Considerations for Using Neuroblastoma Cell Lines to Examine the Roles of Iron and Ferroptosis in Neurodegeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viability Assays
2.2. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR)
2.3. Protein Isolation and Expression Analyses
2.4. Statistics
3. Results
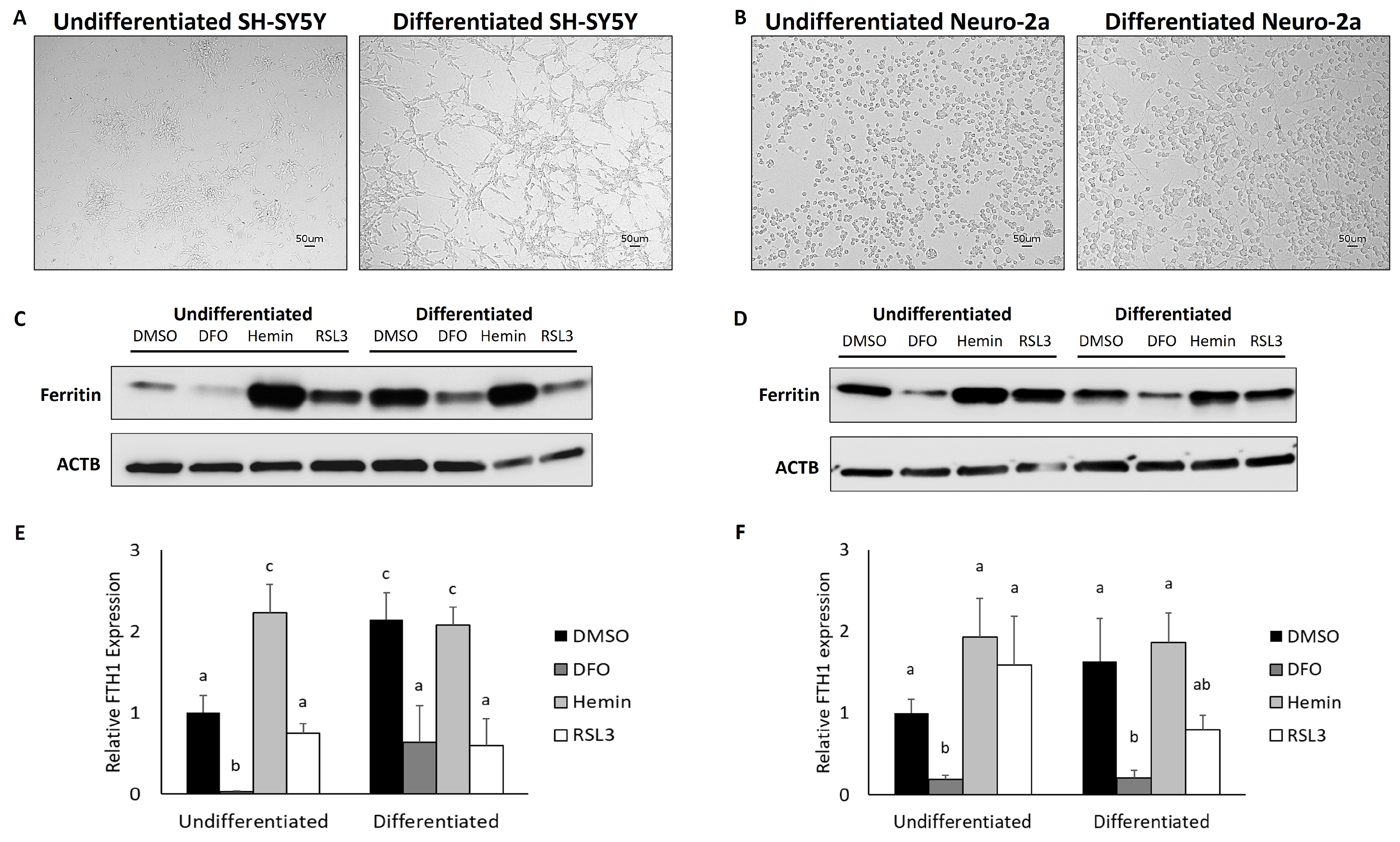
3.1. Differentiated Neuroblastoma Cell Lines Accumulate Ferritin and Are Responsive to Changes in Iron Chelation
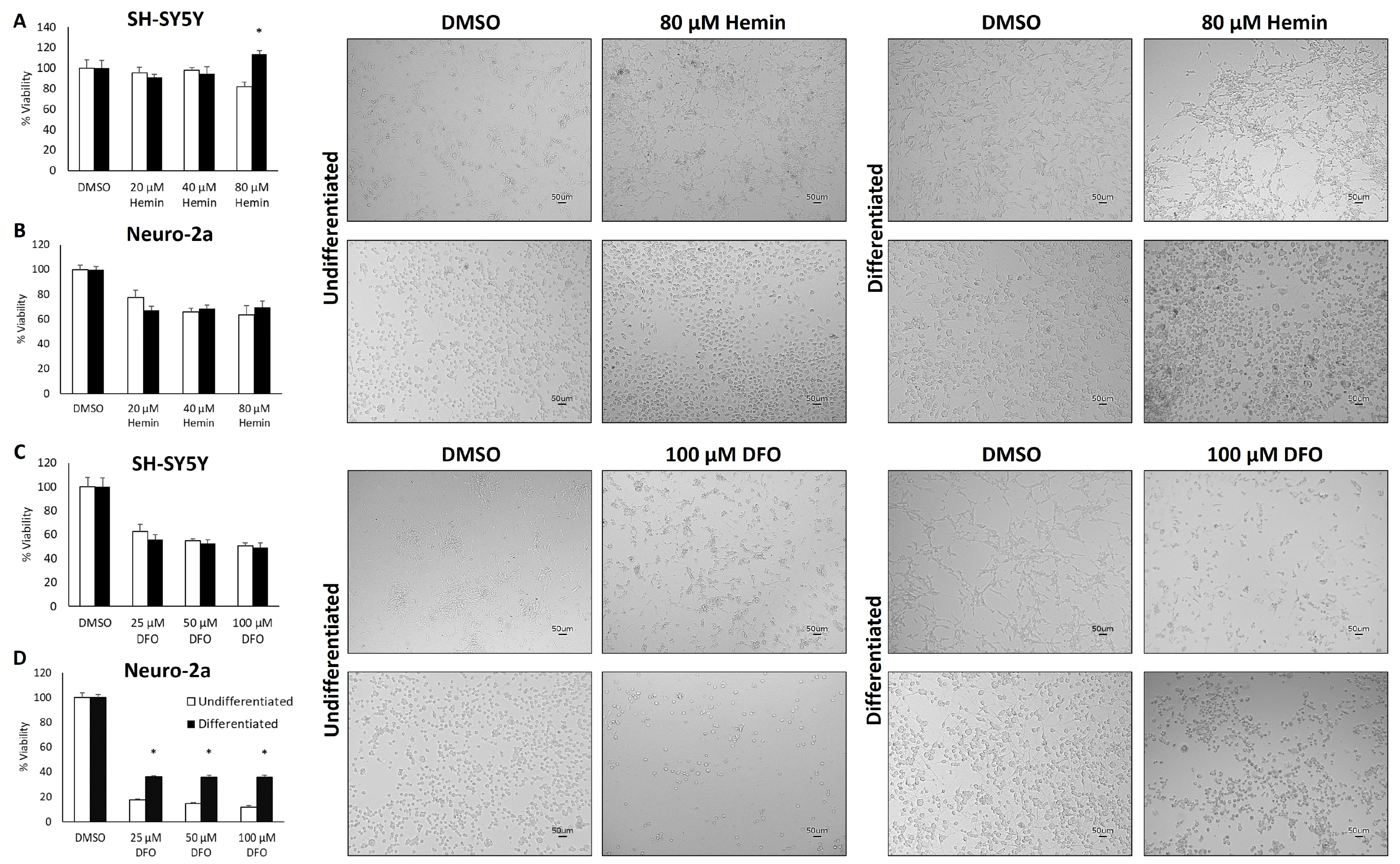
3.2. Neuronal Differentiation Does Not Influence Tolerance to Changes in Iron Availability
3.3. Differentiated Neuroblastoma Cell Lines Are Remarkably Resistant to Ferroptosis
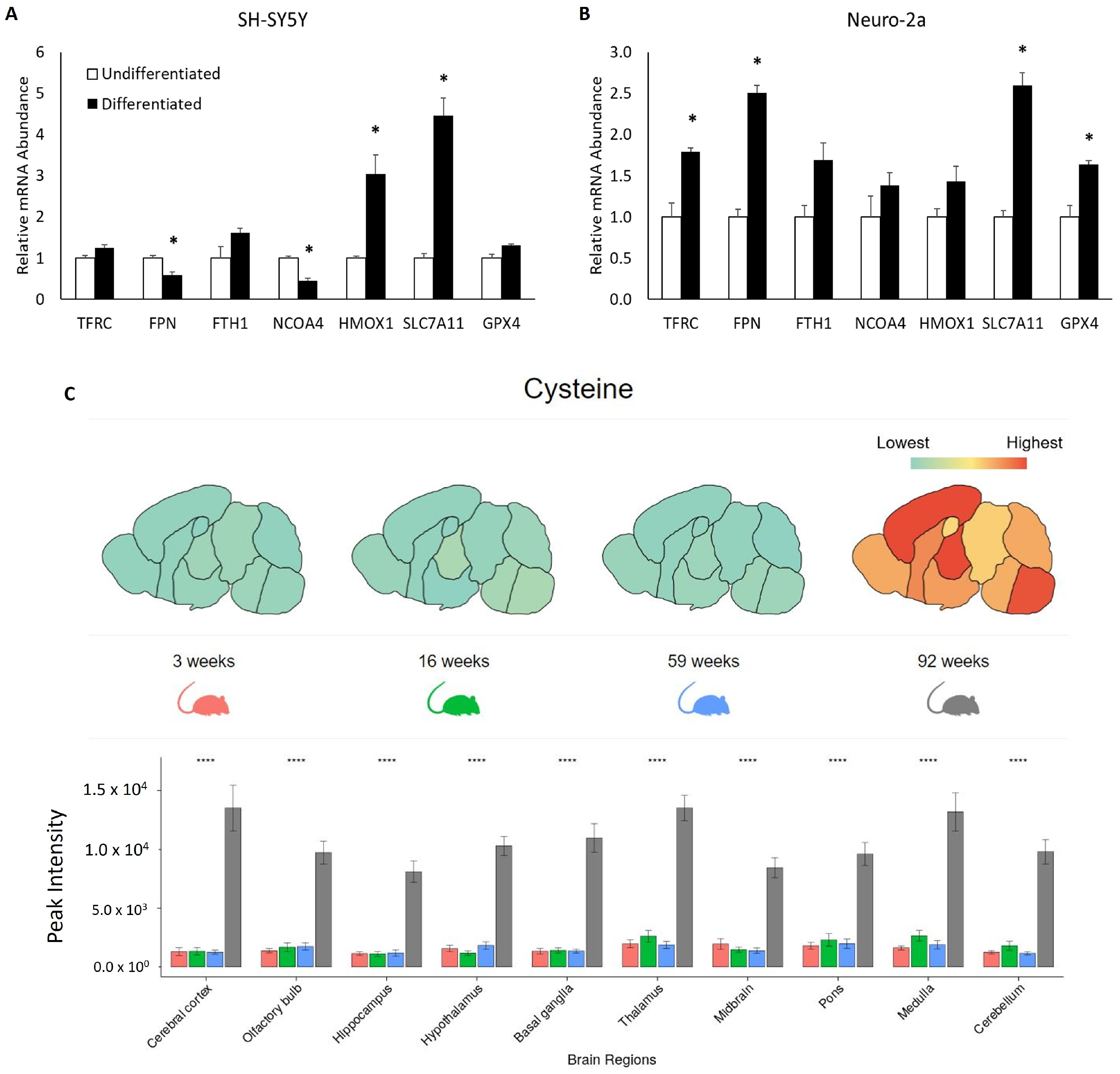
3.4. Neuronal Maturation Increases the Expression of mRNA Associated with Ferroptosis Resistance
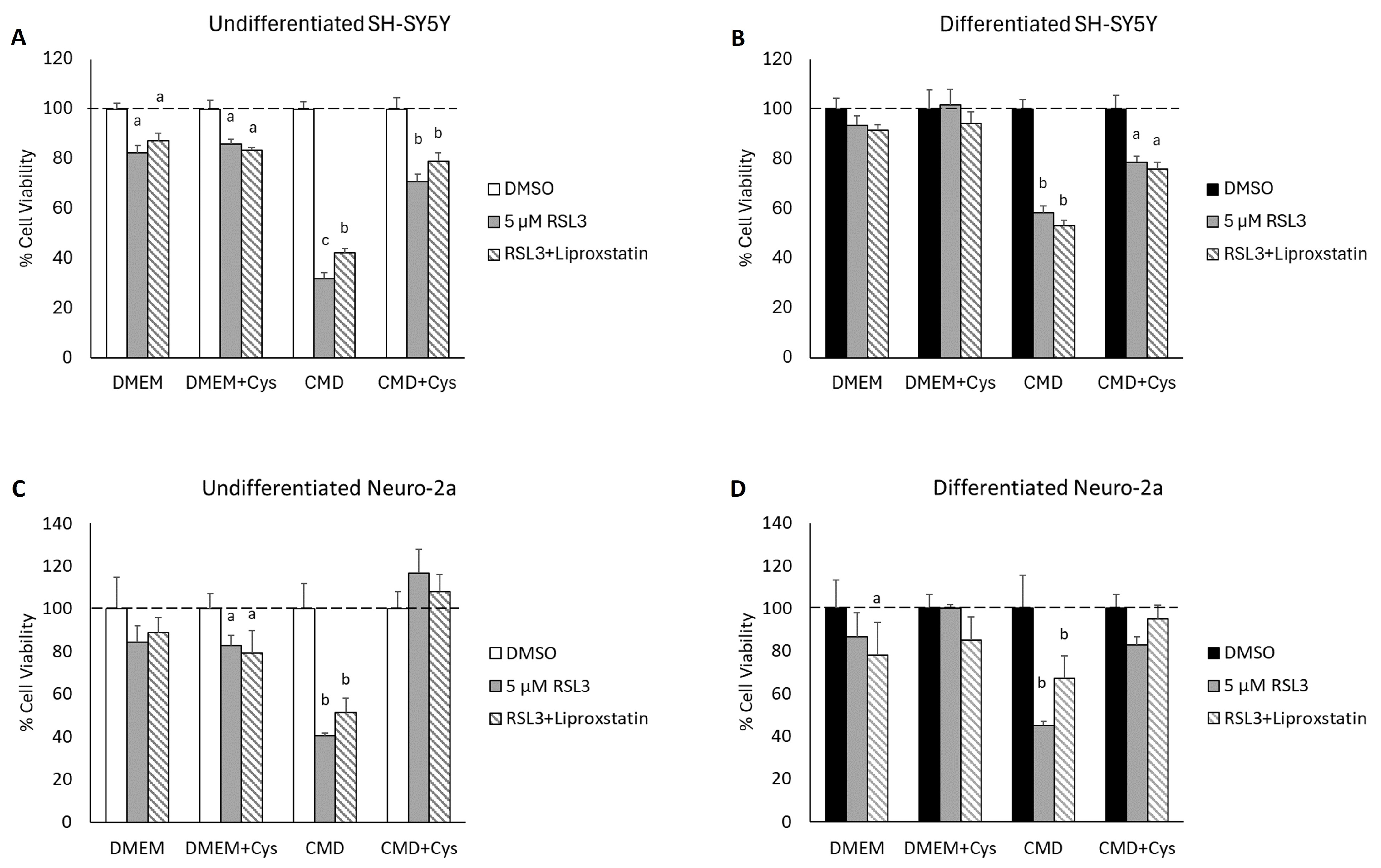
3.5. Cysteine Depletion Sensitizes Differentiated Neurons to RSL3-Mediated Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A Delicate Balance: Iron Metabolism and Diseases of the Brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Iron Levels in the Human Brain: A Post-Mortem Study of Anatomical Region Differences and Age-Related Changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain Iron Is Associated with Accelerated Cognitive Decline in People with Alzheimer Pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Faux, N.G.; Bush, A.I. Alzheimer’s Disease Neuroimaging Initiative Ferritin Levels in the Cerebrospinal Fluid Predict Alzheimer’s Disease Outcomes and Are Regulated by APOE. Nat. Commun. 2015, 6, 6760. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- VanLandingham, J.W.; Levenson, C.W. Effect of Retinoic Acid on Ferritin H Expression during Brain Development and Neuronal Differentiation. Nutr. Neurosci. 2003, 6, 39–45. [Google Scholar] [CrossRef]
- Strother, L.; Miles, G.B.; Holiday, A.R.; Cheng, Y.; Doherty, G.H. Long-Term Culture of SH-SY5Y Neuroblastoma Cells in the Absence of Neurotrophins: A Novel Model of Neuronal Ageing. J. Neurosci. Methods 2021, 362, 109301. [Google Scholar] [CrossRef]
- Traxler, L.; Lagerwall, J.; Eichhorner, S.; Stefanoni, D.; D’Alessandro, A.; Mertens, J. Metabolism Navigates Neural Cell Fate in Development, Aging and Neurodegeneration. Dis. Model. Mech. 2021, 14, dmm048993. [Google Scholar] [CrossRef]
- Tong, Z.-B.; Kim, H.; El Touny, L.; Simeonov, A.; Gerhold, D. LUHMES Dopaminergic Neurons Are Uniquely Susceptible to Ferroptosis. Neurotox. Res. 2022, 40, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Chen, W.; Nguyen, N.; Madhavan, L.; Dodson, M.; Zhang, D.D. α-Syn Overexpression, NRF2 Suppression, and Enhanced Ferroptosis Create a Vicious Cycle of Neuronal Loss in Parkinson’s Disease. Free Radic. Biol. Med. 2022, 192, 130–140. [Google Scholar] [CrossRef]
- Ito, K.; Eguchi, Y.; Imagawa, Y.; Akai, S.; Mochizuki, H.; Tsujimoto, Y. MPP+ Induces Necrostatin-1- and Ferrostatin-1-Sensitive Necrotic Death of Neuronal SH-SY5Y Cells. Cell Death Discov. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Graham, D.M.; Keen, C.L.; Rucker, R.B.; Messerli, M.A. Neurulation and Neurite Extension Require the Zinc Transporter ZIP12 (Slc39a12). Proc. Natl. Acad. Sci. USA 2013, 110, 9903–9908. [Google Scholar] [CrossRef] [PubMed]
- Melino, G.; Thiele, C.J.; Knight, R.A.; Piacentini, M. Retinoids and the Control of Growth/Death Decisions in Human Neuroblastoma Cell Lines. J. Neuro-Oncol. 1997, 31, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Sagara, J.; Miura, K.; Bannai, S. Cystine Uptake and Glutathione Level in Fetal Brain Cells in Primary Culture and in Suspension. J. Neurochem. 1993, 61, 1667–1671. [Google Scholar] [CrossRef]
- dos Santos, C.O.; Dore, L.C.; Valentine, E.; Shelat, S.G.; Hardison, R.C.; Ghosh, M.; Wang, W.; Eisenstein, R.S.; Costa, F.F.; Weiss, M.J. An Iron Responsive Element-like Stem-Loop Regulates α-Hemoglobin-Stabilizing Protein mRNA. J. Biol. Chem. 2008, 283, 26956–26964. [Google Scholar] [CrossRef]
- Sun, L.; Dong, H.; Zhang, W.; Wang, N.; Ni, N.; Bai, X.; Liu, N. Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms. DNA Cell Biol. 2021, 40, 172–183. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Shrestha, Y.; Flavin, R.J.; Regan, M.M.; Penney, K.L.; Mucci, L.A.; Stampfer, M.J.; Hunter, D.J.; Chanock, S.J.; Schafer, E.J.; et al. Analysis of the 10q11 Cancer Risk Locus Implicates MSMB and NCOA4 in Human Prostate Tumorigenesis. PLoS Genet. 2010, 6, e1001204. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The Glutamate/Cystine Antiporter SLC7A11/xCT Enhances Cancer Cell Dependency on Glucose by Exporting Glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Teuffel, R.; Laham, N.; Ehrlich, R.; Decker, P.; Lemonnier, F.A.; Pascolo, S. Expression of Iron Transport Proteins Divalent Metal Transporter-1, Ferroportin-1, HFE and Transferrin Receptor-1 in Human Monocyte-derived Dendritic Cells. Cell Biochem. Funct. 2007, 25, 287–296. [Google Scholar] [CrossRef]
- Wang, W.; Di, X.; D’Agostino, R.B., Jr.; Torti, S.V.; Torti, F.M. Excess Capacity of the Iron Regulatory Protein System. J. Biol. Chem. 2007, 282, 24650–24659. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Watanabe, H.; Shiuchi, T.; Hamano, H.; Horinouchi, Y.; Imanishi, M.; Goda, M.; Zamami, Y.; Takechi, K.; Izawa-Ishizawa, Y.; et al. Deletion of H-Ferritin in Macrophages Alleviates Obesity and Diabetes Induced by High-Fat Diet in Mice. Diabetologia 2020, 63, 1588–1602. [Google Scholar] [CrossRef]
- Grube, J.; Woitok, M.M.; Mohs, A.; Erschfeld, S.; Lynen, C.; Trautwein, C.; Otto, T. ACSL4-Dependent Ferroptosis Does Not Represent a Tumor-Suppressive Mechanism but ACSL4 Rather Promotes Liver Cancer Progression. Cell Death Dis. 2022, 13, 704. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, P.; Zhang, M.; Zhang, X.; Lv, L.; Liu, H.; Liu, Y.; Zhou, Y. Inhibition of SLC7A11 by Sulfasalazine Enhances Osteogenic Differentiation of Mesenchymal Stem Cells by Modulating BMP2/4 Expression and Suppresses Bone Loss in Ovariectomized Mice. J. Bone Miner. Res. 2017, 32, 508–521. [Google Scholar] [CrossRef]
- Troadec, M.-B.; Fautrel, A.; Drénou, B.; Leroyer, P.; Camberlein, E.; Turlin, B.; Guillouzo, A.; Brissot, P.; Loréal, O. Transcripts of Ceruloplasmin but Not Hepcidin, Both Major Iron Metabolism Genes, Exhibit a Decreasing Pattern along the Portocentral Axis of Mouse Liver. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2008, 1782, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D.; Software, P. PBC Dplyr: A Grammar of Data Manipulation; Dplyr: Cairo, Egypt, 2023. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 August 2024).
- Villanueva, R.A.M.; Chen, Z.J. Ggplot2: Elegant Graphics for Data Analysis (2nd Ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. Available online: https://www.tandfonline.com/doi/full/10.1080/15366367.2019.1565254 (accessed on 12 August 2024). [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ge, M.; Xing, P.; Xia, T.; Zhang, C.; Ma, K.; Ma, Y.; Li, S.; Li, W.; Liu, X.; et al. Cystine Deprivation Triggers CD36-Mediated Ferroptosis and Dysfunction of Tumor Infiltrating CD8+ T Cells. Cell Death Dis. 2024, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- L-Cysteine in Cell Culture. Available online: https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/mammalian-cell-culture/cysteine (accessed on 1 August 2024).
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Lee, S.J. Extracellular Concentration of L-Cystine Determines the Sensitivity to System Xc- Inhibitors. Biomol. Ther. 2022, 30, 184–190. [Google Scholar] [CrossRef]
- Cozzi, A.; Orellana, D.I.; Santambrogio, P.; Rubio, A.; Cancellieri, C.; Giannelli, S.; Ripamonti, M.; Taverna, S.; Di Lullo, G.; Rovida, E.; et al. Stem Cell Modeling of Neuroferritinopathy Reveals Iron as a Determinant of Senescence and Ferroptosis during Neuronal Aging. Stem Cell Rep. 2019, 13, 832–846. [Google Scholar] [CrossRef]
- Tschuck, J.; Padmanabhan Nair, V.; Galhoz, A.; Zaratiegui, C.; Tai, H.-M.; Ciceri, G.; Rothenaigner, I.; Tchieu, J.; Stockwell, B.R.; Studer, L.; et al. Suppression of Ferroptosis by Vitamin A or Radical-Trapping Antioxidants Is Essential for Neuronal Development. Nat. Commun. 2024, 15, 7611. [Google Scholar] [CrossRef]
- Lai, X.; Wu, A.; Bing, Y.; Liu, Y.; Luo, J.; Yan, H.; Zheng, P.; Yu, J.; Chen, D. Retinoic Acid Protects against Lipopolysaccharide-Induced Ferroptotic Liver Injury and Iron Disorders by Regulating Nrf2/HO-1 and RARβ Signaling. Free Radic. Biol. Med. 2023, 205, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. CISD1 Inhibits Ferroptosis by Protection against Mitochondrial Lipid Peroxidation. Biochem. Biophys. Res. Commun. 2016, 478, 838–844. [Google Scholar] [CrossRef]
- Novera, W.; Lee, Z.W.; Nin, D.S.; Dai, M.Z.; Binte Idres, S.; Wu, H.; Damen, J.M.A.; Tan, T.Z.; Sim, A.Y.L.; Long, Y.C.; et al. Cysteine Deprivation Targets Ovarian Clear Cell Carcinoma Via Oxidative Stress and Iron-Sulfur Cluster Biogenesis Deficit. Antioxid. Redox Signal. 2020, 33, 1191–1208. [Google Scholar] [CrossRef]
- Li, K.; Tong, W.-H.; Hughes, R.M.; Rouault, T.A. Roles of the Mammalian Cytosolic Cysteine Desulfurase, ISCS, and Scaffold Protein, ISCU, in Iron-Sulfur Cluster Assembly. J. Biol. Chem. 2006, 281, 12344–12351. [Google Scholar] [CrossRef]
- Damulina, A.; Pirpamer, L.; Soellradl, M.; Sackl, M.; Tinauer, C.; Hofer, E.; Enzinger, C.; Gesierich, B.; Duering, M.; Ropele, S.; et al. Cross-Sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease Using 3-T MRI. Radiology 2020, 296, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ji, J.; Rabow, Z.; Shen, T.; Folz, J.; Brydges, C.R.; Fan, S.; Lu, X.; Mehta, S.; Showalter, M.R.; et al. A Metabolome Atlas of the Aging Mouse Brain. Nat. Commun. 2021, 12, 6021. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Accession Number | Forward Primer | Reverse Primer | Source |
|---|---|---|---|---|
| FTH1 | NM_002032.2 | 5′aacatgctgagaaactgatg | 5′gcacactccattgcattcagc | [18] |
| GPX4 | NM_001039847 | 5′acaagaacggctgcgtggtgaa | 5′gccacacacttgtggagctaga | [19] |
| HMOX1 | NM_002133.1 | 5′cgggccagcaacaaagtg | 5′agtgtaaggacccatcggagaa | Primer Express |
| NCOA4 | NM_001145260.1 | 5′cagcagctctactcgttattgg | 5′tctccaggcacacagagact | [20] |
| PPIB | NM_000942.5 | 5′tgccatcgccaaggagtag | 5′tgcacagacggtcactcaaa | Primer Express |
| SLC7A11 | NM_014331.4 | 5′atgcagtggcagtgaccttt | 5′ggcaacaaagatcggaactg | [21] |
| SLC40A1 (FPN) | NM_014585.5 | 5′tgaccagggcgggaga | 5′agaggtcaggtagtcggcca | [22] |
| TFRC | NM_001128148.1 | 5′agttgaacaaagtggcacgagcag | 5′agcagttggctgttgtacctctca | [23] |
| Gene Symbol | Accession Number | Forward Primer | Reverse Primer | Source |
|---|---|---|---|---|
| Fth1 | NM_010239.2 | 5′tgatgaagctgcagaaccag | 5′gtgcacactccattgcattc | [24] |
| Gpx4 | NR_110342.1 | 5′acccactgtggaaatggatga | 5′ctctatcacctggggctcctc | [25] |
| Hmox1 | NM_010442 | 5′tcaggtgtccagagaaggcttt | 5′tcttccagggccgtgtagat | Primer Express |
| Ncoa4 | NM_019744 | 5′cgccagaccatcaccacat | 5′ctcgcgtgagccatcagat | Primer Express |
| Rpl19 | NM_000981 | 5′gacggaagggcaggcatatg | 5′tgtggatgtgctccatgagg | Primer Express |
| Slc7a11 | NM_011990.2 | 5′atctcccccaagggcatact | 5′gcataggacagggctccaaa | [26] |
| Slc40a1 (Fpn) | NM_016917.2 | 5′gctgctagaatcggtctttggt | 5′cagcaactgtgtcaccgtcaa | [27] |
| Tfrc | NM_011638 | 5′ttggacatgctcatctaggaactg | 5′ctgagatggcggaaactgagt | Primer Express |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardona, C.J.; Kim, Y.; Chowanadisai, W.; Montgomery, M.R. Considerations for Using Neuroblastoma Cell Lines to Examine the Roles of Iron and Ferroptosis in Neurodegeneration. Cells 2024, 13, 1541. https://doi.org/10.3390/cells13181541
Cardona CJ, Kim Y, Chowanadisai W, Montgomery MR. Considerations for Using Neuroblastoma Cell Lines to Examine the Roles of Iron and Ferroptosis in Neurodegeneration. Cells. 2024; 13(18):1541. https://doi.org/10.3390/cells13181541
Chicago/Turabian StyleCardona, Cameron J., Yoo Kim, Winyoo Chowanadisai, and McKale R. Montgomery. 2024. "Considerations for Using Neuroblastoma Cell Lines to Examine the Roles of Iron and Ferroptosis in Neurodegeneration" Cells 13, no. 18: 1541. https://doi.org/10.3390/cells13181541
APA StyleCardona, C. J., Kim, Y., Chowanadisai, W., & Montgomery, M. R. (2024). Considerations for Using Neuroblastoma Cell Lines to Examine the Roles of Iron and Ferroptosis in Neurodegeneration. Cells, 13(18), 1541. https://doi.org/10.3390/cells13181541






