Current Approaches for the Prevention and Treatment of Acute and Chronic GVHD
Abstract
:1. Introduction
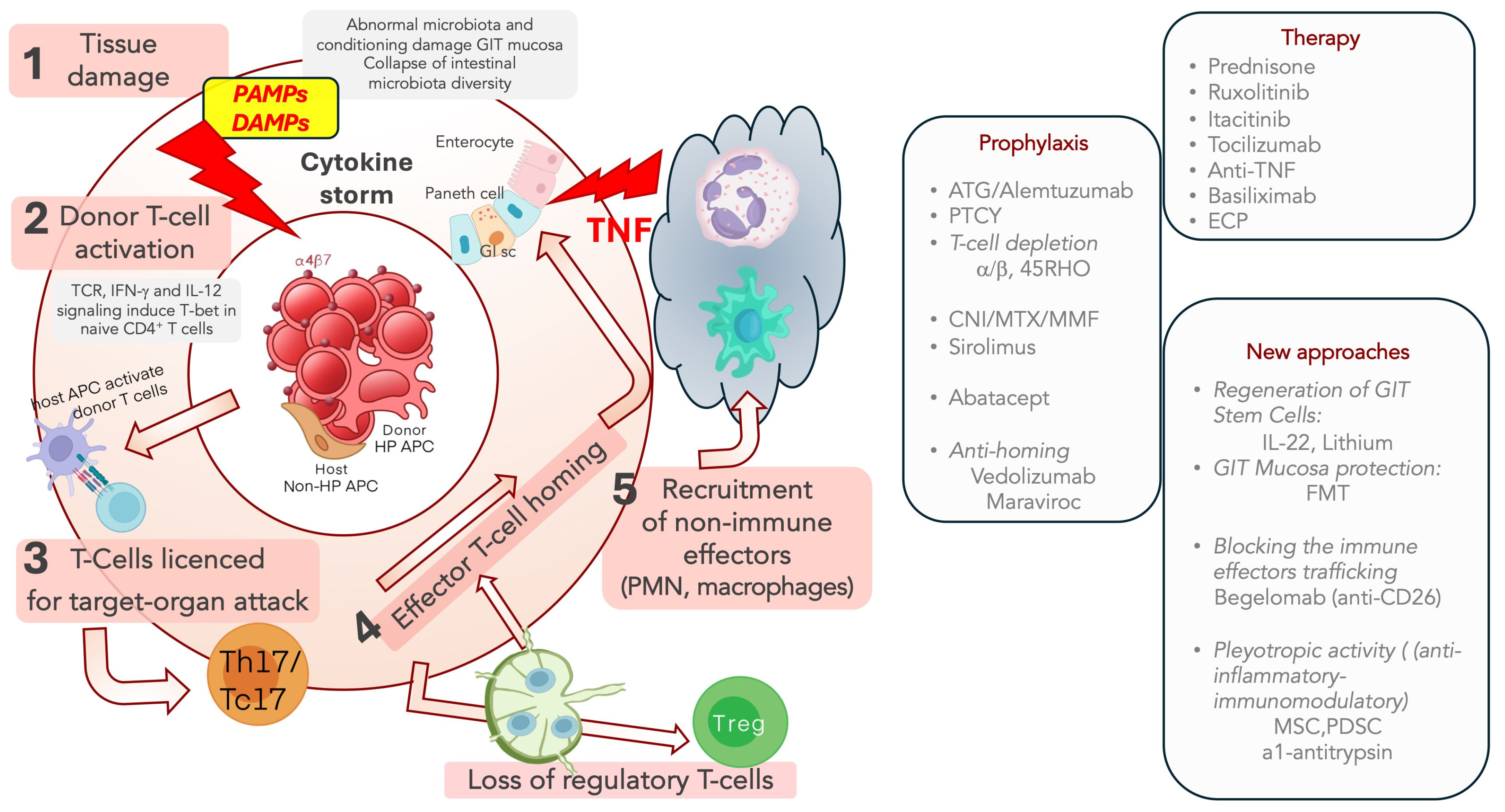
2. Pathophysiology of Acute and Chronic GVHD
3. GVHD Prevention
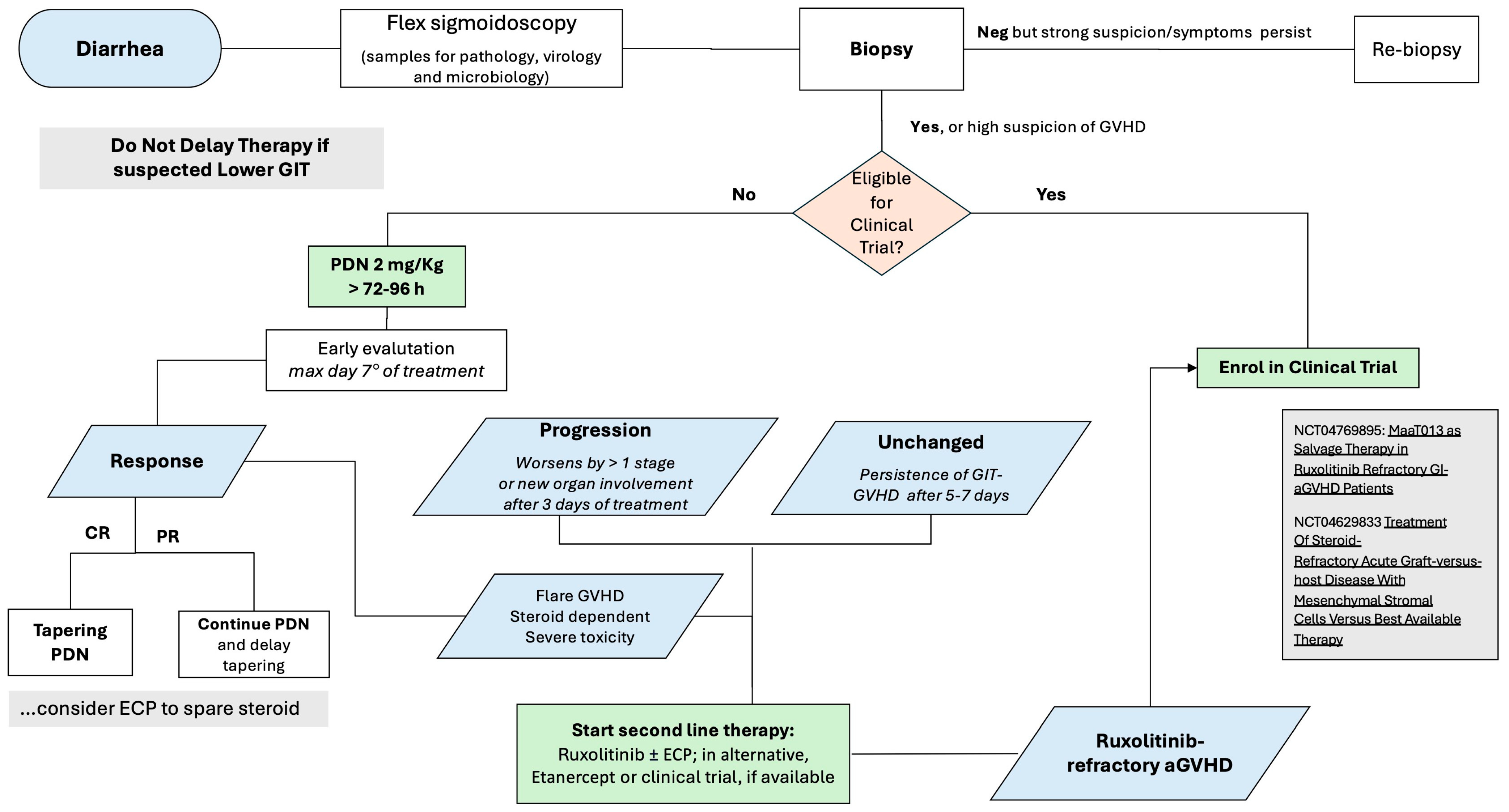
4. Therapy of aGVHD
5. Treatment of SR-aGVHD
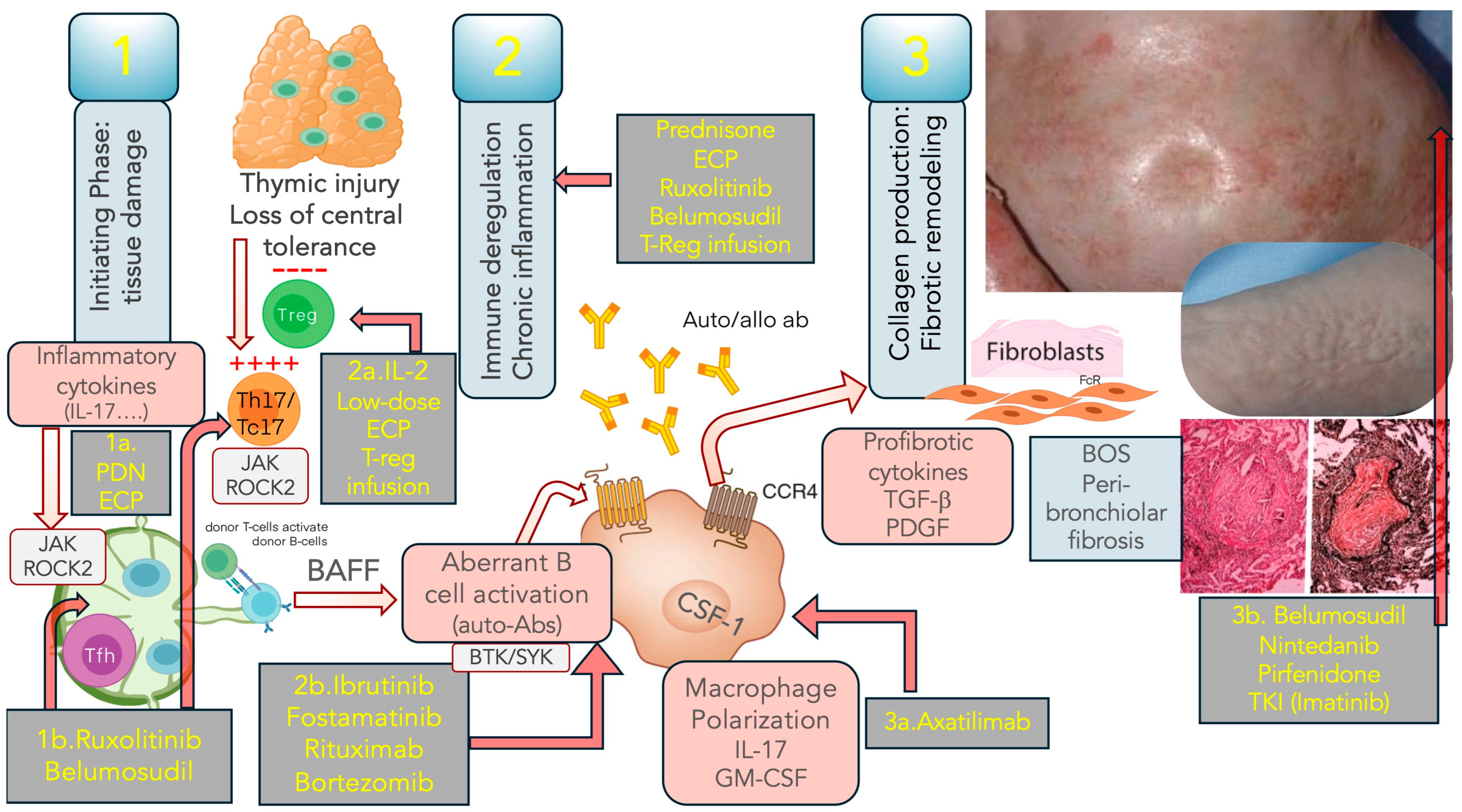
6. Treatment of cGVHD
7. SR-cGVHD
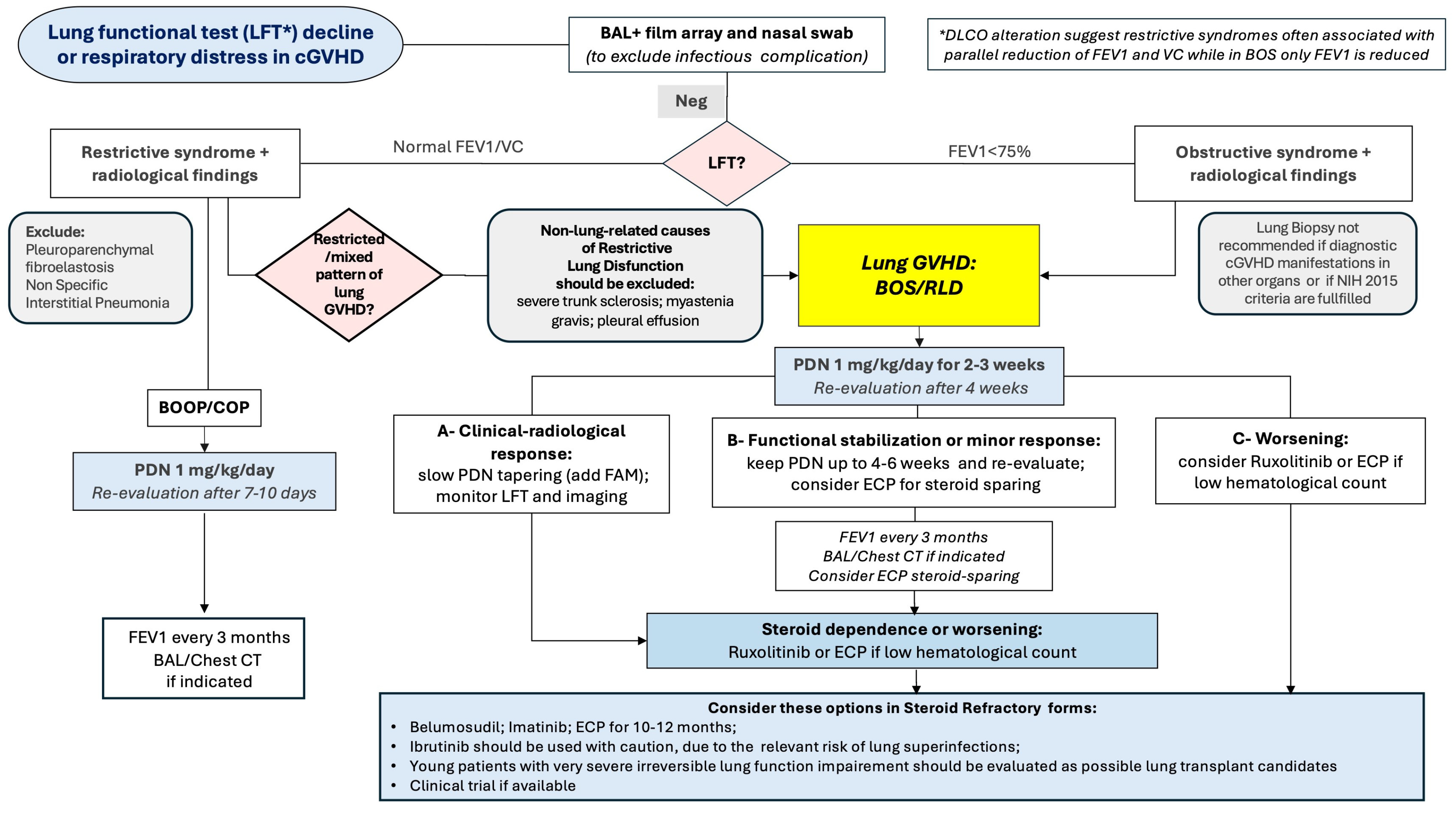
8. Management of Patients with Lung Involvement
9. Management and Topical Treatment of Specific cGVHD Localizations (Skin, Oral, Ocular and Genital cGVHD)
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arai, S.; Arora, M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Teshima, T. Nonclassical manifestations of acute GVHD. Blood 2021, 138, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Flowers, M.E.D. Recognizing and managing chronic graft-versus-host disease. Hematol. Am. Soc. Hematol. Educ. Program 2008, 2008, 134–141. [Google Scholar] [CrossRef]
- On behalf of the EBMT (European Society for Blood and Marrow Transplantation) Transplant Complications Working Party and the “EBMT−NIH (National Institutes of Health)−CIBMTR (Center for International Blood and Marrow Transplant Research) GvHD Task Force”; Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; et al. EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- Pidala, J.; Vogelsang, G.; Martin, P.; Chai, X.; Storer, B.; Pavletic, S.; Weisdorf, D.J.; Jagasia, M.; Cutler, C.; Palmer, J.; et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: A Chronic Graft-versus-Host Disease Consortium study. Haematologica 2012, 97, 451–458. [Google Scholar] [CrossRef]
- Herzog, S.; Weisdorf, D.J.; Shanley, R.; Rayes, A.; Holtan, S.G.; Young, J.-A.; MacMillan, M.L.; El Jurdi, N. Chronic GVHD after steroid-sensitive, -dependent, and -refractory acute GVHD: Incidence and clinical outcomes. Blood Adv. 2023, 7, 3644–3650. [Google Scholar] [CrossRef]
- Flowers, M.E.D.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.-P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Anasetti, C.; Logan, B.R.; Lee, S.J.; Waller, E.K.; Weisdorf, D.J.; Wingard, J.R.; Cutler, C.S.; Westervelt, P.; Woolfrey, A.; Couban, S.; et al. Peripheral-Blood Stem Cells versus Bone Marrow from Unrelated Donors. N. Engl. J. Med. 2012, 367, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.; Logan, B.R.; Confer, D.L.; Haagenson, M.; Wagner, J.E.; Weisdorf, D.J.; Wingard, J.R.; Rowley, S.D.; Stroncek, D.; Gee, A.P.; et al. Peripheral Blood Grafts from Unrelated Donors Are Associated with Increased Acute and Chronic Graft-versus-Host Disease without Improved Survival. Biol. Blood Marrow Transplant. 2007, 13, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, Y.; Spyrou, N.; Hogan, W.J.; Ayuk, F.; DeFilipp, Z.; Weber, D.; Choe, H.K.; Hexner, E.O.; Rösler, W.; Etra, A.M.; et al. Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. 2023, 7, 4479–4491. [Google Scholar] [CrossRef]
- Arai, S.; Jagasia, M.; Storer, B.; Chai, X.; Pidala, J.; Cutler, C.; Arora, M.; Weisdorf, D.J.; Flowers, M.E.D.; Martin, P.J.; et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood 2011, 118, 4242–4249. [Google Scholar] [CrossRef]
- Watkins, B.K.; Horan, J.; Storer, B.; Martin, P.J.; Carpenter, P.A.; Flowers, M.E.D. Recipient and donor age impact the risk of developing chronic GvHD in children after allogeneic hematopoietic transplant. Bone Marrow Transplant. 2017, 52, 625–626. [Google Scholar] [CrossRef]
- Baird, K.; Cooke, K.; Schultz, K.R. Chronic graft-versus-host disease (GVHD) in children. Pediatr. Clin. N. Am. 2010, 57, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef]
- Couriel, D.R.; Saliba, R.M.; Giralt, S.; Khouri, I.; Andersson, B.; De Lima, M.; Hosing, C.; Anderlini, P.; Donato, M.; Cleary, K.; et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol. Blood Marrow Transplant. 2004, 10, 178–185. [Google Scholar] [CrossRef]
- Lee, S.-E.; Cho, B.-S.; Kim, J.-H.; Yoon, J.-H.; Shin, S.-H.; Yahng, S.-A.; Eom, K.-S.; Kim, Y.-J.; Kim, H.-J.; Lee, S.; et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013, 48, 587–592. [Google Scholar] [CrossRef]
- Holtick, U.; Albrecht, M.; Chemnitz, J.M.; Theurich, S.; Skoetz, N.; Scheid, C.; Von Bergwelt-Baildon, M. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst. Rev. 2014, 2014, CD010189. [Google Scholar] [CrossRef]
- O’Donnell, P.V.; Eapen, M.; Horowitz, M.M.; Logan, B.R.; DiGilio, A.; Brunstein, C.; Fuchs, E.J.; Flowers, M.E.D.; Salit, R.; Raj, K.; et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: A matched-pair analysis. Bone Marrow Transplant. 2016, 51, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, M.; Romee, R.; St. Martin, A.; Arora, M.; Al Malki, M.; Antin, J.H.; Bredeson, C.N.; Brunstein, C.G.; Chhabra, S.; Fuchs, E.J.; et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood 2021, 138, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; Pasquini, M.C.; Logan, B.; Soiffer, R.J.; Wu, J.; Devine, S.M.; Geller, N.; Giralt, S.; Heslop, H.E.; Horowitz, M.M.; et al. Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor–Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies. J. Clin. Oncol. 2022, 40, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Bashey, A.; Zhang, X.; Jackson, K.; Brown, S.; Ridgeway, M.; Solh, M.; Morris, L.E.; Holland, H.K.; Solomon, S.R. Comparison of Outcomes of Hematopoietic Cell Transplants from T-Replete Haploidentical Donors Using Post-Transplantation Cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 Allele-Matched Unrelated Donors and HLA-Identical Sibling Donors: A Multivariable Analysis Including Disease Risk Index. Biol. Blood Marrow Transplant. 2016, 22, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.J.; Hamerschlak, N.; Rocha, V.; Bonfim, C.; Kerbauy, M.N. Outcomes after Haploidentical Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide: A Systematic Review and Meta-Analysis Comparing Myeloablative with Reduced-Intensity Conditioning Regimens and Bone Marrow with Peripheral Blood Stem Cell Grafts. Transplant. Cell. Ther. 2021, 27, 782.e1–782.e7. [Google Scholar] [CrossRef]
- Sterling, C.H.; Hughes, M.S.; Tsai, H.-L.; Yarkony, K.; Fuchs, E.J.; Swinnen, L.J.; Paul, S.; Bolaños-Meade, J.; Luznik, L.; Imus, P.H.; et al. Allogeneic Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide for Peripheral T Cell Lymphoma: The Importance of Graft Source. Transplant. Cell. Ther. 2023, 29, 267.e1–267.e5. [Google Scholar] [CrossRef]
- Lacan, C.; Lambert, J.; Forcade, E.; Robin, M.; Chevallier, P.; Loron, S.; Bulabois, C.-É.; Orvain, C.; Ceballos, P.; Daguindau, E.; et al. Bone marrow graft versus peripheral blood graft in haploidentical hematopoietic stem cells transplantation: A retrospective analysis in1344 patients of SFGM-TC registry. J. Hematol. Oncol. 2024, 17, 2. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Socié, G.; Kean, L.S.; Zeiser, R.; Blazar, B.R. Insights from integrating clinical and preclinical studies advance understanding of graft-versus-host disease. J. Clin. Investig. 2021, 131, e149296. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Hill, G.R.; Betts, B.C.; Tkachev, V.; Kean, L.S.; Blazar, B.R. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu. Rev. Immunol. 2021, 39, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Socie, G.; Michonneau, D. Milestones in acute GVHD pathophysiology. Front. Immunol. 2022, 13, 1079708. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.-R.; Sun, Y.; Rossi, C.; et al. Gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Shah, N.N.; Renteria, A.S.; Cutler, C.; Jansson, J.; Akbari, M.; Chen, C.; Quadri, S.; Parfionovas, A.; Devine, S.M. Vedolizumab for prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019, 3, 4136–4146. [Google Scholar] [CrossRef]
- Beilhack, A.; Schulz, S.; Baker, J.; Beilhack, G.F.; Wieland, C.B.; Herman, E.I.; Baker, E.M.; Cao, Y.-A.; Contag, C.H.; Negrin, R.S. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 2005, 106, 1113–1122. [Google Scholar] [CrossRef]
- Mora, J.R.; Bono, M.R.; Manjunath, N.; Weninger, W.; Cavanagh, L.L.; Rosemblatt, M.; Von Andrian, U.H. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 2003, 424, 88–93. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kim, H.T.; McDonough, S.; Bascug, G.; Warshauer, B.; Koreth, J.; Cutler, C.; Ho, V.T.; Alyea, E.P.; Antin, J.H.; et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J. Clin. Investig. 2010, 120, 1479–1493. [Google Scholar] [CrossRef]
- Forcade, E.; Kim, H.T.; Cutler, C.; Wang, K.; Alho, A.C.; Nikiforow, S.; Ho, V.T.; Koreth, J.; Armand, P.; Alyea, E.P.; et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood 2016, 127, 2489–2497. [Google Scholar] [CrossRef]
- McManigle, W.; Youssef, A.; Sarantopoulos, S. B cells in chronic graft-versus-host disease. Hum. Immunol. 2019, 80, 393–399. [Google Scholar] [CrossRef]
- Sarantopoulos, S.; Ritz, J. Aberrant B-cell homeostasis in chronic GVHD. Blood 2015, 125, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Svegliati, S.; Olivieri, A.; Campelli, N.; Luchetti, M.; Poloni, A.; Trappolini, S.; Moroncini, G.; Bacigalupo, A.; Leoni, P.; Avvedimento, E.V.; et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood 2007, 110, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.; Dazzi, F. Chronic GVHD as an autoimmune disease. Best Pract. Res. Clin. Haematol. 2008, 21, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zorn, E.; Miklos, D.B.; Floyd, B.H.; Mattes-Ritz, A.; Guo, L.; Soiffer, R.J.; Antin, J.H.; Ritz, J. Minor Histocompatibility Antigen DBY Elicits a Coordinated B and T Cell Response after Allogeneic Stem Cell Transplantation. J. Exp. Med. 2004, 199, 1133–1142. [Google Scholar] [CrossRef]
- Marinelli Busilacchi, E.; Costantini, A.; Mancini, G.; Tossetta, G.; Olivieri, J.; Poloni, A.; Viola, N.; Butini, L.; Campanati, A.; Goteri, G.; et al. Nilotinib Treatment of Patients Affected by Chronic Graft-versus-Host Disease Reduces Collagen Production and Skin Fibrosis by Downmodulating the TGF-β and p-SMAD Pathway. Biol. Blood Marrow Transplant. 2020, 26, 823–834. [Google Scholar] [CrossRef]
- Olivieri, A.; Mancini, G.; Olivieri, J.; Marinelli Busilacchi, E.; Cimminiello, M.; Pascale, S.P.; Nuccorini, R.; Patriarca, F.; Corradini, P.; Bacigalupo, A.; et al. Nilotinib in steroid-refractory cGVHD: Prospective parallel evaluation of response, according to NIH criteria and exploratory response criteria (GITMO criteria). Bone Marrow Transplant. 2020, 55, 2077–2086. [Google Scholar] [CrossRef]
- Martin, P.J.; Storer, B.E.; Inamoto, Y.; Flowers, M.E.D.; Carpenter, P.A.; Pidala, J.; Palmer, J.; Arora, M.; Jagasia, M.; Arai, S.; et al. An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood 2017, 130, 360–367. [Google Scholar] [CrossRef]
- Dehn, J.; Spellman, S.; Hurley, C.K.; Shaw, B.E.; Barker, J.N.; Burns, L.J.; Confer, D.L.; Eapen, M.; Fernandez-Vina, M.; Hartzman, R.; et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: Guidelines from the NMDP/CIBMTR. Blood 2019, 134, 924–934. [Google Scholar] [CrossRef]
- Pidala, J.; Lee, S.J.; Ahn, K.W.; Spellman, S.; Wang, H.-L.; Aljurf, M.; Askar, M.; Dehn, J.; Fernandez Viña, M.; Gratwohl, A.; et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood 2014, 124, 2596–2606. [Google Scholar] [CrossRef]
- Tsamadou, C.; Engelhardt, D.; Platzbecker, U.; Sala, E.; Valerius, T.; Wagner-Drouet, E.; Wulf, G.; Kröger, N.; Murawski, N.; Einsele, H.; et al. HLA-DRB3/4/5 Matching Improves Outcome of Unrelated Hematopoietic Stem Cell Transplantation. Front. Immunol. 2021, 12, 771449. [Google Scholar] [CrossRef]
- Shono, Y.; Van Den Brink, M.R.M. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Jamy, O.; Zeiser, R.; Chen, Y.-B. Novel developments in the prophylaxis and treatment of acute GVHD. Blood 2023, 142, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.A.; Antin, J.H.; Karanes, C.; Fay, J.W.; Avalos, B.R.; Yeager, A.M.; Przepiorka, D.; Davies, S.; Petersen, F.B.; Bartels, P.; et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000, 96, 2062–2068. [Google Scholar] [PubMed]
- Ratanatharathorn, V.; Nash, R.A.; Przepiorka, D.; Devine, S.M.; Klein, J.L.; Weisdorf, D.; Fay, J.W.; Nademanee, A.; Antin, J.H.; Christiansen, N.P.; et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998, 92, 2303–2314. [Google Scholar]
- Perkins, J.; Field, T.; Kim, J.; Kharfan-Dabaja, M.A.; Fernandez, H.; Ayala, E.; Perez, L.; Xu, M.; Alsina, M.; Ochoa, L.; et al. A Randomized Phase II Trial Comparing Tacrolimus and Mycophenolate Mofetil to Tacrolimus and Methotrexate for Acute Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2010, 16, 937–947. [Google Scholar] [CrossRef]
- Törlén, J.; Ringdén, O.; Garming-Legert, K.; Ljungman, P.; Winiarski, J.; Remes, K.; Itälä-Remes, M.; Remberger, M.; Mattsson, J. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica 2016, 101, 1417–1425. [Google Scholar] [CrossRef]
- Kanda, Y.; Kobayashi, T.; Mori, T.; Tanaka, M.; Nakaseko, C.; Yokota, A.; Watanabe, R.; Kako, S.; Kakihana, K.; Kato, J.; et al. A randomized controlled trial of cyclosporine and tacrolimus with strict control of blood concentrations after unrelated bone marrow transplantation. Bone Marrow Transplant. 2016, 51, 103–109. [Google Scholar] [CrossRef]
- Cutler, C.; Logan, B.; Nakamura, R.; Johnston, L.; Choi, S.; Porter, D.; Hogan, W.J.; Pasquini, M.; MacMillan, M.L.; Hsu, J.W.; et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014, 124, 1372–1377. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; Sainvil, M.; Lange, P.B.; Giardino, A.A.; Bachanova, V.; Devine, S.M.; Waller, E.K.; Jagirdar, N.; Herrera, A.F.; et al. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: A multicentre randomized trial. Br. J. Haematol. 2016, 173, 96–104. [Google Scholar] [CrossRef]
- Sandmaier, B.M.; Kornblit, B.; Storer, B.E.; Olesen, G.; Maris, M.B.; Langston, A.A.; Gutman, J.A.; Petersen, S.L.; Chauncey, T.R.; Bethge, W.A.; et al. Addition of sirolimus to standard cyclosporine plus mycophenolate mofetil-based graft-versus-host disease prophylaxis for patients after unrelated non-myeloablative haemopoietic stem cell transplantation: A multicentre, randomised, phase 3 trial. Lancet Haematol. 2019, 6, e409–e418. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Cassady, K.; Yang, S.; Chen, T.; Wang, L.; Yan, H.; Zhang, X.; Feng, Y. The Addition of Sirolimus to GVHD Prophylaxis After Allogeneic Hematopoietic Stem Cell Transplantation: A Meta-Analysis of Efficacy and Safety. Front. Oncol. 2021, 11, 683263. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kajigaya, S.; Solomou, E.E.; Keyvanfar, K.; Xu, X.; Raghavachari, N.; Munson, P.J.; Herndon, T.M.; Chen, J.; Young, N.S. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood 2008, 111, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Broady, R.; Yu, J.; Levings, M.K. ATG-induced expression of FOXP3 in human CD4(+) T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood 2009, 114, 5003–5006. [Google Scholar] [CrossRef]
- Valdez-Ortiz, R.; Bestard, O.; Llaudó, I.; Franquesa, M.; Cerezo, G.; Torras, J.; Herrero-Fresneda, I.; Correa-Rotter, R.; Grinyó, J.M. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl. Int. 2015, 28, 108–119. [Google Scholar] [CrossRef]
- Boenisch, O.; Lopez, M.; Elyaman, W.; Magee, C.N.; Ahmad, U.; Najafian, N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4+ T cells and requires the presence of monocytes. Am. J. Transplant. 2012, 12, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.; Bethge, W.A.; Schmoor, C.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009, 10, 855–864. [Google Scholar] [CrossRef]
- Kröger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef]
- Soiffer, R.J.; Kim, H.T.; McGuirk, J.; Horwitz, M.E.; Johnston, L.; Patnaik, M.M.; Rybka, W.; Artz, A.; Porter, D.L.; Shea, T.C.; et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti–T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease–Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J. Clin. Oncol. 2017, 35, 4003–4011. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Wu, D.-P.; Lai, Y.-R.; Liu, Q.-F.; Sun, Y.-Q.; Hu, J.; Hu, Y.; Zhou, J.-F.; Li, J.; Wang, S.-Q.; et al. Antithymocyte Globulin for Matched Sibling Donor Transplantation in Patients With Hematologic Malignancies: A Multicenter, Open-Label, Randomized Controlled Study. J. Clin. Oncol. 2020, 38, 3367–3376. [Google Scholar] [CrossRef]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: Final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2020, 7, e100–e111. [Google Scholar] [CrossRef]
- Socié, G.; Schmoor, C.; Bethge, W.A.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti–T-cell globulin ATG-Fresenius. Blood 2011, 117, 6375–6382. [Google Scholar] [CrossRef] [PubMed]
- Chakupurakal, G.; Freudenberger, P.; Skoetz, N.; Ahr, H.; Theurich, S. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst. Rev. 2023, 2023, CD009159. [Google Scholar] [CrossRef]
- Hale, G.; Cobbold, S.; Novitzky, N.; Bunjes, D.; Willemze, R.; Prentice, H.G.; Milligan, D.; MacKinnon, S.; Waldmann, H. CAMPATH Users CAMPATH-1 antibodies in stem-cell transplantation. Cytotherapy 2001, 3, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Lankester, A.C.; Albert, M.H.; Booth, C.; Gennery, A.R.; Güngör, T.; Hönig, M.; Morris, E.C.; Moshous, D.; Neven, B.; Schulz, A.; et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021, 56, 2052–2062. [Google Scholar] [CrossRef]
- Holtzman, N.G.; Curtis, L.M.; Salit, R.B.; Shaffer, B.C.; Pirsl, F.; Ostojic, A.; Steinberg, S.M.; Schulz, E.; Wilder, J.S.; Hughes, T.E.; et al. High-dose Alemtuzumab-Cyclosporine vs Tacrolimus-Methotrexate-Sirolimus for Chronic Graft-versus-Host Disease Prevention. Blood Adv. 2024, 8, 4294–4310. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Fuchs, E.J. Post-Transplantation Cyclophosphamide for Tolerance Induction in HLA-Haploidentical Bone Marrow Transplantation. Semin. Oncol. 2012, 39, 683–693. [Google Scholar] [CrossRef]
- Nunes, N.S.; Kanakry, C.G. Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front. Immunol. 2019, 10, 2668. [Google Scholar] [CrossRef]
- Chiusolo, P.; Bug, G.; Olivieri, A.; Brune, M.; Mordini, N.; Alessandrino, P.E.; Dominietto, A.; Raiola, A.M.; Di Grazia, C.; Gualandi, F.; et al. A Modified Post-Transplant Cyclophosphamide Regimen, for Unmanipulated Haploidentical Marrow Transplantation, in Acute Myeloid Leukemia: A Multicenter Study. Biol. Blood Marrow Transplant. 2018, 24, 1243–1249. [Google Scholar] [CrossRef]
- Bolaños-Meade, J.; Hamadani, M.; Wu, J.; Al Malki, M.M.; Martens, M.J.; Runaas, L.; Elmariah, H.; Rezvani, A.R.; Gooptu, M.; Larkin, K.T.; et al. Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. N. Engl. J. Med. 2023, 388, 2338–2348. [Google Scholar] [CrossRef]
- Aversa, F.; Tabilio, A.; Velardi, A.; Cunningham, I.; Terenzi, A.; Falzetti, F.; Ruggeri, L.; Barbabietola, G.; Aristei, C.; Latini, P.; et al. Treatment of High-Risk Acute Leukemia with T-Cell–Depleted Stem Cells from Related Donors with One Fully Mismatched HLA Haplotype. N. Engl. J. Med. 1998, 339, 1186–1193. [Google Scholar] [CrossRef]
- Peters, C.; Matthes-Martin, S.; Fritsch, G.; Holter, W.; Lion, T.; Witt, V.; Höcker, P.; Fischer, G.; Dieckmann, K.; Handgretinger, R.; et al. Transplantation of highly purified peripheral blood CD34+ cells from HLA-mismatched parental donors in 14 children: Evaluation of early monitoring of engraftment. Leukemia 1999, 13, 2070–2078. [Google Scholar] [CrossRef]
- Zecca, M.; Strocchio, L.; Pagliara, D.; Comoli, P.; Bertaina, A.; Giorgiani, G.; Perotti, C.; Corbella, F.; Brescia, L.; Locatelli, F. HLA-Haploidentical T Cell–Depleted Allogeneic Hematopoietic Stem Cell Transplantation in Children with Fanconi Anemia. Biol. Blood Marrow Transplant. 2014, 20, 571–576. [Google Scholar] [CrossRef]
- Bleakley, M.; Heimfeld, S.; Loeb, K.R.; Jones, L.A.; Chaney, C.; Seropian, S.; Gooley, T.A.; Sommermeyer, F.; Riddell, S.R.; Shlomchik, W.D. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J. Clin. Investig. 2015, 125, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Merli, P.; Pagliara, D.; Li Pira, G.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, K.; Otto, M.; Hematti, P.; Kenkre, V. TCR αβ+/CD19+ cell depletion in haploidentical hematopoietic allogeneic stem cell transplantation: A review of current data. Leuk. Lymphoma 2019, 60, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Di Ianni, M.; Liberatore, C.; Santoro, N.; Ranalli, P.; Guardalupi, F.; Corradi, G.; Villanova, I.; Di Francesco, B.; Lattanzio, S.; Passeri, C.; et al. Cellular Strategies for Separating GvHD from GvL in Haploidentical Transplantation. Cells 2024, 13, 134. [Google Scholar] [CrossRef]
- Koura, D.T.; Horan, J.T.; Langston, A.A.; Qayed, M.; Mehta, A.; Khoury, H.J.; Harvey, R.D.; Suessmuth, Y.; Couture, C.; Carr, J.; et al. In Vivo T Cell Costimulation Blockade with Abatacept for Acute Graft-versus-Host Disease Prevention: A First-in-Disease Trial. Biol. Blood Marrow Transplant. 2013, 19, 1638–1649. [Google Scholar] [CrossRef]
- Watkins, B.; Qayed, M.; McCracken, C.; Bratrude, B.; Betz, K.; Suessmuth, Y.; Yu, A.; Sinclair, S.; Furlan, S.; Bosinger, S.; et al. Phase II Trial of Costimulation Blockade With Abatacept for Prevention of Acute GVHD. J. Clin. Oncol. 2021, 39, 1865–1877. [Google Scholar] [CrossRef]
- New, J.; Li, B.; Koh, W.; Ng, H.; Tan, S.; Yap, E.; Chan, S.; Hu, H. T cell infiltration and chemokine expression: Relevance to the disease localization in murine graft-versus-host disease. Bone Marrow Transplant. 2002, 29, 979–986. [Google Scholar] [CrossRef]
- Wysocki, C.A.; Burkett, S.B.; Panoskaltsis-Mortari, A.; Kirby, S.L.; Luster, A.D.; McKinnon, K.; Blazar, B.R.; Serody, J.S. Differential Roles for CCR5 Expression on Donor T Cells during Graft-versus-Host Disease Based on Pretransplant Conditioning. J. Immunol. 2004, 173, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Reshef, R.; Ganetsky, A.; Acosta, E.P.; Blauser, R.; Crisalli, L.; McGraw, J.; Frey, N.V.; Hexner, E.O.; Hoxie, J.A.; Loren, A.W.; et al. Extended CCR5 Blockade for Graft-versus-Host Disease Prophylaxis Improves Outcomes of Reduced-Intensity Unrelated Donor Hematopoietic Cell Transplantation: A Phase II Clinical Trial. Biol. Blood Marrow Transplant. 2019, 25, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Meade, J.; Reshef, R.; Fraser, R.; Fei, M.; Abhyankar, S.; Al-Kadhimi, Z.; Alousi, A.M.; Antin, J.H.; Arai, S.; Bickett, K.; et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: A randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019, 6, e132–e143. [Google Scholar] [CrossRef]
- Chen, Y.-B.; McDonough, S.; Chen, H.; Kennedy, J.; Illiano, C.; Attar, E.C.; Ballen, K.K.; Dey, B.R.; McAfee, S.L.; Jagasia, M.; et al. Expression of α4β7 integrin on memory CD8+ T cells at the presentation of acute intestinal GVHD. Bone Marrow Transplant. 2013, 48, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Mohty, M.; Zeiser, R.; Teshima, T.; Takanami, Y.; Rossiter, G.; Jansson, J.; Fløisand, Y. Vedolizumab for Prophylaxis of Lower Gastrointestinal (GI) Acute Graft-versus-Host Disease (aGVHD) After Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) From Unrelated Donors: Results of a Phase 3 Randomized, Double-Blind, Placebo-Controlled Multicenter Study (GRAPHITE). Clin. Adv. Hematol. Oncol. 2023, 21, 12–13. [Google Scholar]
- Yazdandoust, E.; Hajifathali, A.; Roshandel, E.; Zarif, M.N.; Pourfathollah, A.A.; Parkhideh, S.; Mehdizadeh, M.; Amini-Kafiabad, S. Gut microbiota intervention by pre and probiotics can induce regulatory T cells and reduce the risk of severe acute GVHD following allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2023, 78, 101836. [Google Scholar] [CrossRef]
- Fang, J.; Hu, C.; Hong, M.; Wu, Q.; You, Y.; Zhong, Z.; Li, W.; Zou, P.; Hu, Y.; Xia, L. Prophylactic Effects of Interleukin-2 Receptor Antagonists against Graft-versus-Host Disease Following Unrelated Donor Peripheral Blood Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 754–762. [Google Scholar] [CrossRef]
- Malard, F.; Huang, X.-J.; Sim, J.P.Y. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia 2020, 34, 1229–1240. [Google Scholar] [CrossRef]
- Huang, R.; Chen, T.; Wang, S.; Wang, J.; Su, Y.; Liu, J.; Zhang, Y.; Ma, X.; Wen, Q.; Kong, P.; et al. Mesenchymal Stem Cells for Prophylaxis of Chronic Graft-vs-Host Disease After Haploidentical Hematopoietic Stem Cell Transplant: An Open-Label Randomized Clinical Trial. JAMA Oncol. 2024, 10, 220–226. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar] [PubMed]
- Rowlings, P.A.; Przepiorka, D.; Klein, J.P.; Gale, R.P.; Passweg, J.R.; Henslee-Downey, P.J.; Cahn, J.Y.; Calderwood, S.; Gratwohl, A.; Socié, G.; et al. IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br. J. Haematol. 1997, 97, 855–864. [Google Scholar] [CrossRef]
- MacMillan, M.L.; Weisdorf, D.J.; Wagner, J.E.; DeFor, T.E.; Burns, L.J.; Ramsay, N.K.C.; Davies, S.M.; Blazar, B.R. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: Comparison of grading systems. Biol. Blood Marrow Transplant. 2002, 8, 387–394. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, M.L.; DeFor, T.E.; Weisdorf, D.J. The best endpoint for acute GVHD treatment trials. Blood 2010, 115, 5412–5417. [Google Scholar] [CrossRef]
- Levine, J.E.; Braun, T.M.; Harris, A.C.; Holler, E.; Taylor, A.; Miller, H.; Magenau, J.; Weisdorf, D.J.; Ho, V.T.; Bolaños-Meade, J.; et al. A prognostic score for acute graft-versus-host disease based on biomarkers: A multicentre study. Lancet Haematol. 2015, 2, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Toubai, T.; Magenau, J. Immunopathology and biology-based treatment of steroid-refractory graft-versus-host disease. Blood 2020, 136, 429–440. [Google Scholar] [CrossRef]
- Zeisbrich, M.; Becker, N.; Benner, A.; Radujkovic, A.; Schmitt, K.; Beimler, J.; Ho, A.D.; Zeier, M.; Dreger, P.; Luft, T. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant. 2017, 52, 1399–1405. [Google Scholar] [CrossRef]
- Ibrahim, R.B.; Abidi, M.H.; Cronin, S.M.; Lum, L.G.; Al-Kadhimi, Z.; Ratanatharathorn, V.; Uberti, J.P. Nonabsorbable Corticosteroids Use in the Treatment of Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2009, 15, 395–405. [Google Scholar] [CrossRef]
- Mielcarek, M.; Furlong, T.; Storer, B.E.; Green, M.L.; McDonald, G.B.; Carpenter, P.A.; Flowers, M.E.D.; Storb, R.; Boeckh, M.; Martin, P.J. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: A randomized controlled trial. Haematologica 2015, 100, 842–848. [Google Scholar] [CrossRef]
- Tarantino, G.; Saraceni, F.; Mancini, G.; Poiani, M.; Maroni, L.; Goteri, G.; Scortechini, I.; Fiorentini, A.; Dubbini, M.V.; Marini, F.; et al. Gastrointestinal Complications after Allogeneic Hematopoietic Stem Cell Transplant: A Multidisciplinary Approach with Early Endoscopic Evaluation. Clin. Hematol. Int. 2021, 3, 161. [Google Scholar] [CrossRef]
- Greinix, H.T.; Ayuk, F.; Zeiser, R. Extracorporeal photopheresis in acute and chronic steroid-refractory graft-versus-host disease: An evolving treatment landscape. Leukemia 2022, 36, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Drexler, B.; Buser, A.; Infanti, L.; Stehle, G.; Halter, J.; Holbro, A. Extracorporeal Photopheresis in Graft-versus-Host Disease. Transfus. Med. Hemother. 2020, 47, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dalle, I.; Reljic, T.; Nishihori, T.; Antar, A.; Bazarbachi, A.; Djulbegovic, B.; Kumar, A.; Kharfan-Dabaja, M.A. Extracorporeal Photopheresis in Steroid-Refractory Acute or Chronic Graft-versus-Host Disease: Results of a Systematic Review of Prospective Studies. Biol. Blood Marrow Transplant. 2014, 20, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Schub, N.; Günther, A.; Schrauder, A.; Claviez, A.; Ehlert, C.; Gramatzki, M.; Repp, R. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011, 46, 143–147. [Google Scholar] [CrossRef]
- Spoerl, S.; Mathew, N.R.; Bscheider, M.; Schmitt-Graeff, A.; Chen, S.; Mueller, T.; Verbeek, M.; Fischer, J.; Otten, V.; Schmickl, M.; et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 2014, 123, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Zeiser, R.; Von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Khoury, H.J.; Jagasia, M.; Ali, H.; Schiller, G.J.; Staser, K.; Choi, J.; Gehrs, L.; Arbushites, M.C.; Yan, Y.; et al. A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv. 2020, 4, 1656–1669. [Google Scholar] [CrossRef]
- Mohty, M.; Holler, E.; Jagasia, M.; Jenq, R.; Malard, F.; Martin, P.; Socié, G.; Zeiser, R. Refractory acute graft-versus-host disease: A new working definition beyond corticosteroid refractoriness. Blood 2020, 136, 1903–1906. [Google Scholar] [CrossRef]
- Zeiser, R.; Socié, G.; Schroeder, M.A.; Abhyankar, S.; Vaz, C.P.; Kwon, M.; Clausen, J.; Volodin, L.; Giebel, S.; Chacon, M.J.; et al. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): A randomised, multicentre, double-blind, phase 3 trial. Lancet Haematol. 2022, 9, e14–e25. [Google Scholar] [CrossRef]
- Drobyski, W.R.; Pasquini, M.; Kovatovic, K.; Palmer, J.; Douglas Rizzo, J.; Saad, A.; Saber, W.; Hari, P. Tocilizumab for the Treatment of Steroid Refractory Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2011, 17, 1862–1868. [Google Scholar] [CrossRef]
- Gergis, U.; Arnason, J.; Yantiss, R.; Shore, T.; Wissa, U.; Feldman, E.; Woodworth, T. Effectiveness and Safety of Tocilizumab, an Anti–Interleukin-6 Receptor Monoclonal Antibody, in a Patient With Refractory GI Graft-Versus-Host Disease. J. Clin. Oncol. 2010, 28, e602–e604. [Google Scholar] [CrossRef] [PubMed]
- Magenau, J.M.; Goldstein, S.C.; Peltier, D.; Soiffer, R.J.; Braun, T.; Pawarode, A.; Riwes, M.M.; Kennel, M.; Antin, J.H.; Cutler, C.S.; et al. α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood 2018, 131, 1372–1379. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Angelucci, E.; Raiola, A.M.; Varaldo, R.; Di Grazia, C.; Gualandi, F.; Benedetti, E.; Risitano, A.; Musso, M.; Zallio, F.; et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody—Begelomab. Bone Marrow Transplant. 2020, 55, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Samson, L.; Ensbey, K.S.; Takahashi, S.; Clouston, A.D.; Martin, P.J.; Hill, G.R. Lithium attenuates graft-versus-host disease via effects on the intestinal stem cell niche. Blood 2023, 141, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef]
- Martin, P.J. How I treat steroid-refractory acute graft-versus-host disease. Blood 2020, 135, 1630–1638. [Google Scholar] [CrossRef]
- Malard, F.; Loschi, M.; Huynh, A.; Cluzeau, T.; Guenounou, S.; Legrand, F.; Magro, L.; Orvain, C.; Charbonnier, A.; Panz-Klapuch, M.; et al. Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: A single-arm, multicentre phase 2 trial. eClinicalMedicine 2023, 62, 102111. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Hu, B.; Liu, J.; Kong, P.; Lou, S.; Su, Y.; Yang, T.; Li, H.; Liu, Y.; et al. Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy and Safety of Umbilical Cord–Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 2843–2850. [Google Scholar] [CrossRef]
- Baron, F.; Lechanteur, C.; Willems, E.; Bruck, F.; Baudoux, E.; Seidel, L.; Vanbellinghen, J.-F.; Hafraoui, K.; Lejeune, M.; Gothot, A.; et al. Cotransplantation of Mesenchymal Stem Cells Might Prevent Death from Graft-versus-Host Disease (GVHD) without Abrogating Graft-versus-Tumor Effects after HLA-Mismatched Allogeneic Transplantation following Nonmyeloablative Conditioning. Biol. Blood Marrow Transplant. 2010, 16, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Baron, F.; Storb, R. Mesenchymal Stromal Cells: A New Tool against Graft-versus-Host Disease? Biol. Blood Marrow Transplant. 2012, 18, 822–840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, J.; Hu, Z.; Yang, Y.-G.; Zhou, Q.; Sun, L.; Wu, J. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: A systematic review. Stem Cell Res. Ther. 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Ringden, O.; Sadeghi, B.; Gonen-Yaacovi, G.; Segurado, O.G. Novel therapies for graft versus host disease with a focus on cell therapies. Front. Immunol. 2023, 14, 1241068. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Fisher, S.A.; Cutler, A.; Doree, C.; Brunskill, S.J.; Stanworth, S.J.; Navarrete, C.; Girdlestone, J. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst. Rev. 2019, 2019, CD009768. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Prockop, S.; Chaudhury, S.; Horn, B.; Nemecek, E.; Prasad, V.; Satwani, P.; Teira, P.; Hayes, J.; Burke, E.; et al. Study 275: Updated Expanded Access Program for Remestemcel-L in Steroid-Refractory Acute Graft-versus-Host Disease in Children. Biol. Blood Marrow Transplant. 2020, 26, 855–864. [Google Scholar] [CrossRef]
- Daly, A. Remestemcel-L, the first cellular therapy product for the treatment of graft-versus-host disease. Drugs Today Barc. Spain 1998 2012, 48, 773–783. [Google Scholar] [CrossRef]
- Kebriaei, P.; Hayes, J.; Daly, A.; Uberti, J.; Marks, D.I.; Soiffer, R.; Waller, E.K.; Burke, E.; Skerrett, D.; Shpall, E.; et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 835–844. [Google Scholar] [CrossRef]
- Kuçi, Z.; Bönig, H.; Kreyenberg, H.; Bunos, M.; Jauch, A.; Janssen, J.W.G.; Škifić, M.; Michel, K.; Eising, B.; Lucchini, G.; et al. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: A multicenter survey. Haematologica 2016, 101, 985–994. [Google Scholar] [CrossRef]
- Bader, P.; Kuçi, Z.; Bakhtiar, S.; Basu, O.; Bug, G.; Dennis, M.; Greil, J.; Barta, A.; Kállay, K.M.; Lang, P.; et al. Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 2018, 53, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, A.; Farge, D.; Kili, S.; Forte, M.; Weiss, D.J.; Grignon, F.; Boelens, J.J. International Society for Cell and Gene Therapy Clinical Translation Committee recommendations on mesenchymal stromal cells in graft-versus-host disease: Easy manufacturing is faced with standardizing and commercialization challenges. Cytotherapy 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Ringdén, O.; Sadeghi, B. Placenta-Derived Decidua Stromal Cells: A New Frontier in the Therapy of Acute Graft-Versus-Host Disease. Stem Cells Dayt. Ohio 2024, 42, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Martin, P.J.; Storer, B.E.; Palmer, J.; Weisdorf, D.J.; Pidala, J.; Flowers, M.E.D.; Arora, M.; Jagasia, M.; Arai, S.; et al. Association of severity of organ involvement with mortality and recurrent malignancy in patients with chronic graft-versus-host disease. Haematologica 2014, 99, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Sullivan, K.M.; Weiden, P.L.; McDonald, G.B.; Striker, G.E.; Sale, G.E.; Hackman, R.; Tsoi, M.-S.; Storb, R.; Donnall Thomas, E. Chronic graft-versus-host syndrome in man. Am. J. Med. 1980, 69, 204–217. [Google Scholar] [CrossRef]
- Flowers, M.E.D.; Martin, P.J. How we treat chronic graft-versus-host disease. Blood 2015, 125, 606–615. [Google Scholar] [CrossRef]
- Koc, S.; Leisenring, W.; Flowers, M.E.D.; Anasetti, C.; Deeg, H.J.; Nash, R.A.; Sanders, J.E.; Witherspoon, R.P.; Storb, R.; Appelbaum, F.R.; et al. Therapy for chronic graft-versus-host disease: A randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002, 100, 48–51. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Witherspoon, R.P.; Storb, R.; Weiden, P.; Flournoy, N.; Dahlberg, S.; Deeg, H.J.; Sanders, J.E.; Doney, K.C.; Appelbaum, F.R. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: Prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood 1988, 72, 546–554. [Google Scholar] [CrossRef]
- Wolff, D.; Gerbitz, A.; Ayuk, F.; Kiani, A.; Hildebrandt, G.C.; Vogelsang, G.B.; Elad, S.; Lawitschka, A.; Socie, G.; Pavletic, S.Z.; et al. Consensus Conference on Clinical Practice in Chronic Graft-versus-Host Disease (GVHD): First-Line and Topical Treatment of Chronic GVHD. Biol. Blood Marrow Transplant. 2010, 16, 1611–1628. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Couriel, D.R.; Lazaryan, A.; Bhatt, V.R.; Buxbaum, N.P.; Alousi, A.M.; Olivieri, A.; Pulanic, D.; Halter, J.P.; Henderson, L.A.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2020 Treatment of Chronic GVHD Report. Transplant. Cell. Ther. 2021, 27, 729–737. [Google Scholar] [CrossRef]
- Olivieri, J.; Manfredi, L.; Postacchini, L.; Tedesco, S.; Leoni, P.; Gabrielli, A.; Rambaldi, A.; Bacigalupo, A.; Olivieri, A.; Pomponio, G. Consensus recommendations for improvement of unmet clinical needs—The example of chronic graft-versus-host disease: A systematic review and meta-analysis. Lancet Haematol. 2015, 2, e297–e305. [Google Scholar] [CrossRef] [PubMed]
- Maas-Bauer, K.; Kiote-Schmidt, C.; Bertz, H.; Apostolova, P.; Wäsch, R.; Ihorst, G.; Finke, J.; Zeiser, R. Ruxolitinib–ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021, 56, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Lee, S.J. Three US Food and Drug Administration–approved therapies for chronic GVHD. Blood 2022, 139, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.J.; Chen, Y.-B.; DeFilipp, Z. Recent FDA Approvals in the Treatment of Graft-Versus-Host Disease. Oncologist 2022, 27, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Choi, J.; Staser, K.; DiPersio, J.F. The Role of Janus Kinase Signaling in Graft-Versus-Host Disease and Graft Versus Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 1125–1134. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Weiss, J.M.; Nyuydzefe, M.S.; Chen, W.; Scher, J.U.; Mo, R.; Depoil, D.; Rao, N.; Liu, B.; Wei, J.; et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 16814–16819. [Google Scholar] [CrossRef]
- Flynn, R.; Paz, K.; Du, J.; Reichenbach, D.K.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Vulic, A.; Luznik, L.; MacDonald, K.K.P.; Hill, G.R.; et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood 2016, 127, 2144–2154. [Google Scholar] [CrossRef]
- Riches, D.W.H.; Backos, D.S.; Redente, E.F. ROCK and Rho. Am. J. Pathol. 2015, 185, 909–912. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Kim, H.T.; Yang, Z.; Noonan, J.; Blazar, B.R.; Lee, S.J.; Pavletic, S.Z.; Cutler, C. Clinical response to belumosudil in bronchiolitis obliterans syndrome: A combined analysis from 2 prospective trials. Blood Adv. 2022, 6, 6263–6270. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Poe, J.C.; Su, H.; Anand, S.; Matsushima, G.K.; Rathmell, J.C.; Maillard, I.; Radojcic, V.; Imai, K.; Reyes, N.J.; et al. BAFF promotes heightened BCR responsiveness and manifestations of chronic GVHD after allogeneic stem cell transplantation. Blood 2021, 137, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, S.; Stevenson, K.E.; Kim, H.T.; Washel, W.S.; Bhuiya, N.S.; Cutler, C.S.; Alyea, E.P.; Ho, V.T.; Soiffer, R.J.; Antin, J.H.; et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood 2011, 117, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Poe, J.C.; Jia, W.; Di Paolo, J.A.; Reyes, N.J.; Kim, J.Y.; Su, H.; Sundy, J.S.; Cardones, A.R.; Perez, V.L.; Chen, B.J.; et al. SYK inhibitor entospletinib prevents ocular and skin GVHD in mice. JCI Insight 2018, 3, e122430. [Google Scholar] [CrossRef]
- Kloehn, J.; Kruchen, A.; Schütze, K.; Wustrau, K.; Schrum, J.; Müller, I. Immune Ablation and Stem Cell Rescue in Two Pediatric Patients with Progressive Severe Chronic Graft-Versus-Host Disease. Int. J. Mol. Sci. 2022, 23, 15403. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Cimminiello, M.; Corradini, P.; Mordini, N.; Fedele, R.; Selleri, C.; Onida, F.; Patriarca, F.; Pavone, E.; Svegliati, S.; et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 2013, 122, 4111–4118. [Google Scholar] [CrossRef]
- Du, J.; Paz, K.; Flynn, R.; Vulic, A.; Robinson, T.M.; Lineburg, K.E.; Alexander, K.A.; Meng, J.; Roy, S.; Panoskaltsis-Mortari, A.; et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production. Blood 2017, 129, 2570–2580. [Google Scholar] [CrossRef]
- Tang, W.; Yu, T.; Dong, T.; Liu, T.; Ji, J. Nintedanib in Bronchiolitis Obliterans Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Chest 2020, 158, e89–e91. [Google Scholar] [CrossRef]
- Kitko, C.L.; Arora, M.; DeFilipp, Z.; Zaid, M.A.; Di Stasi, A.; Radojcic, V.; Betts, C.B.; Coussens, L.M.; Meyers, M.L.; Qamoos, H.; et al. Axatilimab for Chronic Graft-Versus-Host Disease After Failure of at Least Two Prior Systemic Therapies: Results of a Phase I/II Study. J. Clin. Oncol. 2023, 41, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; MacDonald, K.P.A.; Hill, G.R. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood 2018, 131, 2651–2660. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Lees, C.J.; Blazar, B.R. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002, 99, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Brunstein, C.G.; Miller, J.S.; McKenna, D.H.; Hippen, K.L.; DeFor, T.E.; Sumstad, D.; Curtsinger, J.; Verneris, M.R.; MacMillan, M.L.; Levine, B.L.; et al. Umbilical cord blood–derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood 2016, 127, 1044–1051. [Google Scholar] [CrossRef]
- Bergeron, A.; Cheng, G.-S. Bronchiolitis Obliterans Syndrome and Other Late Pulmonary Complications After Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 607–621. [Google Scholar] [CrossRef]
- Gazourian, L.; Rogers, A.J.; Ibanga, R.; Weinhouse, G.L.; Pinto-Plata, V.; Ritz, J.; Soiffer, R.J.; Antin, J.H.; Washko, G.R.; Baron, R.M.; et al. Factors associated with bronchiolitis obliterans syndrome and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Am. J. Hematol. 2014, 89, 404–409. [Google Scholar] [CrossRef]
- Verleden, G.M.; Glanville, A.R.; Lease, E.D.; Fisher, A.J.; Calabrese, F.; Corris, P.A.; Ensor, C.R.; Gottlieb, J.; Hachem, R.R.; Lama, V.; et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transplant. 2019, 38, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, F.; Chevret, S.; Lorillon, G.; De Bazelaire, C.; Peffault de Latour, R.; Meignin, V.; Michallet, M.; Hermet, E.; Wyplosz, B.; Houdouin, V.; et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir. Med. 2014, 108, 1525–1533. [Google Scholar] [CrossRef]
- Pang, Y.; Charya, A.V.; Keller, M.B.; Sirajuddin, A.; Fu, Y.-P.; Holtzman, N.G.; Pavletic, S.Z.; Agbor-Enoh, S. The ISHLT chronic lung allograft dysfunction consensus criteria are applicable to pulmonary chronic graft-versus-host disease. Blood Adv. 2022, 6, 4196–4207. [Google Scholar] [CrossRef]
- Glanville, A.R.; Benden, C.; Bergeron, A.; Cheng, G.-S.; Gottlieb, J.; Lease, E.D.; Perch, M.; Todd, J.L.; Williams, K.M.; Verleden, G.M. Bronchiolitis obliterans syndrome after lung or haematopoietic stem cell transplantation: Current management and future directions. ERJ Open Res. 2022, 8, 00185–02022. [Google Scholar] [CrossRef] [PubMed]
- Meignin, V.; Thivolet-Bejui, F.; Kambouchner, M.; Hussenet, C.; Bondeelle, L.; Mitchell, A.; Chagnon, K.; Begueret, H.; Segers, V.; Cottin, V.; et al. Lung histopathology of non-infectious pulmonary complications after allogeneic haematopoietic stem cell transplantation. Histopathology 2018, 73, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Holbro, A.; Lehmann, T.; Girsberger, S.; Stern, M.; Gambazzi, F.; Lardinois, D.; Heim, D.; Passweg, J.R.; Tichelli, A.; Bubendorf, L.; et al. Lung Histology Predicts Outcome of Bronchiolitis Obliterans Syndrome after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef]
- Hefazi, M.; Langer, K.J.; Khera, N.; Adamski, J.; Roy, V.; Winters, J.L.; Gastineau, D.A.; Jacob, E.K.; Kreuter, J.D.; Gandhi, M.J.; et al. Extracorporeal Photopheresis Improves Survival in Hematopoietic Cell Transplant Patients with Bronchiolitis Obliterans Syndrome without Significantly Impacting Measured Pulmonary Functions. Biol. Blood Marrow Transplant. 2018, 24, 1906–1913. [Google Scholar] [CrossRef]
- Yanik, G.A.; Mineishi, S.; Levine, J.E.; Kitko, C.L.; White, E.S.; Vander Lugt, M.T.; Harris, A.C.; Braun, T.; Cooke, K.R. Soluble Tumor Necrosis Factor Receptor: Enbrel (Etanercept) for Subacute Pulmonary Dysfunction Following Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1044–1054. [Google Scholar] [CrossRef]
- Li, H.; Zhou, X.; Tan, H.; Hu, Y.; Zhang, L.; Liu, S.; Dai, M.; Li, Y.; Li, Q.; Mao, Z.; et al. Neutrophil extracellular traps contribute to the pathogenesis of acid-aspiration-induced ALI/ARDS. Oncotarget 2018, 9, 1772–1784. [Google Scholar] [CrossRef]
- Bos, S.; Murray, J.; Marchetti, M.; Cheng, G.-S.; Bergeron, A.; Wolff, D.; Sander, C.; Sharma, A.; Badawy, S.M.; Peric, Z.; et al. ERS/EBMT clinical practice guidelines on treatment of pulmonary chronic graft- versus -host disease in adults. Eur. Respir. J. 2024, 63, 2301727. [Google Scholar] [CrossRef]
- Strong Rodrigues, K.; Oliveira-Ribeiro, C.; de Abreu Fiuza Gomes, S.; Knobler, R. Cutaneous Graft-Versus-Host Disease: Diagnosis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 33–50. [Google Scholar] [CrossRef]
- Kitajima, T.; Imamura, S. Graft-versus-host reaction enhanced by ultraviolet radiation. Arch. Dermatol. Res. 1993, 285, 499–501. [Google Scholar] [CrossRef]
- Rostagno, E.; Campanati, A.; Mordini, N.; Cannici, C.; Cioce, M.; De Cecco, V.; Samarani, E.; Foà, R.; Olivieri, A.; Botti, S. Phototherapy and topical treatments for cutaneous graft vs. host disease in haematopoietic stem cell transplantation patients: A scoping review. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Li, S.; Kim, H.; Lerman, M.; Sultan, A.; Alyea, E.P.; Armand, P.; Cutler, C.; Ho, V.; Koreth, J.; et al. An Open-Label Phase II Randomized Trial of Topical Dexamethasone and Tacrolimus Solutions for the Treatment of Oral Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2016, 22, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, P.A.; Kitko, C.L.; Elad, S.; Flowers, M.E.D.; Gea-Banacloche, J.C.; Halter, J.P.; Hoodin, F.; Johnston, L.; Lawitschka, A.; McDonald, G.B.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Gianfreda, F.; Carlotta, D.; Armati, S.; Barlattani, A.; Bollero, P. Oral Manifestations of Graft vs. Host Disease: A Comprehensive Review for Best Practice in Dentistry. Med. Kaunas Lith. 2023, 59, 1937. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Mahdavi Sharif, P.; Cheraqpour, K.; Koganti, R.; Masoumi, A.; Baharnoori, S.M.; Salabati, M.; Djalilian, A.R. Ocular graft-versus-host disease (oGVHD): From A to Z. Surv. Ophthalmol. 2023, 68, 697–712. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Rosenthal, P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea 2007, 26, 1195–1199. [Google Scholar] [CrossRef]
- Preston, M.; Richards, A. Vulvar and Vaginal Graft Versus Host Disease After Allogeneic Stem Cell Transplant-A Systematic Review. J. Low. Genit. Tract Dis. 2023, 27, 266–274. [Google Scholar] [CrossRef]
- Hirsch, P.; Leclerc, M.; Rybojad, M.; Petropoulou, A.D.; Robin, M.; Ribaud, P.; de la Tour, R.P.; Cavelier-Balloy, B.; Socié, G.; Vexiau-Robert, D. Female genital chronic graft-versus-host disease: Importance of early diagnosis to avoid severe complications. Transplantation 2012, 93, 1265–1269. [Google Scholar] [CrossRef]
- Sobel, J.D.; Reichman, O.; Misra, D.; Yoo, W. Prognosis and Treatment of Desquamative Inflammatory Vaginitis. Obstet. Gynecol. 2011, 117, 850–855. [Google Scholar] [CrossRef]
- Spiryda, L.B.; Laufer, M.R.; Soiffer, R.J.; Antin, J.A. Graft-versus-host disease of the vulva and/or vagina: Diagnosis and treatment. Biol. Blood Marrow Transplant. 2003, 9, 760–765. [Google Scholar] [CrossRef]
- Kean, L.S.; Burns, L.J.; Kou, T.D.; Kapikian, R.; Tang, X.-Y.; Zhang, M.-J.; Hemmer, M.; Connolly, S.E.; Polinsky, M.; Gavin, B.; et al. Improved Overall Survival of Patients Treated with Abatacept in Combination with a Calcineurin Inhibitor and Methotrexate Following 7/8 HLA-Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplantation: Analysis of the Center for International Blood and Marrow Transplant Research Database. Blood 2021, 138, 3912. [Google Scholar] [CrossRef]
- FDA. Approves Abatacept for Prophylaxis of Acute Graft versus Host Disease. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abatacept-prophylaxis-acute-graft-versus-host-disease (accessed on 15 December 2021).
- Choe, H.; Ferrara, J.L.M. New therapeutic targets and biomarkers for acute graft-versus-host disease (GVHD). Expert Opin. Ther. Targets 2021, 25, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.; Nador, R.G.; English, J.C.; Yee, J.; Levy, R.; Bergeron, C.; Swiston, J.R.; Mets, O.M.; Muller, N.L.; Bilawich, A.-M. Chronic Lung Allograft Dysfunction: Review of CT and Pathologic Findings. Radiol. Cardiothorac. Imaging 2021, 3, e200314. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Sarwal, M.; Roedder, S.; Lee, S.J. Biologic markers of chronic GVHD. Bone Marrow Transplant. 2014, 49, 324–331. [Google Scholar] [CrossRef]
| Type of GVHD | Classical or Late aGVHD | Classic cGVHD | Overlap cGVHD | Indefinite Other cGVHD |
|---|---|---|---|---|
| Characteristics and Organs involved | Skin: maculopapular rash Liver: elevated bilirubin GIT: anorexia with weight loss, nausea, vomiting, diarrhea, severe pain, GI bleeding and/or ileus | Skin, nails, scalp and body hair Mouth Eyes Esophagus Lungs Muscles, joints and fascia Genitalia | Simultaneous presence of acute and chronic GVHD manifestations | Atypical signs and symptoms of alloreactivity outside NIH diagnostic criteria |
| Risk Factor | aGVHD | cGVHD | References |
|---|---|---|---|
| Stem cell source (PBSC) | Debated Yes | Yes | [10,11,12] [13] |
| Donor characteristics: Female-to-male graft Older donor Mismatched and unrelated donors | Yes Yes Yes | Yes Yes Not significant | [10,11,14,15,16] |
| Patient age | Not significant | >12 years | [17] |
| DLI | Yes | // | [11,15] |
| Severe infections during the peri-transplant period | Yes | Yes | [10,11,18] |
| Conditioning (Myeloablative regimen TBI/irradiation-based regimen) | Yes | Yes | [11,15,16,17,18,19] |
| Strategy/Transplant Setting | Agent/Mechanism | Results | References |
|---|---|---|---|
| Donor selection/MUD | Matching based on high-definition HLA-typing | Mismatches at HLA-DQB1, HDRB3/4/5 increases the risk of aGVHD HLA-B dimorphisms associated with higher GVHD Non-permissive HLA-DPB1 mismatch increases NRM | [48,49,50] |
| Inactivate alloreactive T-cells MRD/MUD | CNI; MMF Inhibition of TCR Inhibition of alloreactive T-cell proliferation | CyA/MTX: aGVHD II–IV: 44–76%; cGVHD 56% Tac/MTX: aGVHD II–IV: 56% (III–IV 18%); cGVHD 2y 76% Tac/MTX: aGVHD II–IV 31.9% (III–IV 13%); cGVHD 56% Tac/MTX: aGVHD II–IV 74% (III–IV 4%); cGVHD 1y 45% Tac/MMF: aGVHD II–IV 79% (III–IV 19%); cGVHD 1y 38% | [32,53,54] [53] [54] [55] |
In vivo T-cell depletion and modulation after HSCT Haploidentical_NMA/MAC Haploidentical_PTCy/Tac/MMF vs. Tac/MTX (RIC/NMA) Haploidentical vs. MUD vs. MRD Haploidentical vs. MUD (RIC/MAC) Haploidentical_BM vs. PBSC (RIC/MAC) Haploidentical_BM vs. PBSC (NMA) Haploidentical MAC vs. RIC_BM vs. PB vs. BM | PTCy Elimination/inhibition of alloreactive T cells; peripheral tolerance; central deletion of alloreactive T cells in the thymus | PTCy with bone marrow source aGVHD II–IV: 34% (III–IV: 6%); cGVHD extensive: 5 *–25% ** (NMA) aGVHD II–IV 17% (III–IV 5%); cGVHD moderate/severe: 15% (MAC) aGVHD II–IV 37.6% (III–IV 10.1%); cGVHD severe 2y: 27% (MAC) PTCy with peripheral blood stem cells source aGVHD II–IV: 53.8% (III–IV: 6.3%); cGVHD 1y: 21.9% vs. aGVHD II–IV: 51.9% (III–IV: 14.7%); cGVHD 1y: 35.1% PTCy with bone marrow or blood stem cells source aGVHD II–IV: 41% (III–IV: 17%); cGVHD mod/sev 2y: 31% vs. aGVHD II–IV: 48% (III–IV: 18%); cGVHD mod/sev 2y: 47% vs. aGVHD II–IV: 28% (III–IV: 9%); cGVHD mod/sev 2y: 44% aGVHD II–IV: 29–33% (III–IV: 9–10%); cGVHD 2y: 27–33% vs. aGVHD II–IV: 29–32% (III–IV: 4%); cGVHD 2y: 29–25% aGVHD II–IV: 27.9% (III–IV: 7.7%); cGVHD extensive 2y: 14% vs. aGVHD II–IV: 38.3% (III–IV: 15.9%); cGVHD extensive 2y: 14% aGVHD II–IV: 33% (III–IV: 0%); cGVHD 1y: 21; 2y: 23% vs. aGVHD II–IV: 40% (III–IV: 5%); cGVHD 1y: 14; 2y: 19% HR aGVHD II–IV 1.01 (III–IV: 1.38); cGVHD: 1.05; extensive 1.11 HR aGVHD II–IV 1.67 (III–IV: 1.82); cGVHD: 1.46; extensive 1.44 | [76] [79] [26] [80] [54] [52] [57] [51] [26] |
| Immunomediated in vivo T-cell depletion MRD/MUD MUD_RIC | ATG: polyclonal heterologous antibodies Targets: CD2,CD3,CD4, CD8, CD11a, CD107a, CD28 Alemtuzumab (humanized anti-CD-3 monoclonal antibody) | aGVHD II–IV 18% (range 7–34%) cGVHD 28% (range 16–28%)
Alemtuzumab/CyA vs. TMS: severe cGVHD 1y 0% vs 10.3%, 5y 4.5% vs. 28.5% | [32] [67] [68] [69] [70] [71] [75] |
| Blocking alloreactivity and augmenting TReg MRD/MAC_Tac/Sir vs. Tac/MTX MRD/MUD_RIC TMS vs. CyA/MMF MUD_NMA Sir/CyA/MMF vs. CyA/MMF | Sirolimus mTOR inhibition | Tac/Sir: GVHD II–IV 26%; cGVHD: 53% vs. Tac/MTX: GVHD II–IV 34%; cGVHD: 45% TMS aGVHD II–IV: 9% (III–IV: 3%); cGVHD 2y 59% CyA/MMF aGVHD II–IV: 25% (III–IV: 4%) cGVHD 2y 63% Sir/CyA/MMF aGVHD II–IV: 26% (III–IV: 2%); cGVHD 1y: 49% vs. CyA/MMF aGVHD II–IV: 52%; (III–IV: 8%); cGVHD 1y: 50% | [58] [59] [60] |
| Ex-vivo alloreactive T-Cell Depletion Haploidentical | selection of CD34+ cells Immune depletion of alpha/beta T cells | aGVHD III–IV 3%; cGVHD 0% aGVHD II–IV: 18% (range 11–28%); cGVHD: 8% aGVHD I–II: 26%; III–IV: 6%; cGVHD: 6% | [81] [85] [86] |
| Ex-vivo alloreactive T-Cell Depletion Pediatric patients | selection of CD34+ cells CD34+ Megadose (19–21 × 106/kg) + higher T cell dose (1.4–4.7 × 104/kg) | aGVHD 0%; cGVHD 0% aGVHD II–IV: 17%; cGVHD: 35% | [82] [83] |
| Selective depletion of alloreactive T cells Matched related (adults) Haploidentical (pediatrics) Developmental | Depletion of CD45RA naïve T cells, preserving the CD34+ and CD45RO fractions (better activity vs. infections and GVL) | aGVHD II–IV 66%; cGVHD 7–9% aGVHD II–IV 18%; cGVHD 35% | [84] |
| ↓ T-Cell Co-stimulatory/ Co-inhibitory signal MUD_ CNI/MTX + ABA vs. CNI/MTX + placebo Mismatched CNI/MTX + ABA vs. control IBMTR | Abatacept (CD28/CTLA-4 inhibitor) blockade of costimulatory T cell signaling and inhibition of T cell activation | aGVHD II–IV: 43.1% (III–IV: 6.8%); cGVHD moderate–severe 1y: 44.8% vs. aGVHD II–IV: 62.1% (III–IV: 14.8%) cGVHD moderate–severe 1y: 36% aGVHD II–IV: 41.9% (III–IV: 2.3%); cGVHD moderate–severe: 57.9% vs. aGVHD II–IV: 53.2% (III–IV: 30.2%) | [89] |
| Blocking T-Cell trafficking (anti-homing compounds) MUD or Mismatched | Vedolizumab α4β7integrins inhibitor: mediates migration of T-cells to intestinal mucosa | aGVHD II–IV: 19% (III–IV: 5%) Lower GI aGvHD: 7.1% vs. 18.8% in the placebo group | [35] [95] |
| Blocking T-Cell trafficking MRD/MUD RIC | Maraviroc CCR5 inhibitor blocks alloreactive lymphocyte chemotaxis to intestinal mucosa | 3 prophylaxis regimens compared in 273 randomized patients 1-TAC + MMF + PTCy; 2-TAC + MTX + BOR; 3-TAC + MTX + Maraviroc No sign of reduction of both acute and cGVHD with Maraviroc | [93] |
| Modulation of microbiota Developmental | Microbiota Transplantation induces modifications of alloreactivity against intestinal mucosa antigens | aGVHD III/IV: 0% vs. 25% in the control group | [96] |
| Overall Glucksberg Criteria/MAGIC criteria | Original Glucksberg Criteria | Modified Glucksberg or Keystone Criteria | Minnesota Criteria | MAGIC Criteria | IBMTR Criteria | Overall IBMTR Grade |
|---|---|---|---|---|---|---|
| 0 | no organ involvement (skin = 0; and liver = 0; and GI = 0) corresponds to the absence of aGVHD | O | ||||
| I | Skin = stage 1–2, without liver/GI involvement or decrease in performance status/fever | skin = stage 1–2, without liver/GI involvement | Skin = stage 1–2, without liver/GI involvement | Skin = stage 1–2, without liver/GI involvement | Skin = stage 1–2, without liver/GI involvement | A |
| II | Skin = stage 1–2 and (liver and/or GI involvement = stage 1–2) with a mild decrease in performance status | Skin = stage 3 and/or liver = 1 and/or GI = 1 | Skin = stage 3 and/or liver = stage 1 and/or GI = stage 1 | Skin = stage 3 and/or liver = stage 1 and/or GI = stage | Skin = stage 2 and/or liver = stage 1–2 and/or GI = stage 1–2 | B |
| III | (skin and/or liver and/or GI = stage 2–4) with marked decrease in performance status | Liver = stage 2–3 and/or GI = 2–4 | Liver = stage 2–4 and/or GI = 2–3 | Liver = stage 2–3 and/or lower GI = 2–3, with skin stage 0–3 and/or upper GI stage 0–1 | Skin = stage 3 and/or liver = stage 3 and/or GI = stage 3 | C |
| IV | (skin and/or liver and/or GI = stage 2–4) with Karnofsky < 30% | Skin = stage 4 and/or liver = stage 4 | Skin = stage 4 and/or GI = stage 4 | Skin = stage 4; and/or liver = stage 4; and/or lower GI = stage 4, with stage 0–1 upper GI | Skin = stage 4; and/or liver = stage 4; and/or GI = stage 4 | D |
| Original Seattle Classification [144] | NIH 2005 [3] | NIH 2015 [4] |
|---|---|---|
| Limited One or both of: Localized skin involvement Hepatic dysfunction due to cGVHD Extensive One of: Generalized skin involvement Localized skin involvement and/or hepatic dysfunction due to GVHD, AND: Liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis, or: Involvement of eye (Schirmer’s test with <5 mm wetting), OR: Involvement of minor salivary glands or oral mucosa demonstrated on labial biopsy, OR: Involvement of any other target organ | Mild cGVHD: only 1 or 2 organs (except the lung), with a score of 1 Moderate cGVHD:
| Mild cGVHD: 1 or 2 organs with a maximum score of 1 AND Lung score of 0 Moderate cGVHD
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivieri, A.; Mancini, G. Current Approaches for the Prevention and Treatment of Acute and Chronic GVHD. Cells 2024, 13, 1524. https://doi.org/10.3390/cells13181524
Olivieri A, Mancini G. Current Approaches for the Prevention and Treatment of Acute and Chronic GVHD. Cells. 2024; 13(18):1524. https://doi.org/10.3390/cells13181524
Chicago/Turabian StyleOlivieri, Attilio, and Giorgia Mancini. 2024. "Current Approaches for the Prevention and Treatment of Acute and Chronic GVHD" Cells 13, no. 18: 1524. https://doi.org/10.3390/cells13181524






