Mild Heat Stress Alters the Physical State and Structure of Membranes in Triacylglycerol-Deficient Fission Yeast, Schizosaccharomyces pombe
Abstract
1. Introduction
| Name | Genotype | Reference |
|---|---|---|
| BRC1 | h-; leu1-32; ura4-D18 | [22] |
| BRC48 | h-; leu1-32; ura4-D18; dga1::ura4; plh1::ura4 | [11] |
| BRC40 | h-; leu1-32; ura4-D18; hsp16:GFP(KmR) | [8] |
| BRC62 | h-; leu1-32; ura4-D18; hsp16:GFP(KmR); dga1::ura4; plh1::ura4 | this study |
| BRC98 | h-; pBip1-GFP-AHEL::leu1 | [23] |
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Heat Shock
2.3. Quantitative and Qualitative Analysis of Organelles
2.4. Generalized Polarization
2.5. HSP16 Induction
2.6. Intracellular Trehalose Levels
2.7. Statistical Analysis
3. Results
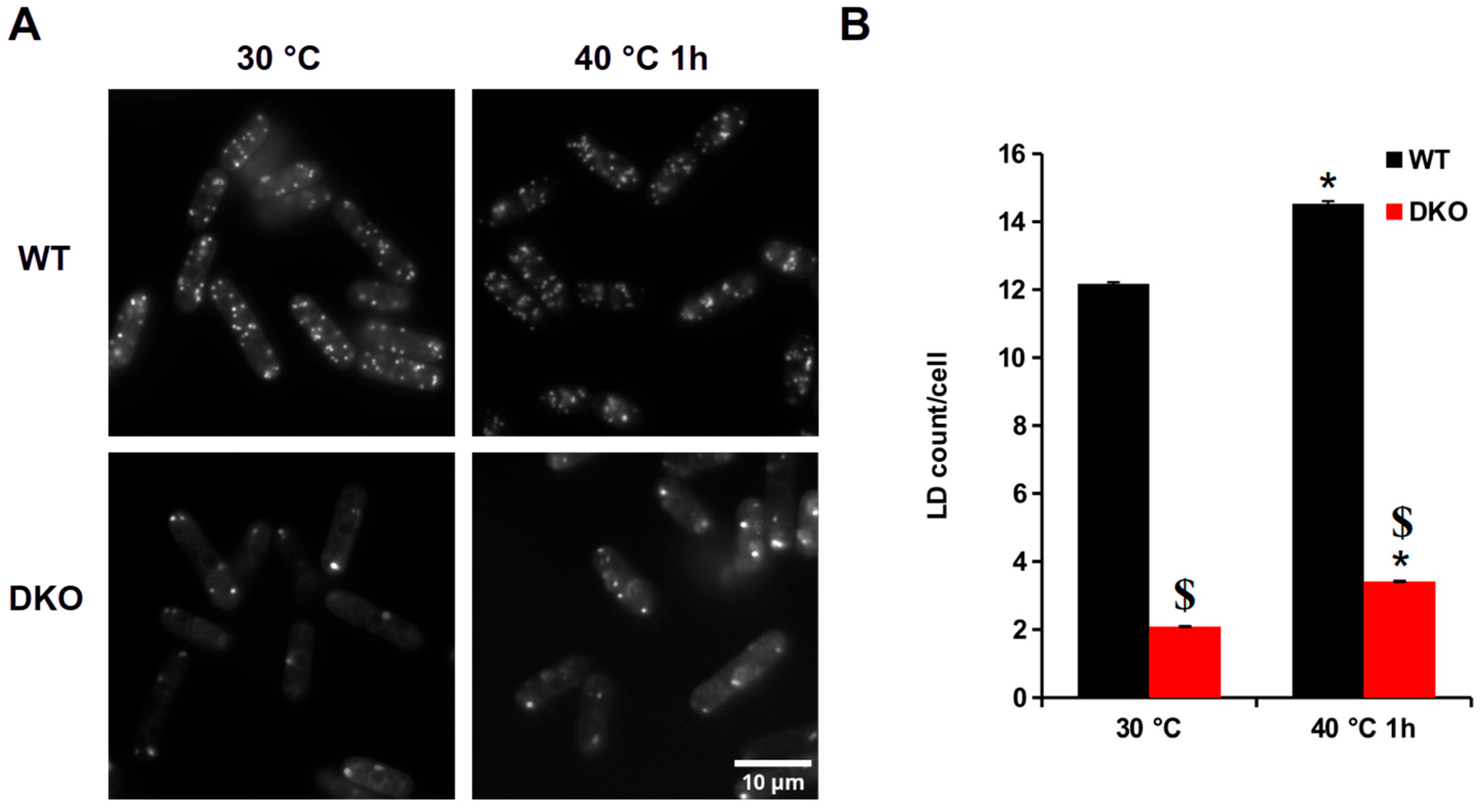
3.1. Heat Shock-Induced Changes in LDs in Wild-Type and DKO Cells
3.2. Heat Shock Induces Enlargement of Vacuoles in DKO Cells
3.3. Heat Stress-Induced Morphological Changes in the Mitochondrial Networks and the Cortical Endoplasmic Reticulum
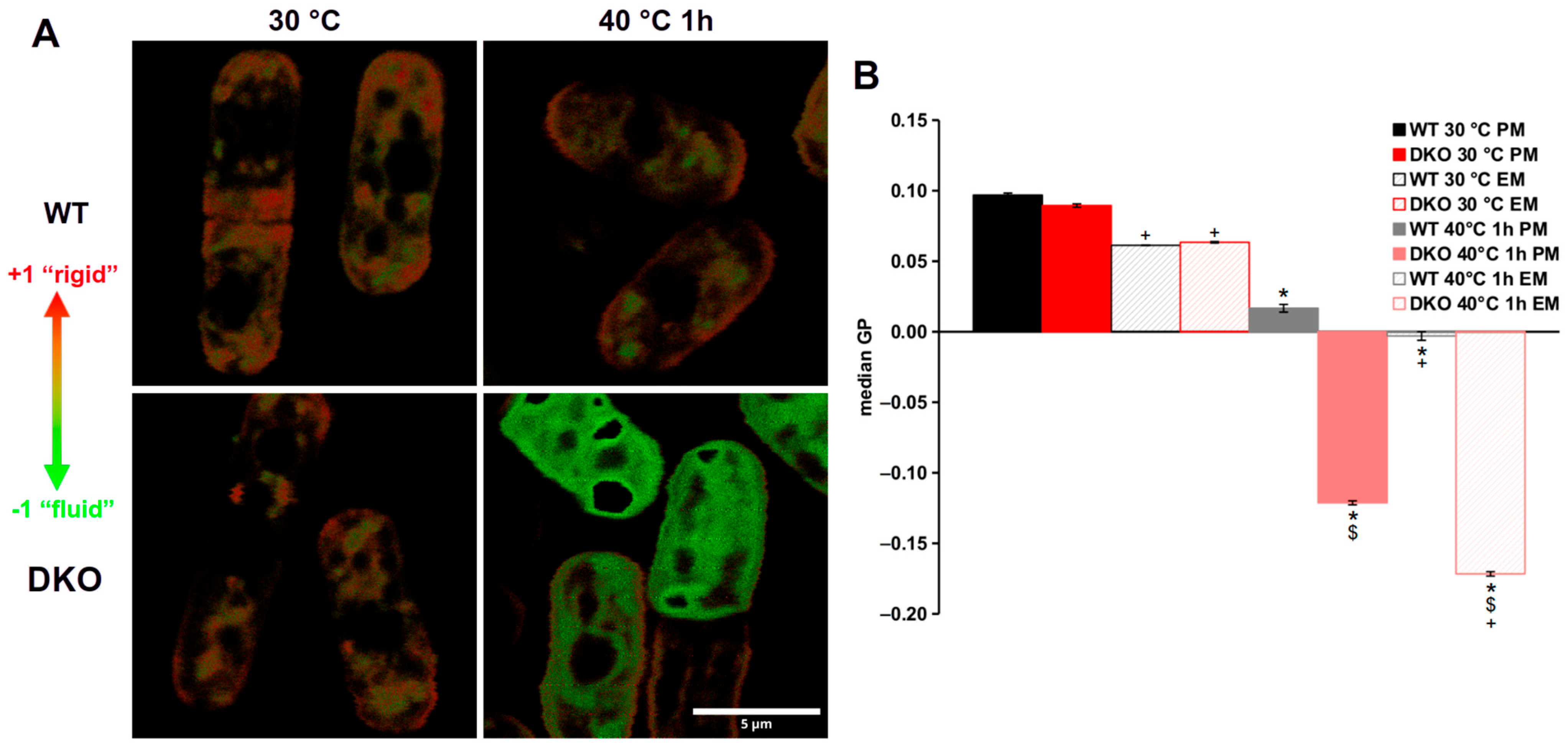
3.4. Highly Altered Membrane Physical State in Heat-Stressed DKO Cells
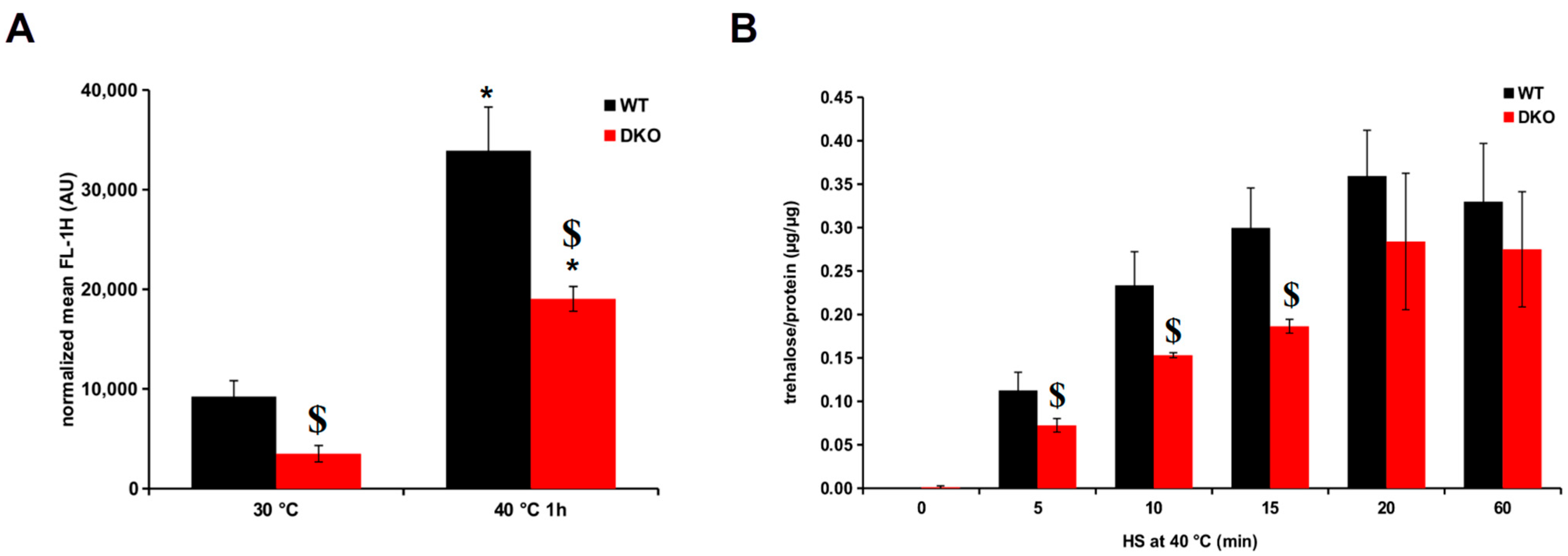
3.5. Impaired Trehalose and HSP16 Production in the Heat-Stressed DKO Mutant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigh, L.; Maresca, B.; Harwood, J.L. Does the Membrane’s Physical State Control the Expression of Heat Shock and Other Genes? Trends Biochem. Sci. 1998, 23, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Glatz, A.; Varvasovszki, V.; Torok, Z.; Pali, T.; Balogh, G.; Kovacs, E.; Nadasdi, L.; Benko, S.; Joo, F.; et al. Membrane Physical State Controls the Signaling Mechanism of the Heat Shock Response in Synechocystis PCC 6803: Identification of Hsp17 as a “Fluidity Gene”. Proc. Natl. Acad. Sci. USA 1998, 95, 3513–3518. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.; Péter, M.; Glatz, A.; Gombos, I.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Key Role of Lipids in Heat Stress Management. FEBS Lett. 2013, 587, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Török, Z.; Crul, T.; Maresca, B.; Schütz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Péter, M.; et al. Plasma Membranes as Heat Stress Sensors: From Lipid-Controlled Molecular Switches to Therapeutic Applications. BBA-Biomembranes 2014, 1838, 1594–1618. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New Insights on Trehalose: A Multifunctional Molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Török, Z.; Goloubinoff, P.; Horváth, I.; Tsvetkova, N.M.; Glatz, A.; Balogh, G.; Varvasovszki, V.; Los, D.A.; Vierling, E.; Crowe, J.H.; et al. Synechocystis HSP17 Is an Amphitropic Protein That Stabilizes Heat-Stressed Membranes and Binds Denatured Proteins for Subsequent Chaperone-Mediated Refolding. Proc. Natl. Acad. Sci. USA 2001, 98, 3098–3103. [Google Scholar] [CrossRef]
- Horváth, I.; Multhoff, G.; Sonnleitner, A.; Vígh, L. Membrane-Associated Stress Proteins: More than Simply Chaperones. BBA-Biomembranes 2008, 1778, 1653–1664. [Google Scholar] [CrossRef]
- Glatz, A.; Pilbat, A.-M.; Németh, G.L.; Vince-Kontár, K.; Jósvay, K.; Hunya, Á.; Udvardy, A.; Gombos, I.; Péter, M.; Balogh, G.; et al. Involvement of Small Heat Shock Proteins, Trehalose, and Lipids in the Thermal Stress Management in Schizosaccharomyces pombe. Cell Stress Chaperons 2016, 21, 327–338. [Google Scholar] [CrossRef]
- Péter, M.; Gudmann, P.; Kóta, Z.; Török, Z.; Vígh, L.; Glatz, A.; Balogh, G. Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe. Int. J. Mol. Sci. 2021, 22, 13272. [Google Scholar] [CrossRef]
- Meyers, A.; Chourey, K.; Weiskittel, T.M.; Pfiffner, S.; Dunlap, J.R.; Hettich, R.L.; Dalhaimer, P. The Protein and Neutral Lipid Composition of Lipid Droplets Isolated from the Fission Yeast, Schizosaccharomyces pombe. J. Microbiol. 2017, 55, 112–122. [Google Scholar] [CrossRef]
- Péter, M.; Glatz, A.; Gudmann, P.; Gombos, I.; Török, Z.; Horváth, I.; Vígh, L.; Balogh, G. Metabolic Crosstalk between Membrane and Storage Lipids Facilitates Heat Stress Management in Schizosaccharomyces pombe. PLoS ONE 2017, 12, e0173739. [Google Scholar] [CrossRef] [PubMed]
- Wilfling, F.; Haas, J.T.; Walther, T.C.; Jr, R.V.F. Lipid Droplet Biogenesis. Curr. Opin. Cell Biol. 2014, 29, 39–45. [Google Scholar] [CrossRef]
- Welte, M.A. Expanding Roles for Lipid Droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef] [PubMed]
- Gubern, A.; Barceló-Torns, M.; Casas, J.; Barneda, D.; Masgrau, R.; Picatoste, F.; Balsinde, J.; Balboa, M.A.; Claro, E. Lipid Droplet Biogenesis Induced by Stress Involves Triacylglycerol Synthesis That Depends on Group VIA Phospholipase A2. J. Biol. Chem. 2009, 284, 5697–5708. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Benning, C. Triacylglycerol Accumulation in Photosynthetic Cells in Plants and Algae. Subcell. Biochem. 2016, 86, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, Y.; Wang, J.; Singer, S.D.; Chen, G. The Role of Triacylglycerol in Plant Stress Response. Plants 2020, 9, 472. [Google Scholar] [CrossRef]
- Zhang, Q.; Chieu, H.K.; Low, C.P.; Zhang, S.; Heng, C.K.; Yang, H. Schizosaccharomyces pombe Cells Deficient in Triacylglycerols Synthesis Undergo Apoptosis upon Entry into the Stationary Phase. J. Biol. Chem. 2003, 278, 47145–47155. [Google Scholar] [CrossRef]
- Dahlqvist, A.; Ståhl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid: Diacylglycerol Acyltransferase: An Enzyme That Catalyzes the Acyl-CoA-Independent Formation of Triacylglycerol in Yeast and Plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef]
- Oelkers, P.; Cromley, D.; Padamsee, M.; Billheimer, J.T.; Sturley, S.L. The DGA1 Gene Determines a Second Triglyceride Synthetic Pathway in Yeast. J. Biol. Chem. 2002, 277, 8877–8881. [Google Scholar] [CrossRef]
- Campomanes, P.; Zoni, V.; Vanni, S. Local Accumulation of Diacylglycerol Alters Membrane Properties Nonlinearly Due to Its Transbilayer Activity. Commun. Chem. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Li, D.; Yang, S.G.; He, C.W.; Zhang, Z.T.; Liang, Y.; Li, H.; Zhu, J.; Su, X.; Gong, Q.; Xie, Z. Excess Diacylglycerol at the Endoplasmic Reticulum Disrupts Endomembrane Homeostasis and Autophagy. BMC Biol. 2020, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, K.; Shiozaki, M.; Russell, P. Mcs4 Mitotic Catastrophe Suppressor Regulates the Fission Yeast Cell Cycle through the Wik1-Wis1-Spc1 Kinase Cascade. Mol. Biol. Cell 1997, 8, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.; Gu, Y.; Chen, J.-S.; Beckley, J.R.; Gould, K.L.; Oliferenko, S. Temporal Regulation of Lipin Activity Diverged to Account for Differences in Mitotic Programs. Curr. Biol. 2016, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Forsburg, S.L. Growth and Manipulation of S. pombe. Curr. Protoc. Mol. Biol. 2003, 64, 13–16. [Google Scholar] [CrossRef]
- Adomshick, V.; Pu, Y.; Veiga-Lopez, A. Automated Lipid Droplet Quantification System for Phenotypic Analysis of Adipocytes Using CellProfiler. Toxicol. Mech. Methods 2020, 30, 378–387. [Google Scholar] [CrossRef]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative Imaging of Membrane Lipid Order in Cells and Organisms. Nat. Protoc. 2011, 7, 24–35. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio) Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Spandl, J.; White, D.J.; Peychl, J.; Thiele, C. Live Cell Multicolor Imaging of Lipid Droplets with a New Dye, LD540. Traffic 2009, 10, 1579–1584. [Google Scholar] [CrossRef]
- Rains, A.; Bryant, Y.; Dorsett, K.A.; Culver, A.; Egbaria, J.; Williams, A.; Barnes, M.; Lamere, R.; Rossi, A.R.; Waldrep, S.C.; et al. Ypt4 and Lvs1 Regulate Vacuolar Size and Function in Schizosaccharomyces pombe. Cell. Logist. 2017, 7, e1335270. [Google Scholar] [CrossRef]
- Arai, S.; Lee, S.-C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A Molecular Fluorescent Probe for Targeted Visualization of Temperature at the Endoplasmic Reticulum. Sci. Rep. 2014, 4, 6701. [Google Scholar] [CrossRef]
- Ishii, A.; Kawai, M.; Noda, H.; Kato, H.; Takeda, K.; Asakawa, K.; Ichikawa, Y.; Sasanami, T.; Tanaka, K.; Kimura, Y. Accelerated invagination of vacuoles as a stress response in chronically heat-stressed yeasts. Sci. Rep. 2018, 8, 2644. [Google Scholar] [CrossRef]
- Richards, A.; Veses, V.; Gow, N.A.R. Vacuole Dynamics in Fungi. Fungal Biol. Rev. 2010, 24, 93–105. [Google Scholar] [CrossRef]
- Keuenhof, K.; Berglund, L.L.; Hill, S.M.; Schneider, K.L.; Widlund, P.O.; Nyström, T.; Höög, J.L. Large Organellar Changes Occur during Mild Heat Shock in Yeast. J. Cell Sci. 2022, 1, jcs258325. [Google Scholar] [CrossRef]
- Poot, M.; Zhang, Y.Z.; Krämer, J.A.; Wells, K.S.; Jones, L.J.; Hanzel, D.K.; Lugade, A.G.; Singer, V.L.; Haugland, R.P. Analysis of Mitochondrial Morphology and Function with Novel Fixable Fluorescent Stains. J. Histochem. Cytochem. 1996, 44, 1363–1372. [Google Scholar] [CrossRef]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker Green TM and CMXrosamine to Measure Changes in Mitochondrial Membrane Potentials in Living Cells and Tissues. Cytom. Part A 2004, 61, 162–169. [Google Scholar] [CrossRef]
- Uehara, L.; Saitoh, S.; Mori, A.; Sajiki, K.; Toyoda, Y.; Masuda, F.; Soejima, S.; Tahara, Y.; Yanagida, M. Multiple Nutritional Phenotypes of Fission Yeast Mutants Defective in Genes Encoding Essential Mitochondrial Proteins. Open Biol. 2021, 11, 200369. [Google Scholar] [CrossRef]
- Zhang, D.; Vjestica, A.; Oliferenko, S. The Cortical ER Network Limits the Permissive Zone for Actomyosin Ring Assembly. Curr. Biol. 2010, 20, 1029–1034. [Google Scholar] [CrossRef]
- Zhang, D.; Vjestica, A.; Oliferenko, S. Plasma Membrane Tethering of the Cortical ER Necessitates Its Finely Reticulated Architecture. Curr. Biol. 2012, 22, 2048–2052. [Google Scholar] [CrossRef]
- Dinic, J.; Biverståhl, H.; Mäler, L.; Parmryd, I. Laurdan and Di-4-ANEPPDHQ Do Not Respond to Membrane-Inserted Peptides and Are Good Probes for Lipid Packing. BBA-Biomembranes 2011, 1808, 298–306. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “Chemical Chaperone”. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks; Advances in Experimental Medicine and Biology; Csermely, P., Vígh, L., Eds.; Springer: New York, NY, USA, 2007; Volume 594, pp. 143–158. ISBN 978-0-387-39974-4. [Google Scholar]
- Singer, M.A.; Lindquist, S. Multiple Effects of Trehalose on Protein Folding In Vitro and In Vivo. Mol. Cell 1998, 1, 639–648. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Spivey, W.W.; Rustgi, S.; Welti, R.; Roth, M.R.; Burow, M.D.; Bridges, W.C.; Narayanan, S. Lipid Modulation Contributes to Heat Stress Adaptation in Peanut. Front. Plant Sci. 2023, 14, 1299371. [Google Scholar] [CrossRef]
- Kohlwein, S.D.; Veenhuis, M.; van der Klei, I.J. Lipid Droplets and Peroxisomes: Key Players in Cellular Lipid Homeostasis or A Matter of Fat—Store ’em Up or Burn ’Em Down. Genetics 2013, 193, 1–50. [Google Scholar] [CrossRef]
- Bone, N.; Millar, J.B.A.; Toda, T.; Armstrong, J. Regulated Vacuole Fusion and Fission in Schizosaccharomyces pombe: An Osmotic Response Dependent on MAP Kinases. Curr. Biol. 1998, 8, 135–144. [Google Scholar] [CrossRef][Green Version]
- Hariri, H.; Rogers, S.; Ugrankar, R.; Liu, Y.L.; Feathers, J.R.; Henne, W.M. Lipid Droplet Biogenesis Is Spatially Coordinated at ER–Vacuole Contacts under Nutritional Stress. EMBO Rep. 2018, 19, 57–72. [Google Scholar] [CrossRef]
- Girik, V.; Feng, S.; Hariri, H.; Henne, W.M.; Riezman, H. Vacuole-Specific Lipid Release for Tracking Intracellular Lipid Metabolism and Transport in Saccharomyces cerevisiae. ACS Chem. Biol. 2022, 17, 1485–1494. [Google Scholar] [CrossRef]
- Meyers, A.; del Rio, Z.P.; Beaver, R.A.; Morris, R.M.; Weiskittel, T.M.; Alshibli, A.K.; Mannik, J.; Morrell-Falvey, J.; Dalhaimer, P. Lipid Droplets Form from Distinct Regions of the Cell in the Fission Yeast Schizosaccharomyces pombe: Fission Yeast Lipid Droplet Formation. Traffic 2016, 17, 657–669. [Google Scholar] [CrossRef]
- He, J.; Liu, K.; Zheng, S.; Wu, Y.; Zhao, C.; Yan, S.; Liu, L.; Ruan, K.; Ma, X.; Fu, C. The acyl-CoA-binding Protein Acb1 Regulates Mitochondria, Lipid Droplets, and Cell Proliferation. FEBS Lett. 2022, 596, 1795–1808. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Q.; Peng, X.; Choi, S.-Y.; Sarma, K.; Ren, H.; Morris, A.J.; Frohman, M.A. piRNA-Associated Germline Nuage Formation and Spermatogenesis Require MitoPLD Profusogenic Mitochondrial-Surface Lipid Signaling. Dev. Cell 2011, 20, 376–387. [Google Scholar] [CrossRef]
- Gallardo, P.; Real-Calderón, P.; Flor-Parra, I.; Salas-Pino, S.; Daga, R.R. Acute Heat Stress Leads to Reversible Aggregation of Nuclear Proteins into Nucleolar Rings in Fission Yeast. Cell Rep. 2020, 33, 108377. [Google Scholar] [CrossRef]
- Balogh, G.; Maulucci, G.; Gombos, I.; Horváth, I.; Török, Z.; Péter, M.; Fodor, E.; Páli, T.; Benkő, S.; Parasassi, T.; et al. Heat Stress Causes Spatially-Distinct Membrane Re-Modelling in K562 Leukemia Cells. PLoS ONE 2011, 6, e21182. [Google Scholar] [CrossRef] [PubMed]
- Makarova, M.; Peter, M.; Balogh, G.; Glatz, A.; MacRae, J.I.; Lopez Mora, N.; Booth, P.; Makeyev, E.; Vigh, L.; Oliferenko, S. Delineating the Rules for Structural Adaptation of Membrane-Associated Proteins to Evolutionary Changes in Membrane Lipidome. Curr. Biol. 2020, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.; Reina, F.; Hof, M.; Eggeling, C.; Sezgin, E. Laurdan and Di-4-ANEPPDHQ Probe Different Properties of the Membrane. J. Phys. D Appl. Phys. 2017, 50, 134004. [Google Scholar] [CrossRef] [PubMed]
- Makushok, T.; Alves, P.; Huisman, S.M.; Kijowski, A.R.; Brunner, D. Sterol-Rich Membrane Domains Define Fission Yeast Cell Polarity. Cell 2016, 165, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Wachtler, V.; Rajagopalan, S.; Balasubramanian, M.K. Sterol-Rich Plasma Membrane Domains in the Fission Yeast Schizosaccharomyces pombe. J. Cell Sci. 2003, 116, 867–874. [Google Scholar] [CrossRef]
- De Virgilio, C.; Simmen, U.; Hottiger, T.; Boiler, T.; Wiemken, A. Heat Shock Induces Enzymes of Trehalose Metabolism, Trehalose Accumulation, and Thermotolerance in Schizosaccharomyces pombe, Even in the Presence of Cycloheximide. FEBS Lett. 1990, 273, 107–110. [Google Scholar] [CrossRef]


| Organelle | Dye | Final Conc | Incubation, min | Reference |
|---|---|---|---|---|
| lipid droplets | LD540 | 50 ng/mL | 15 min | Limes-Institut-Bonn [28] |
| vacuoles | MDY-64 | 10 µM | 15 min | Invitrogen, Thermo Fisher [29] |
| cortical ER | ER-thermo-Yellow | 1 µM | 25 min | [30] |
| mitochondria | MitoTracker Red CMXRos | 350 nM | 15 min | Invitrogen, Thermo Fisher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudmann, P.; Gombos, I.; Péter, M.; Balogh, G.; Török, Z.; Vígh, L.; Glatz, A. Mild Heat Stress Alters the Physical State and Structure of Membranes in Triacylglycerol-Deficient Fission Yeast, Schizosaccharomyces pombe. Cells 2024, 13, 1543. https://doi.org/10.3390/cells13181543
Gudmann P, Gombos I, Péter M, Balogh G, Török Z, Vígh L, Glatz A. Mild Heat Stress Alters the Physical State and Structure of Membranes in Triacylglycerol-Deficient Fission Yeast, Schizosaccharomyces pombe. Cells. 2024; 13(18):1543. https://doi.org/10.3390/cells13181543
Chicago/Turabian StyleGudmann, Péter, Imre Gombos, Mária Péter, Gábor Balogh, Zsolt Török, László Vígh, and Attila Glatz. 2024. "Mild Heat Stress Alters the Physical State and Structure of Membranes in Triacylglycerol-Deficient Fission Yeast, Schizosaccharomyces pombe" Cells 13, no. 18: 1543. https://doi.org/10.3390/cells13181543
APA StyleGudmann, P., Gombos, I., Péter, M., Balogh, G., Török, Z., Vígh, L., & Glatz, A. (2024). Mild Heat Stress Alters the Physical State and Structure of Membranes in Triacylglycerol-Deficient Fission Yeast, Schizosaccharomyces pombe. Cells, 13(18), 1543. https://doi.org/10.3390/cells13181543







