Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W
Abstract
:1. Introduction
2. Material and Methods
2.1. Structural Analysis
2.2. Cell Culture, Treatment and Harvest
2.3. Genome-Editing with CRISPR-Cas9
2.4. Western Blot
2.5. RNA Analysis
2.6. Immunofluorescence
2.7. Sandwich ELISA
2.8. Flow Cytometry
2.9. Protein Sequential Extraction
2.10. Human Brain Samples
2.11. Statistical Analysis
3. Results
3.1. Structural Analysis of PRKN p.R275W
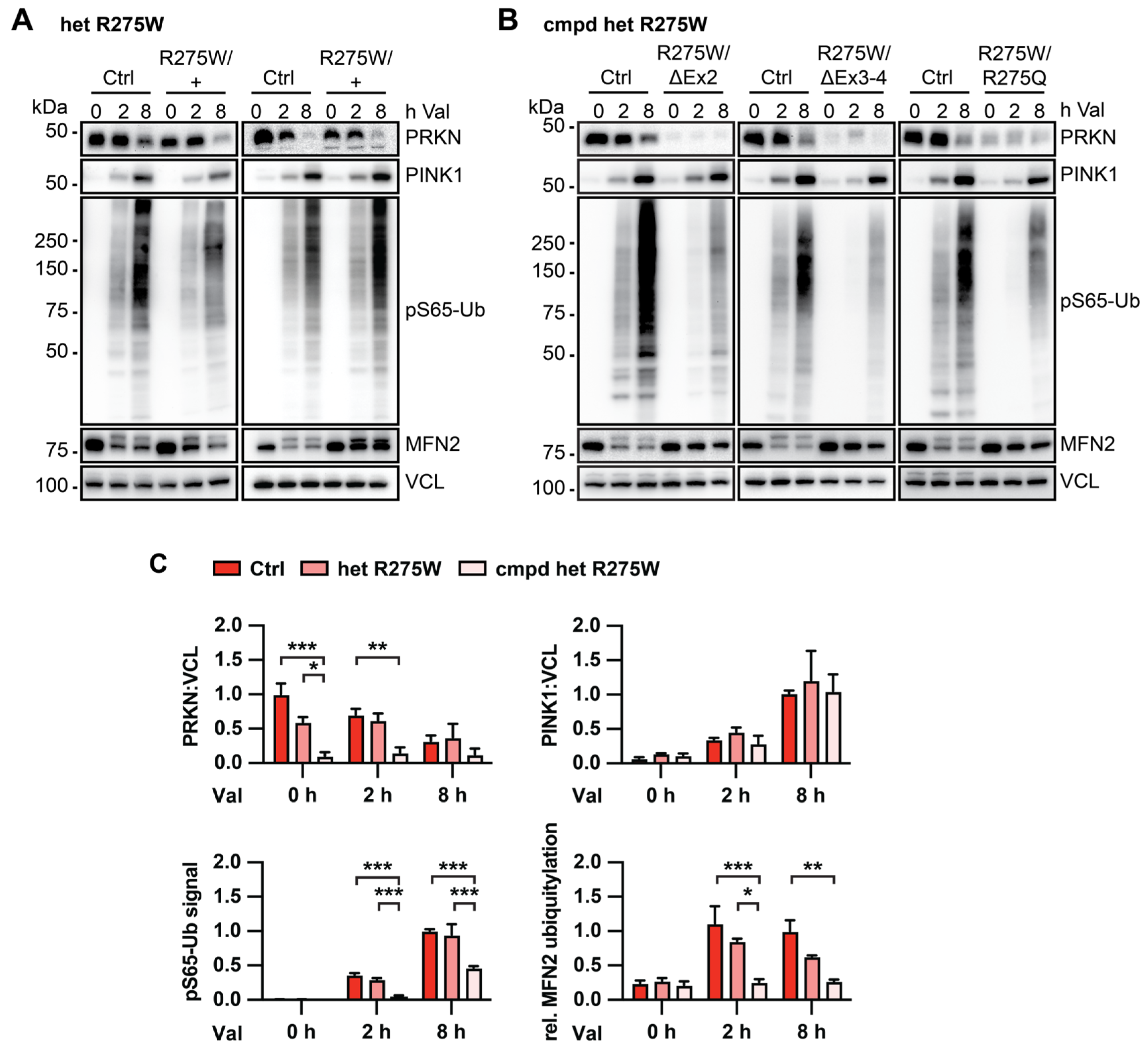
3.2. Skin Fibroblasts with PRKN p.R275W Substitution Have Reduced PRKN Protein Levels
3.3. PRKN p.R275W Presents with Substantially Reduced Protein Levels in Isogenic DA Neurons
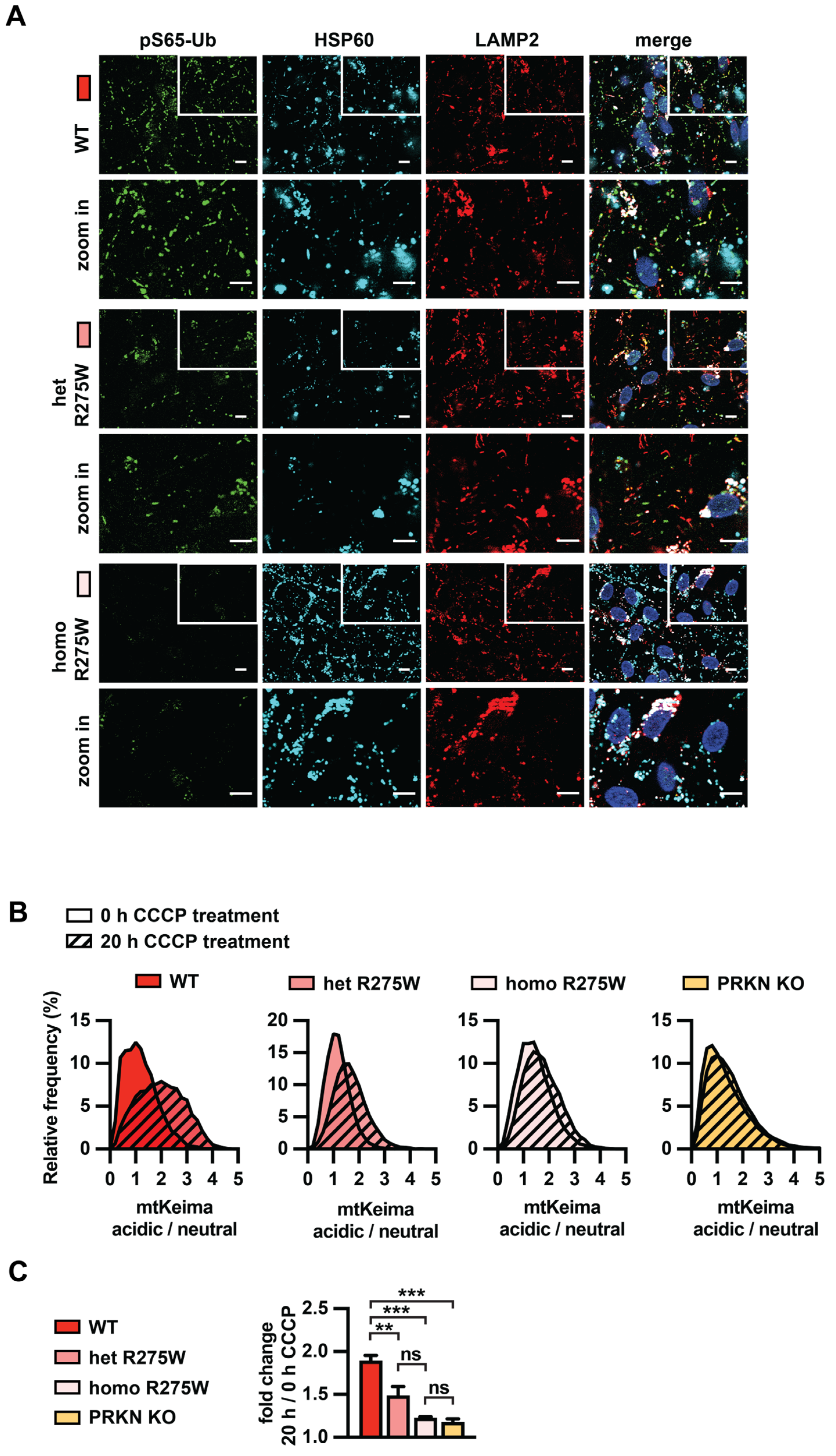
3.4. PRKN p.R275W Fails to Mediate Mitophagy in Dopamine Neurons
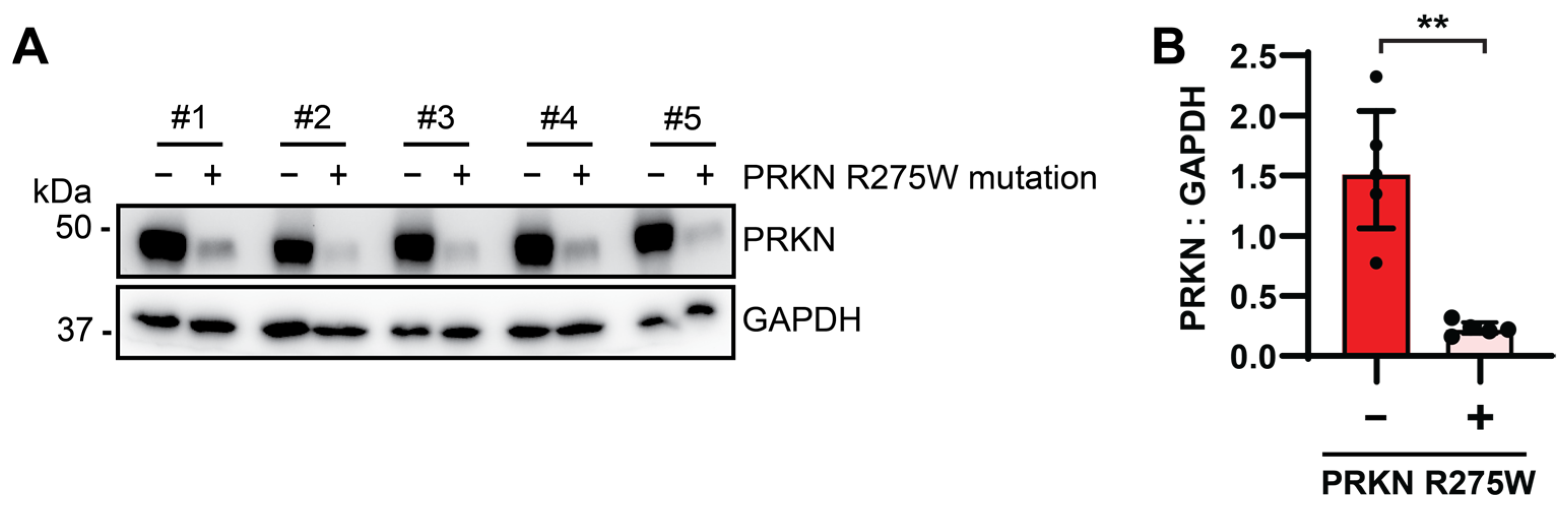
3.5. PRKN Protein Levels Are Reduced in Human Autopsy Brain from p.R275W Mutation Carriers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCCP | carbonyl cyanide m-chlorophenyl hydrazone |
| DA | dopamine |
| ELISA | enzyme-linked immunosorbent assay |
| KO | knock-out |
| LB | Lewy body |
| MFN2 | mitofusin 2 |
| MSD | Meso Scale Discovery |
| MitoKeima | mitochondrially targeted Keima red protein |
| PD | Parkinson disease |
| pS65-Ub | phosphorylated ubiquitin at Serine 65 |
| RING | really interesting new gene |
| Ub | ubiquitin |
| WT | wild-type |
References
- Dickson, D.W. Neuropathology of Parkinson disease. Park. Relat. Disord. 2018, 46 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Holmstrom, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; de Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2010, 107, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.A.; Lazarou, M.; Fogel, A.I.; Li, Y.; Yamano, K.; Sarraf, S.A.; Banerjee, S.; Youle, R.J. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 2014, 205, 143–153. [Google Scholar] [CrossRef]
- Kazlauskaite, A.; Kondapalli, C.; Gourlay, R.; Campbell, D.G.; Ritorto, M.S.; Hofmann, K.; Alessi, D.R.; Knebel, A.; Trost, M.; Muqit, M.M. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem. J. 2014, 460, 127–139. [Google Scholar] [CrossRef]
- Kondapalli, C.; Kazlauskaite, A.; Zhang, N.; Woodroof, H.I.; Campbell, D.G.; Gourlay, R.; Burchell, L.; Walden, H.; Macartney, T.J.; Deak, M.; et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012, 2, 120080. [Google Scholar] [CrossRef]
- Iguchi, M.; Kujuro, Y.; Okatsu, K.; Koyano, F.; Kosako, H.; Kimura, M.; Suzuki, N.; Uchiyama, S.; Tanaka, K.; Matsuda, N. Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J. Biol. Chem. 2013, 288, 22019–22032. [Google Scholar] [CrossRef]
- Shiba-Fukushima, K.; Imai, Y.; Yoshida, S.; Ishihama, Y.; Kanao, T.; Sato, S.; Hattori, N. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci. Rep. 2012, 2, 1002. [Google Scholar] [CrossRef]
- Okatsu, K.; Koyano, F.; Kimura, M.; Kosako, H.; Saeki, Y.; Tanaka, K.; Matsuda, N. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol. 2015, 209, 111–128. [Google Scholar] [CrossRef]
- Truban, D.; Hou, X.; Caulfield, T.R.; Fiesel, F.C.; Springer, W. PINK1, Parkin, and Mitochondrial Quality Control: What can we Learn about Parkinson’s Disease Pathobiology? J. Parkinsons Dis. 2017, 7, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Makarious, M.B.; Bandres-Ciga, S.; Gibbs, J.R.; Ding, J.; Hernandez, D.G.; Brooks, J.; Grenn, F.P.; Iwaki, H.; Singleton, A.B.; et al. The Parkinson’s Disease DNA Variant Browser. Mov. Disord. 2021, 36, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.J.; Sambin, S.; Criniere-Boizet, B.; Courtin, T.; Tesson, C.; Casse, F.; Ferrien, M.; Mariani, L.L.; Carvalho, S.; Lejeune, F.X.; et al. Genotype-phenotype correlation in PRKN-associated Parkinson’s disease. NPJ Parkinsons Dis. 2024, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, E.; Thobois, S.; Lesage, S.; Broussolle, E.; du Montcel, S.T.; Ribeiro, M.J.; Remy, P.; Pelissolo, A.; Dubois, B.; Mallet, L.; et al. A multidisciplinary study of patients with early-onset PD with and without parkin mutations. Neurology 2009, 72, 110–116. [Google Scholar] [CrossRef]
- Farrer, M.; Chan, P.; Chen, R.; Tan, L.; Lincoln, S.; Hernandez, D.; Forno, L.; Gwinn-Hardy, K.; Petrucelli, L.; Hussey, J.; et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 2001, 50, 293–300. [Google Scholar] [CrossRef]
- Doherty, K.M.; Silveira-Moriyama, L.; Parkkinen, L.; Healy, D.G.; Farrell, M.; Mencacci, N.E.; Ahmed, Z.; Brett, F.M.; Hardy, J.; Quinn, N.; et al. Parkin disease: A clinicopathologic entity? JAMA Neurol. 2013, 70, 571–579. [Google Scholar] [CrossRef]
- Cornejo-Olivas, M.R.; Torres, L.; Mata, I.F.; Mazzetti, P.; Rivas, D.; Cosentino, C.; Inca-Martinez, M.; Cuba, J.M.; Zabetian, C.P.; Leverenz, J.B. A Peruvian family with a novel PARK2 mutation: Clinical and pathological characteristics. Park. Relat. Disord. 2015, 21, 444–448. [Google Scholar] [CrossRef]
- Chung, K.K.; Zhang, Y.; Lim, K.L.; Tanaka, Y.; Huang, H.; Gao, J.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: Implications for Lewy-body formation in Parkinson disease. Nat. Med. 2001, 7, 1144–1150. [Google Scholar] [CrossRef]
- Sriram, S.R.; Li, X.; Ko, H.S.; Chung, K.K.; Wong, E.; Lim, K.L.; Dawson, V.L.; Dawson, T.M. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum. Mol. Genet. 2005, 14, 2571–2586. [Google Scholar] [CrossRef]
- Cookson, M.R.; Lockhart, P.J.; McLendon, C.; O’Farrell, C.; Schlossmacher, M.; Farrer, M.J. RING finger 1 mutations in Parkin produce altered localization of the protein. Hum. Mol. Genet. 2003, 12, 2957–2965. [Google Scholar] [CrossRef]
- Fiesel, F.C.; Caulfield, T.R.; Moussaud-Lamodiere, E.L.; Ogaki, K.; Dourado, D.F.; Flores, S.C.; Ross, O.A.; Springer, W. Structural and Functional Impact of Parkinson Disease-Associated Mutations in the E3 Ubiquitin Ligase Parkin. Hum. Mutat. 2015, 36, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Broadway, B.J.; Boneski, P.K.; Bredenberg, J.M.; Kolicheski, A.; Hou, X.; Soto-Beasley, A.I.; Ross, O.A.; Springer, W.; Fiesel, F.C. Systematic Functional Analysis of PINK1 and PRKN Coding Variants. Cells 2022, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Lohmann-Hedrich, K.; Rogaeva, E.; Schlossmacher, M.G.; Lang, A.E. Deciphering the role of heterozygous mutations in genes associated with parkinsonism. Lancet Neurol. 2007, 6, 652–662. [Google Scholar] [CrossRef]
- Trempe, J.F.; Gehring, K. Structural Mechanisms of Mitochondrial Quality Control Mediated by PINK1 and Parkin. J. Mol. Biol. 2023, 435, 168090. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; MacDougall, E.J.; Tang, M.Y.; Krahn, A.I.; Gan-Or, Z.; Trempe, J.F.; Fon, E.A. The landscape of Parkin variants reveals pathogenic mechanisms and therapeutic targets in Parkinson’s disease. Hum. Mol. Genet. 2019, 28, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.N.; Weber, C.A.; Coban, M.; Cocker, L.T.; Fiesel, F.C.; Springer, W.; Caulfield, T.R. In Silico Investigation of Parkin-Activating Mutations Using Simulations and Network Modeling. Biomolecules 2024, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. Methods Mol. Biol. 2018, 1685, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Abraham, M.J.; Gready, J.E. Optimization of parameters for molecular dynamics simulation using smooth particle-mesh Ewald in GROMACS 4.5. J. Comput. Chem. 2011, 32, 2031–2040. [Google Scholar] [CrossRef]
- Polak, E.; Ribiere, G. Note sur la convergence de méthodes de directions conjuguées. AFCET 1969, 3, 35–43. [Google Scholar] [CrossRef]
- Bhadra, P.; Siu, S.W.I. Refined Empirical Force Field to Model Protein-Self-Assembled Monolayer Interactions Based on AMBER14 and GAFF. Langmuir 2019, 35, 9622–9633. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, R.T.; Beauchamp, K.A.; Harrigan, M.P.; Klein, C.; Swails, J.M.; Hernandez, C.X.; Schwantes, C.R.; Wang, L.P.; Lane, T.J.; Pande, V.S. MDTraj: A Modern Open Library for the Analysis of Molecular Dynamics Trajectories. Biophys. J. 2015, 109, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Watzlawik, J.O.; Hou, X.; Fricova, D.; Ramnarine, C.; Barodia, S.K.; Gendron, T.F.; Heckman, M.G.; DeTure, M.; Siuda, J.; Wszolek, Z.K.; et al. Sensitive ELISA-based detection method for the mitophagy marker p-S65-Ub in human cells, autopsy brain, and blood samples. Autophagy 2021, 17, 2613–2628. [Google Scholar] [CrossRef]
- Fiesel, F.C.; Fricova, D.; Hayes, C.S.; Coban, M.A.; Hudec, R.; Bredenberg, J.M.; Broadway, B.J.; Markham, B.N.; Yan, T.; Boneski, P.K.; et al. Substitution of PINK1 Gly411 modulates substrate receptivity and turnover. Autophagy 2023, 19, 1711–1732. [Google Scholar] [CrossRef]
- Gegg, M.E.; Cooper, J.M.; Chau, K.Y.; Rojo, M.; Schapira, A.H.; Taanman, J.W. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum. Mol. Genet. 2010, 19, 4861–4870. [Google Scholar] [CrossRef]
- Donato, R.; Miljan, E.A.; Hines, S.J.; Aouabdi, S.; Pollock, K.; Patel, S.; Edwards, F.A.; Sinden, J.D. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007, 8, 36. [Google Scholar] [CrossRef]
- Rubio de la Torre, E.; Luzon-Toro, B.; Forte-Lago, I.; Minguez-Castellanos, A.; Ferrer, I.; Hilfiker, S. Combined kinase inhibition modulates parkin inactivation. Hum. Mol. Genet. 2009, 18, 809–823. [Google Scholar] [CrossRef]
- Sun, N.; Malide, D.; Liu, J.; Rovira, I.I.; Combs, C.A.; Finkel, T. A fluorescence-based imaging method to measure in vitro and in vivo mitophagy using mt-Keima. Nat. Protoc. 2017, 12, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Kogure, T.; Mizushima, N.; Yoshimori, T.; Miyawaki, A. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem. Biol. 2011, 18, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Galasko, D.; Kosaka, K.; Perry, E.K.; Dickson, D.W.; Hansen, L.A.; Salmon, D.P.; Lowe, J.; Mirra, S.S.; Byrne, E.J.; et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 1996, 47, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Narendra, D.; Kane, L.A.; Hauser, D.N.; Fearnley, I.M.; Youle, R.J. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 2010, 6, 1090–1106. [Google Scholar] [CrossRef]
- Wang, C.; Lu, R.; Ouyang, X.; Ho, M.W.; Chia, W.; Yu, F.; Lim, K.L. Drosophila overexpressing parkin R275W mutant exhibits dopaminergic neuron degeneration and mitochondrial abnormalities. J. Neurosci. 2007, 27, 8563–8570. [Google Scholar] [CrossRef]
- Clausen, L.; Voutsinos, V.; Cagiada, M.; Johansson, K.E.; Gronbaek-Thygesen, M.; Nariya, S.; Powell, R.L.; Have, M.K.N.; Oestergaard, V.H.; Stein, A.; et al. A mutational atlas for Parkin proteostasis. Nat. Commun. 2024, 15, 1541. [Google Scholar] [CrossRef]
- Weissbach, A.; Konig, I.R.; Huckelheim, K.; Pramstaller, P.P.; Werner, E.; Bruggemann, N.; Tadic, V.; Lohmann, K.; Baumer, T.; Munchau, A.; et al. Influence of L-dopa on subtle motor signs in heterozygous Parkin- and PINK1 mutation carriers. Park. Relat. Disord. 2017, 42, 95–99. [Google Scholar] [CrossRef]
- Huttenlocher, J.; Stefansson, H.; Steinberg, S.; Helgadottir, H.T.; Sveinbjornsdottir, S.; Riess, O.; Bauer, P.; Stefansson, K. Heterozygote carriers for CNVs in PARK2 are at increased risk of Parkinson’s disease. Hum. Mol. Genet. 2015, 24, 5637–5643. [Google Scholar] [CrossRef]
- Yu, E.; Rudakou, U.; Krohn, L.; Mufti, K.; Ruskey, J.A.; Asayesh, F.; Estiar, M.A.; Spiegelman, D.; Surface, M.; Fahn, S.; et al. Analysis of Heterozygous PRKN Variants and Copy-Number Variations in Parkinson’s Disease. Mov. Disord. 2021, 36, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huang, X.; Yoon, E.; Bandres-Ciga, S.; Blauwendraat, C.; Billingsley, K.J.; Cade, J.H.; Wu, B.P.; Williams, V.H.; Schindler, A.B.; et al. Heterozygous PRKN mutations are common but do not increase the risk of Parkinson’s disease. Brain 2022, 145, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Lubbe, S.J.; Bustos, B.I.; Hu, J.; Krainc, D.; Joseph, T.; Hehir, J.; Tan, M.; Zhang, W.; Escott-Price, V.; Williams, N.M.; et al. Assessing the relationship between monoallelic PRKN mutations and Parkinson’s risk. Hum. Mol. Genet. 2021, 30, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.F.; Wang, L.; He, D.; Yang, Q.H.; Duan, Z.X.; Zhang, X.W.; Nie, L.L.; Yan, X.X.; Tang, B.S. Clinical features and [11C]-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurol. Sci. 2011, 32, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hilker, R.; Klein, C.; Ghaemi, M.; Kis, B.; Strotmann, T.; Ozelius, L.J.; Lenz, O.; Vieregge, P.; Herholz, K.; Heiss, W.D.; et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the parkin gene. Ann. Neurol. 2001, 49, 367–376. [Google Scholar] [CrossRef]
- Hilker, R.; Klein, C.; Hedrich, K.; Ozelius, L.J.; Vieregge, P.; Herholz, K.; Pramstaller, P.P.; Heiss, W.D. The striatal dopaminergic deficit is dependent on the number of mutant alleles in a family with mutations in the parkin gene: Evidence for enzymatic parkin function in humans. Neurosci. Lett. 2002, 323, 50–54. [Google Scholar] [CrossRef]
- Pavese, N.; Khan, N.L.; Scherfler, C.; Cohen, L.; Brooks, D.J.; Wood, N.W.; Bhatia, K.P.; Quinn, N.P.; Lees, A.J.; Piccini, P. Nigrostriatal dysfunction in homozygous and heterozygous parkin gene carriers: An 18F-dopa PET progression study. Mov. Disord. 2009, 24, 2260–2266. [Google Scholar] [CrossRef]
- Khan, N.L.; Brooks, D.J.; Pavese, N.; Sweeney, M.G.; Wood, N.W.; Lees, A.J.; Piccini, P. Progression of nigrostriatal dysfunction in a parkin kindred: An [18F]dopa PET and clinical study. Brain 2002, 125, 2248–2256. [Google Scholar] [CrossRef]
- Pitz, V.; Makarious, M.B.; Bandres-Ciga, S.; Iwaki, H.; 23andMe Research Team; Singleton, A.B.; Nalls, M.; Heilbron, K.; Blauwendraat, C. Analysis of rare Parkinson’s disease variants in millions of people. NPJ Park. Dis. 2024, 10, 11. [Google Scholar] [CrossRef]
- Watzlawik, J.O.; Fiesel, F.C.; Fiorino, G.; Bustillos, B.A.; Baninameh, Z.; Markham, B.N.; Hou, X.; Hayes, C.S.; Bredenberg, J.M.; Kurchaba, N.W.; et al. Basal activity of PINK1 and PRKN in cell models and rodent brain. Autophagy 2023, 1–12. [Google Scholar] [CrossRef]
- Tokarew, J.M.; El-Kodsi, D.N.; Lengacher, N.A.; Fehr, T.K.; Nguyen, A.P.; Shutinoski, B.; O’Nuallain, B.; Jin, M.; Khan, J.M.; Ng, A.C.H.; et al. Age-associated insolubility of parkin in human midbrain is linked to redox balance and sequestration of reactive dopamine metabolites. Acta Neuropathol. 2021, 141, 725–754. [Google Scholar] [CrossRef] [PubMed]
- Madsen, D.A.; Schmidt, S.I.; Blaabjerg, M.; Meyer, M. Interaction between Parkin and alpha-Synuclein in PARK2-Mediated Parkinson’s Disease. Cells 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Erskine, D.; Koss, D.; Korolchuk, V.I.; Outeiro, T.F.; Attems, J.; McKeith, I. Lipids, lysosomes and mitochondria: Insights into Lewy body formation from rare monogenic disorders. Acta Neuropathol. 2021, 141, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castano-Diez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, T.H.; Koga, S.; Bredenberg, J.M.; Faroqi, A.H.; Delenclos, M.; Bu, G.; Wszolek, Z.K.; Carr, J.A.; Ross, O.A.; et al. Alpha-synuclein-associated changes in PINK1-PRKN-mediated mitophagy are disease context dependent. Brain Pathol. 2023, e13175. [Google Scholar] [CrossRef]
- Tufi, R.; Clark, E.H.; Hoshikawa, T.; Tsagkaraki, C.; Stanley, J.; Takeda, K.; Staddon, J.M.; Briston, T. High-content phenotypic screen to identify small molecule enhancers of Parkin-dependent ubiquitination and mitophagy. SLAS Discov. 2023, 28, 73–87. [Google Scholar] [CrossRef]
- Tsefou, E.; Walker, A.S.; Clark, E.H.; Hicks, A.R.; Luft, C.; Takeda, K.; Watanabe, T.; Ramazio, B.; Staddon, J.M.; Briston, T.; et al. Investigation of USP30 inhibition to enhance Parkin-mediated mitophagy: Tools and approaches. Biochem. J. 2021, 478, 4099–4118. [Google Scholar] [CrossRef]


| Cell Line | PRKN Genotype | Age at Onset | Age at Biopsy | Sex |
|---|---|---|---|---|
| Ctrl 1 | WT | n/a | 55 | F |
| Ctrl 2 | WT | n/a | 67 | F |
| Ctrl 3 | WT | n/a | 64 | M |
| Ctrl 4 | WT | n/a | 46 | M |
| Het 1 | R275W/+ | 43 | 61 | F |
| Het 2 | R275W/+ | 44 | 48 | M |
| Cmpd Het 1 | R275W/ΔEx2 | 25 | 51 | M |
| Cmpd Het 2 | R275W/ΔEx3-4 | 37 | 72 | M |
| Cmpd Het 3 | R275W/R275Q | 47 | 51 | F |
| Pair | p.R275W Substitution | Age | Sex | Pathological Diagnosis | Braak Stage | Thal Phase |
|---|---|---|---|---|---|---|
| #1 | − | 71 | M | iLBD | III | 2 |
| + | 72 | M | DLBD | II | 0 | |
| #2 | − | 85 | M | AD, DLBD | IV | 5 |
| + | 94 | M | DLBD | IV | 2 | |
| #3 | − | 86 | F | AD | V | 3 |
| + | 88 | F | AD | V | n/a | |
| #4 | − | 90 | F | AD | VI | 5 |
| + | 97 | F | AD | VI | 5 | |
| #5 | − | 93 | F | AD | V | 5 |
| + | 99 | F | AD | V | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustillos, B.A.; Cocker, L.T.; Coban, M.A.; Weber, C.A.; Bredenberg, J.M.; Boneski, P.K.; Siuda, J.; Slawek, J.; Puschmann, A.; Narendra, D.P.; et al. Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W. Cells 2024, 13, 1540. https://doi.org/10.3390/cells13181540
Bustillos BA, Cocker LT, Coban MA, Weber CA, Bredenberg JM, Boneski PK, Siuda J, Slawek J, Puschmann A, Narendra DP, et al. Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W. Cells. 2024; 13(18):1540. https://doi.org/10.3390/cells13181540
Chicago/Turabian StyleBustillos, Bernardo A., Liam T. Cocker, Mathew A. Coban, Caleb A. Weber, Jenny M. Bredenberg, Paige K. Boneski, Joanna Siuda, Jaroslaw Slawek, Andreas Puschmann, Derek P. Narendra, and et al. 2024. "Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W" Cells 13, no. 18: 1540. https://doi.org/10.3390/cells13181540








