MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Cell Induction, siRNA Synthesis, and Cell Transfection
2.3. Oil Red O, Bodipy Staining, and Triglyceride Content Determination
2.4. CCK-8 Assay
2.5. Analysis by Flow Cytometry
2.6. RNA Extraction and Reverse Transcription–Quantitative PCR
2.7. RNA Sequencing (RNA-Seq)
2.8. Dual-Luciferase Reporter Assay
2.9. Western Blot
2.10. Statistical Analysis
3. Results
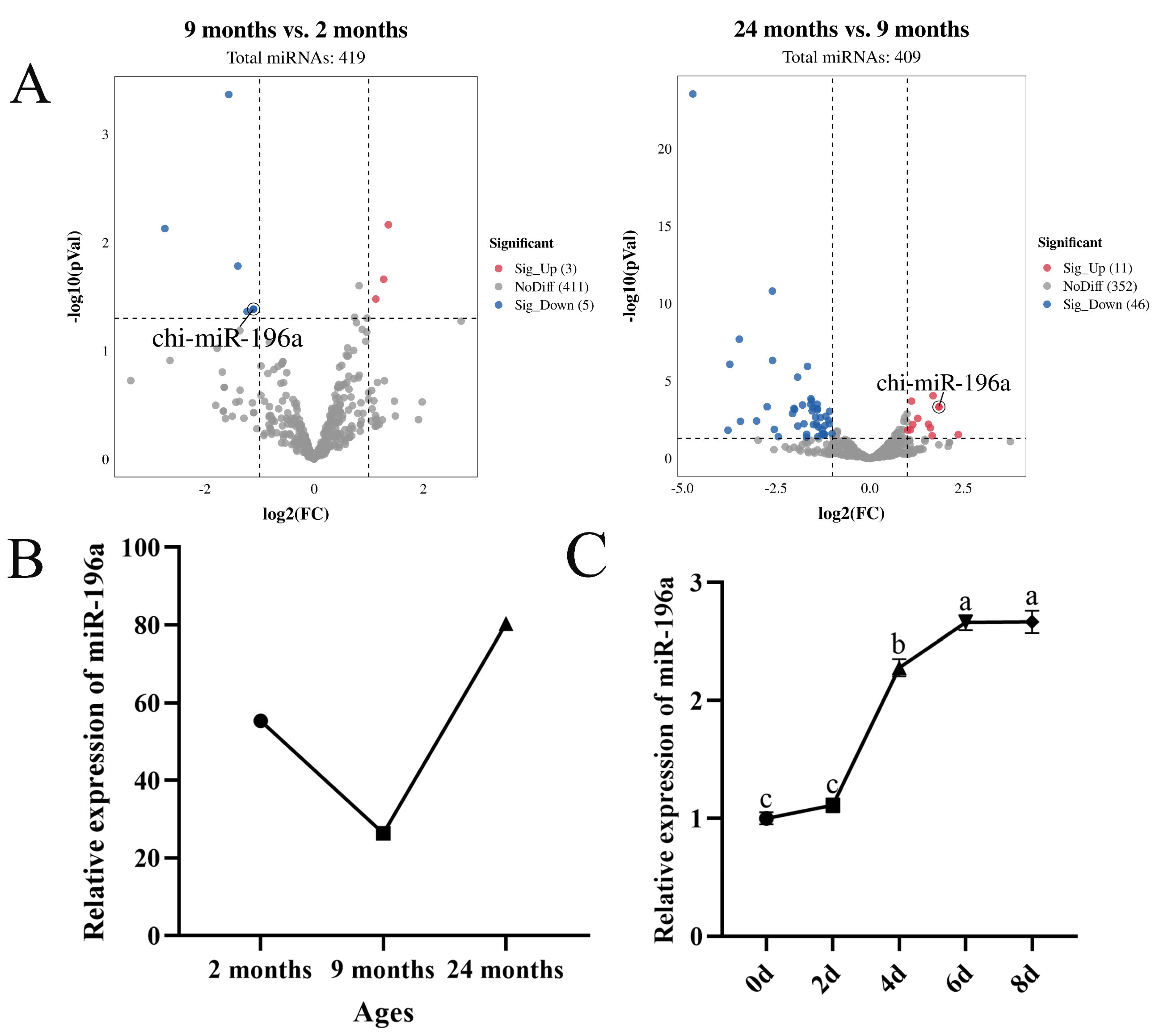
3.1. miR-196a Is Associated with Intramuscular Fat Deposition
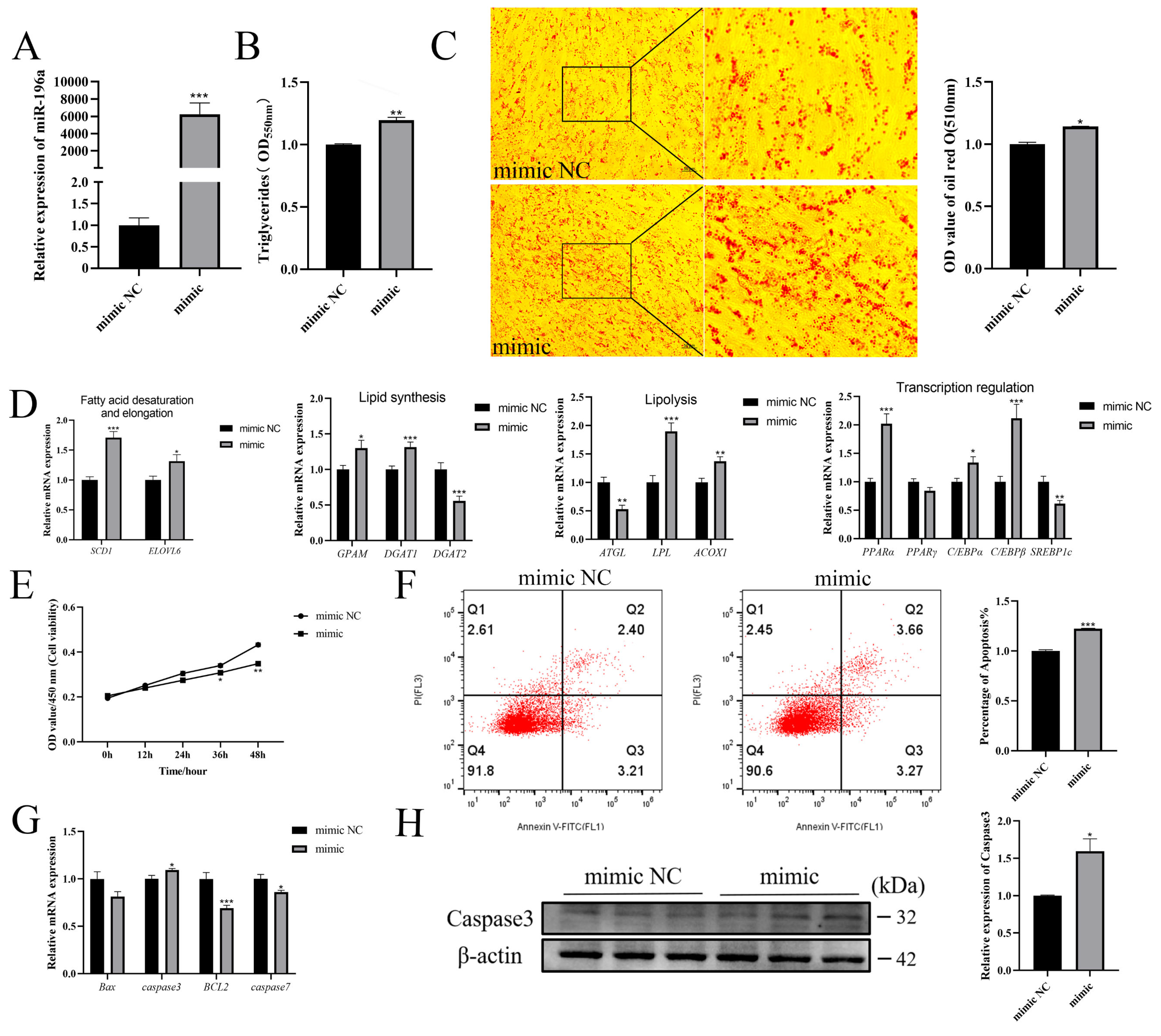
3.2. Overexpression of miR-196a Promotes Adipogenesis and Inhibits Proliferation of Goat Intramuscular Preadipocytes
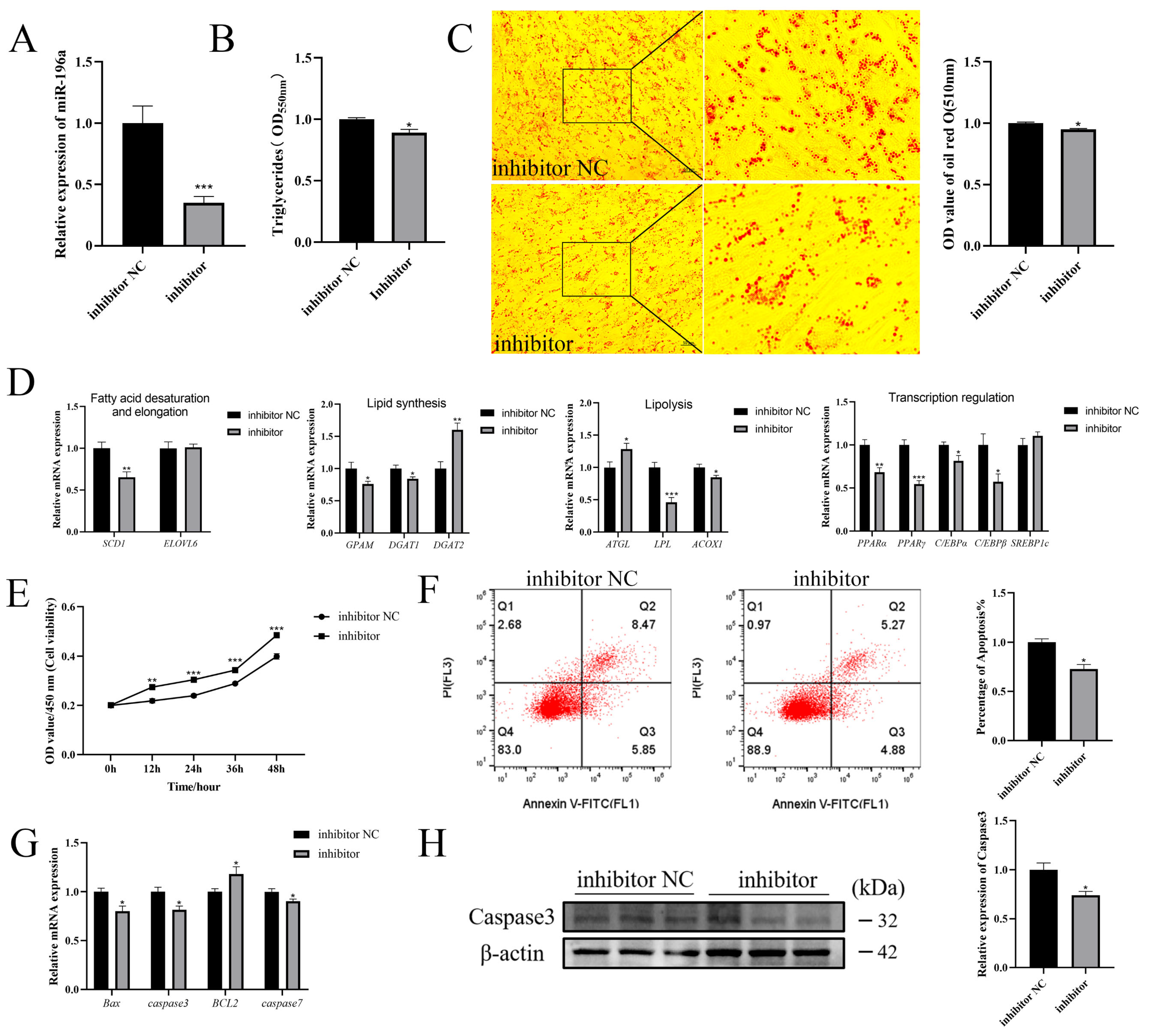
3.3. Knockdown of miR-196a Inhibits Adipogenesis and Promotes Proliferation of Goat Intramuscular Preadipocytes
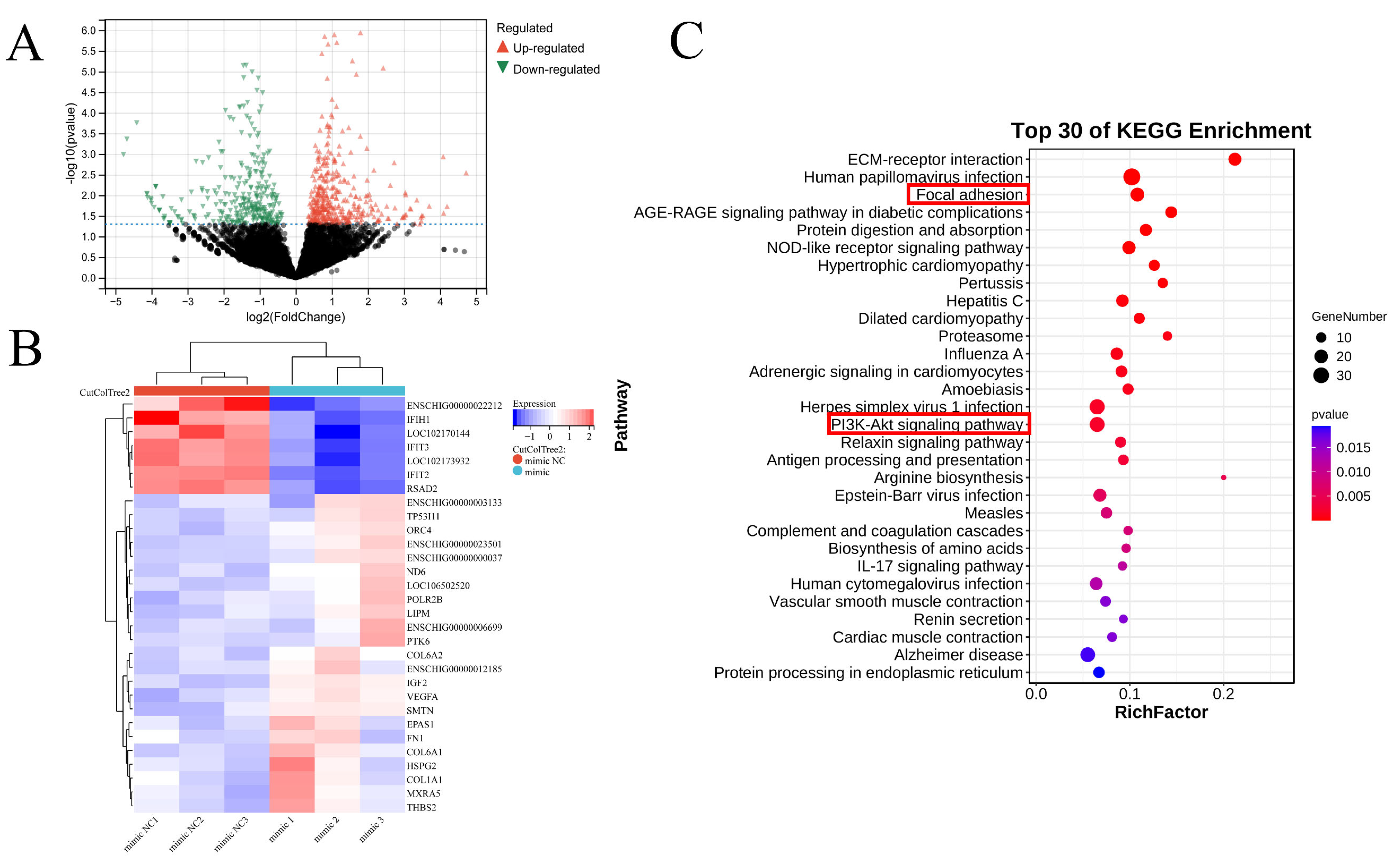
3.4. Identification and Analysis of Differentially Expressed Genes after miR-196a Overexpression
3.5. miR-196a Promotes Lipid Accumulation by Activating PI3K-Akt Pathway
3.6. MAP3K1 Is a Direct Target Gene of miR-196a in Goat Intramuscular Preadipocytes
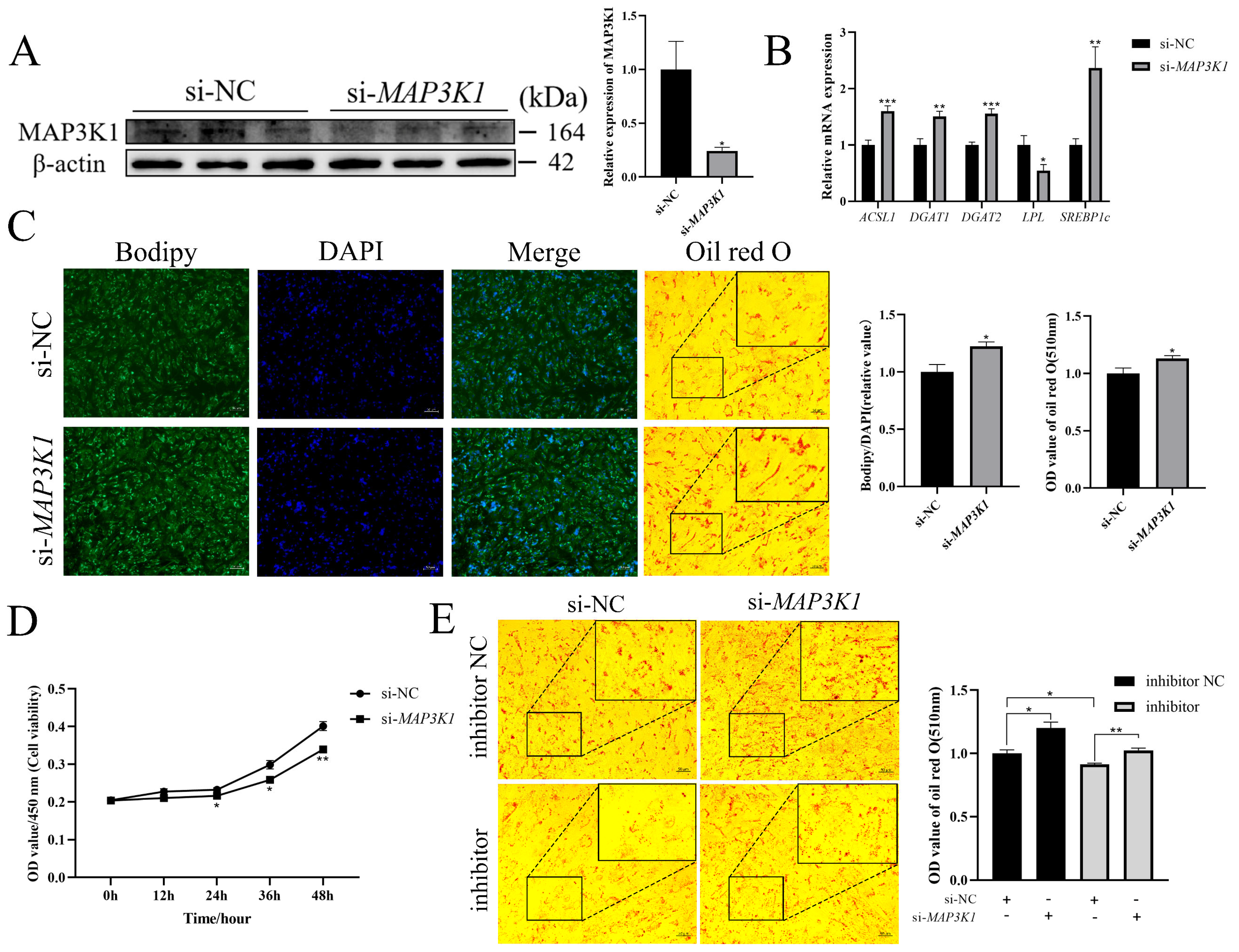
3.7. miR-196a Promotes Adipogenesis and Inhibits Proliferation by Targeting MAP3K1 in Goat Intramuscular Preadipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Anim. Int. J. Anim. Biosci. 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Hausman, G.J.; Dodson, M.V.; Ajuwon, K.; Azain, M.; Barnes, K.M.; Guan, L.L.; Jiang, Z.; Poulos, S.P.; Sainz, R.D.; Smith, S.; et al. Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci. 2009, 87, 1218–1246. [Google Scholar] [CrossRef]
- Berry, R.; Jeffery, E.; Rodeheffer, M.S. Weighing in on adipocyte precursors. Cell Metab. 2014, 19, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Xiong, Y.; Peng, Y.; Gao, Y.; Qin, J.; Chu, G.Y.; Pang, W.J.; Yang, G.S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. Int. J. Mol. Sci. 2017, 18, 2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Du, J.; Wang, L.; Niu, L.; Zhao, Y.; Tang, G.; Jiang, Y.; Shuai, S.; Bai, L.; Li, X.; et al. MicroRNA-143a-3p modulates preadipocyte proliferation and differentiation by targeting MAPK7. Biomed. Pharmacother. 2018, 108, 531–539. [Google Scholar] [CrossRef]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shao, J.; Zhang, X.; Liu, Z.; Tang, T.; Chen, G.; Xia, S.; Zhao, K.; Kang, Z.; Sun, W.; et al. miR-383-5p Regulates Preadipocyte Proliferation and Differentiation by Targeting RAD51AP1. Int. J. Mol. Sci. 2023, 24, 14025. [Google Scholar] [CrossRef]
- Zhang, W.; Raza, S.H.A.; Li, B.; Sun, B.; Wang, S.; Pant, S.D.; Al-Abbas, N.S.; Shaer, N.A.; Zan, L. miR-33a Inhibits the Differentiation of Bovine Preadipocytes through the IRS2-Akt Pathway. Genes 2023, 14, 529. [Google Scholar] [CrossRef]
- Chen, X.; Raza, S.H.A.; Ma, X.; Wang, J.; Wang, X.; Liang, C.; Yang, X.; Mei, C.; Suhail, S.M.; Zan, L. Bovine Pre-adipocyte Adipogenesis Is Regulated by bta-miR-150 Through mTOR Signaling. Front. Genet. 2021, 12, 636550. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Li, Y.; Qu, C.; Zhang, Y.; Liu, H.; Zhang, X. MicroRNA-378 regulates adipogenic differentiation in bovine intramuscular preadipocytes by targeting CaMKK2. Adipocyte 2021, 10, 483–492. [Google Scholar] [CrossRef]
- Li, M.; Zhang, N.; Li, J.; Zhang, W.; Hei, W.; Ji, M.; Yang, Y.; Cao, G.; Guo, X.; Li, B. MiR-23b Promotes Porcine Preadipocyte Differentiation via SESN3 and ACSL4. Cells 2022, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qiu, H.; Chen, Z.; Li, L.; Zeng, Y.; Luo, J.; Gou, D. miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta. J. Anim. Sci. Biotechnol. 2018, 9, 48. [Google Scholar] [CrossRef]
- Ning, X.; Liu, S.; Qiu, Y.; Li, G.; Li, Y.; Li, M.; Yang, G. Expression Profiles and Biological Roles of miR-196a in Swine. Genes 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, L.; Gu, H.; Du, J.; Liu, J.; Zhang, S.; Zhu, L. The Effect of miR-196a-5p on Proliferation and Differentiation of 3T3-L1 Preadipocyte. China Biotechnol. 2018, 38, 9–17. [Google Scholar] [CrossRef]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef]
- Romao, J.M.; Jin, W.; He, M.; McAllister, T.; Guan, L.L. Altered microRNA expression in bovine subcutaneous and visceral adipose tissues from cattle under different diet. PLoS ONE 2012, 7, e40605. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, Y.; Shao, P.; Yang, Y.; Huang, L.; Du, Z.; Zhang, C.; Wang, Y.; Lin, Y.; Zhu, J. Integrated analysis of differently expressed microRNAs and mRNAs at different postnatal stages reveals intramuscular fat deposition regulation in goats (Capra hircus). Anim. Genet. 2024, 55, 238–248. [Google Scholar] [CrossRef]
- Mutlu, A.S.; Duffy, J.; Wang, M.C. Lipid metabolism and lipid signals in aging and longevity. Dev. Cell 2021, 56, 1394–1407. [Google Scholar] [CrossRef]
- Liao, X.; Song, L.; Zhang, L.; Wang, H.; Tong, Q.; Xu, J.; Yang, G.; Yang, S.; Zheng, H. LAMP3 regulates hepatic lipid metabolism through activating PI3K/Akt pathway. Mol. Cell. Endocrinol. 2018, 470, 160–167. [Google Scholar] [CrossRef]
- Zhao, T.; Xiang, Q.; Lie, B.; Chen, D.; Li, M.; Zhang, X.; Yang, J.; He, B.; Zhang, W.; Dong, R.; et al. Yishen Huashi granule modulated lipid metabolism in diabetic nephropathy via PI3K/AKT/mTOR signaling pathways. Heliyon 2023, 9, e14171. [Google Scholar] [CrossRef]
- Dong, X.; Tang, S.; Zhang, W.; Gao, W.; Chen, Y. GPR39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the PI3K/AKT cell signaling pathway. J. Recept. Signal Transduct. Res. 2016, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, M.; Zhang, A.; Liu, Z.; Jiang, G.; Tang, T.; Wang, J.; Jia, X.; Lai, S. Interference of a mammalian circRNA regulates lipid metabolism reprogramming by targeting miR-24-3p/Igf2/PI3K-AKT-mTOR and Igf2bp2/Ucp1 axis. Cell. Mol. Life Sci. CMLS 2023, 80, 252. [Google Scholar] [CrossRef]
- Tashiro, E.; Nagasawa, Y.; Itoh, S.; Imoto, M. Involvement of miR-3180-3p and miR-4632-5p in palmitic acid-induced insulin resistance. Mol. Cell. Endocrinol. 2021, 534, 111371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, H.; Shi, X.; Warren, C.R.; Lotta, L.A.; Friesen, M.; Meissner, T.B.; Langenberg, C.; Wabitsch, M.; Wareham, N.; et al. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ. Res. 2020, 126, 330–346. [Google Scholar] [CrossRef]
- Xie, H.L.; Zhang, Y.H.; Tan, X.D.; Zheng, Y.; Ni, H.Y.; Dong, L.P.; Zheng, J.L.; Diao, J.Z.; Yin, Y.J.; Zhang, J.B.; et al. miR-375 Induced the Formation and Transgenerational Inheritance of Fatty Liver in Poultry by Targeting MAP3K1. DNA Cell Biol. 2022, 41, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Illa, S.K.; Mukherjee, S.; Nath, S.; Mukherjee, A. Genome-Wide Scanning for Signatures of Selection Revealed the Putative Genomic Regions and Candidate Genes Controlling Milk Composition and Coat Color Traits in Sahiwal Cattle. Front. Genet. 2021, 12, 699422. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Lin, Y.; Wang, Y.; Shi, H.; Huang, L.; Zhao, W.; Xiang, H.; Zhu, J. MCD Inhibits Lipid Deposition in Goat Intramuscular Preadipocytes. Genes 2023, 14, 440. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Zhu, J.; Zhao, Y.; Lin, Y. Molecular characterization of GTP binding protein overexpressed in skeletal muscle (GEM) and its role in promoting adipogenesis in goat intramuscular preadipocytes. Anim. Biotechnol. 2020, 31, 17–24. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis. Int. J. Mol. Sci. 2022, 23, 12295. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, W.; Wang, Y.; Li, H.; Zhang, C.; Wang, Y.; Lin, Y.; Shi, H.; Xiang, H.; Huang, L.; et al. Expression Variation of CPT1A Induces Lipid Reconstruction in Goat Intramuscular Precursor Adipocytes. Int. J. Mol. Sci. 2023, 24, 13415. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, J.; Wang, Y.; Li, Q.; Lin, S. Identification of differentially expressed genes through RNA sequencing in goats (Capra hircus) at different postnatal stages. PLoS ONE 2017, 12, e0182602. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, B.Y.; Sun, J.; Zhou, P.Y.; Guan, R.Y.; Zhou, C.; Yang, Z.F.; Wang, Z.T.; Zhou, J.; Fan, J.; et al. BRG1 regulates lipid metabolism in hepatocellular carcinoma through the PIK3AP1/PI3K/AKT pathway by mediating GLMP expression. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2022, 54, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Wang, L.; Bai, L.; Yao, R.; Jia, Z.; Ma, Y.; Chen, Y.; Hao, H.; Wu, X.; et al. Stearic acid promotes lipid synthesis through CD36/Fyn/FAK/mTORC1 axis in bovine mammary epithelial cells. Int. J. Biol. Macromol. 2023, 253, 127324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cai, R.; Tang, G.; Zhang, W.; Pang, W. MiR-146a-5p targeting SMAD4 and TRAF6 inhibits adipogenensis through TGF-β and AKT/mTORC1 signal pathways in porcine intramuscular preadipocytes. J. Anim. Sci. Biotechnol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Brandão, B.B.; Guerra, B.A.; Mori, M.A. Shortcuts to a functional adipose tissue: The role of small non-coding RNAs. Redox Biol. 2017, 12, 82–102. [Google Scholar] [CrossRef]
- Hu, C.M.; Peng, J.; Lv, L.; Wang, X.H.; Huo, J.R.; Liu, D.L. MiR-196a promotes the proliferation and migration of esophageal cancer via the UHRF2/TET2 axis. Mol. Cell. Biochem. 2022, 477, 537–547. [Google Scholar] [CrossRef]
- Chan, S.C.; Tung, C.W.; Lin, C.W.; Tung, Y.S.; Wu, P.M.; Cheng, P.H.; Chen, C.M.; Yang, S.H. miR-196a provides antioxidative neuroprotection via USP15/Nrf2 regulation in Huntington’s disease. Free. Radic. Biol. Med. 2023, 209, 292–300. [Google Scholar] [CrossRef]
- Hilton, C.; Neville, M.J.; Wittemans, L.B.L.; Todorcevic, M.; Pinnick, K.E.; Pulit, S.L.; Luan, J.; Kulyté, A.; Dahlman, I.; Wareham, N.J.; et al. MicroRNA-196a links human body fat distribution to adipose tissue extracellular matrix composition. EBioMedicine 2019, 44, 467–475. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- Pope, E.D., 3rd; Kimbrough, E.O.; Vemireddy, L.P.; Surapaneni, P.K.; Copland, J.A., 3rd; Mody, K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin. Ther. Targets 2019, 23, 473–483. [Google Scholar] [CrossRef]
- Sorisky, A. From preadipocyte to adipocyte: Differentiation-directed signals of insulin from the cell surface to the nucleus. Crit. Rev. Clin. Lab. Sci. 1999, 36, 1–34. [Google Scholar] [CrossRef]
- Boone, C.; Mourot, J.; Grégoire, F.; Remacle, C. The adipose conversion process: Regulation by extracellular and intracellular factors. Reprod. Nutr. Dev. 2000, 40, 325–358. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. TEM 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Blank, H.M.; Maitra, N.; Polymenis, M. Lipid biosynthesis: When the cell cycle meets protein synthesis? Cell Cycle 2017, 16, 905–906. [Google Scholar] [CrossRef]
- Yang, M.; Gao, X.; Hu, C.; Wang, S.; Sheng, H.; Ma, Y. Bta-miR-484 Targets SFRP1 and Affects Preadipocytes Proliferation, Differentiation, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 12710. [Google Scholar] [CrossRef]
- Hecht, E.; Zago, M.; Sarill, M.; Rico de Souza, A.; Gomez, A.; Matthews, J.; Hamid, Q.; Eidelman, D.H.; Baglole, C.J. Aryl hydrocarbon receptor-dependent regulation of miR-196a expression controls lung fibroblast apoptosis but not proliferation. Toxicol. Appl. Pharmacol. 2014, 280, 511–525. [Google Scholar] [CrossRef]
- Haneke, K.; Schott, J.; Lindner, D.; Hollensen, A.K.; Damgaard, C.K.; Mongis, C.; Knop, M.; Palm, W.; Ruggieri, A.; Stoecklin, G. CDK1 couples proliferation with protein synthesis. J. Cell Biol. 2020, 219, e201906147. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Hemming, M.L.; Lundberg, M.Z.; Serrata, M.P.; Goldaracena, I.; Liu, N.; Yin, P.; Paulo, J.A.; Gygi, S.P.; George, S.; et al. Concurrent inhibition of CDK2 adds to the anti-tumour activity of CDK4/6 inhibition in GIST. Br. J. Cancer 2022, 127, 2072–2085. [Google Scholar] [CrossRef]
- Wang, S.C. PCNA: A silent housekeeper or a potential therapeutic target? Trends Pharmacol. Sci. 2014, 35, 178–186. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Yan, Y.; Wang, H.; Yang, J.; Zheng, Z.; Zha, J.; Bo, P.; Tang, Y.; Guo, X.; et al. CircDOCK1 suppresses cell apoptosis via inhibition of miR-196a-5p by targeting BIRC3 in OSCC. Oncol. Rep. 2018, 39, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Huang, J.; Li, Z.; Gong, Y.; Zou, B.; Liu, X.; Ding, L.; Li, P.; Zhu, Z.; et al. HIF-2α upregulation mediated by hypoxia promotes NAFLD-HCC progression by activating lipid synthesis via the PI3K-AKT-mTOR pathway. Aging 2019, 11, 10839–10860. [Google Scholar] [CrossRef]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, C.C.; Frerichs, K.; Rahman, F.; Berger, M.; Lang, H.; Galle, P.R.; Moehler, M.; Gockel, I. High miR-196a levels promote the oncogenic phenotype of colorectal cancer cells. World J. Gastroenterol. 2009, 15, 2089–2096. [Google Scholar] [CrossRef]
- Wu, X.; Pu, L.; Chen, W.; Zhao, Q.; Wu, G.; Li, D.; Zhu, H. LY294002 attenuates inflammatory response in endotoxin-induced uveitis by downregulating JAK3 and inactivating the PI3K/Akt signaling. Immunopharmacol. Immunotoxicol. 2022, 44, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Pardons, M.; Cole, B.; Lambrechts, L.; van Snippenberg, W.; Rutsaert, S.; Noppe, Y.; De Langhe, N.; Dhondt, A.; Vega, J.; Eyassu, F.; et al. Potent latency reversal by Tat RNA-containing nanoparticle enables multi-omic analysis of the HIV-1 reservoir. Nat. Commun. 2023, 14, 8397. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, W.; Li, D.; Zeng, J.; Tang, Q.; Zhang, Y.; Luan, F.; Zeng, N. Cepharanthine Suppresses Herpes Simplex Virus Type 1 Replication Through the Downregulation of the PI3K/Akt and p38 MAPK Signaling Pathways. Front. Microbiol. 2021, 12, 795756. [Google Scholar] [CrossRef]
- Tang, Q.; Luan, F.; Yuan, A.; Sun, J.; Rao, Z.; Wang, B.; Liu, Y.; Zeng, N. Sophoridine Suppresses Herpes Simplex Virus Type 1 Infection by Blocking the Activation of Cellular PI3K/Akt and p38 MAPK Pathways. Front. Microbiol. 2022, 13, 872505. [Google Scholar] [CrossRef] [PubMed]
- Filiberti, A.; Gmyrek, G.B.; Berube, A.N.; Royer, D.J.; Carr, D.J.J. An intact complement system dampens cornea inflammation during acute primary HSV-1 infection. Sci. Rep. 2021, 11, 10247. [Google Scholar] [CrossRef] [PubMed]
- Lafaille, F.G.; Harschnitz, O.; Lee, Y.S.; Zhang, P.; Hasek, M.L.; Kerner, G.; Itan, Y.; Ewaleifoh, O.; Rapaport, F.; Carlile, T.M.; et al. Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis. Nat. Med. 2019, 25, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kimura, E.; Mongan, M.; Xia, Y. Genetic Control of MAP3K1 in Eye Development and Sex Differentiation. Cells 2021, 11, 34. [Google Scholar] [CrossRef]
- Lee, J.T., Jr.; McCubrey, J.A. The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia 2002, 16, 486–507. [Google Scholar] [CrossRef]


| Gene | Full Name | Sequence | Tm/°C | Application |
|---|---|---|---|---|
| SCD1 | Stearoyl-CoA desaturase 1 | TGGCGTTCCAGAATGACGTT | 60 | RT-qPCR |
| ACCCCATAGATACCACGGCA | ||||
| ELOVL6 | Long-chain fatty acid family member 6 | GGAAGCCTTTAGTGCTCTGGTC | 60 | RT-qPCR |
| ATTGTATCTCCTAGTTCGGGTGC | ||||
| GPAM | Glycerol-3-phosphate acyltransferase, mitochondrial | GCAGGTTTATCCAGTATGGCATT | 60 | RT-qPCR |
| GGACTGATATCTTCCTGATCATCTTG | ||||
| DGAT1 | Diacylglycerol O-acyltransferase 1 | CCACTGGGACCTGAGGTGTC | 60 | RT-qPCR |
| GCATCACCACACACCAATTCA | ||||
| DGAT2 | Diacylglycerol O-acyltransferase 2 | CATGTACACATTCTGCACCGATT | 60 | RT-qPCR |
| TGACCTCCTGCCACCTTTCT | ||||
| ATGL | Adipose triglyceride lipase | GGAGCTTATCCAGGCCAATG | 60 | RT-qPCR |
| TGCGGGCAGATGTCACTCT | ||||
| LPL | Lipoprotein lipase | AGGACACTTGCCACCTCATTC | 60 | RT-qPCR |
| TTGGAGTCTGGTTCCCTCTTGTA | ||||
| ACOX1 | Acyl-CoA oxidase 1 | CGAGTTCATTCTCAACAGTCCT | 60 | RT-qPCR |
| GCATCTTCAAGTAGCCATTATCC | ||||
| PPARα | Peroxisome proliferator-activated receptor alpha | AGGTCCGCATCTTCCACT | 60 | RT-qPCR |
| GCTTCGTAAACGCCATACTT | ||||
| PPARγ | Peroxisome proliferator-activated receptor gamma | AAGCGTCAGGGTTCCACTATG | 60 | RT-qPCR |
| GAACCTGATGGCGTTATGAGAC | ||||
| C/EBPα | Enhancer-binding protein alpha | GCGGCAAAGCCAAGAAGTCC | 60 | RT-qPCR |
| CGGCTCAGTTGTTCCACCC | ||||
| C/EBPβ | Enhancer-binding protein beta | GCCTGTCCACGTCCTCGTCGTCCAGC | 60 | RT-qPCR |
| CGGATCTTGTACTCGTCGCTGTGCTTGTCC | ||||
| SREBP1c | Sterol regulatory element-binding protein 1c | ACGCCATCGAGAAACGCTAC | 60 | RT-qPCR |
| GTGCGCAGACTCAGGTTCTC | ||||
| Bax | BCL2-associated X | TTTCCGACGGCAACTTCAA | 60 | RT-qPCR |
| TGAGCACTCCAGCCACAAA | ||||
| Caspase3 | Caspase3 | GACGTGGATGCAGCAAACCTCA | 60 | RT-qPCR |
| TTCACCATGGCTTAGAAGCACG | ||||
| Caspase7 | Caspase7 | GGAACAGATGGCAAGACAGCAATAAAG | 60 | RT-qPCR |
| GCCTGAATGAAGAAGAGTTTGGGTTTC | ||||
| BCL2 | Cell lymphoma-2 | ATGTGTGTGGAGAGCGTCAA | 60 | RT-qPCR |
| CCTTCAGAGACAGCCAGGAG | ||||
| PCNA | Proliferating cell nuclear antigen | ATCAGCTCAAGTGGCGTGAA | 60 | RT-qPCR |
| TGCCAAGGTGTCCGCATTAT | ||||
| CDK4 | Cyclin-dependent kinase 4 | AAGTGGTGGGACAGTCAAGC | 60 | RT-qPCR |
| ACAGAAGAGAGGCTTTCGACG | ||||
| CDK1 | Cyclin-dependent kinase 1 | AGATTTTGGCCTTGCCAGAG | 60 | RT-qPCR |
| AGCTGACCCCAGCAATACTT | ||||
| ACSL1 | Acyl-CoA synthetase long-chain family member 1 | TGACTGTTGCTGGAGACTGG | 60 | RT-qPCR |
| CAGCCGTCTTTATCCAGAGC | ||||
| MAP3K1 | Mitogen-activated protein kinase kinase kinase 1 | AGGCTCAAGATGTGGGAACTG | 60 | RT-qPCR |
| ATCCTGATGATGTTTGGGTGAT | ||||
| chi-miR-196a | chi-miR-196a | TCGGCAGGTAGGTAGTTTCATG | 60 | RT-qPCR |
| TGGAGTCGGCAATTCAGTTGA | ||||
| U6 | U6 | GCTTCGGCAGCACATATACTAAAAT | 60 | RT-qPCR |
| CGCTTCACGAATTTGCGTGTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, W.; Li, H.; Xiang, H.; Zhang, C.; Du, Z.; Huang, L.; Zhu, J. MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway. Cells 2024, 13, 1459. https://doi.org/10.3390/cells13171459
Yang Y, Zhang W, Li H, Xiang H, Zhang C, Du Z, Huang L, Zhu J. MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway. Cells. 2024; 13(17):1459. https://doi.org/10.3390/cells13171459
Chicago/Turabian StyleYang, Yuling, Wenyang Zhang, Haiyang Li, Hua Xiang, Changhui Zhang, Zhanyu Du, Lian Huang, and Jiangjiang Zhu. 2024. "MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway" Cells 13, no. 17: 1459. https://doi.org/10.3390/cells13171459
APA StyleYang, Y., Zhang, W., Li, H., Xiang, H., Zhang, C., Du, Z., Huang, L., & Zhu, J. (2024). MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway. Cells, 13(17), 1459. https://doi.org/10.3390/cells13171459





