] [CrossRef]
- Sisek-Šprem, M.; Križaj, A.; Jukić, V.; Milošević, M.; Petrović, Z.; Herceg, M. Testosterone levels and clinical features of schizophrenia with emphasis on negative symptoms and aggression. Nord. J. Psychiatry 2015, 69, 102–109. [Google Scholar] [CrossRef]
- Goldman, M.B.; Gnerlich, J.; Hussain, N. Neuroendocrine responses to a cold pressor stimulus in polydipsic hyponatremic and in matched schizophrenic patients. Neuropsychopharmacology 2007, 32, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Brenner, K.; Liu, A.; Laplante, D.P.; Lupien, S.; Pruessner, J.C.; Ciampi, A.; Joober, R.; King, S. Cortisol response to a psychosocial stressor in schizophrenia: Blunted, delayed, or normal? Psychoneuroendocrinology 2009, 34, 859–868. [Google Scholar] [CrossRef]
- van Venrooij, J.A.; Fluitman, S.B.; Lijmer, J.G.; Kavelaars, A.; Heijnen, C.J.; Westenberg, H.G.; Kahn, R.S.; Gispen-de Wied, C.C. Impaired neuroendocrine and immune response to acute stress in medication-naive patients with a first episode of psychosis. Schizophr. Bull. 2012, 38, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Memic, A.; Hasandedić, L.; Rietschel, L.; Frank, J.; Lang, M.; Witt, S.H.; Forstner, A.J.; Degenhardt, F.; Wüst, S.; et al. Perceived stress and hair cortisol: Differences in bipolar disorder and schizophrenia. Psychoneuroendocrinology 2016, 69, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; González-Rodríguez, A.; Huerta-Ramos, E.; Usall, J.; Cobo, J.; Bioque, M.; Barbero, J.D.; García-Rizo, C.; Tost, M.; Monreal, J.A.; et al. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: A systematic review and narrative synthesis. Psychoneuroendocrinology 2018, 93, 8–19. [Google Scholar] [CrossRef]
- Krstic, M.D.; Rogatsky, I.; Yamamoto, K.R.; Garabedian, M.J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 1997, 17, 3947–3954. [Google Scholar] [CrossRef]
- Davies, T.H.; Ning, Y.M.; Sánchez, E.R. A new first step in activation of steroid receptors: Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002, 277, 4597–4600. [Google Scholar] [CrossRef] [PubMed]
- Criado-Marrero, M.; Rein, T.; Binder, E.B.; Porter, J.T.; Koren, J., 3rd; Blair, L.J. Hsp90 and FKBP51: Complex regulators of psychiatric diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160532. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef]
- Ismaili, N.; Garabedian, M.J. Modulation of glucocorticoid receptor function via phosphorylation. Ann. N. Y. Acad. Sci. 2004, 1024, 86–101. [Google Scholar] [CrossRef]
- Galliher-Beckley, A.J.; Cidlowski, J.A. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life 2009, 61, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.J.; Knable, M.B.; O’Grady, J.; Orthmann, J.; Weickert, C.S. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry 2002, 7, 985–994, 924. [Google Scholar] [CrossRef]
- Perlman, W.R.; Webster, M.J.; Kleinman, J.E.; Weickert, C.S. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol. Psychiatry 2004, 56, 844–852. [Google Scholar] [CrossRef]
- Sinclair, D.; Tsai, S.Y.; Woon, H.G.; Weickert, C.S. Abnormal glucocorticoid receptor mRNA and protein isoform expression in the prefrontal cortex in psychiatric illness. Neuropsychopharmacology 2011, 36, 2698–2709. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Li, C.; Tao, H.; Yang, X.; Zhang, X.; Wang, X. Altered Expression of Glucocorticoid Receptor and Neuron-Specific Enolase mRNA in Peripheral Blood in First-Episode Schizophrenia and Chronic Schizophrenia. Front. Psychiatry 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Budziszewska, B.; Leśkiewicz, M.; Fijał, K.; Regulska, M.; Kubera, M.; Wędzony, K.; Lasoń, W. Hyperactivity of the hypothalamus-pituitary-adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: Effects of antipsychotic drugs. Eur. J. Pharmacol. 2011, 650, 586–595. [Google Scholar] [CrossRef]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Scammell, J.G.; Denny, W.B.; Valentine, D.L.; Smith, D.F. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 2001, 124, 152–165. [Google Scholar] [CrossRef]
- Matosin, N.; Arloth, J.; Czamara, D.; Edmond, K.Z.; Maitra, M.; Fröhlich, A.S.; Martinelli, S.; Kaul, D.; Bartlett, R.; Curry, A.R.; et al. Associations of psychiatric disease and ageing with FKBP5 expression converge on superficial layer neurons of the neocortex. Acta Neuropathol. 2023, 145, 439–459. [Google Scholar] [CrossRef]
- Holmes, M.C.; Seckl, J.R. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol. Cell. Endocrinol. 2006, 248, 9–14. [Google Scholar] [CrossRef]
- Freedman, R. Schizophrenia. N. Engl. J. Med. 2003, 349, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Relationships between fat and bone. Osteoporos. Int. 2008, 19, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; van Staa, T.P.; Egberts, A.C.; Leufkens, H.G.; Cooper, C.; de Vries, F. Antipsychotic use and the risk of hip/femur fracture: A population-based case-control study. Osteoporos. Int. 2009, 20, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Ballon, J.S.; Pajvani, U.; Freyberg, Z.; Leibel, R.L.; Lieberman, J.A. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol. Metab. 2014, 25, 593–600. [Google Scholar] [CrossRef]
- Prestwood, T.R.; Asgariroozbehani, R.; Wu, S.; Agarwal, S.M.; Logan, R.W.; Ballon, J.S.; Hahn, M.K.; Freyberg, Z. Roles of inflammation in intrinsic pathophysiology and antipsychotic drug-induced metabolic disturbances of schizophrenia. Behav. Brain Res. 2021, 402, 113101. [Google Scholar] [CrossRef]
- Mondelli, V.; Pariante, C.M.; Navari, S.; Aas, M.; D’Albenzio, A.; Di Forti, M.; Handley, R.; Hepgul, N.; Marques, T.R.; Taylor, H.; et al. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr. Res. 2010, 119, 75–78. [Google Scholar] [CrossRef]
- Wang, C.; McInnis, J.; Ross-Sanchez, M.; Shinnick-Gallagher, P.; Wiley, J.L.; Johnson, K.M. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: Implications for schizophrenia. Neuroscience 2001, 107, 535–550. [Google Scholar] [CrossRef]
- Radonjić, N.V.; Knezević, I.D.; Vilimanovich, U.; Kravić-Stevović, T.; Marina, L.V.; Nikolić, T.; Todorović, V.; Bumbasirević, V.; Petronijević, N.D. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Neuropharmacology 2010, 58, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, T.; Petronijević, M.; Sopta, J.; Velimirović, M.; Stojković, T.; Jevtić Dožudić, G.; Aksić, M.; Radonjić, N.V.; Petronijević, N. Haloperidol affects bones while clozapine alters metabolic parameters—Sex specific effects in rats perinatally treated with phencyclidine. BMC Pharmacol. Toxicol. 2017, 18, 65. [Google Scholar] [CrossRef]
- Malik, J.A.; Yaseen, Z.; Thotapalli, L.; Ahmed, S.; Shaikh, M.F.; Anwar, S. Understanding translational research in schizophrenia: A novel insight into animal models. Mol. Biol. Rep. 2023, 50, 3767–3785. [Google Scholar] [CrossRef]
- Lipska, B.K.; Weinberger, D.R. To model a psychiatric disorder in animals: Schizophrenia as a reality test. Neuropsychopharmacology 2000, 23, 223–239. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.; Fone, K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Grayson, B.; Barnes, S.A.; Markou, A.; Piercy, C.; Podda, G.; Neill, J.C. Postnatal Phencyclidine (PCP) as a Neurodevelopmental Animal Model of Schizophrenia Pathophysiology and Symptomatology: A Review. Curr. Top. Behav. Neurosci. 2016, 29, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Białoń, M.; Wąsik, A. Advantages and Limitations of Animal Schizophrenia Models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef] [PubMed]
- Bey, T.; Patel, A. Phencyclidine intoxication and adverse effects: A clinical and pharmacological review of an illicit drug. Cal. J. Emerg. Med. 2007, 8, 9–14. [Google Scholar] [PubMed]
- McKibben, C.E.; Reynolds, G.P.; Jenkins, T.A. Concurrent Risperidone Administration Attenuates the Development of Locomotor Sensitization Following Sub-Chronic Phencyclidine in Rats. Pharmacopsychiatry 2016, 49, 62–65. [Google Scholar] [CrossRef]
- Andersen, J.D.; Pouzet, B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology 2004, 29, 1080–1090. [Google Scholar] [CrossRef]
- Wang, C.; McInnis, J.; West, J.B.; Bao, J.; Anastasio, N.; Guidry, J.A.; Ye, Y.; Salvemini, D.; Johnson, K.M. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J. Pharmacol. Exp. Ther. 2003, 304, 266–271. [Google Scholar] [CrossRef]
- Radonjić, N.V.; Petronijević, N.D.; Vucković, S.M.; Prostran, M.S.; Nesić, Z.I.; Todorović, V.R.; Paunović, V.R. Baseline temperature in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Physiol. Behavi. 2008, 93, 437–443. [Google Scholar] [CrossRef]
- Stojković, T.; Radonjić, N.V.; Velimirović, M.; Jevtić, G.; Popović, V.; Doknić, M.; Petronijević, N.D. Risperidone reverses phencyclidine induced decrease in glutathione levels and alterations of antioxidant defense in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 192–199. [Google Scholar] [CrossRef]
- Radonjić, N.V.; Jakovcevski, I.; Bumbaširević, V.; Petronijević, N.D. Perinatal phencyclidine administration decreases the density of cortical interneurons and increases the expression of neuregulin-1. Psychopharmacology 2013, 227, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, G.; Nikolić, T.; Mirčić, A.; Stojković, T.; Velimirović, M.; Trajković, V.; Marković, I.; Trbovich, A.M.; Radonjić, N.V.; Petronijević, N.D. Mitochondrial impairment, apoptosis and autophagy in a rat brain as immediate and long-term effects of perinatal phencyclidine treatment—Influence of restraint stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Petronijevic, N.; Sopta, J.; Doknic, M.; Radonjic, N.; Petronijevic, M.; Pekic, S.; Maric, N.; Jasovic-Gasic, M.; Popovic, V. Chronic risperidone exposure does not show any evidence of bone mass deterioration in animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Pechnick, R.N.; George, R.; Poland, R.E.; Hiramatsu, M.; Cho, A.K. Characterization of the effects of the acute and chronic administration of phencyclidine on the release of adrenocorticotropin, corticosterone and prolactin in the rat: Evidence for the differential development of tolerance. J. Pharmacol. Exp. Ther. 1989, 250, 534–540. [Google Scholar] [PubMed]
- Ikonomidou, C.; Bosch, F.; Miksa, M.; Bittigau, P.; Vöckler, J.; Dikranian, K.; Tenkova, T.I.; Stefovska, V.; Turski, L.; Olney, J.W. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999, 283, 70–74. [Google Scholar] [CrossRef]
- Adams, S.M.; de Rivero Vaccari, J.C.; Corriveau, R.A. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. J. Neuroscisci. 2004, 24, 9441–9450. [Google Scholar] [CrossRef]
- Shelnutt, S.R.; Gunnell, M.; Owens, S.M. Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J. Pharmacol. Exp. Ther. 1999, 290, 1292–1298. [Google Scholar]
- Nabeshima, T.; Yamaguchi, K.; Yamada, K.; Hiramatsu, M.; Kuwabara, Y.; Furukawa, H.; Kameyama, T. Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur. J. Pharmacol. 1984, 97, 217–227. [Google Scholar] [CrossRef]
- Olney, J.W.; Labruyere, J.; Wang, G.; Wozniak, D.F.; Price, M.T.; Sesma, M.A. NMDA antagonist neurotoxicity: Mechanism and prevention. Science 1991, 254, 1515–1518. [Google Scholar] [CrossRef]
- Wessinger, W.D. Sexual dimorphic effects of chronic phencyclidine in rats. Eur. J. Pharmacol. 1995, 277, 107–112. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.; Gommeren, W.; Luyten, W.H.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Kapur, S.; VanderSpek, S.C.; Brownlee, B.A.; Nobrega, J.N. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: A suggested solution based on in vivo occupancy. J. Pharmacol. Exp. Ther. 2003, 305, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Gearhart, D.A.; Warner, S.E.; Zhang, G.; Bartlett, M.G.; Middlemore, M.L.; Beck, W.D., Jr.; Mahadik, S.P.; Waller, J.L. Oral haloperidol or risperidone treatment in rats: Temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience 2007, 146, 1316–1332. [Google Scholar] [CrossRef][Green Version]
- Steward, L.J.; Kennedy, M.D.; Morris, B.J.; Pratt, J.A. Chronic phencyclidine (PCP)-induced modulation of muscarinic receptor mRNAs in rat brain: Impact of antipsychotic drug treatment. Neuropharmacology 2012, 62, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier: San Diego, CA, USA, 2005. [Google Scholar]
- Myin-Germeys, I.; van Os, J. Stress-reactivity in psychosis: Evidence for an affective pathway to psychosis. Clin. Psychol. Rev. 2007, 27, 409–424. [Google Scholar] [CrossRef] [PubMed]
- van Winkel, R.; Stefanis, N.C.; Myin-Germeys, I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr. Bull. 2008, 34, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Amani, M.; Samadi, H.; Doosti, M.H.; Azarfarin, M.; Bakhtiari, A.; Majidi-Zolbanin, N.; Mirza-Rahimi, M.; Salari, A.A. Neonatal NMDA receptor blockade alters anxiety- and depression-related behaviors in a sex-dependent manner in mice. Neuropharmacology 2013, 73, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.M.; Muguruza, C.; Sierra, S.; Moreno, J.L.; Callado, L.F.; Meana, J.J.; Beardsley, P.M.; González-Maeso, J. Glucocorticoid receptor dysregulation underlies 5-HT2AR-dependent synaptic and behavioral deficits in a mouse neurodevelopmental disorder model. J. Biol. Chem. 2022, 298, 102481. [Google Scholar] [CrossRef]
- Zimmermann, C.A.; Arloth, J.; Santarelli, S.; Löschner, A.; Weber, P.; Schmidt, M.V.; Spengler, D.; Binder, E.B. Stress dynamically regulates co-expression networks of glucocorticoid receptor-dependent MDD and SCZ risk genes. Transl. Psychiatry 2019, 9, 41. [Google Scholar] [CrossRef]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P.; et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef]
- Rogatsky, I.; Logan, S.K.; Garabedian, M.J. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc. Nat. Acad. Sci. USA 1998, 95, 2050–2055. [Google Scholar] [CrossRef]
- Miller, A.L.; Webb, M.S.; Copik, A.J.; Wang, Y.; Johnson, B.H.; Kumar, R.; Thompson, E.B. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: Correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol. Endocrinol. 2005, 19, 1569–1583. [Google Scholar] [CrossRef]
- Wang, Z.; Frederick, J.; Garabedian, M.J. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J. Biol. Chem. 2002, 277, 26573–26580. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Kono, E.; Dang, T.; Garabedian, M.J. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol. Endocrinol. 2007, 21, 625–634. [Google Scholar] [CrossRef]
- Chen, W.; Dang, T.; Blind, R.D.; Wang, Z.; Cavasotto, C.N.; Hittelman, A.B.; Rogatsky, I.; Logan, S.K.; Garabedian, M.J. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol. Endocrinol. 2008, 22, 1754–1766. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Adachi, M.; Yasui, H.; Takekawa, M.; Tanaka, H.; Imai, K. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol. Endocrinol. 2002, 16, 2382–2392. [Google Scholar] [CrossRef] [PubMed]
- Ishima, T.; Iyo, M.; Hashimoto, K. Neurite outgrowth mediated by the heat shock protein Hsp90α: A novel target for the antipsychotic drug aripiprazole. Transl. Psychiatry 2012, 2, e170. [Google Scholar] [CrossRef] [PubMed]
- Loones, M.T.; Chang, Y.; Morange, M. The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation. Cell Stress Chaperones 2000, 5, 291–305. [Google Scholar] [CrossRef]
- Benitez, M.J.; Sanchez-Ponce, D.; Garrido, J.J.; Wandosell, F. Hsp90 activity is necessary to acquire a proper neuronal polarization. Biochim. Biophys. Acta 2014, 1843, 245–252. [Google Scholar] [CrossRef]
- Luo, W.; Sun, W.; Taldone, T.; Rodina, A.; Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 24. [Google Scholar] [CrossRef]
- Schmidt, U.; Buell, D.R.; Ionescu, I.A.; Gassen, N.C.; Holsboer, F.; Cox, M.B.; Novak, B.; Huber, C.; Hartmann, J.; Schmidt, M.V.; et al. A role for synapsin in FKBP51 modulation of stress responsiveness: Convergent evidence from animal and human studies. Psychoneuroendocrinology 2015, 52, 43–58. [Google Scholar] [CrossRef]
- Hashimoto, K.; Tomitaka, S.; Narita, N.; Minabe, Y.; Iyo, M.; Fukui, S. Induction of heat shock protein (HSP)-70 in posterior cingulate and retrosplenial cortex of rat brain by dizocilpine and phencyclidine: Lack of protective effects of sigma receptor ligands. Addict. Biol. 1996, 1, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Minabe, Y.; Iyo, M. Expression of cyclooxygenase-2 mRNA in rat retrosplenial cortex following administration of phencyclidine. Brain Res. 1997, 762, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Arion, D.; Unger, T.; Lewis, D.A.; Levitt, P.; Mirnics, K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol. Psychiatry 2007, 62, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.J.; Riedel, M.; Gruber, R.; Ackenheil, M.; Müller, N. Antibodies to heat shock proteins in schizophrenic patients: Implications for the mechanism of the disease. Am. J. Psychiatry 1999, 156, 1103–1104. [Google Scholar] [CrossRef]
- Sun, H.; Wu, M.; Wang, M.; Zhang, X.; Zhu, J. The regulatory role of endoplasmic reticulum chaperone proteins in neurodevelopment. Front. Neurosci. 2022, 16, 1032607. [Google Scholar] [CrossRef]
- Mihaljevic, M.; Zeljic, K.; Soldatovic, I.; Andric, S.; Mirjanic, T.; Richards, A.; Mantripragada, K.; Pekmezovic, T.; Novakovic, I.; Maric, N.P. The emerging role of the FKBP5 gene polymorphisms in vulnerability-stress model of schizophrenia: Further evidence from a Serbian population. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 527–539. [Google Scholar] [CrossRef]
- Sinclair, D.; Fillman, S.G.; Webster, M.J.; Weickert, C.S. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci. Rep. 2013, 3, 3539. [Google Scholar] [CrossRef]
- Hertzberg, L.; Zohar, A.H.; Yitzhaky, A. Gene Expression Meta-Analysis of Cerebellum Samples Supports the FKBP5 Gene-Environment Interaction Model for Schizophrenia. Life 2021, 11, 190. [Google Scholar] [CrossRef]
- Debs, S.R.; Rothmond, D.A.; Zhu, Y.; Weickert, C.S.; Purves-Tyson, T.D. Molecular evidence of altered stress responsivity related to neuroinflammation in the schizophrenia midbrain. J. Psychiatr. Res. 2024, 177, 118–128. [Google Scholar] [CrossRef]
- Borges, S.; Gayer-Anderson, C.; Mondelli, V.A. Systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 2013, 38, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhou, D.F.; Cao, L.Y.; Wu, G.Y.; Shen, Y.C. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: Association with psychopathology and response to antipsychotics. Neuropsychopharmacology 2005, 30, 1532–1538. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Pivac, N.; Mihaljevic-Peles, A.; Mustapic, M.; Relja, M.; Ljubicic, D.; Marcinko, D.; Muck-Seler, D. The effects of olanzapine and fluphenazine on plasma cortisol, prolactin and muscle rigidity in schizophrenic patients: A double blind study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 399–402. [Google Scholar] [CrossRef]
- Popovic, V.; Doknic, M.; Maric, N.; Pekic, S.; Damjanovic, A.; Miljic, D.; Popovic, S.; Miljic, N.; Djurovic, M.; Jasovic-Gasic, M.; et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology 2007, 85, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Isikhan, S.Y.; Ansari, M.H.K.; Samadi, M.; Sabuncuoglu, S. Effects of clozapine and haloperidol treatment on plasma concentrations of androgen hormones and androgendependent organ changes in rats. Indian J. Pharmacol. 2019, 51, 269–275. [Google Scholar] [CrossRef]
- García-Osta, A.; Frechilla, D.; Del Río, J. Reduced basal and phencyclidine-induced expression of heat shock protein-70 in rat prefrontal cortex by the atypical antipsychotic abaperidone. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.; Roh, S.; Yang, B.H.; Lee, J.S.; Chai, Y.G.; Choi, M.R.; Park, Y.C.; Kim, D.J.; Kim, D.; Choi, J.; et al. Effects of haloperidol and risperidone on the expression of heat shock protein 70 in MK-801-treated rat C6 glioma cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1793–1797. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Binder, E.B. Schizophrenia in the spectrum of gene-stress interactions: The FKBP5 example. Schizophr. Bull. 2015, 41, 323–329. [Google Scholar] [CrossRef]
- Hartmann, J.; Wagner, K.V.; Liebl, C.; Scharf, S.H.; Wang, X.D.; Wolf, M.; Hausch, F.; Rein, T.; Schmidt, U.; Touma, C.; et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 2012, 62, 332–339. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Harbich, D.; Schmid, B.; Lucassen, P.J.; Wagner, K.V.; Schmidt, M.V.; Hartmann, J. Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS ONE 2014, 9, e95796. [Google Scholar] [CrossRef]
- Gaali, S.; Kirschner, A.; Cuboni, S.; Hartmann, J.; Kozany, C.; Balsevich, G.; Namendorf, C.; Fernandez-Vizarra, P.; Sippel, C.; Zannas, A.S.; et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat. Chem. Biol. 2015, 11, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zannas, A.S.; Chrousos, G.P. Epigenetic programming by stress and glucocorticoids along the human lifespan. Mol. Psychiatry 2017, 22, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Mourtzi, N.; Sertedaki, A.; Charmandari, E. Glucocorticoid Signaling and Epigenetic Alterations in Stress-Related Disorders. Int. J. Mol. Sci. 2021, 22, 5964. [Google Scholar] [CrossRef] [PubMed]



| NaCl | PCP | NaCl-H | PCP-H | NaCl-C | PCP-C | |
|---|---|---|---|---|---|---|
| Corticosterone (ng/mL) | 51 ± 8.5 | 67.8 ± 11.3 | 55.6 ± 10.4 | 37.6 ± 2.8 # | 61.7 ± 5.6 | 39.9 ± 3.4 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, T.; Bogosavljević, M.V.; Stojković, T.; Kanazir, S.; Lončarević-Vasiljković, N.; Radonjić, N.V.; Popić, J.; Petronijević, N. Effects of Antipsychotics on the Hypothalamus–Pituitary–Adrenal Axis in a Phencyclidine Animal Model of Schizophrenia. Cells 2024, 13, 1425. https://doi.org/10.3390/cells13171425
Nikolić T, Bogosavljević MV, Stojković T, Kanazir S, Lončarević-Vasiljković N, Radonjić NV, Popić J, Petronijević N. Effects of Antipsychotics on the Hypothalamus–Pituitary–Adrenal Axis in a Phencyclidine Animal Model of Schizophrenia. Cells. 2024; 13(17):1425. https://doi.org/10.3390/cells13171425
Chicago/Turabian StyleNikolić, Tatjana, Milica Velimirović Bogosavljević, Tihomir Stojković, Selma Kanazir, Nataša Lončarević-Vasiljković, Nevena V. Radonjić, Jelena Popić, and Nataša Petronijević. 2024. "Effects of Antipsychotics on the Hypothalamus–Pituitary–Adrenal Axis in a Phencyclidine Animal Model of Schizophrenia" Cells 13, no. 17: 1425. https://doi.org/10.3390/cells13171425
APA StyleNikolić, T., Bogosavljević, M. V., Stojković, T., Kanazir, S., Lončarević-Vasiljković, N., Radonjić, N. V., Popić, J., & Petronijević, N. (2024). Effects of Antipsychotics on the Hypothalamus–Pituitary–Adrenal Axis in a Phencyclidine Animal Model of Schizophrenia. Cells, 13(17), 1425. https://doi.org/10.3390/cells13171425





