Microgravity Effect on Pancreatic Islets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Culture of Pancreatic Islets (PIs) and Boundary Cap Neural Crest Stem Cells (BCs)
2.2. Three-Dimensional Printing of Pancreatic Islets (PIs) and Boundary Cap Neural Crest Stem Cells (BCs)
2.3. Preparation and Loading Pancreatic Islets and Boundary Cap Neural Crest Stem Cells into Sounding Rocket
2.4. Post-Flight Processing of Pancreatic Islets (PIs) and Boundary Cap Neural Crest Stem Cells (BCs)
2.5. Image Analysis
2.6. ELISA
2.7. Statistical Analysis
3. Results
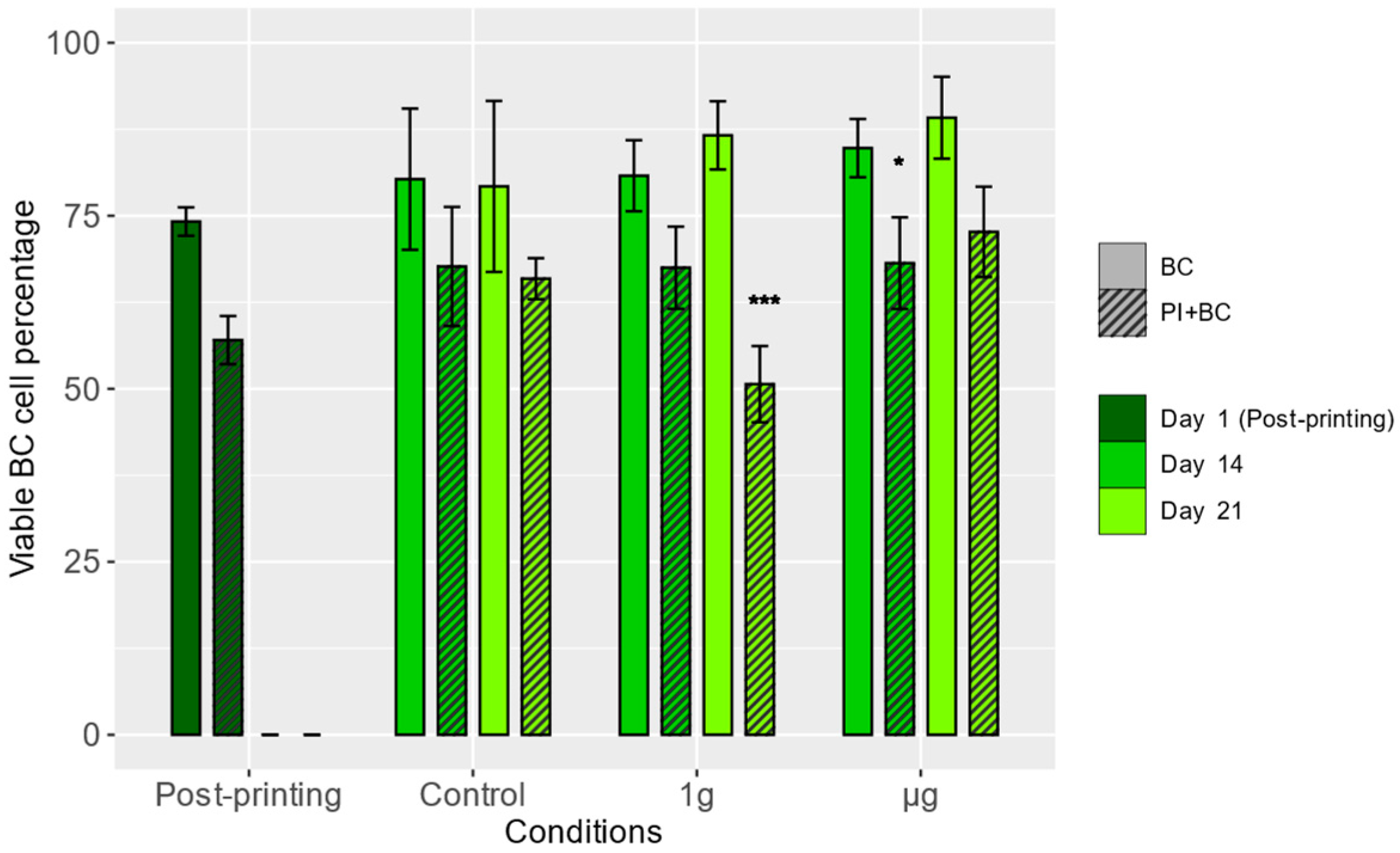
3.1. Viability of Boundary Cap Neural Crest Stem Cells (BCs)
3.2. Proliferation of Boundary Cap Neural Crest Stem Cells (BCs)
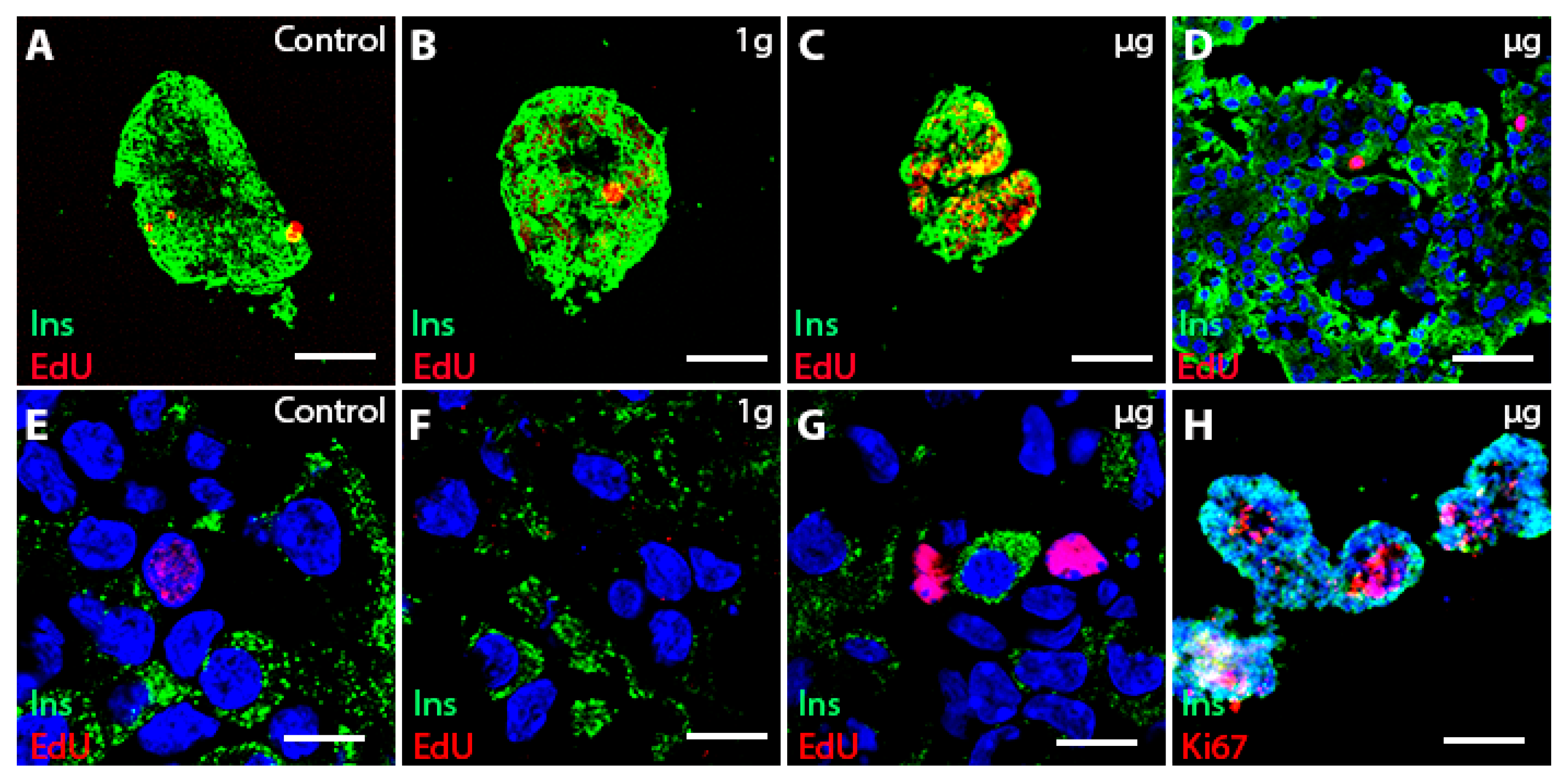
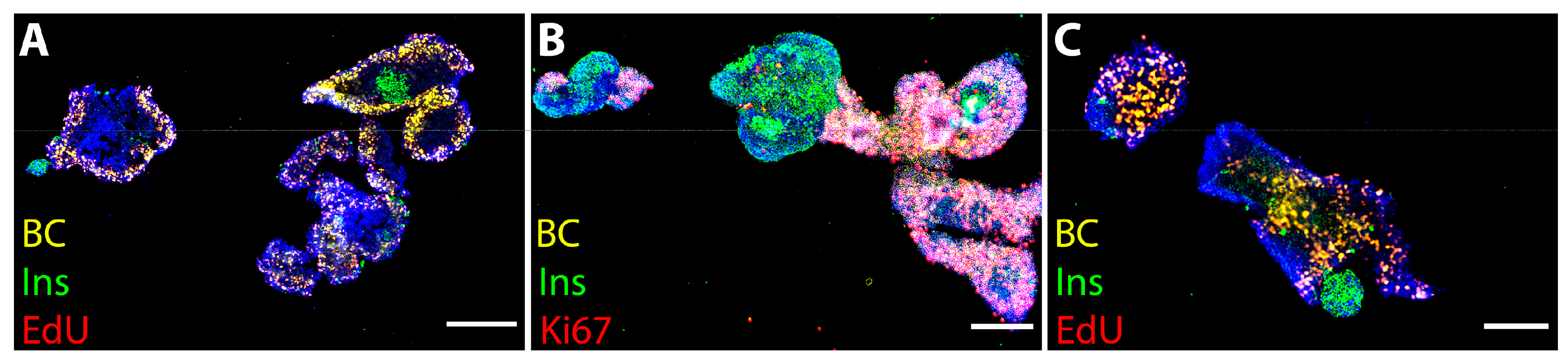
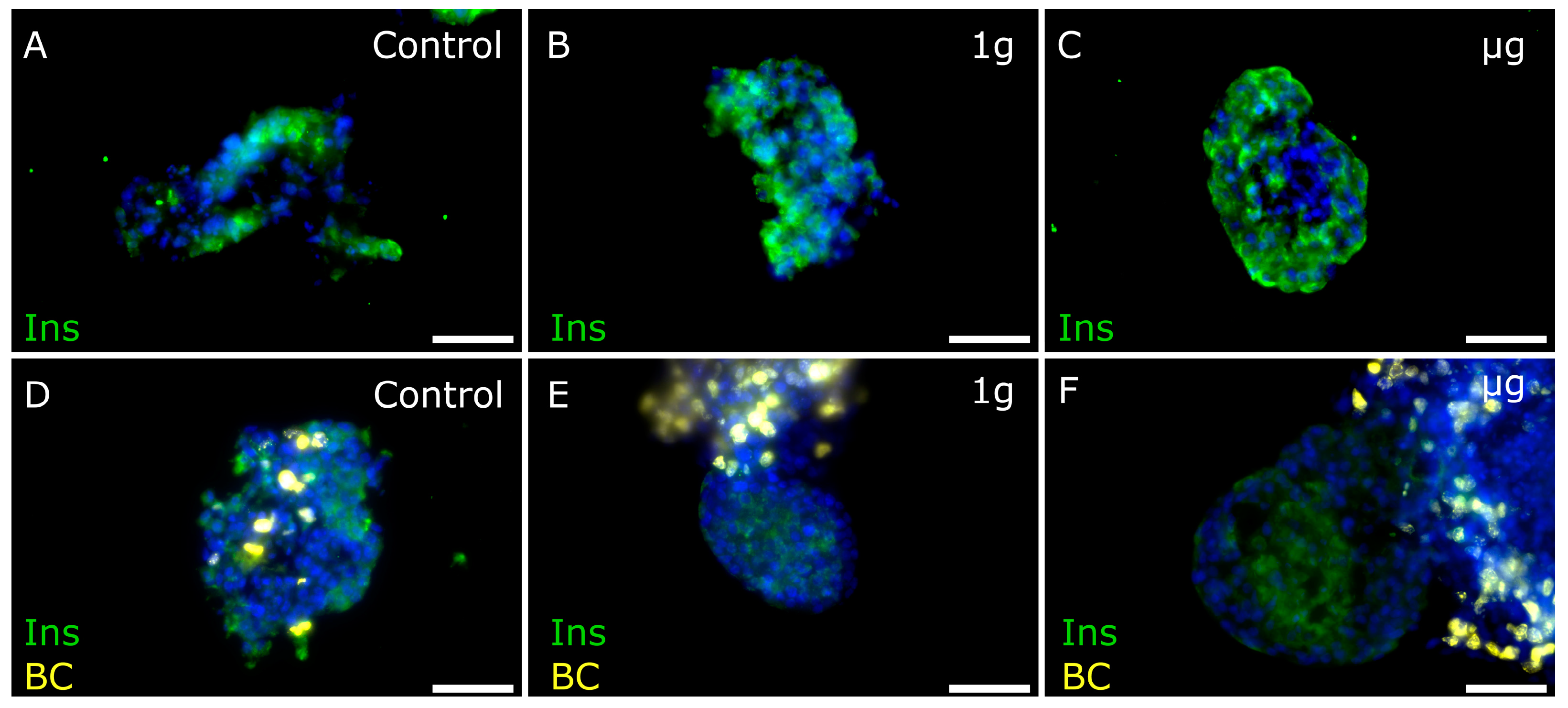
3.3. Islet Cell Proliferation in Free-Floating Culture after µg Exposure
3.4. Cell Proliferation Is Increased in 3D-Printed Cultures Two–Three Weeks after µg Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Mandrup-Poulsen, T. A Choice of Death—The Signal-Transduction of Immune-Mediated Beta-Cell Apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Imagawa, A.; Okita, K.; Sayama, K.; Moriwaki, M.; Iwahashi, H.; Yamagata, K.; Tamura, S.; Matsuzawa, Y.; Hanafusa, T.; et al. Macrophages and Dendritic Cells Infiltrating Islets with or without Beta Cells Produce Tumour Necrosis Factor-α in Patients with Recent-Onset Type 1 Diabetes. Diabetologia 2007, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Docherty, F.M.; Sussel, L. Islet Regeneration: Endogenous and Exogenous Approaches. Int. J. Mol. Sci. 2021, 22, 3306. [Google Scholar] [CrossRef] [PubMed]
- Plank, J.L.; Mundell, N.A.; Frist, A.Y.; LeGrone, A.W.; Kim, T.; Musser, M.A.; Walter, T.J.; Labosky, P.A. Influence and Timing of Arrival of Murine Neural Crest on Pancreatic Beta Cell Development and Maturation. Dev. Biol. 2011, 349, 321–330. [Google Scholar] [CrossRef]
- Grouwels, G.; Vasylovska, S.; Olerud, J.; Leuckx, G.; Ngamjariyawat, A.; Yuchi, Y.; Jansson, L.; Van de Casteele, M.; Kozlova, E.N.; Heimberg, H. Differentiating Neural Crest Stem Cells Induce Proliferation of Cultured Rodent Islet Beta Cells. Diabetologia 2012, 55, 2016–2025. [Google Scholar] [CrossRef]
- Maro, G.S.; Vermeren, M.; Voiculescu, O.; Melton, L.; Cohen, J.; Charnay, P.; Topilko, P. Neural Crest Boundary Cap Cells Constitute a Source of Neuronal and Glial Cells of the PNS. Nat. Neurosci. 2004, 7, 930–938. [Google Scholar] [CrossRef]
- Hjerling-Leffler, J.; Marmigère, F.; Heglind, M.; Cederberg, A.; Koltzenburg, M.; Enerbäck, S.; Ernfors, P. The Boundary Cap: A Source of Neural Crest Stem Cells That Generate Multiple Sensory Neuron Subtypes. Development 2005, 132, 2623–2632. [Google Scholar] [CrossRef]
- Aquino, J.B.; Hjerling-Leffler, J.; Koltzenburg, M.; Edlund, T.; Villar, M.J.; Ernfors, P. In Vitro and in Vivo Differentiation of Boundary Cap Neural Crest Stem Cells into Mature Schwann Cells. Exp. Neurol. 2006, 198, 438–449. [Google Scholar] [CrossRef]
- Zujovic, V.; Thibaud, J.; Bachelin, C.; Vidal, M.; Coulpier, F.; Charnay, P.; Topilko, P.; Evercooren, A.B.-V. Boundary Cap Cells Are Highly Competitive for CNS Remyelination: Fast Migration and Efficient Differentiation in PNS and CNS Myelin-Forming Cells. Stem Cells 2010, 28, 470–479. [Google Scholar] [CrossRef]
- Zujovic, V.; Thibaud, J.; Bachelin, C.; Vidal, M.; Deboux, C.; Coulpier, F.; Stadler, N.; Charnay, P.; Topilko, P.; Baron-Van Evercooren, A. Boundary Cap Cells Are Peripheral Nervous System Stem Cells That Can Be Redirected into Central Nervous System Lineages. Proc. Natl. Acad. Sci. USA 2011, 108, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Trolle, C.; Konig, N.; Abrahamsson, N.; Vasylovska, S.; Kozlova, E.N. Boundary Cap Neural Crest Stem Cells Homotopically Implanted to the Injured Dorsal Root Transitional Zone Give Rise to Different Types of Neurons and Glia in Adult Rodents. BMC Neurosci. 2014, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Radomska, K.J.; Topilko, P. Boundary Cap Cells in Development and Disease. Curr. Opin. Neurobiol. 2017, 47, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Olerud, J.; Kanaykina, N.; Vasilovska, S.; King, D.; Sandberg, M.; Jansson, L.; Kozlova, E.N. Neural Crest Stem Cells Increase Beta Cell Proliferation and Improve Islet Function in Co-Transplanted Murine Pancreatic Islets. Diabetologia 2009, 52, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Grapensparr, L.; Vasylovska, S.; Li, Z.; Olerud, J.; Jansson, L.; Kozlova, E.; Carlsson, P.-O. Co-Transplantation of Human Pancreatic Islets With Post-Migratory Neural Crest Stem Cells Increases β-Cell Proliferation and Vascular And Neural Regrowth. J. Clin. Endocrinol. Metab. 2015, 100, E583–E590. [Google Scholar] [CrossRef]
- Aggarwal, T.; Hoeber, J.; Ivert, P.; Vasylovska, S.; Kozlova, E.N. Boundary Cap Neural Crest Stem Cells Promote Survival of Mutant SOD1 Motor Neurons. Neurotherapeutics 2017, 14, 773–783. [Google Scholar] [CrossRef]
- Lau, J.; Vasylovska, S.; Kozlova, E.N.; Carlsson, P.-O. Surface Coating of Pancreatic Islets with Neural Crest Stem Cells Improves Engraftment and Function after Intraportal Transplantation. Cell Transpl. 2015, 24, 2263–2272. [Google Scholar] [CrossRef]
- Trolle, C.; Han, Y.; Mutt, S.J.; Christoffersson, G.; Kozlova, E.N. Boundary Cap Neural Crest Stem Cells Promote Angiogenesis after Transplantation to Avulsed Dorsal Roots in Mice and Induce Migration of Endothelial Cells in 3D Printed Scaffolds. Neurosci. Lett. 2024, 826, 137724. [Google Scholar] [CrossRef]
- Han, Y.; Barasa, P.; Zeger, L.; Salomonsson, S.B.; Zanotti, F.; Egli, M.; Zavan, B.; Trentini, M.; Florin, G.; Vaerneus, A.; et al. Effects of Microgravity on Neural Crest Stem Cells. Front. Neurosci. 2024, 18, 1379076. [Google Scholar] [CrossRef]
- Huang, H.-H.; Farmer, K.; Windscheffel, J.; Yost, K.; Power, M.; Wright, D.E.; Stehno-Bittel, L. Exercise Increases Insulin Content and Basal Secretion in Pancreatic Islets in Type 1 Diabetic Mice. J. Diabetes Res. 2011, 2011, 481427. [Google Scholar] [CrossRef]
- Webb, M.A.; Platton, S.L.; Dennison, A.R.; James, R.F.L. Immunohistochemical Evidence That Culture in the High Aspect Rotating Vessel Can Up-Regulate Hormone Expression in Growth Dedifferentiated PHHI-Derived Islet Cells. Vitr. Cell. Dev. Biol. Anim. 2007, 43, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tanaka, S.; Sekine, K.; Kita, S.; Okamura, A.; Takebe, T.; Zheng, Y.-W.; Ueno, Y.; Tanaka, J.; Taniguchi, H. The Generation of Pancreatic β-Cell Spheroids in a Simulated Microgravity Culture System. Biomaterials 2013, 34, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Luca, G.; Calvitti, M.; Nastruzzi, C.; Macchiarulo, G.; Becchetti, E.; Neri, L.M.; Capitani, S.; Basta, G.; Brunetti, P.; Calafiore, R.; et al. Effects of Simulated Microgravity on the Morphology and Function of Neonatal Porcine Cell Clusters Cultured with and without Sertoli Cells. Cell Transpl. 2006, 15, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hostens, K.; Ling, Z.; Van Schravendijk, C.; Pipeleers, D. Prolonged Exposure of Human β-Cells to High Glucose Increases Their Release of Proinsulin during Acute Stimulation with Glucose or Arginine1. J. Clin. Endocrinol. Metab. 1999, 84, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Salg, G.A.; Poisel, E.; Neulinger-Munoz, M.; Gerhardus, J.; Cebulla, D.; Bludszuweit-Philipp, C.; Vieira, V.; Nickel, F.; Herr, I.; Blaeser, A. Toward 3D-Bioprinting of an Endocrine Pancreas: A Building-Block Concept for Bioartificial Insulin-Secreting Tissue. J. Tissue Eng. 2022, 13, 20417314221091033. [Google Scholar] [CrossRef]
- Tobin, B.W.; Leeper-Woodford, S.K.; Hashemi, B.B.; Smith, S.M.; Sams, C.F. Altered TNF-α, Glucose, Insulin, and Amino Acids in Islets of Langerhans Cultured in a Microgravity Model System. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E92–E102. [Google Scholar] [CrossRef]
- Tobin, B.W.; Uchakin, P.N.; Leeper-Woodford, S.K. Insulin Secretion and Sensitivity in Space Flight: Diabetogenic Effects. Nutrition 2002, 18, 842–848. [Google Scholar] [CrossRef]
- Chun Song; Xiu-Qing Duan; Xi Li; Li-Ou Han; Ping Xu; Chun-Fang Song; Lian-Hong Jin Experimental Study of Rat Beta Islet Cells Cultured under Simulated Microgravity Conditions. Acta Biochim. Et Biophys. Sin. 2004, 36, 47–50. [CrossRef]
- Song, Y.; Wei, Z.; Song, C.; Xie, S.; Feng, J.; Fan, J.; Zhang, Z.; Shi, Y. Simulated Microgravity Combined with Polyglycolic Acid Scaffold Culture Conditions Improves the Function of Pancreatic Islets. BioMed Res. Int. 2013, 2013, 150739. [Google Scholar] [CrossRef]
- Rutzky, L.P.; Bilinski, S.; Kloc, M.; Phan, T.; Zhang, H.; Katz, S.M.; Stepkowski, S.M. Microgravity Culture Condition Reduces Immunogenicity and Improves Function of Pancreatic Islets1. Transplantation 2002, 74, 13–21. [Google Scholar] [CrossRef]
- Hou, Y.; Song, C.; Xie, W.J.; Wei, Z.; Huang, R.P.; Liu, W.; Zhang, Z.L.; Shi, Y.B. Excellent Effect of Three-Dimensional Culture Condition on Pancreatic Islets. Diabetes Res. Clin. Pract. 2009, 86, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Lin, H.; Cheng, Y.-C.; Yen, C.-H.; Huang, R.-N.; Lin, K.-H. Beta-Adrenoceptor Pathway Enhances Mitochondrial Function in Human Neural Stem Cells via Rotary Cell Culture System. J. Neurosci. Methods 2012, 207, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Brungs, S.; Henry, M.; Rotshteyn, T.; Singh Yaduvanshi, N.; Wegener, L.; Jentzsch, S.; Hescheler, J.; Hemmersbach, R.; Boeuf, H. Modulation of Differentiation Processes in Murine Embryonic Stem Cells Exposed to Parabolic Flight-Induced Acute Hypergravity and Microgravity. Stem Cells Dev. 2018, 27, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chiu, S.-L.; Liu, B.; Wu, P.-H.; Delannoy, M.; Lin, D.-T.; Wirtz, D.; Huganir, R.L. Differential Vesicular Sorting of AMPA and GABAA Receptors. Proc. Natl. Acad. Sci. USA 2016, 113, E922–E931. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zeger, L.; Tripathi, R.; Egli, M.; Ille, F.; Lockowandt, C.; Florin, G.; Atic, E.; Redwan, I.N.; Fredriksson, R.; et al. Molecular Genetic Analysis of Neural Stem Cells after Space Flight and Simulated Microgravity on Earth. Biotechnol. Bioeng. 2021, 118, 3832–3846. [Google Scholar] [CrossRef]
- Taguchi, M.; Yoshimoto, S.; Suyama, K.; Sumi, S.; Ohki, S.; Ogata, K.; Fujimoto, R.; Murata, D.; Nakayama, K.; Oka, K. Creating 3D Constructs with Cranial Neural Crest-Derived Cell Lines Using a Bio-3D Printer. J. Oral Biosci. 2024, 66, 339–348. [Google Scholar] [CrossRef]
- Tattikota, S.G.; Sury, M.D.; Rathjen, T.; Wessels, H.-H.; Pandey, A.K.; You, X.; Becker, C.; Chen, W.; Selbach, M.; Poy, M.N. Argonaute2 Regulates the Pancreatic β-Cell Secretome. Mol. Cell. Proteom. 2013, 12, 1214–1225. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeger, L.; Barasa, P.; Han, Y.; Hellgren, J.; Redwan, I.N.; Reiche, M.E.; Florin, G.; Christoffersson, G.; Kozlova, E.N. Microgravity Effect on Pancreatic Islets. Cells 2024, 13, 1588. https://doi.org/10.3390/cells13181588
Zeger L, Barasa P, Han Y, Hellgren J, Redwan IN, Reiche ME, Florin G, Christoffersson G, Kozlova EN. Microgravity Effect on Pancreatic Islets. Cells. 2024; 13(18):1588. https://doi.org/10.3390/cells13181588
Chicago/Turabian StyleZeger, Lukas, Povilas Barasa, Yilin Han, Josefin Hellgren, Itedale Namro Redwan, Myrthe E. Reiche, Gunnar Florin, Gustaf Christoffersson, and Elena N. Kozlova. 2024. "Microgravity Effect on Pancreatic Islets" Cells 13, no. 18: 1588. https://doi.org/10.3390/cells13181588
APA StyleZeger, L., Barasa, P., Han, Y., Hellgren, J., Redwan, I. N., Reiche, M. E., Florin, G., Christoffersson, G., & Kozlova, E. N. (2024). Microgravity Effect on Pancreatic Islets. Cells, 13(18), 1588. https://doi.org/10.3390/cells13181588





