Gestational CBD Shapes Insular Cortex in Adulthood
Abstract
:1. Introduction
2. Materials and Methods
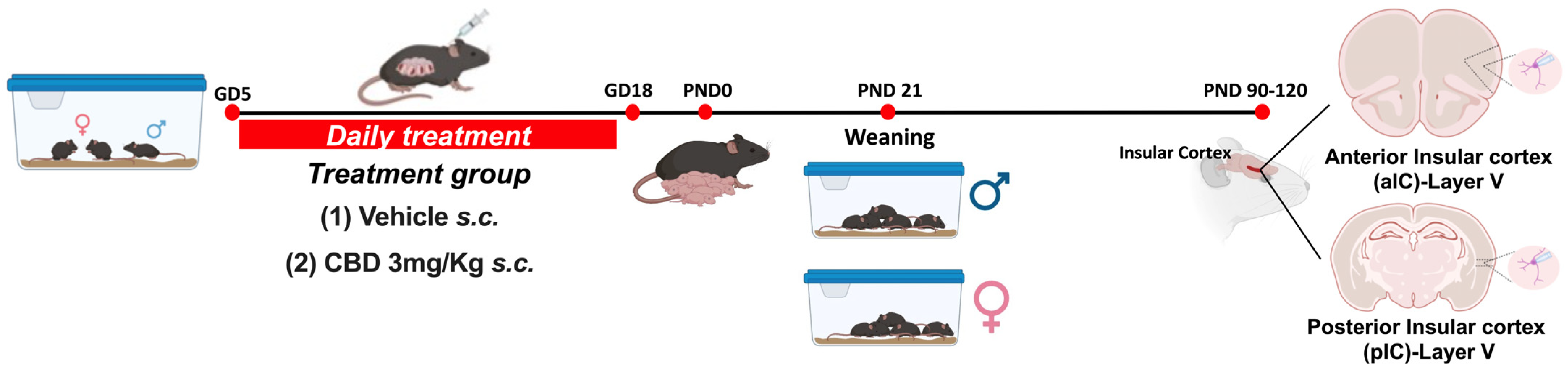
2.1. Animals
2.2. Slice Preparation
2.3. Electrophysiology
2.4. Data Analysis and Statistics
2.5. Statistical Output for Main Figures
3. Results
3.1. Gestational CBD-Induced Sex- and Territory-Specific Alterations in the Properties of IC Pyramidal Neurons

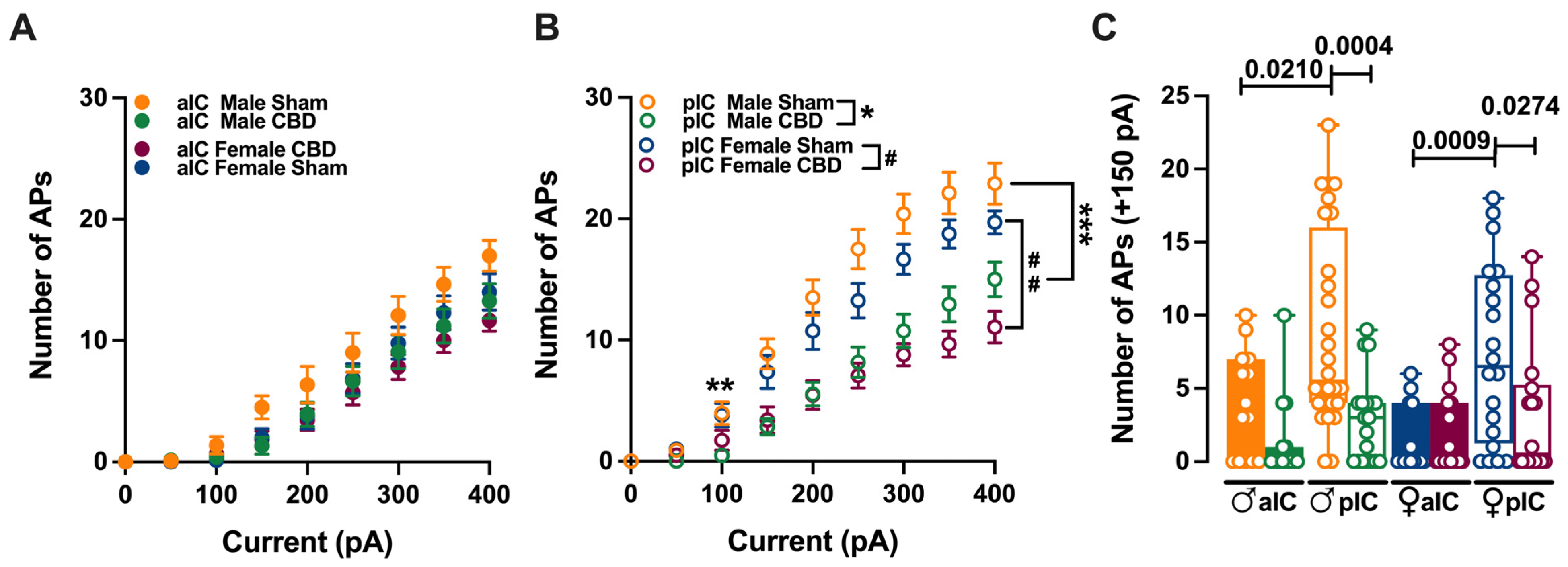
3.2. Gestational Exposure to CBD Altered the Excitability of Pyramidal Neurons in Specific Subregions of the IC
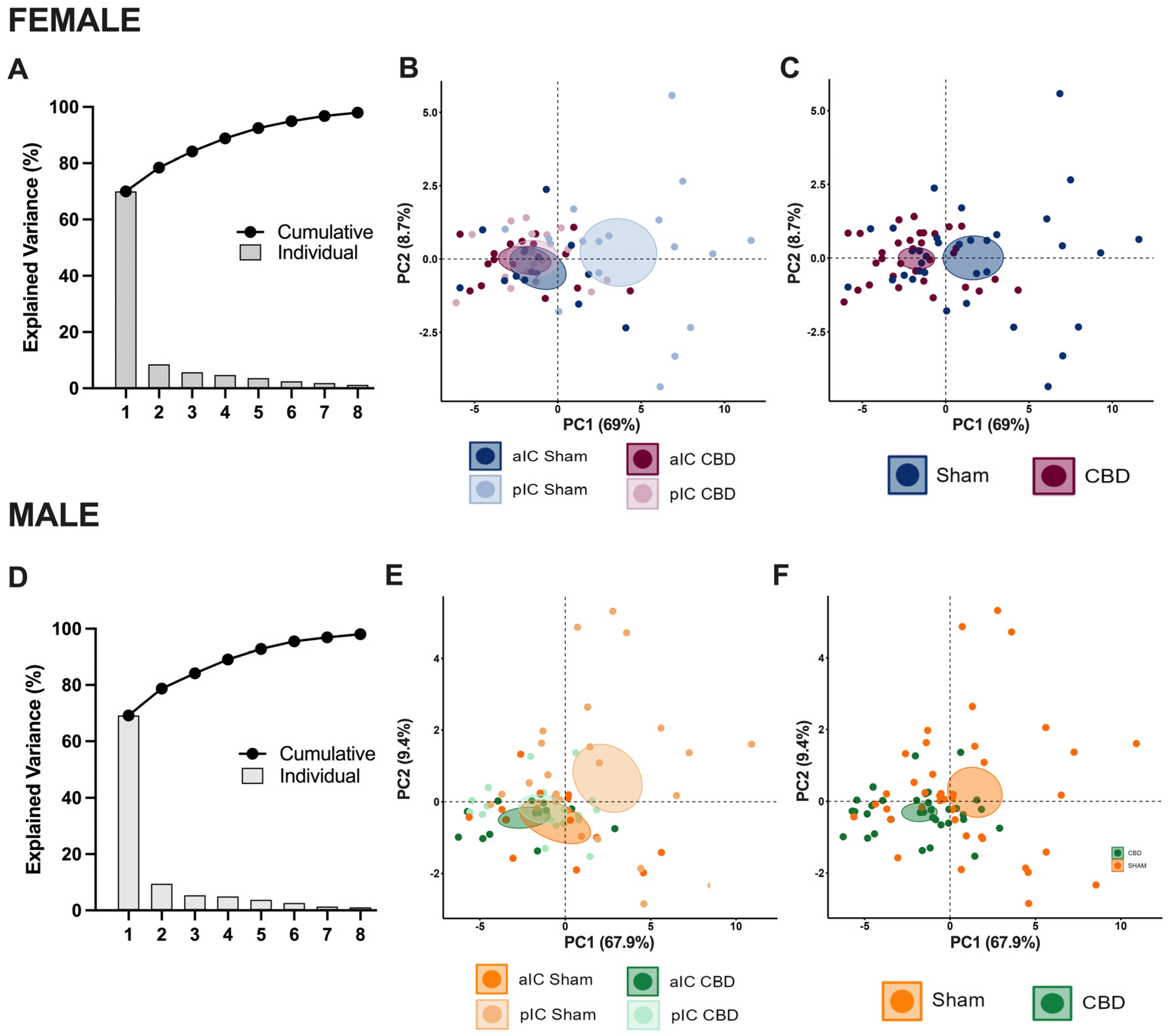
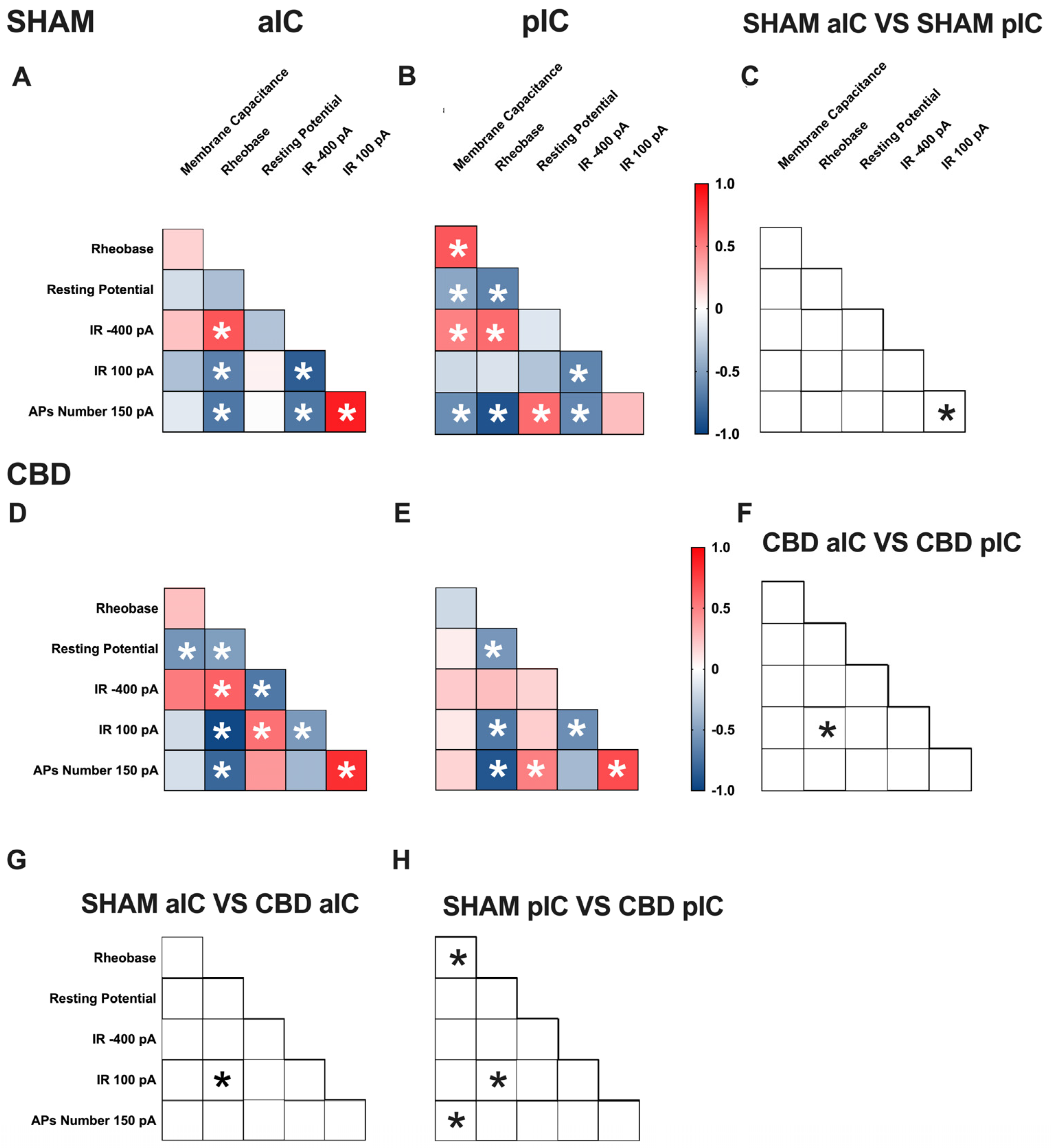
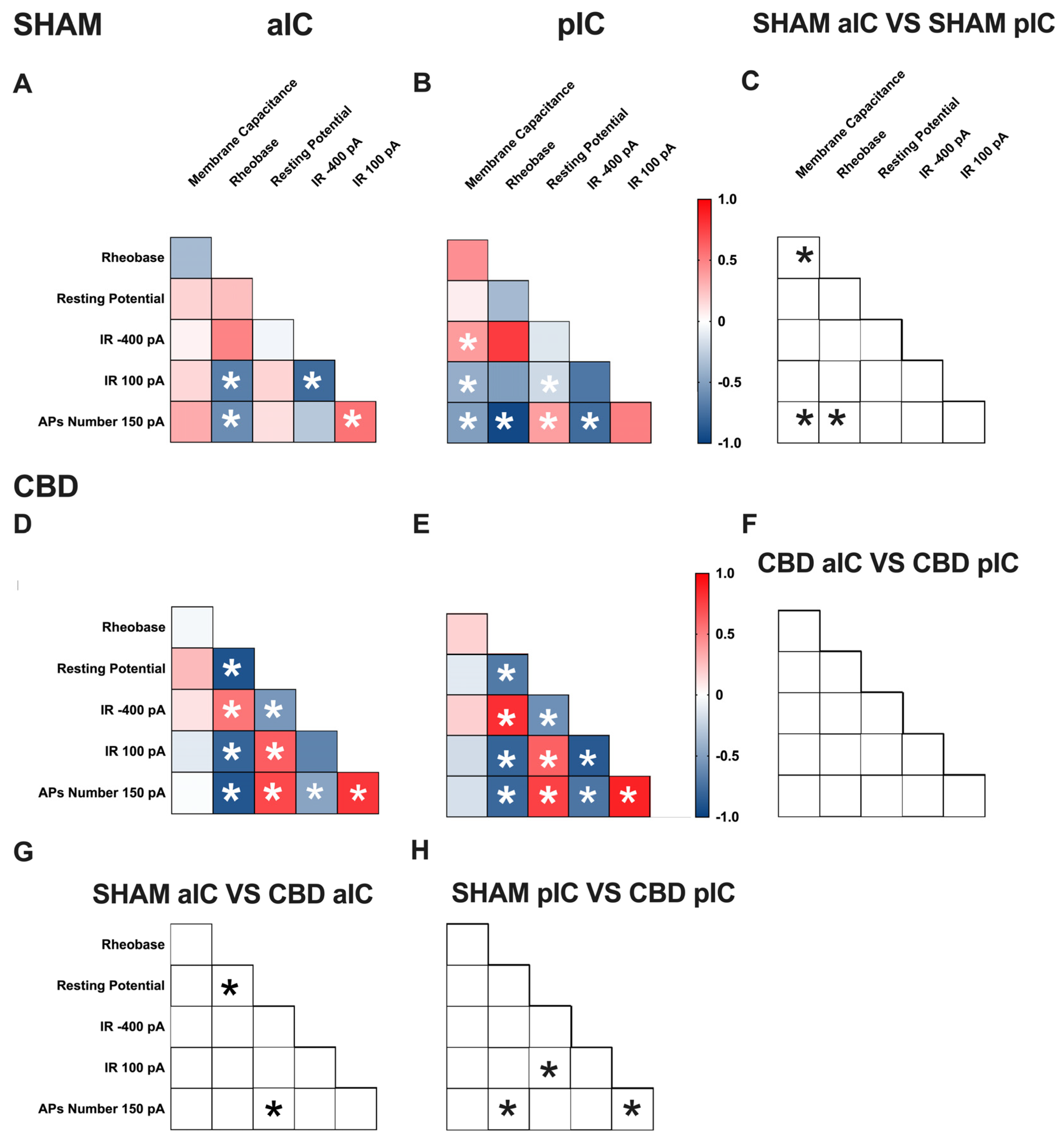
3.3. Territory-Specific Changes in Electrophysiological Correlations Due to Gestational CBD Exposure: Insights from Multivariate Analysis
3.4. Prenatal Cannabidiol Treatment Equalized Excitatory and Inhibitory Synaptic Transmission of the Adult Offspring in a Sex- and Territory-Specific Manner
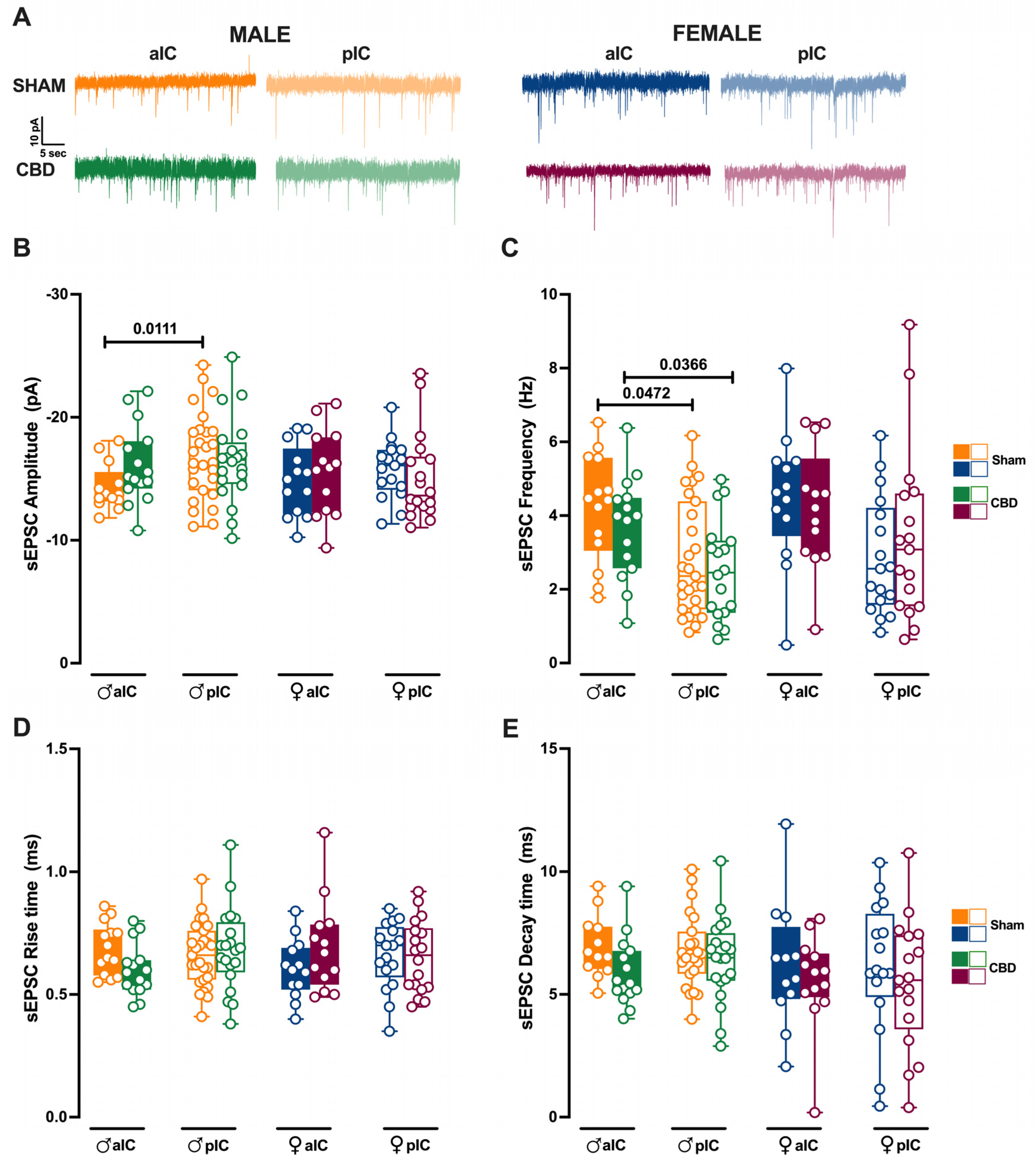
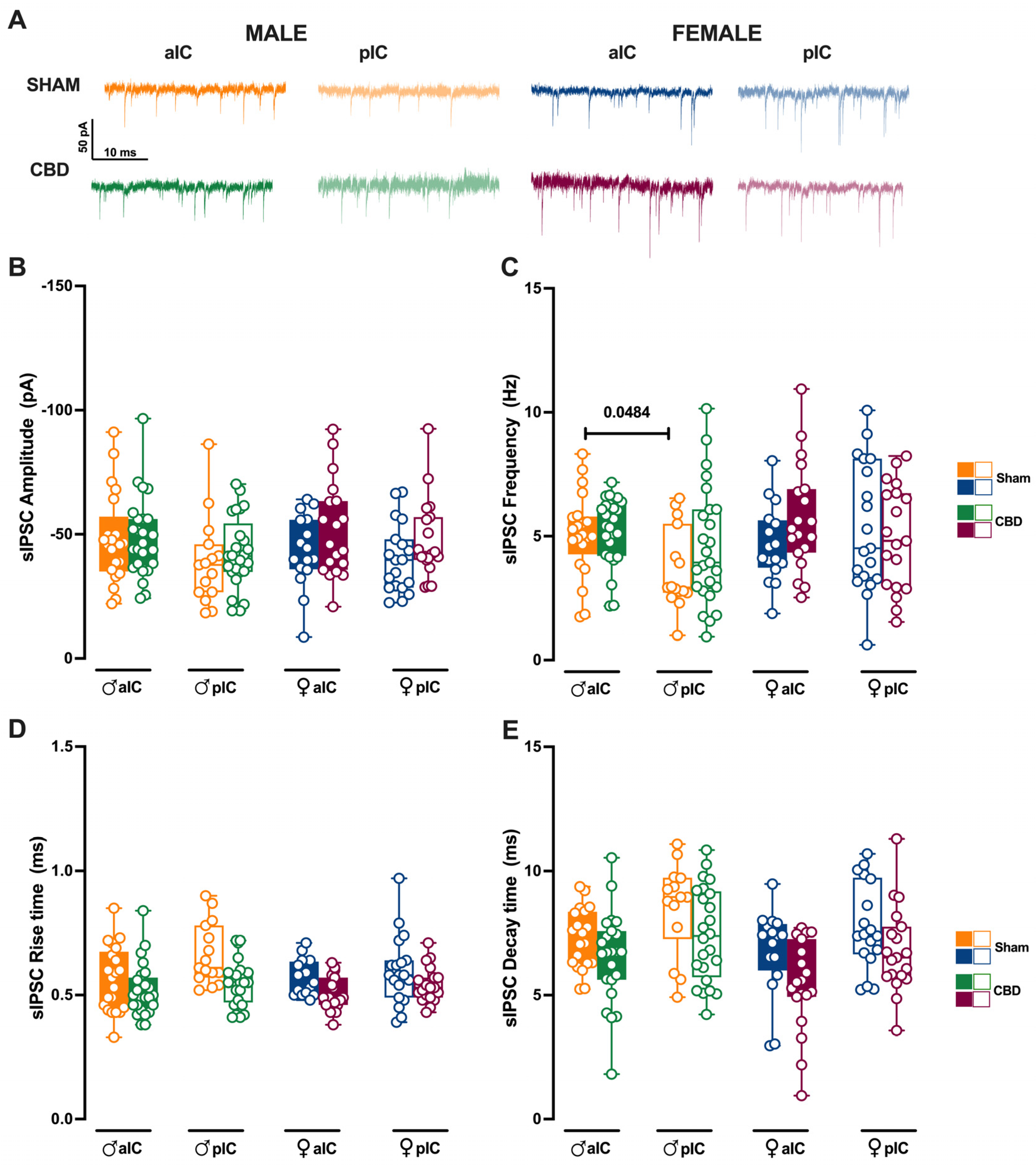
3.5. Prenatal Cannabidiol Exposure Altered the IC’s Excitatory–Inhibitory Balance IC in a Sex- and Territory-Specific Manner

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, S.; Delker, E.; Bandoli, G. The prevalence of cannabis use reported among pregnant individuals in the United States is increasing, 2002–2020. J. Perinatol. 2023, 43, 387. [Google Scholar] [CrossRef] [PubMed]
- Corsi, D.J.; Hsu, H.; Weiss, D.; Fell, D.B.; Walker, M. Trends and correlates of cannabis use in pregnancy: A population-based study in Ontario, Canada from 2012 to 2017. Can J. Public Health 2019, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.Y.; Avalos, L.A.; Alexeeff, S.; Conway, A.; Armstrong, M.A.; Weisner, C.; Campbell, C.I.; Goler, N. Trends in marijuana use among pregnant women with and without nausea and vomiting in pregnancy, 2009–2016. Drug Alcohol Depend. 2019, 196, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Tucker, L.Y.; Alexeeff, S.; Armstrong, M.A.; Conway, A.; Weisner, C.; Goler, N. Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009–2016. JAMA 2017, 318, 2490–2491. [Google Scholar] [CrossRef]
- Petrangelo, A.; Czuzoj-Shulman, N.; Balayla, J.; Abenhaim, H.A. Cannabis Abuse or Dependence During Pregnancy: A Population-Based Cohort Study on 12 Million Births. J. Obstet. Gynaecol. Can. 2019, 41, 623–630. [Google Scholar] [CrossRef]
- Brown, Q.L.; Sarvet, A.L.; Shmulewitz, D.; Martins, S.S.; Wall, M.M.; Hasin, D.S. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002-2014. JAMA 2017, 317, 207–209. [Google Scholar] [CrossRef]
- Volkow, N.D.; Han, B.; Compton, W.M.; McCance-Katz, E.F. Self-reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States. JAMA 2019, 322, 167. [Google Scholar] [CrossRef]
- Rokeby, A.C.E.; Natale, B.V.; Natale, D.R.C. Cannabinoids and the placenta: Receptors, signaling and outcomes. Placenta 2023, 135, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Abuhasira, R.; Shbiro, L.; Landschaft, Y. Medical use of cannabis and cannabinoids containing products—Regulations in Europe and North America. Eur. J. Intern. Med. 2018, 49, 2–6. [Google Scholar] [CrossRef]
- Sarrafpour, S.; Urits, I.; Powell, J.; Nguyen, D.; Callan, J.; Orhurhu, V.; Simopoulos, T.; Viswanath, O.; Kaye, A.D.; Kaye, R.J.; et al. Considerations and Implications of Cannabidiol Use During Pregnancy. Curr. Pain. Headache Rep. 2020, 24, 38. [Google Scholar] [CrossRef]
- Feinshtein, V.; Ben-Zvi, Z.; Eshkoli, T.; Sheizaf, B.; Sheiner, E.; Holcberg, G. Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: An ex vivo study. Am. J. Obstet. Gynecol. 2013, 209, 573.e1–573.e15. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. Cannabidiol disrupts apoptosis, autophagy and invasion processes of placental trophoblasts. Arch. Toxicol. 2021, 95, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.J.; Bushlin, I.; Kazmierczak, S.; Koop, D.; Hendrickson, R.G.; Zuckerman, K.E.; Grigsby, T.M. Cannabis use and measurement of cannabinoids in plasma and breast milk of breastfeeding mothers. Pediatr Res. 2021, 90, 861–868. [Google Scholar] [CrossRef]
- Bekman, E.; Barata, T.; Miranda, C.; Vaz, S.; Ferreira, C.; Quintas, A. Might synthetic cannabinoids influence neural differentiation? Ann. Med. 2021, 53 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Ochiai, W.; Kitaoka, S.; Kawamura, T.; Hatogai, J.; Harada, S.; Iizuka, M.; Ariumi, M.; Takano, S.; Nagai, T.; Sasatsu, M.; et al. Maternal and fetal pharmacokinetic analysis of cannabidiol during pregnancy in mice. Drug Metab. Dispos. 2021, 49, 337–343. [Google Scholar] [CrossRef]
- Iezzi, D.; Caceres-Rodriguez, A.; Chavis, P.; Manzoni, O.J.J. In utero exposure to cannabidiol disrupts select early-life behaviors in a sex-specific manner. Transl. Psychiatry 2022, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Maciel, I.d.S.; de Abreu, G.H.D.; Johnson, C.T.; Bonday, R.; Bradshaw, H.B.; Mackie, K.; Lu, H.C. Perinatal CBD or THC Exposure Results in Lasting Resistance to Fluoxetine in the Forced Swim Test: Reversal by Fatty Acid Amide Hydrolase Inhibition. Cannabis Cannabinoid Res. 2022, 7, 318–327. [Google Scholar] [CrossRef]
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Lamm, C.; Singer, T. The role of anterior insular cortex in social emotions. Brain Struct. Funct. 2010, 214, 579–591. [Google Scholar] [CrossRef]
- Rolls, E.T. Limbic Structures, Emotion, and Memory. Curated Ref. Collect. Neurosci. Biobehav. Psychol. 2017. [Google Scholar] [CrossRef]
- Ibrahim, C.; Le Foll, B.; French, L. Transcriptomic Characterization of the Human Insular Cortex and Claustrum. Front. Neuroanat. 2019, 13, 94. [Google Scholar] [CrossRef]
- Gehrlach, D.A.; Weiand, C.; Gaitanos, T.N.; Cho, E.; Klein, A.S.; Hennrich, A.A.; Conzelmann, K.K.; Gogolla, N. A whole-brain connectivity map of mouse insular cortex. eLife 2020, 9, 1–78. [Google Scholar] [CrossRef]
- Nagai, M.; Kishi, K.; Kato, S. Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur. Psychiatry 2007, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ruette, M.; Linsambarth, S.; Moraga-Amaro, R.; Quintana-Donoso, D.; Méndez, L.; Tamburini, G.; Cornejo, F.; Torres, R.F.; Stehberg, J. The role of the rodent insula in anxiety. Front Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.; Ju, A.; Wu, Y.; Eldirdiri, H.; Delcasso, S.; Couderc, Y.; Fornari, C.; Mitra, A.; Supiot, L.; Vérité, A.; et al. Linking emotional valence and anxiety in a mouse insula-amygdala circuit. Nat. Commun. 2023, 14, 5073. [Google Scholar] [CrossRef]
- Pressman, P.; Rosen, H.J. Disorders of Frontal Lobe Function. Neurobiol. Brain Disord. Biol. Basis Neurol. Psychiatr. Disord. 2015, 542–557. [Google Scholar] [CrossRef]
- Iezzi, D.; Cáceres-Rodríguez, A.; Strauss, B.; Chavis, P.; Manzoni, O.J. Sexual differences in neuronal and synaptic properties across subregions of the mouse insular cortex. Biol. Sex Differ. 2024, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Diedenhofen, B.; Musch, J. cocor: A Comprehensive Solution for the Statistical Comparison of Correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 22 April 2022).
- Doi, M.; Usui, N.; Shimada, S. Prenatal Environment and Neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- Bhatia, D.; Battula, S.; Mikulich-Gilbertson, S.; Sakai, J.; Hammond, D. Cannabidiol-Only Product Use in Pregnancy in the United States and Canada: Findings From the International Cannabis Policy Study. Obstet. Gynecol. 2024, 144, 156–159. [Google Scholar] [CrossRef]
- Catala, M. Development of the Central Nervous System. In Textbook of Pediatric Neurosurgery; Springer Nature: Cham, Switzerland, 2019; pp. 3–77. [Google Scholar]
- Afif, A.; Bouvier, R.; Buenerd, A.; Trouillas, J.; Mertens, P. Development of the human fetal insular cortex: Study of the gyration from 13 to 28 gestational weeks. Brain Struct. Funct. 2007, 212, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.Y.S.; Kalani, M.A.; Gwinn, R.; Keogh, B.; Tse, V.C.K. Embryological development of the human insula and its implications for the spread and resection of insular gliomas. Neurosurg. Focus. 2009, 27, 2. [Google Scholar] [CrossRef]
- Evrard, H.C.; (Bud) Craig, A.D. Insular Cortex. In Brain Mapping: An Encyclopedic Reference; Academic Press: Cambridge, MA, USA, 2023; Volume 2, pp. 387–393. [Google Scholar]
- Brummelte, S.; Mc Glanaghy, E.; Bonnin, A.; Oberlander, T.F. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 2017, 342, 212. [Google Scholar] [CrossRef] [PubMed]
- Hanswijk, S.I.; Spoelder, M.; Shan, L.; Verheij, M.M.M.; Muilwijk, O.G.; Li, W.; Liu, C.; Kolk, S.M.; Homberg, J.R. Gestational Factors throughout Fetal Neurodevelopment: The Serotonin Link. Int. J. Mol. Sci. 2020, 21, 5850. [Google Scholar] [CrossRef] [PubMed]
- Ju, A.; Fernandez-Arroyo, B.; Wu, Y.; Jacky, D.; Beyeler, A. Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Mol. Brain. 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.S.; Gomez Wulschner, L.E.; Hoelscher, V.M.; Folts, L.; Korth, K.M.; Oh, W.C.; Bates, E.A. Fetal cannabidiol (CBD) exposure alters thermal pain sensitivity, problem-solving, and prefrontal cortex excitability. Mol. Psychiatry 2023, 28, 3397–3413. [Google Scholar] [CrossRef] [PubMed]
- Hunnicutt, B.J.; Jongbloets, B.C.; Birdsong, W.T.; Gertz, K.J.; Zhong, H.; Mao, T. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife 2016, 5, e19103. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.L.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Heim, S.; Zilles, K.; Amunts, K. A systems perspective on the effective connectivity of overt speech production. Philos. Trans. A Math. Phys. Eng. Sci. 2009, 367, 2399–2421. [Google Scholar] [CrossRef]
- Oh, A.; Duerden, E.G.; Pang, E.W. The role of the insula in speech and language processing. Brain Lang. 2014, 135, 96. [Google Scholar] [CrossRef]
- Bamiou, D.E.; Musiek, F.E.; Luxon, L.M. The insula (Island of Reil) and its role in auditory processing: Literature review. Brain Res. Rev. 2003, 42, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J. Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain 2016, 157, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Rosso, I.M.; Weiner, M.R.; Crowley, D.J.; Silveri, M.M.; Rauch, S.L.; Jensen, J.E. Insula and anterior cingulate GABA levels in post-traumatic stress disorder: Preliminary findings using magnetic resonance spectroscopy. Depress. Anxiety 2014, 31, 115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iezzi, D.; Cáceres-Rodríguez, A.; Pereira-Silva, J.; Chavis, P.; Manzoni, O.J.J. Gestational CBD Shapes Insular Cortex in Adulthood. Cells 2024, 13, 1486. https://doi.org/10.3390/cells13171486
Iezzi D, Cáceres-Rodríguez A, Pereira-Silva J, Chavis P, Manzoni OJJ. Gestational CBD Shapes Insular Cortex in Adulthood. Cells. 2024; 13(17):1486. https://doi.org/10.3390/cells13171486
Chicago/Turabian StyleIezzi, Daniela, Alba Cáceres-Rodríguez, Jessica Pereira-Silva, Pascale Chavis, and Olivier Jacques José Manzoni. 2024. "Gestational CBD Shapes Insular Cortex in Adulthood" Cells 13, no. 17: 1486. https://doi.org/10.3390/cells13171486





