Prolactin Modulates the Proliferation and Secretion of Goat Mammary Epithelial Cells via Regulating Sodium-Coupled Neutral Amino Acid Transporter 1 and 2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Cell Isolation and Culture
2.3. Hormone Administration
2.4. Cell Proliferation Analysis
2.5. Quantitative Real-Time PCR
2.6. Western Blotting
2.7. Dual-Luciferase Reporter Gene Assay
2.8. RNA Interference
2.9. Serum PRL Quantification
2.10. Cell Fatty Acids Quantification
2.11. SNAT1 and SNAT2 Overexpression
2.12. Transcriptome Analysis of GMECs Treated with PRL
2.13. Statistical Analysis
3. Results
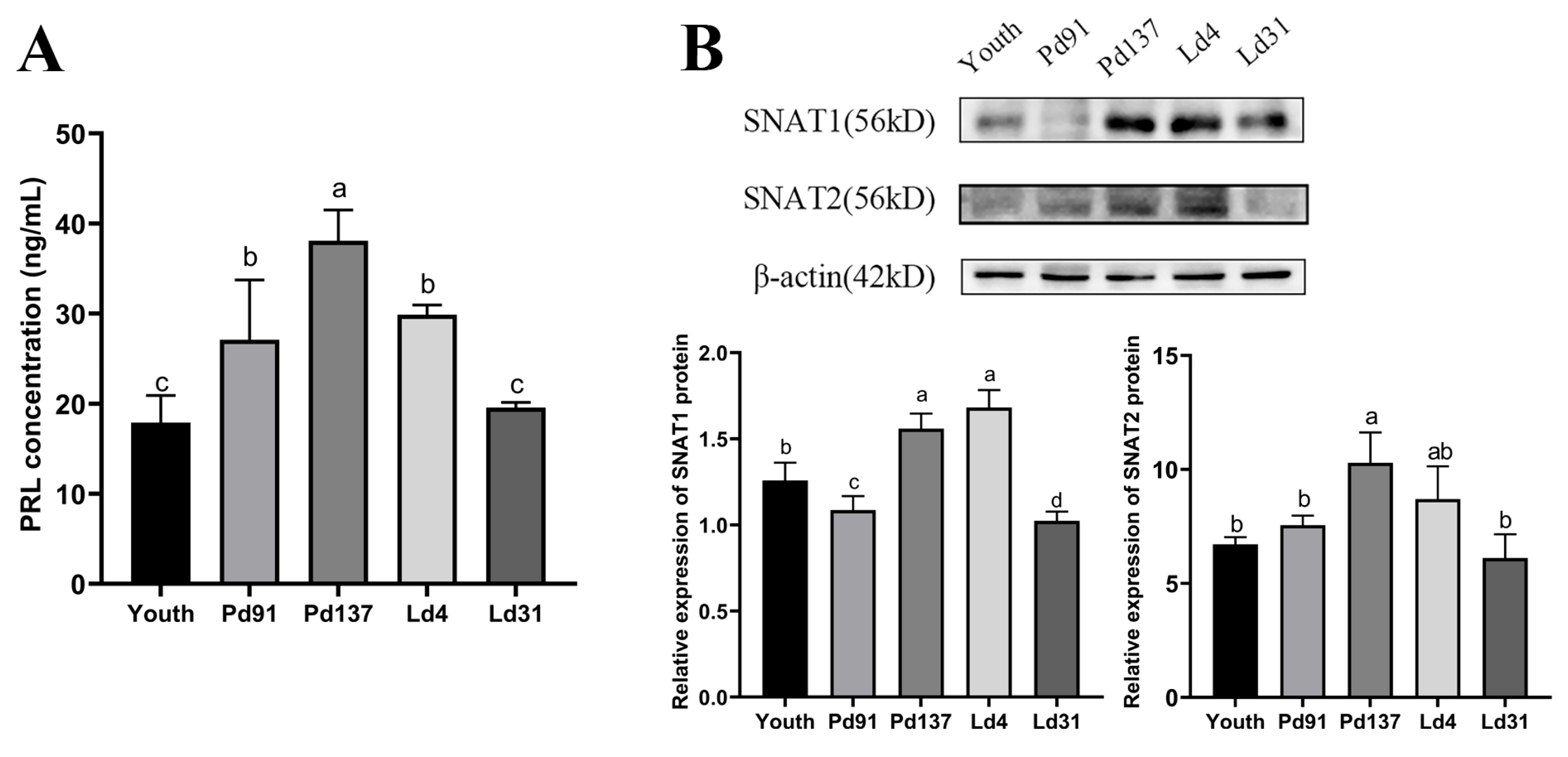
3.1. Protein Expression of SNAT1/2 Are Up-Regulated in Goat Mammary Glands during Late Pregnancy and Early Lactation
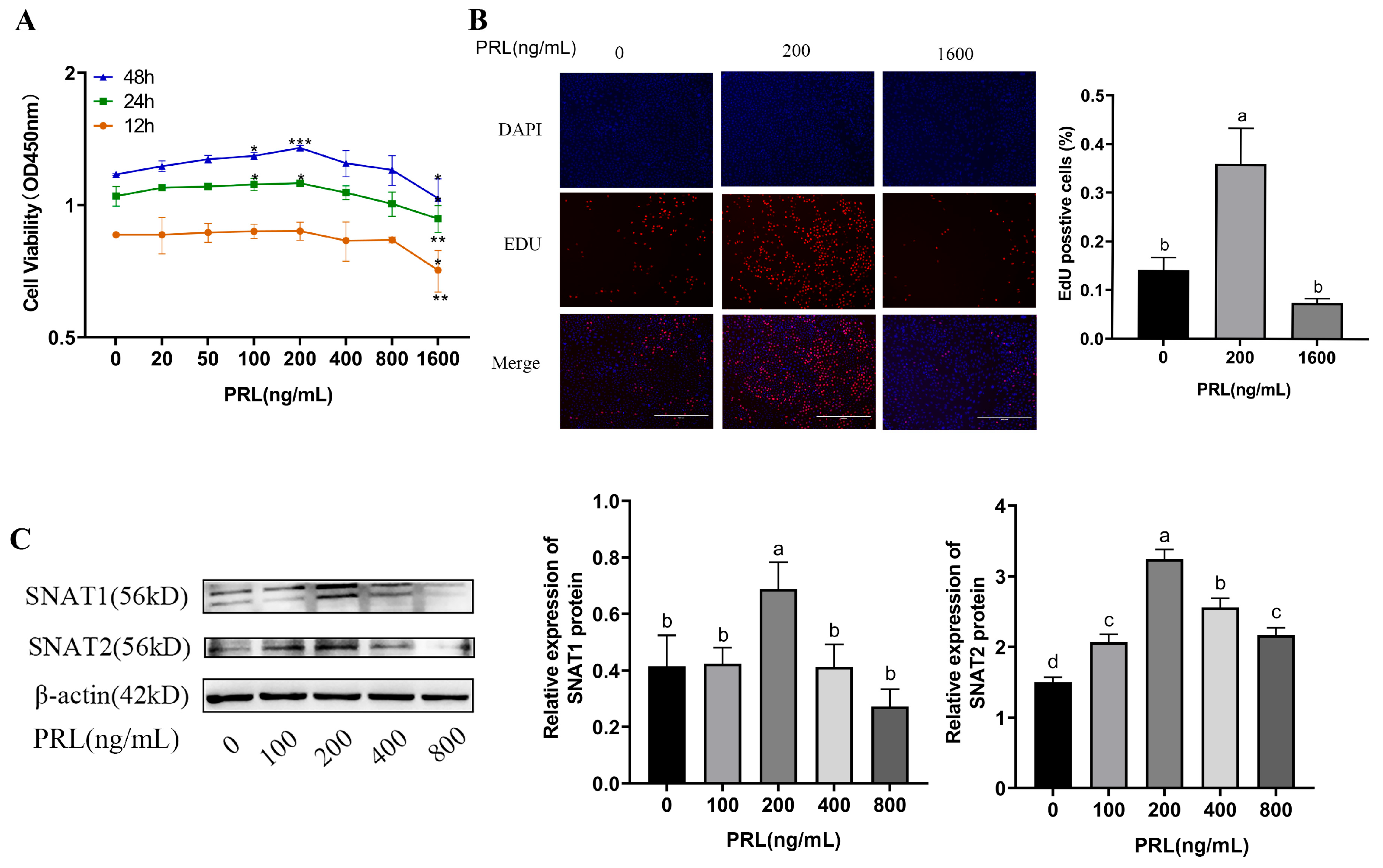
3.2. SNAT1/2 Proteins Are Up-Regulated by Specific Concentrations of PRL Which Promote the Proliferation of GMECs
3.3. SNAT1/2 Proteins Are Up-Regulated by Specific Concentrations of PRL Which Promote the Lactation of GMECs
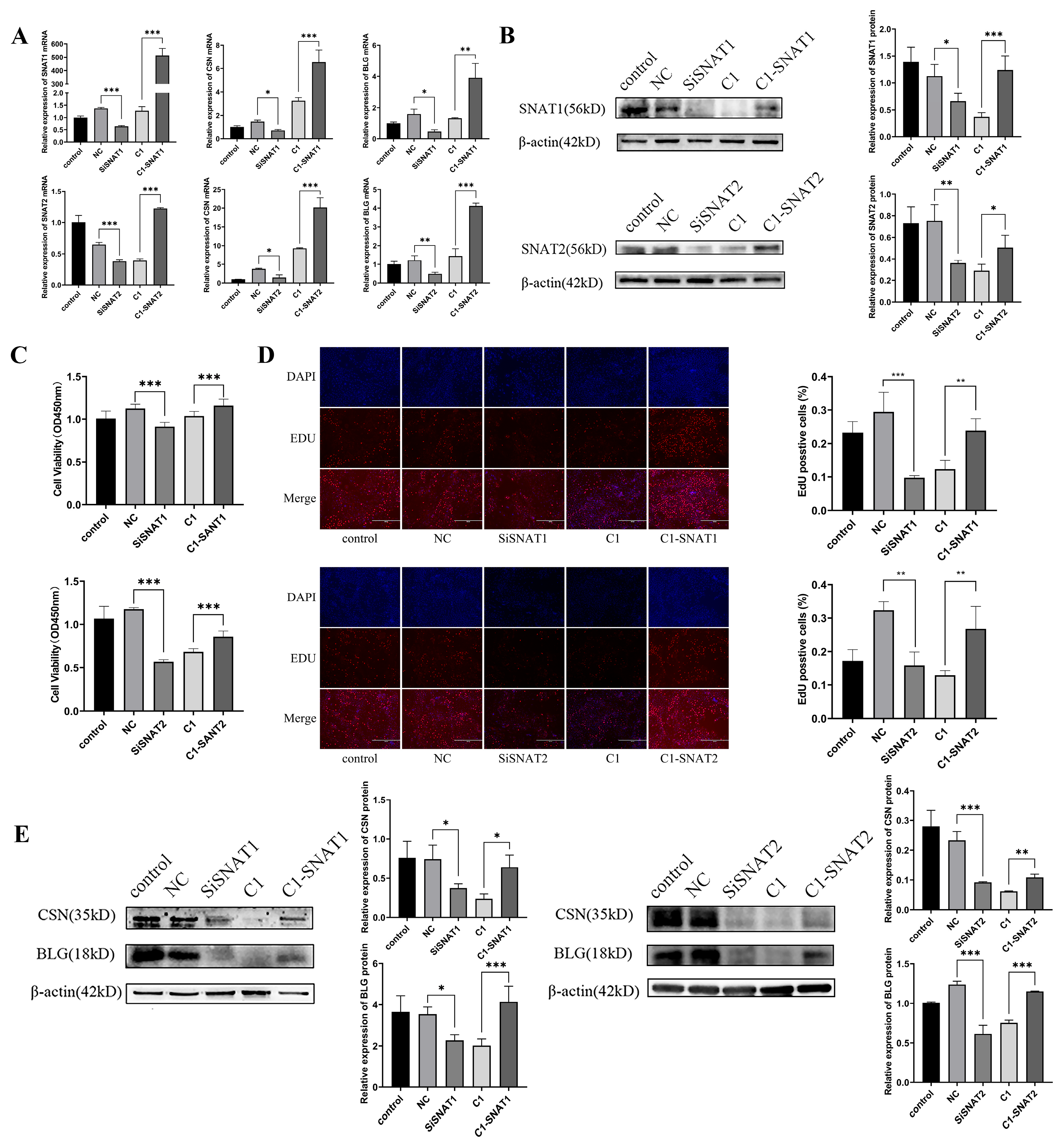
3.4. SNAT1/2 Are Involved in PRL Modulation of the Proliferation and Lactation of GMECs
3.5. SNAT1/2 mRNAs Are Up-Regulated in Response to PRL Stimulation in GMECs
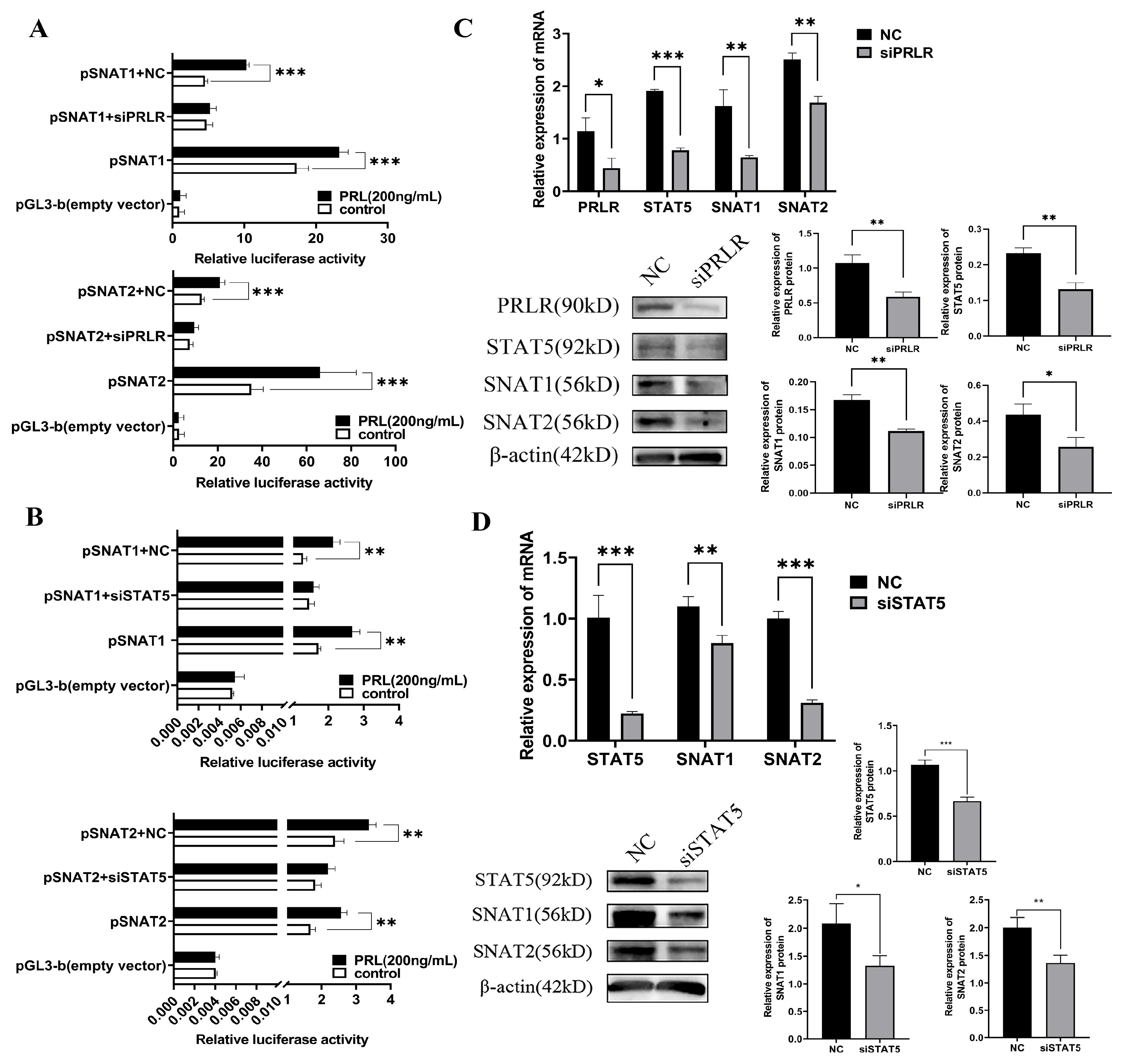
3.6. Promoter Regions of SNAT1 and SNAT2 Are Activated by PRL
3.7. PRL Activates SNAT1/2 through the PRLR-STAT5-Dependent Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D. Prolactin and mammary gland development. J. Mammary Gland Biol. Neoplasia 1999, 4, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, M.; Shi, Y.; Song, S.; Hou, X.; Lin, Y. Prolactin regulates LAT1 expression via STAT5 (signal transducer and activator of transcription 5) signaling in mammary epithelial cells of dairy cows. J. Dairy Sci. 2020, 103, 6627–6634. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, C.A.; Rice, M.S.; Collins, L.C.; Chen, D.; Frank, D.A.; Walker, S.; Clevenger, C.V.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Prolactin levels and breast cancer risk by tumor expression of prolactin-related markers. Breast Cancer Res. 2023, 25, 24. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations. Available online: https://www.fao.org/home/zh (accessed on 7 August 2024).
- National Bureau of Statistics. Available online: https://www.stats.gov.cn/english/ (accessed on 7 August 2024).
- Xiong, Q.; Chai, J.; Li, X.; Suo, X.; Zhang, N.; Tao, H.; Liu, Y.; Yang, Q.; Jiang, S.; Chen, M.J.L.S. Two tagSNPs in the prolactin receptor gene are associated with growth and litter traits in Boer and Macheng Black crossbred goats. Livest. Sci. 2016, 193, 71–77. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Xiong, Q.; Suo, X.; Zhang, N.; Yang, Q.; Chen, M. Genetic Polymorphism of Ten Microsatellites in Two Goat Breeds and Its Relationship with Heterosis. Agric. Sci. Technol. 2013, 14, 1078. [Google Scholar]
- Limin, W.; Ruiping, S.; Yan, L.; Ruina, L.; Quanwei, L.; Xinli, Z.; Feng, W.; Lili, H.; Manping, X.J.A.H.; Science, F. Slaughtering Performance and Meat Quality of Hainan Black Goat and Its Hybrid Offsprings with Nubian Black Goat. Anim. Husb. Feed Sci. 2019, 11, 49–52. [Google Scholar]
- Rosales-Nieto, C.A.; Thompson, A.N.; Cuevas-Reyes, V.; Hérnandez-Arteaga, L.E.; Greeff, J.C.; Ehrhardt, R.; Veiga-Lopez, A.; Martin, G.B.J.T. Utilising male stimulus to improve the reproductive efficiency of 8-month-old nulliparous ewes and adult parous ewes. Theriogenology 2024, 217, 143–150. [Google Scholar] [CrossRef]
- Rezaei, R.; Wu, Z.; Hou, Y.; Bazer, F.W.; Wu, G. Amino acids and mammary gland development: Nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 2016, 7, 20. [Google Scholar] [CrossRef]
- Bröer, S. The SLC38 family of sodium–amino acid co-transporters. Pflügers Arch.-Eur. J. Physiol. 2014, 466, 155–172. [Google Scholar] [CrossRef]
- Mackenzie, B.; Erickson, J.D. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflügers Arch.-Eur. J. Physiol. 2004, 447, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Puertes, I.R.; Saez, G.T.; Viña, J.R. Role of prolactin in amino acid uptake by the lactating mammary gland of the rat. FEBS Lett. 1981, 126, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Zois, C.E.; El-Ansari, R.; Craze, M.L.; Rakha, E.A.; Fan, S.J.; Valli, A.; Haider, S.; Goberdhan, D.C.I.; Green, A.R.; et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br. J. Cancer 2021, 124, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, O.R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony, R.V.; Brown, T.L.; Hillman, S.L.; Spencer, R.; David, A.L.; Rosario, F.J.; et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
- Qureshi, T.; Bjørkmo, M.; Nordengen, K.; Gundersen, V.; Utheim, T.P.; Watne, L.O.; Storm-Mathisen, J.; Hassel, B.; Chaudhry, F.A. Slc38a1 Conveys Astroglia-Derived Glutamine into GABAergic Interneurons for Neurotransmitter GABA Synthesis. Cells 2020, 9, 1686. [Google Scholar] [CrossRef]
- Desforges, M.; Greenwood, S.L.; Glazier, J.D.; Westwood, M.; Sibley, C.P. The contribution of SNAT1 to system A amino acid transporter activity in human placental trophoblast. Biochem. Biophys. Res. Commun. 2010, 398, 130–134. [Google Scholar] [CrossRef]
- Ortiz, V.; Alemán, G.; Escamilla-Del-Arenal, M.; Recillas-Targa, F.; Torres, N.; Tovar, A.R. Promoter characterization and role of CRE in the basal transcription of the rat SNAT2 gene. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E1092–E1102. [Google Scholar] [CrossRef]
- Takarada, T.; Ogura, M.; Nakamichi, N.; Kakuda, T.; Nakazato, R.; Kokubo, H.; Ikeno, S.; Nakamura, S.; Kutsukake, T.; Hinoi, E.; et al. Upregulation of Slc38a1 Gene Along with Promotion of Neurosphere Growth and Subsequent Neuronal Specification in Undifferentiated Neural Progenitor Cells Exposed to Theanine. Neurochem. Res. 2016, 41, 5–15. [Google Scholar] [CrossRef]
- Feng, H.G.; Wu, C.X.; Zhong, G.C.; Gong, J.P.; Miao, C.M.; Xiong, B. Integrative analysis reveals that SLC38A1 promotes hepatocellular carcinoma development via PI3K/AKT/mTOR signaling via glutamine mediated energy metabolism. J. Cancer Res. Clin. Oncol. 2023, 149, 15879–15898. [Google Scholar] [CrossRef]
- López, A.; Torres, N.; Ortiz, V.; Alemán, G.; Hernández-Pando, R.; Tovar, A.R. Characterization and regulation of the gene expression of amino acid transport system A (SNAT2) in rat mammary gland. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E1059–E1066. [Google Scholar] [CrossRef]
- Qi, H.; Meng, C.; Jin, X.; Li, X.; Li, P.; Gao, X. Methionine promotes milk protein and fat synthesis and cell proliferation via the SNAT2-PI3K signaling pathway in bovine mammary epithelial cells. J. Agric. Food Chem. 2018, 66, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Villegas, L.A.; López-Barradas, A.M.; Torres, N.; Hernández-Pando, R.; León-Contreras, J.C.; Granados, O.; Ortíz, V.; Tovar, A.R. Prolactin and the dietary protein/carbohydrate ratio regulate the expression of SNAT2 amino acid transporter in the mammary gland during lactation. Biochim. Biophys. Acta 2015, 1848, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Kaur, S.; Chavarria, T.E.; Binart, N.; Sutherland, R.L.; Weinberg, R.A.; Kelly, P.A.; Ormandy, C.J. Prolactin Controls Mammary Gland Development via Direct and Indirect Mechanisms. Dev. Biol. 1999, 210, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Q.; Zhang, Y.; Liu, D.; Yang, D.; Han, L.; Chen, J.; Ding, Y. Regulation of Tight Junctions by Sex Hormones in Goat Mammary Epithelial Cells. Animals 2022, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ma, X.; Liu, H.; Jia, Q.; Chen, J.; Ding, Y.; Sun, M.; Zhu, H. SNAT2-mediated regulation of estrogen and progesterone in the proliferation of goat mammary epithelial cells. Amino Acids 2024, 56, 17. [Google Scholar] [CrossRef] [PubMed]
- De Dios, N.; Orrillo, S.; Irizarri, M.; Theas, M.S.; Boutillon, F.; Candolfi, M.; Seilicovich, A.; Goffin, V.; Pisera, D.; Ferraris, J. JAK2/STAT5 Pathway Mediates Prolactin-Induced Apoptosis of Lactotropes. Neuroendocrinology 2019, 108, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, H.; Liu, Q.; Gao, Q.; Gao, F.; Han, Y.; Yuan, Z.; Zhang, H.; Weng, Q. Seasonal expressions of prolactin, prolactin receptor and STAT5 in the scented glands of the male muskrats (Ondatra zibethicus). Eur. J. Histochem. 2019, 63, 2991. [Google Scholar] [CrossRef] [PubMed]
- Hart, I.C. The relationship between lactation and the release of prolactin and growth hormone in the goat. J. Reprod. Fertil. 1974, 39, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Baldwin, R.L.; Troutt, H.F.; Bruss, M.L.; Galland, J.C.; Farver, T.B.; Rostami, J.; Weaver, L.D.; Holmberg, C.A. Impact of bovine somatotropin administration beginning at day 70 of lactation on serum metabolites, milk constituents, and production in cows previously exposed to exogenous somatotropin. Am. J. Vet. Res. 1992, 53, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Peel, C.J.; Bauman, D.E. Somatotropin and lactation. J. Dairy Sci. 1987, 70, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Chao, T.; Zhang, C.; Liu, Z.; Hou, L.; Wang, J.; Wang, A.; Wang, Y.; Zhou, J.; Xuan, R.; et al. Transcriptome Analysis of Dairy Goat Mammary Gland Tissues from Different Lactation Stages. DNA Cell Biol. 2019, 38, 129–143. [Google Scholar] [CrossRef]
- Li, Z.; Lan, X.; Guo, W.; Sun, J.; Huang, Y.; Wang, J.; Huang, T.; Lei, C.; Fang, X.; Chen, H. Comparative transcriptome profiling of dairy goat microRNAs from dry period and peak lactation mammary gland tissues. PLoS ONE 2012, 7, e52388. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, J.; Luo, J.; Cao, W.; Shi, H.; Yao, D.; Li, J.; Sun, Y.; Xu, H.; Yu, K.; et al. Genes regulating lipid and protein metabolism are highly expressed in mammary gland of lactating dairy goats. Funct. Integr. Genom. 2015, 15, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Meyer, M.J.; Li, R.W.; Van Amburgh, M.E.; Boisclair, Y.R.; Capuco, A.V. Regulation of gene expression in the bovine mammary gland by ovarian steroids. J. Dairy Sci. 2007, 90 (Suppl. S1), E55–E65. [Google Scholar] [CrossRef]
- Velázquez-Villegas, L.A.; Ortíz, V.; Ström, A.; Torres, N.; Engler, D.A.; Matsunami, R.; Ordaz-Rosado, D.; García-Becerra, R.; López-Barradas, A.M.; Larrea, F.; et al. Transcriptional regulation of the sodium-coupled neutral amino acid transporter (SNAT2) by 17β-estradiol. Proc. Natl. Acad. Sci. USA 2014, 111, 11443–11448. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.; Beveridge, M.; Kilberg, M.; Novak, D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am. J. Physiol.-Cell Physiol. 2002, 282, C153–C160. [Google Scholar] [CrossRef]
- Naylor, M.J.; Lockefeer, J.A.; Horseman, N.D.; Ormandy, C.J. Prolactin regulates mammary epithelial cell proliferation via autocrine/paracrine mechanism. Endocrine 2003, 20, 111–114. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Zhang, C.; Ma, Y.; Sun, S.; Zhang, T.; Loor, J.J. Mechanism of prolactin inhibition of miR-135b via methylation in goat mammary epithelial cells. J. Cell Physiol. 2018, 233, 651–662. [Google Scholar] [CrossRef]
- Groner, B.; Gouilleux, F. Prolactin-mediated gene activation in mammary epithelial cells. Curr. Opin. Genet. Dev. 1995, 5, 587–594. [Google Scholar] [CrossRef]
- Lopez-Pulido, E.I.; Muñoz-Valle, J.F.; Del Toro-Arreola, S.; Jave-Suárez, L.F.; Bueno-Topete, M.R.; Estrada-Chávez, C.; Pereira-Suárez, A.L. High expression of prolactin receptor is associated with cell survival in cervical cancer cells. Cancer Cell Int. 2013, 13, 103. [Google Scholar] [CrossRef]
- Stefanoska, I.; Krivokuća, M.J.; Vasilijić, S.; Ćujić, D.; Vićovac, L.J.P. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta 2013, 34, 775–783. [Google Scholar] [CrossRef]
- Lee, H.; Heo, Y.; Lee, S.; Hwang, K.; Lee, H.; Choi, S.; Kim, N.J. Retinoic acid plus prolactin to synergistically increase specific casein gene expression in MAC-T cells. J. Dairy Sci. 2013, 96, 3835–3839. [Google Scholar] [CrossRef]
- Kwon, H.C.; Jung, H.S.; Kim, D.H.; Han, J.H.; Han, S.G.J.A. The role of progesterone in Elf5 activation and milk component synthesis for cell-cultured milk production in MAC-T cells. Animals 2024, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 2007, 9, 204. [Google Scholar] [CrossRef]
- Axel, D.I.; Spyridopoulos, I.; Riessen, R.; Runge, H.; Viebahn, R.; Karsch, K.R. Toxicity, uptake kinetics and efficacy of new transfection reagents: Increase of oligonucleotide uptake. J. Vasc. Res. 2000, 37, 221–234, discussion 303–224. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Hu, R.; Hua, X.M.; Ni, Y.X.; Ge, L.; Zhang, L.; Yu, W.; Hao, N.X.; Xia, H.; Fang, Q.; et al. Construction and functional verification of size-reduced plasmids based on TMP resistance gene dfrB10. Microbiol. Spectr. 2023, 11, e0120623. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, J.; Aledo, J.C.; Cwiklinski, E.; Hyde, R.; Taylor, P.M.; Hundal, H.S. SNAT2 transceptor signalling via mTOR: A role in cell growth and proliferation? Front. Biosci. 2011, 3, 1289–1299. [Google Scholar] [CrossRef]
- Martinez, C.S.; Piazza, V.G.; Díaz, M.E.; Boparai, R.K.; Arum, O.; Ramírez, M.C.; González, L.; Becú-Villalobos, D.; Bartke, A.; Turyn, D. Growth hormone (GH)/STAT5 signaling during the growth period in liver of mice overexpressing GH. J. Mol. Endocrinol. 2015, 54, 171. [Google Scholar] [CrossRef]
- Valle-Mendiola, A.; Weiss-Steider, B.; Rocha-Zavaleta, L.; Soto-Cruz, I. IL-2 enhances cervical cancer cells proliferation and JAK3/STAT5 phosphorylation at low doses, while at high doses IL-2 has opposite effects. Cancer Investig. 2014, 32, 115–125. [Google Scholar] [CrossRef]
- Okutani, Y.; Kitanaka, A.; Tanaka, T.; Kamano, H.; Ohnishi, H.; Kubota, Y.; Ishida, T.; Takahara, J. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene 2001, 20, 6643–6650. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr. Reflections on STAT3, STAT5, and STAT6 as fat STATs. Proc. Natl. Acad. Sci. USA 1996, 93, 6221–6224. [Google Scholar] [CrossRef] [PubMed]
- Iavnilovitch, E.; Groner, B.; Barash, I. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 2002, 1, 32–47. [Google Scholar] [PubMed]
- Sinha, S.; Sharma, S.; Vora, J.; Shah, H.; Srivastava, A.; Shrivastava, N. Mucuna pruriens (L.) DC chemo sensitize human breast cancer cells via downregulation of prolactin-mediated JAK2/STAT5A signaling. J. Ethnopharmacol. 2018, 217, 23–35. [Google Scholar] [CrossRef]
- Hayes, H.; Le Chalony, C.; Goubin, G.; Mercier, D.; Payen, E.; Bignon, C.; Kohno, K. Localization of ZNF164, ZNF146, GGTA1, SOX2, PRLR and EEF2 on homoeologous cattle, sheep and goat chromosomes by fluorescent in situ hybridization and comparison with the human gene map. Cytogenet. Cell Genet. 1996, 72, 342–346. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008, 22, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Groner, B. Transcription factor regulation in mammary epithelial cells. Domest. Anim. Endocrinol. 2002, 23, 25–32. [Google Scholar] [CrossRef]
- White, U.A.; Maier, J.; Zhao, P.; Richard, A.J.; Stephens, J.M. The modulation of adiponectin by STAT5-activating hormones. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E129–E136. [Google Scholar] [CrossRef]
- Zeng, X.; Willi, M.; Shin, H.Y.; Hennighausen, L.; Wang, C. Lineage-specific and non-specific cytokine-sensing genes respond differentially to the master regulator STAT5. Cell Rep. 2016, 17, 3333–3346. [Google Scholar] [CrossRef]
- Pinz, S.; Unser, S.; Rascle, A. Signal transducer and activator of transcription STAT5 is recruited to c-Myc super-enhancer. BMC Mol. Biol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Ding, Z.C.; Shi, H.; Aboelella, N.S.; Fesenkova, K.; Park, E.J.; Liu, Z.; Pei, L.; Li, J.; McIndoe, R.A.; Xu, H.; et al. Persistent STAT5 activation reprograms the epigenetic landscape in CD4+ T cells to drive polyfunctionality and antitumor immunity. Sci. Immunol. 2020, 5, eaba5962. [Google Scholar] [CrossRef]
- Feuermann, Y.; Kang, K.; Shamay, A.; Robinson, G.W.; Hennighausen, L. MiR-21 is under control of STAT5 but is dispensable for mammary development and lactation. PLoS ONE 2014, 9, e85123. [Google Scholar] [CrossRef]
- Rädler, P.D.; Wehde, B.L.; Wagner, K.-U. Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells. Mol. Cell. Endocrinol. 2017, 451, 31–39. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Liu, H.; Li, W.; Chen, J.; Cui, Z.; Wang, Z.; Hu, C.; Ding, Y.; Zhu, H. Prolactin Modulates the Proliferation and Secretion of Goat Mammary Epithelial Cells via Regulating Sodium-Coupled Neutral Amino Acid Transporter 1 and 2. Cells 2024, 13, 1461. https://doi.org/10.3390/cells13171461
Ma X, Liu H, Li W, Chen J, Cui Z, Wang Z, Hu C, Ding Y, Zhu H. Prolactin Modulates the Proliferation and Secretion of Goat Mammary Epithelial Cells via Regulating Sodium-Coupled Neutral Amino Acid Transporter 1 and 2. Cells. 2024; 13(17):1461. https://doi.org/10.3390/cells13171461
Chicago/Turabian StyleMa, Xiaoyue, Hanling Liu, Wentao Li, Jianguo Chen, Zhenliang Cui, Zixia Wang, Changmin Hu, Yi Ding, and Hongmei Zhu. 2024. "Prolactin Modulates the Proliferation and Secretion of Goat Mammary Epithelial Cells via Regulating Sodium-Coupled Neutral Amino Acid Transporter 1 and 2" Cells 13, no. 17: 1461. https://doi.org/10.3390/cells13171461
APA StyleMa, X., Liu, H., Li, W., Chen, J., Cui, Z., Wang, Z., Hu, C., Ding, Y., & Zhu, H. (2024). Prolactin Modulates the Proliferation and Secretion of Goat Mammary Epithelial Cells via Regulating Sodium-Coupled Neutral Amino Acid Transporter 1 and 2. Cells, 13(17), 1461. https://doi.org/10.3390/cells13171461






