Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in S. mansoni-Infected Hamsters
Abstract
1. Introduction
2. Materials and Methods
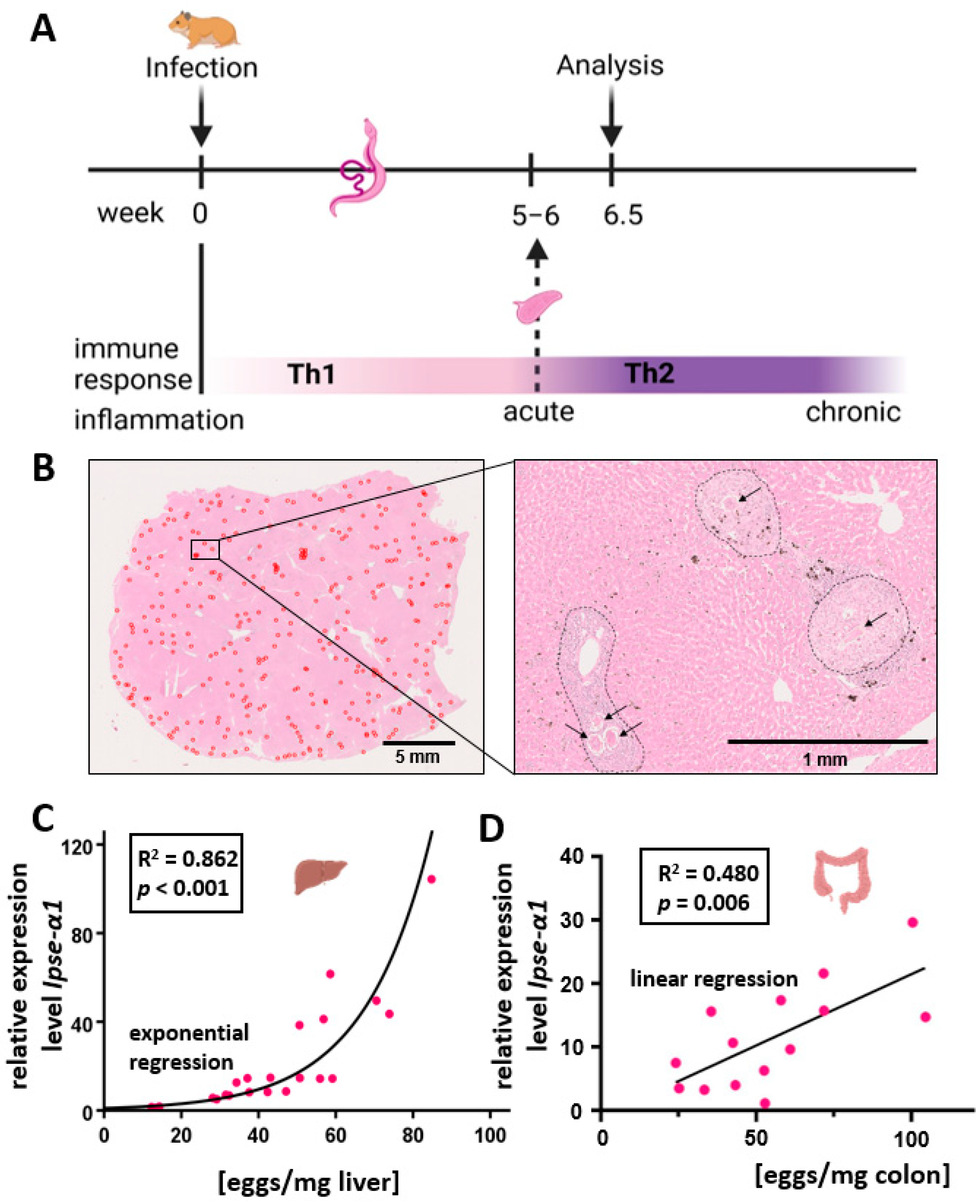
2.1. Animal Model
2.2. Hematoxylin and Eosin Staining
2.3. Quantitative Real-Time PCR
2.4. Determination of the Hepatic and Colon Egg Load
2.5. Statistical Analysis
3. Results
3.1. Quantification and Characterization of the Hepatic and Colon Egg Load
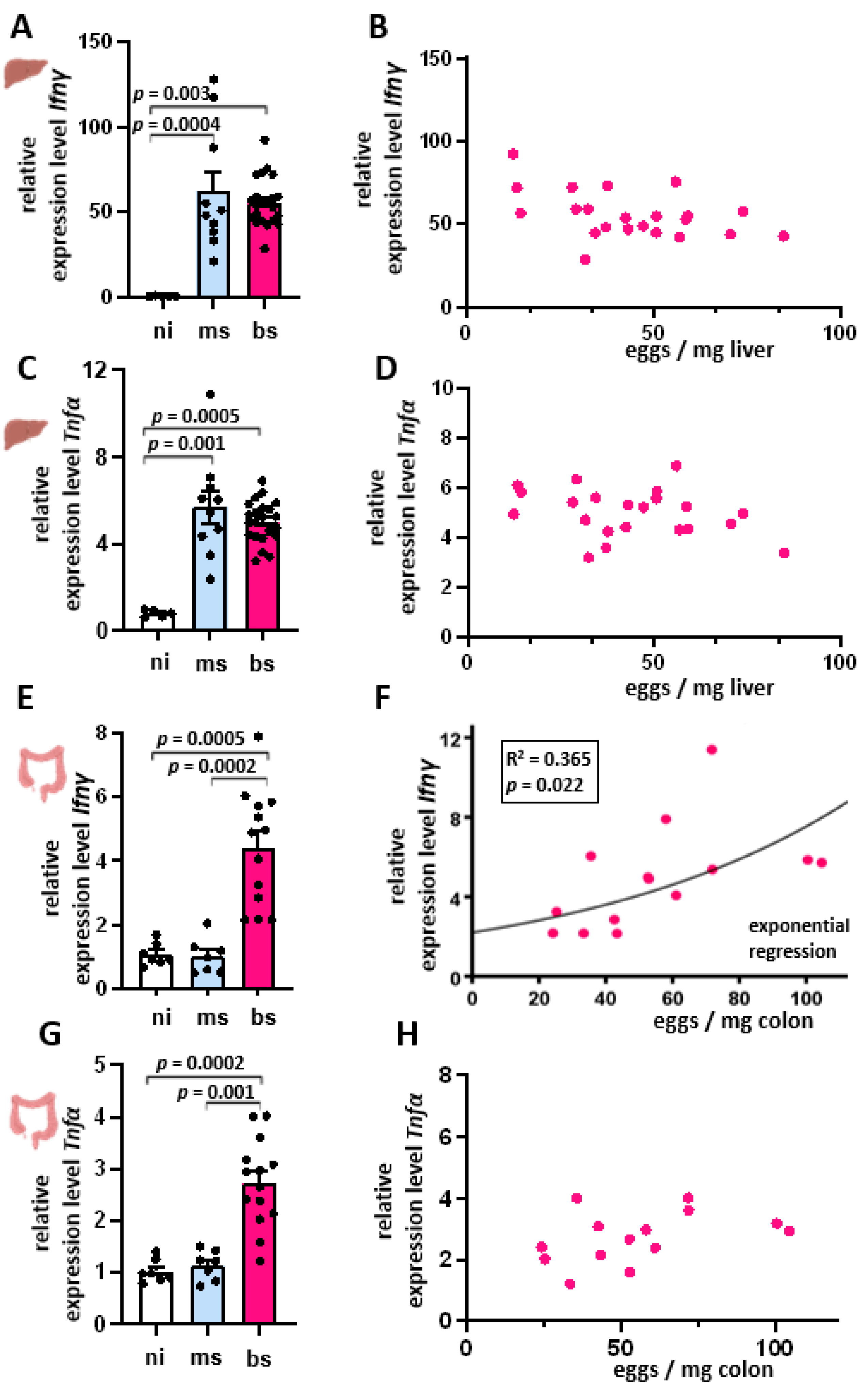
3.2. S. mansoni Infection-Induced Th1 Cytokine Response Is Independent of Hepatic Egg Burden
3.3. Th2 Cytokine Expression Inversely Correlated with the Hepatic Egg Burden
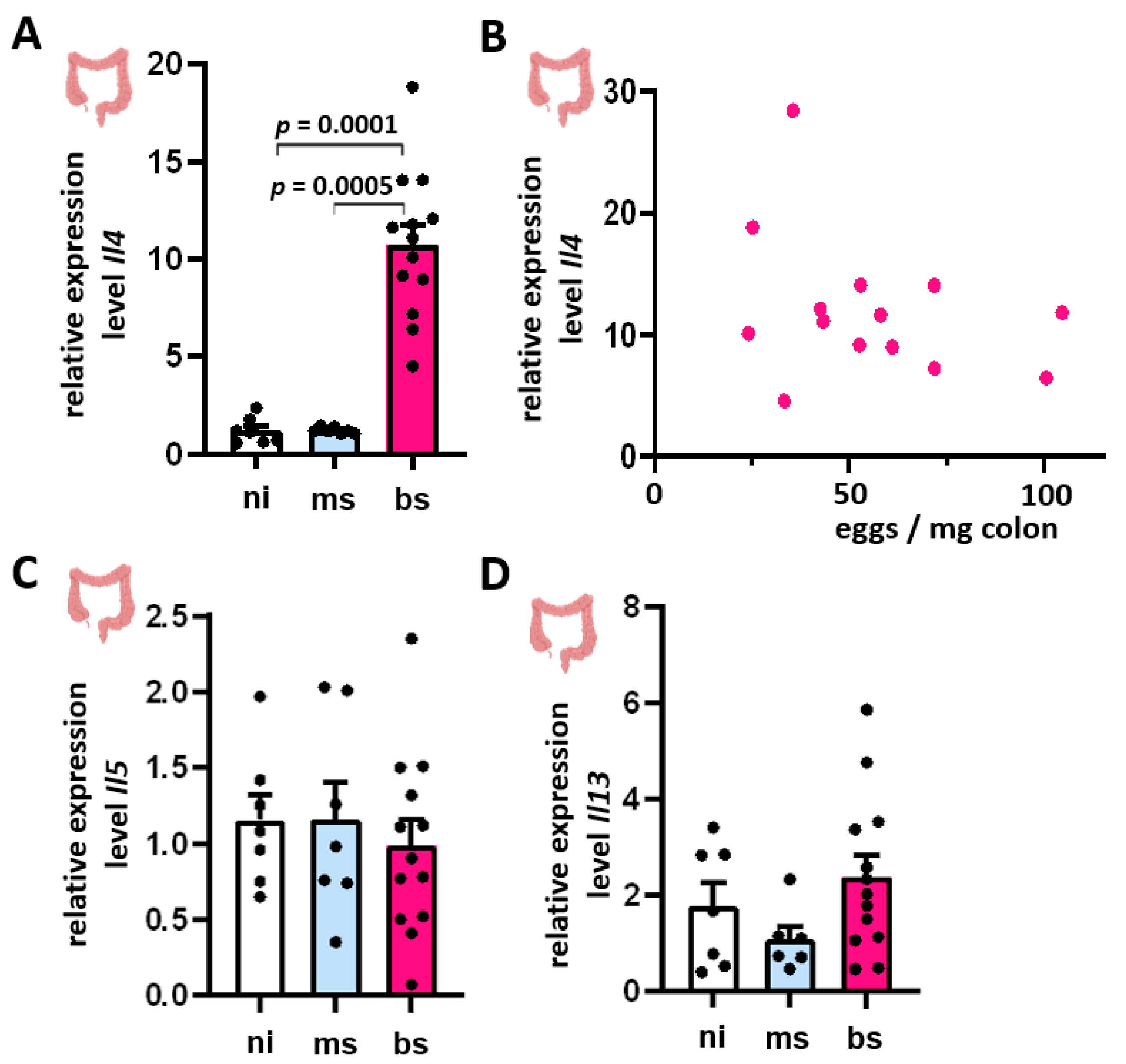
3.4. Induction of Il4, Il5, and Il13 mRNA Expression in Colon Tissue Showed No Dependence on Intestinal Egg Burden
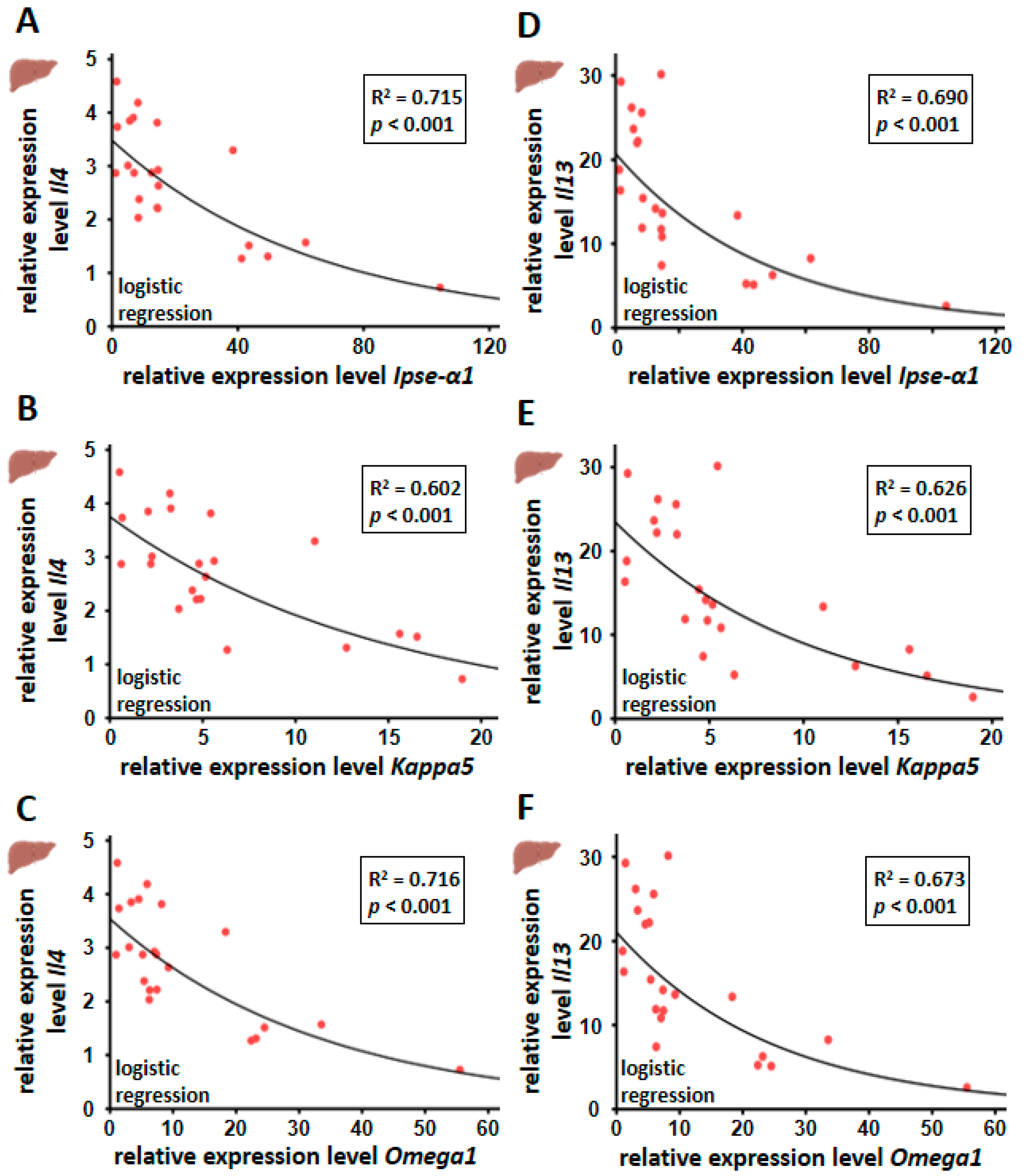
3.5. Hepatic Il4 and Il13 mRNA Expression Correlated Inversely with Egg-Derived Factors of S. mansoni Eggs
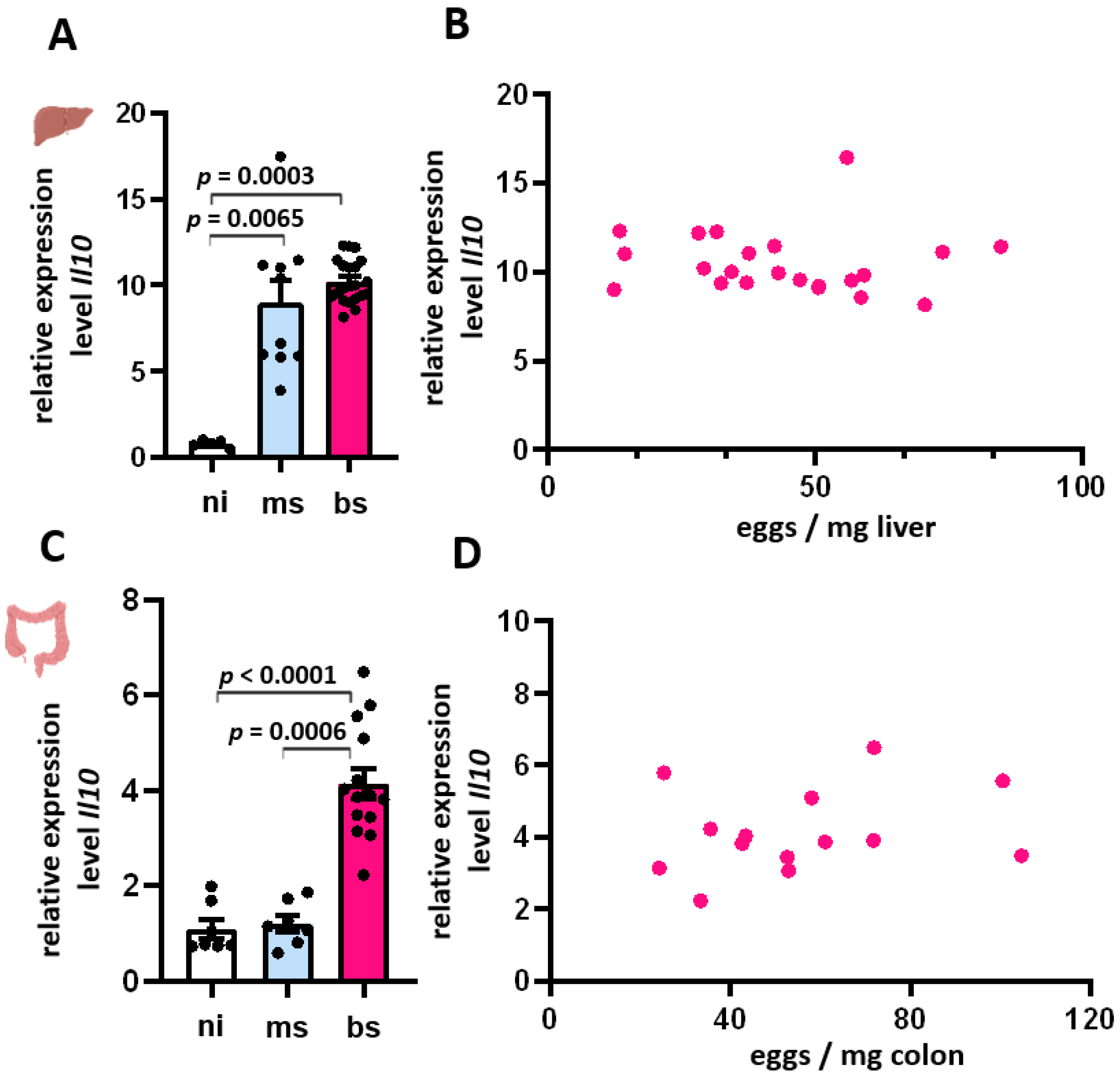
3.6. Expression of Il-10 in Liver and Colon Was Independent of the Egg Burden
4. Discussion
4.1. Egg Load-Independent Th1 Immune Response
4.2. Induction of the Th2 Immune Response in Bs-Infection in S. mansoni
4.3. Reduction of the Th2 Immune Response with Increasing Egg Load
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Bottazzi, M.E.; Strych, U.; Chang, L.-Y.; Lim, Y.A.L.; Goodenow, M.M.; AbuBakar, S. Neglected tropical diseases among the Association of Southeast Asian Nations (ASEAN): Overview and update. PLoS Negl. Trop. Dis. 2015, 9, e0003575. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, E.; Midiri, A.; Manno, A.; Pastorello, M.; Biondo, C.; Mancuso, G. Insights into the epidemiology, pathogenesis, and differential diagnosis of schistosomiasis. Eur. J. Microbiol. Immunol. 2024, 14, 86–96. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Requena-Méndez, A.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248. [Google Scholar] [CrossRef]
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Stensgaard, A.-S.; Vounatsou, P.; Sengupta, M.E.; Utzinger, J. Schistosomes, snails and climate change: Current trends and future expectations. Acta Trop. 2019, 190, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Bergquist, R. Potential Impact of Climate Change on Schistosomiasis: A Global Assessment Attempt. Trop. Med. Infect. Dis. 2018, 3. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Communicable Disease Threats Report, 5–11 July 2015, Week 28. 2015. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-5-11-july-2015-week-28 (accessed on 15 April 2024).
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis-from immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef]
- Schwartz, C.; Fallon, P.G. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Front. Immunol. 2018, 9, 2492. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Abdul-Ghani, R.A.; Hassan, A.A. Murine schistosomiasis as a model for human schistosomiasis mansoni: Similarities and discrepancies. Parasitol. Res. 2010, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deslyper, G.; Doherty, D.G.; Carolan, J.C.; Holland, C.V. The role of the liver in the migration of parasites of global significance. Parasites Vectors 2019, 12, 531. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, C.; Akarid, K.; Chau, F.; Sinet, M.; Verola, O.; Carbon, C.; Derouin, F. The Th1 to Th2 shift induced by Schistosoma mansoni does not exacerbate murine aids (MAIDS). Parasite Immunol. 1998, 20, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Schramm, G.; Haas, H. Th2 immune response against Schistosoma mansoni infection. Microbes Infect. 2010, 12, 881–888. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, V.; Lichtenberger, J.; Grevelding, C.G.; Falcone, F.H.; Roeb, E.; Roderfeld, M. Does Schistosoma mansoni Facilitate Carcinogenesis? Cells 2021, 10, 1982. [Google Scholar] [CrossRef]
- Zaghloul, M.S.; Zaghloul, T.M.; Bishr, M.K.; Baumann, B.C. Urinary schistosomiasis and the associated bladder cancer: Update. J. Egypt. Natl. Canc. Inst. 2020, 32, 44. [Google Scholar] [CrossRef]
- Sombetzki, M.; Rabes, A.; Bischofsberger, M.; Winkelmann, F.; Koslowski, N.; Schulz, C.; Reisinger, E.C. Preventive CTLA-4-Ig Treatment Reduces Hepatic Egg Load and Hepatic Fibrosis in Schistosoma mansoni-Infected Mice. BioMed Res. Int. 2019, 2019, 1704238. [Google Scholar] [CrossRef]
- Schramm, G.; Hamilton, J.V.; Balog, C.I.A.; Wuhrer, M.; Gronow, A.; Beckmann, S.; Wippersteg, V.; Grevelding, C.G.; Goldmann, T.; Weber, E.; et al. Molecular characterisation of kappa-5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol. Biochem. Parasitol. 2009, 166, 4–14. [Google Scholar] [CrossRef]
- Grevelding, C.G. Genomic instability in Schistosoma mansoni. Mol. Biochem. Parasitol. 1999, 101, 207–216. [Google Scholar] [CrossRef]
- Dettman, C.D.; Higgins-Opitz, S.B.; Saikoolal, A. Enhanced efficacy of the paddling method for schistosome infection of rodents by a four-step pre-soaking procedure. Parasitol. Res. 1989, 76, 183–184. [Google Scholar] [CrossRef]
- Lu, Z.; Sessler, F.; Holroyd, N.; Hahnel, S.; Quack, T.; Berriman, M.; Grevelding, C.G. Schistosome sex matters: A deep view into gonad-specific and pairing-dependent transcriptomes reveals a complex gender interplay. Sci. Rep. 2016, 6, 31150. [Google Scholar] [CrossRef] [PubMed]
- Roderfeld, M.; Rath, T.; Pasupuleti, S.; Zimmermann, M.; Neumann, C.; Churin, Y.; Dierkes, C.; Voswinckel, R.; Barth, P.J.; Zahner, D.; et al. Bone marrow transplantation improves hepatic fibrosis in Abcb4-/- mice via Th1 response and matrix metalloproteinase activity. Gut 2012, 61, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Miao, J.; Wang, J.; Robl, N.; Guo, H.; Song, S.; Peng, Y.; Wang, Y.; Huang, S.; Li, X. Validation of Stable Housekeeping Genes for Quantitative Real Time PCR in Golden Syrian Hamster. Indian J. Anim. Res. 2020, 55, 1127–1131. [Google Scholar] [CrossRef]
- Congiu, M.; Slavin, J.L.; Desmond, P.V. Expression of common housekeeping genes is affected by disease in human hepatitis C virus-infected liver. Liver Int. 2011, 31, 386–390. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Cheever, A.W. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull. World Health Organ. 1968, 39, 328–331. [Google Scholar]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Mathieson, W.; Wilson, R.A. A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: The miracidium, hatch fluid and secretions. Int. J. Parasitol. 2010, 40, 617–628. [Google Scholar] [CrossRef]
- Everts, B.; Perona-Wright, G.; Smits, H.H.; Hokke, C.H.; van der Ham, A.J.; Fitzsimmons, C.M.; Doenhoff, M.J.; van der Bosch, J.; Mohrs, K.; Haas, H.; et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 2009, 206, 1673–1680. [Google Scholar] [CrossRef]
- Carson, J.P.; Gobert, G.N. Modulation of the Host Immune Response by Schistosome Egg-Secreted Proteins Is a Critical Avenue of Host-Parasite Communication. Pathogens 2021, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, V.; Gindner, S.; Baier, A.; Hehr, L.; Buss, N.; Russ, L.; Wrobel, S.; Wirth, V.; Tabatabai, K.; Quack, T.; et al. Metabolic reprogramming of hepatocytes by Schistosoma mansoni eggs. JHEP Rep. 2023, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.B.; Senger, M.R.; Vasques, L.M.; Gasparotto, J.; dos Santos, J.P.A.; Pasquali, M.A.d.B.; Moreira, J.C.F.; Silva, F.P.; Gelain, D.P. Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation endproduct (RAGE) and tau levels in multiple organs in mice. Int. J. Parasitol. 2013, 43, 371–379. [Google Scholar] [CrossRef]
- Dunne, D.W.; Jones, F.M.; Doenhoff, M.J. The purification, characterization, serological activity and hepatotoxic properties of two cationic glycoproteins (alpha 1 and omega 1) from Schistosoma mansoni eggs. Parasitology 1991, 103 Pt 2, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.-H.; Lim, K.-C.; McKerrow, J.H.; Caffrey, C.R. Proteomic identification of IPSE/alpha-1 as a major hepatotoxin secreted by Schistosoma mansoni eggs. PLoS Negl. Trop. Dis. 2011, 5, e1368. [Google Scholar] [CrossRef]
- McKenzie, G.J.; Fallon, P.G.; Emson, C.L.; Grencis, R.K.; McKenzie, A.N. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 1999, 189, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhang, J.; Chen, H.; Nie, H.; Miller, H.; Gong, Q.; Liu, C. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front. Immunol. 2020, 11, 61. [Google Scholar] [CrossRef]
- Acharya, S.; Da’dara, A.A.; Skelly, P.J. Schistosome immunomodulators. PLoS Pathog. 2021, 17, e1010064. [Google Scholar] [CrossRef]
- de Oliveira Fraga, L.A.; Torrero, M.N.; Tocheva, A.S.; Mitre, E.; Davies, S.J. Induction of type 2 responses by schistosome worms during prepatent infection. J. Infect. Dis. 2010, 201, 464–472. [Google Scholar] [CrossRef]
- Leptak, C.L.; McKerrow, J.H. Schistosome egg granulomas and hepatic expression of TNF-alpha are dependent on immune priming during parasite maturation. J. Immunol. 1997, 158, 301–307. [Google Scholar] [CrossRef]
- Jenkins, S.J.; Mountford, A.P. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect. Immun. 2005, 73, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G.; Richardson, E.J.; McKenzie, G.J.; McKenzie, A.N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 2000, 164, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and pathogenic Th2 cells in inflammation, tissue repair, and fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, Z. Liver-Mediated Adaptive Immune Tolerance. Front. Immunol. 2019, 10, 2525. [Google Scholar] [CrossRef]
- Fallon, P.G. Immunopathology of schistosomiasis: A cautionary tale of mice and men. Immunol. Today 2000, 21, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Peterková, K.; Konečný, L.; Macháček, T.; Jedličková, L.; Winkelmann, F.; Sombetzki, M.; Dvořák, J. Winners vs. losers: Schistosoma mansoni intestinal and liver eggs exhibit striking differences in gene expression and immunogenicity. PLoS Pathog. 2024, 20, e1012268. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.I.; Lopes, A.A.; Medeiros, M.; Cruz, A.A.; Sousa-Atta, L.; Solé, D.; Carvalho, E.M. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 2000, 123, 145–148. [Google Scholar] [CrossRef]
- Nyan, O.A.; Walraven, G.E.; Banya, W.A.; Milligan, P.; van der Sande, M.; Ceesay, S.M.; Del Prete, G.; McAdam, K.P. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin. Exp. Allergy 2001, 31, 1672–1678. [Google Scholar] [CrossRef]
- Mueller, S.N.; Ahmed, R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2009, 106, 8623–8628. [Google Scholar] [CrossRef]
- Lundy, S.K.; Lerman, S.P.; Boros, D.L. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL(+) T and B cells during murine Schistosoma mansoni infection. Infect. Immun. 2001, 69, 271–280. [Google Scholar] [CrossRef]
- Cheever, A.W.; Macedonia, J.G.; Deb, S.; Cheever, E.A.; Mosimann, J.E. Persistence of eggs and hepatic fibrosis after treatment of Schistosoma mansoni-infected mice. Am. J. Trop. Med. Hyg. 1992, 46, 752–758. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russ, L.; von Bülow, V.; Wrobel, S.; Stettler, F.; Schramm, G.; Falcone, F.H.; Grevelding, C.G.; Roderfeld, M.; Roeb, E. Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in S. mansoni-Infected Hamsters. Cells 2024, 13, 1579. https://doi.org/10.3390/cells13181579
Russ L, von Bülow V, Wrobel S, Stettler F, Schramm G, Falcone FH, Grevelding CG, Roderfeld M, Roeb E. Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in S. mansoni-Infected Hamsters. Cells. 2024; 13(18):1579. https://doi.org/10.3390/cells13181579
Chicago/Turabian StyleRuss, Lena, Verena von Bülow, Sarah Wrobel, Frederik Stettler, Gabriele Schramm, Franco H. Falcone, Christoph G. Grevelding, Martin Roderfeld, and Elke Roeb. 2024. "Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in S. mansoni-Infected Hamsters" Cells 13, no. 18: 1579. https://doi.org/10.3390/cells13181579
APA StyleRuss, L., von Bülow, V., Wrobel, S., Stettler, F., Schramm, G., Falcone, F. H., Grevelding, C. G., Roderfeld, M., & Roeb, E. (2024). Inverse Correlation of Th2-Specific Cytokines with Hepatic Egg Burden in S. mansoni-Infected Hamsters. Cells, 13(18), 1579. https://doi.org/10.3390/cells13181579





