Liver Fibrosis Is Enhanced by a Higher Egg Burden in Younger Mice Infected with S. mansoni
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Material
2.2. Animal Model
2.3. Histologic Staining and Immunohistochemistry
2.4. Assessment of Granuloma Extent and Fibrotic Areas
2.5. Quantitative Real-Time PCR
2.6. Serum Analysis, Hydroxyproline Assay, and Potassium-Hydroxide Digestion
2.7. Cell Culture
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
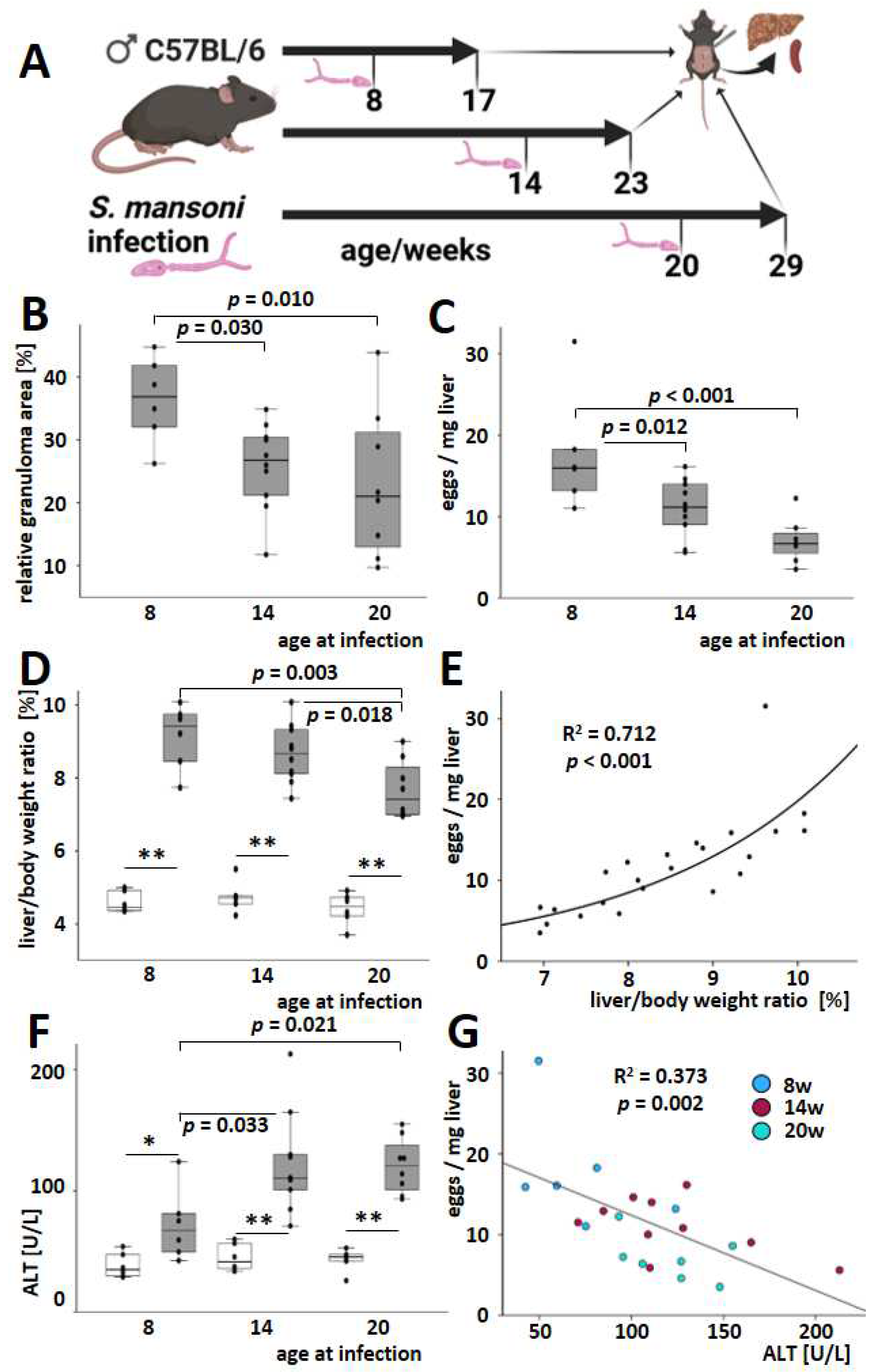
3.1. An Older Age at the Time of Infection Reduced Hepatic Egg Load, Granuloma Extent, and Liver-to-Body Weight Ratio but Increased Serum ALT
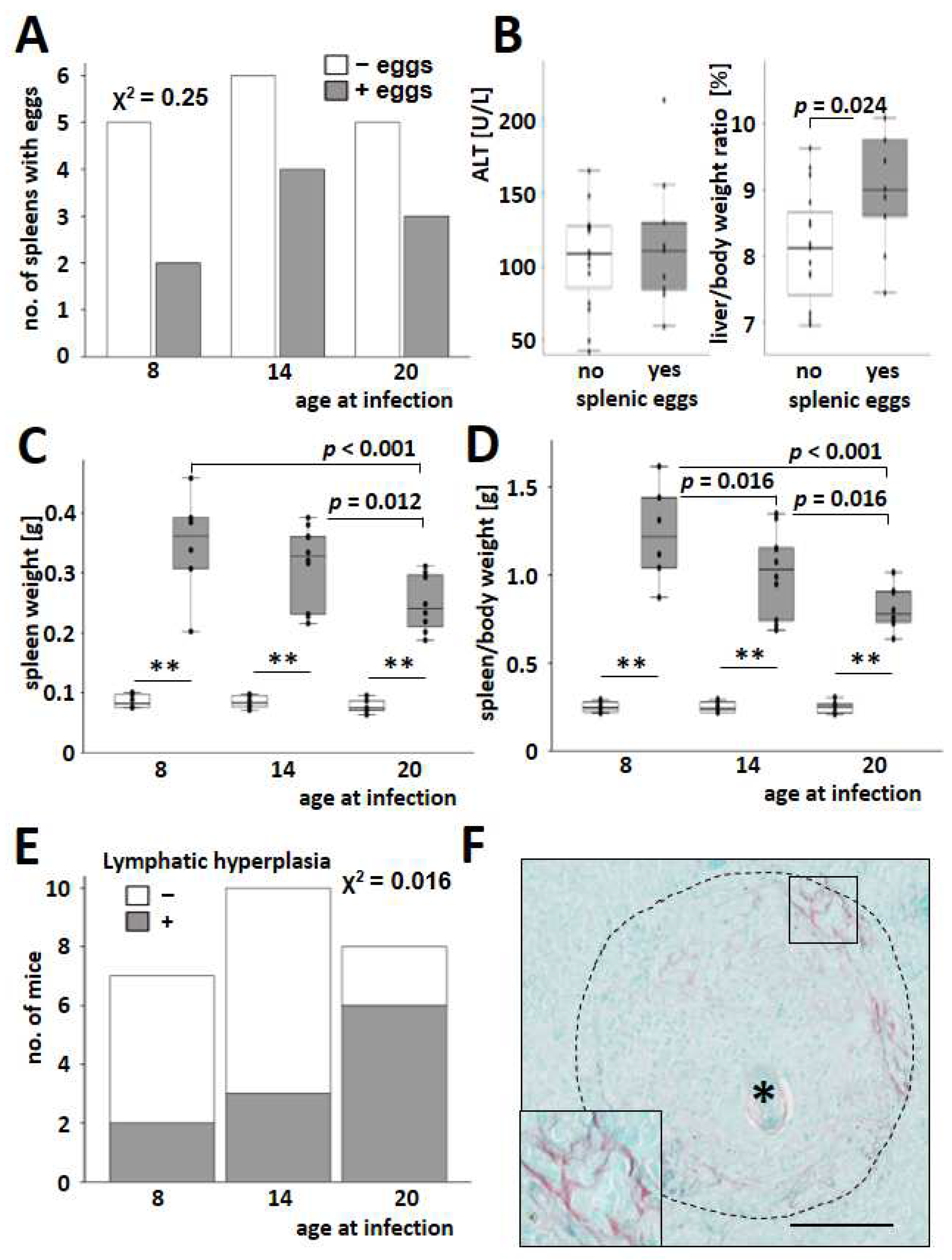
3.2. S. mansoni-Induced Splenomegaly Was More Pronounced in Young Animals
3.3. S. mansoni Induced Granulomatous Hepatic Inflammation Impaired Younger Animals with More Intensity
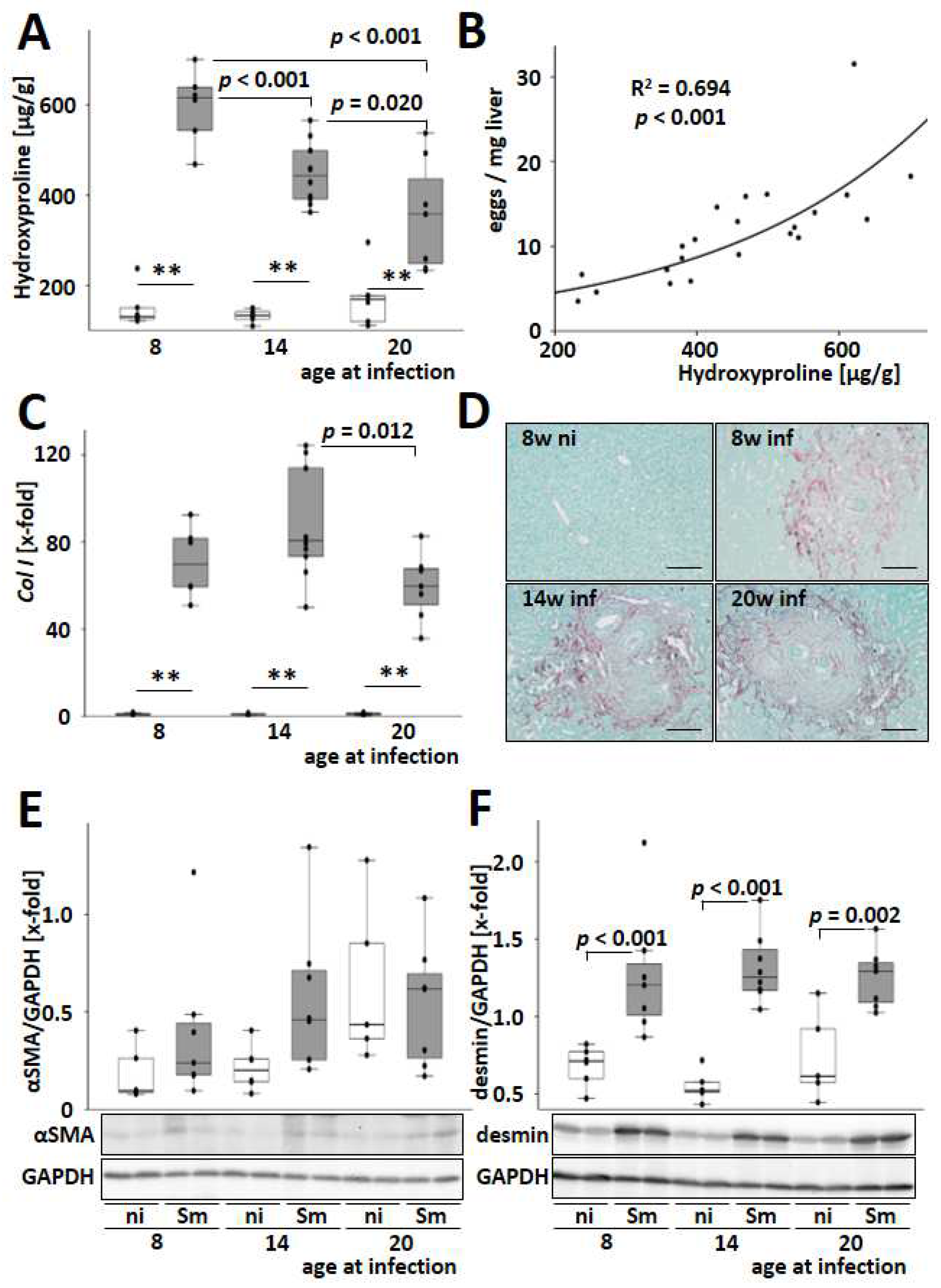
3.4. S. mansoni-Induced Hepatic Fibrosis Is Most Pronounced in Young Infected Animals
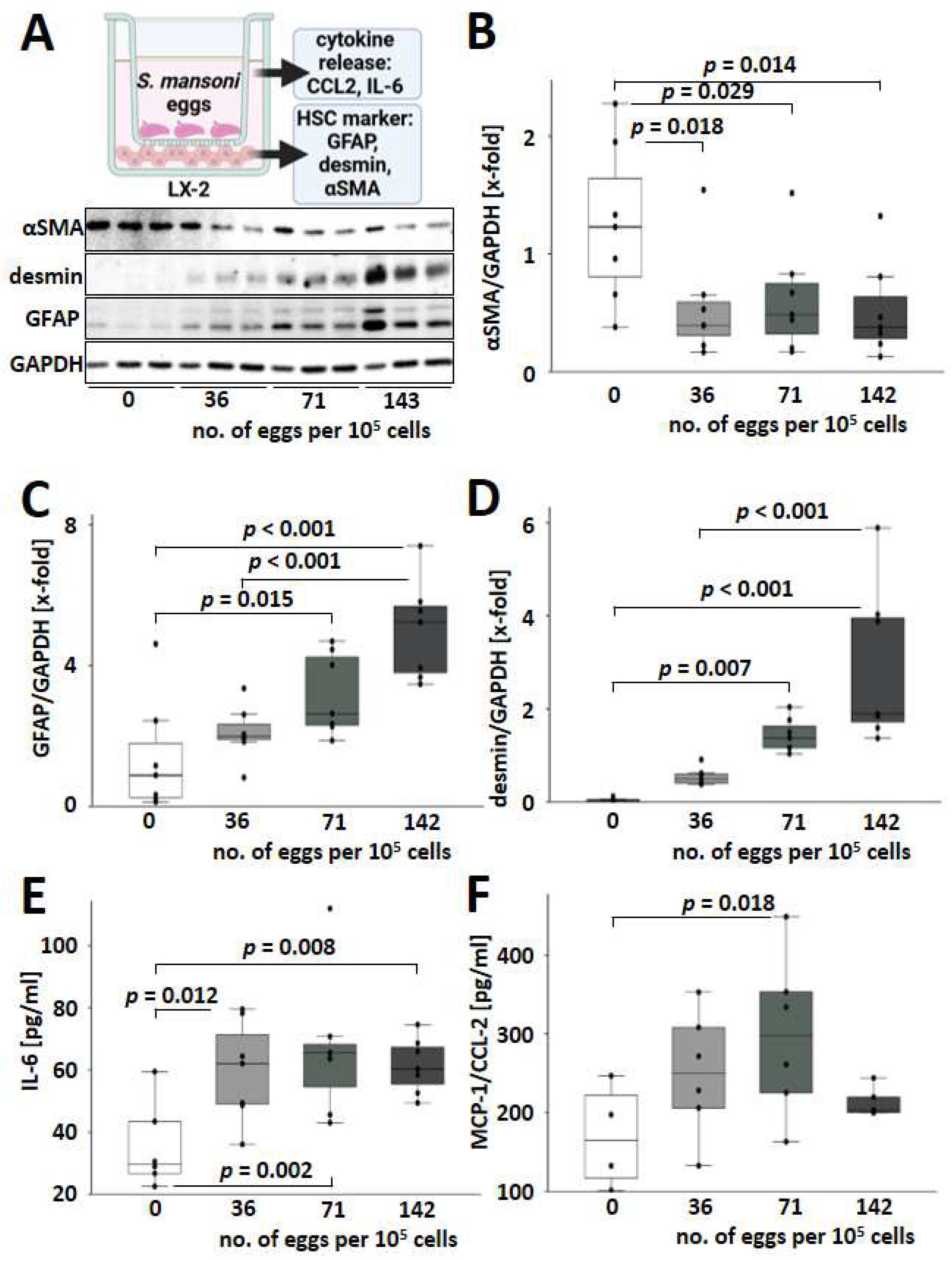
3.5. S. mansoni Egg Activated HSCs towards a GFAP+/desmin+/αSMA− Phenotype Expressing IL-6 and MCP-1
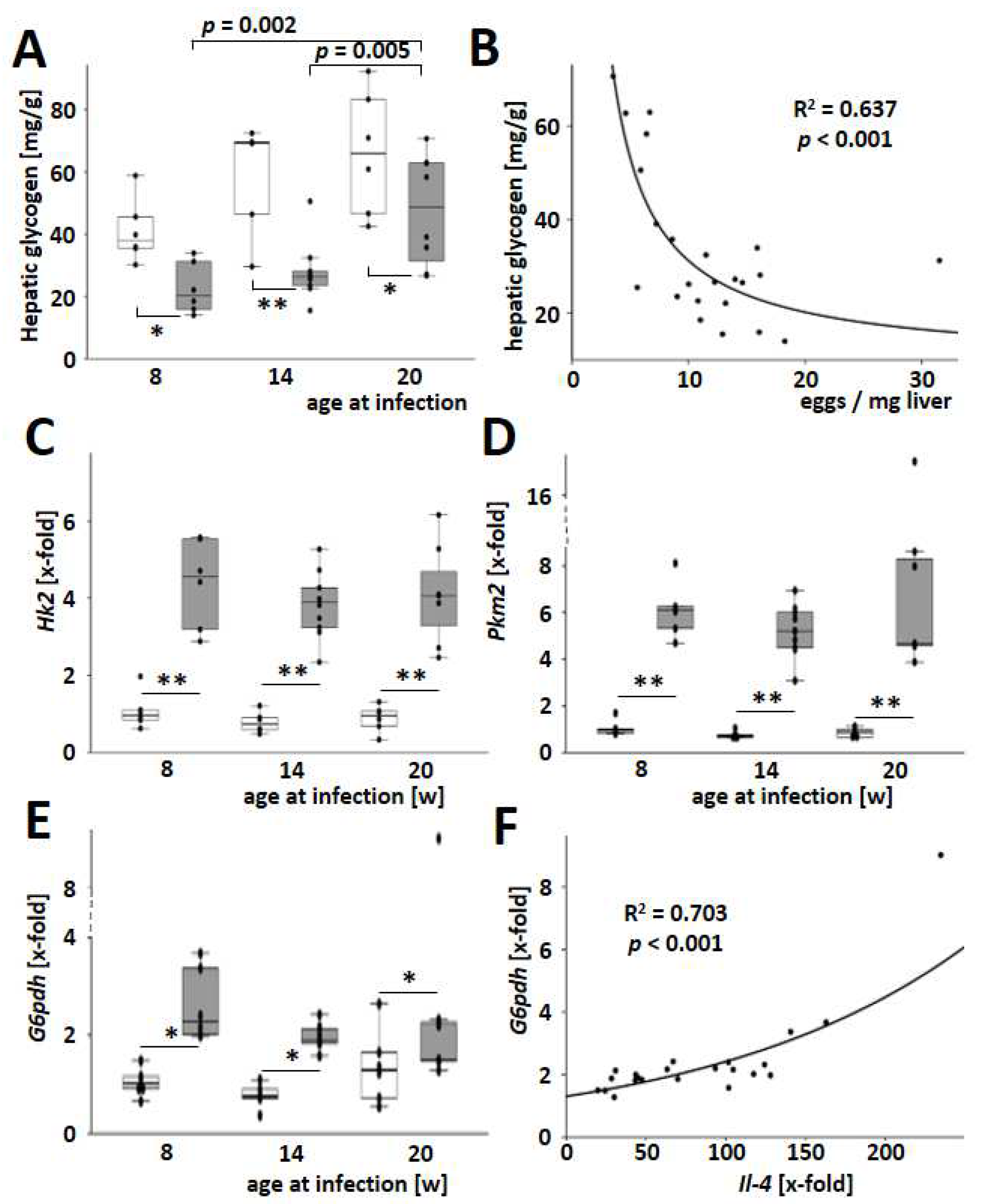
3.6. S. mansoni Egg-Exhausted Glycogen Stores Improved with Increasing Host Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO: Fact-Sheets Schistosomiasis 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 21 March 2024).
- Rothe, C.; Zimmer, T.; Schunk, M.; Wallrauch, C.; Helfrich, K.; Gültekin, F.; Bretzel, G.; Allienne, J.-F.; Boissier, J. Developing Endemicity of Schistosomiasis, Corsica, France. Emerg. Infect. Dis. 2021, 27, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Salas-Coronas, J.; Bargues, M.D.; Lozano-Serrano, A.B.; Artigas, P.; Martínez-Ortí, A.; Mas-Coma, S.; Merino-Salas, S.; Abad Vivas-Pérez, J.I. Evidence of autochthonous transmission of urinary schistosomiasis in Almeria (southeast Spain): An outbreak analysis. Travel Med. Infect. Dis. 2021, 44, 102165. [Google Scholar] [CrossRef] [PubMed]
- von Bülow, V.; Gindner, S.; Baier, A.; Hehr, L.; Buss, N.; Russ, L.; Wrobel, S.; Wirth, V.; Tabatabai, K.; Quack, T.; et al. Metabolic reprogramming of hepatocytes by Schistosoma mansoni eggs. JHEP Rep. 2023, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- Weglage, J.; Wolters, F.; Hehr, L.; Lichtenberger, J.; Wulz, C.; Hempel, F.; Baier, A.; Quack, T.; Köhler, K.; Longerich, T.; et al. Schistosoma mansoni eggs induce Wnt/β-catenin signaling and activate the protooncogene c-Jun in human and hamster colon. Sci. Rep. 2020, 10, 2492. [Google Scholar] [CrossRef]
- Mazigo, H.D.; Dunne, D.W.; Morona, D.; Lutufyo, T.E.; Kinung’hi, S.M.; Kaatano, G.; Nuwaha, F. Periportal fibrosis, liver and spleen sizes among S. mansoni mono or co-infected individuals with human immunodeficiency virus-1 in fishing villages along Lake Victoria shores, North-Western, Tanzania. Parasit. Vectors 2015, 8, 260. [Google Scholar] [CrossRef]
- Fulford, A.J.; Butterworth, A.E.; Sturrock, R.F.; Ouma, J.H. On the use of age-intensity data to detect immunity to parasitic infections, with special reference to Schistosoma mansoni in Kenya. Parasitology 1992, 105, 219–227. [Google Scholar] [CrossRef]
- Woolhouse, M.E. Patterns in parasite epidemiology: The peak shift. Parasitol. Today (Regul. Ed.) 1998, 14, 428–434. [Google Scholar] [CrossRef]
- Freer, J.B.; Bourke, C.D.; Durhuus, G.H.; Kjetland, E.F.; Prendergast, A.J. Schistosomiasis in the first 1000 days. Lancet Infect. Dis. 2018, 18, e193–e203. [Google Scholar] [CrossRef]
- Booth, M.; Mwatha, J.K.; Joseph, S.; Jones, F.M.; Kadzo, H.; Ireri, E.; Kazibwe, F.; Kemijumbi, J.; Kariuki, C.; Kimani, G.; et al. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J. Immunol. 2004, 172, 1295–1303. [Google Scholar] [CrossRef]
- Agnew, A.; Fulford, A.J.; Mwanje, M.T.; Gachuhi, K.; Gutsmann, V.; Krijger, F.W.; Sturrock, R.F.; Vennervald, B.J.; Ouma, J.H.; Butterworth, A.E.; et al. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am. J. Trop. Med. Hyg. 1996, 55, 338–343. [Google Scholar] [CrossRef]
- Cheever, A.W.; Lenzi, J.A.; Lenzi, H.L.; Andrade, Z.A. Experimental models of Schistosoma mansoni infection. Mem. Inst. Oswaldo Cruz 2002, 97, 917–940. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, A.M.; Webbe, G. A study of the death of Schistosoma mansoni cercariae during penetration of mammalian host skin: The influence of the ages of the cercariae and of the host. Int. J. Parasitol. 1973, 3, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Purnell, R.E. Host-parasite relationships in schistosomiasis. Ann. Trop. Med. Parasitol. 1966, 60, 94–99. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Nalugwa, A.; Nuwaha, F.; Tukahebwa, E.M.; Olsen, A. Schistosoma mansoni-Associated Morbidity among Preschool-Aged Children along the Shores of Lake Victoria in Uganda. Trop. Med. Infect. Dis. 2017, 2, 58. [Google Scholar] [CrossRef]
- Dettman, C.D.; Higgins-Opitz, S.B.; Saikoolal, A. Enhanced efficacy of the paddling method for schistosome infection of rodents by a four-step pre-soaking procedure. Parasitol. Res. 1989, 76, 183–184. [Google Scholar] [CrossRef]
- Du Percie Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- López-De León, A.; Rojkind, M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J. Histochem. Cytochem. 1985, 33, 737–743. [Google Scholar] [CrossRef]
- Irungbam, K.; Churin, Y.; Matono, T.; Weglage, J.; Ocker, M.; Glebe, D.; Hardt, M.; Koeppel, A.; Roderfeld, M.; Roeb, E. Cannabinoid receptor 1 knockout alleviates hepatic steatosis by downregulating perilipin 2. Lab. Investig. 2020, 100, 454–465. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Stegemann, H.; Stalder, K. Determination of hydroxyproline. Clin. Chim. Acta 1967, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K.E.-D.M.; Baiuomy, I.R.; Sabry, A.E.-H.A.; Mohammed, M.M.; El-Dardiry, M.A. In vivo assessment of the antischistosomal activity of curcumin loaded nanoparticles versus praziquantel in the treatment of Schistosoma mansoni. Sci. Rep. 2020, 10, 15742. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weimer, J.; Meurer, S.K.; Kron, A.; Seipel, B.; Vater, I.; Arnold, N.; Siebert, R.; Xu, L.; Friedman, S.L.; et al. Genetic characteristics of the human hepatic stellate cell line LX-2. PLoS ONE 2013, 8, e75692. [Google Scholar] [CrossRef]
- Geerts, A.; Eliasson, C.; Niki, T.; Wielant, A.; Vaeyens, F.; Pekny, M. Formation of normal desmin intermediate filaments in mouse hepatic stellate cells requires vimentin. Hepatology 2001, 33, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Hosseini, M.; Liu, X.; Kisseleva, T.; Brenner, D.A. Human hepatic stellate cell isolation and characterization. J. Gastroenterol. 2018, 53, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Bodin, A.; Lagente, V.; Boichot, E. Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1β or TNF-α Release from Human Hepatic Stellate Cells. PLoS ONE 2016, 11, e0153118. [Google Scholar] [CrossRef]
- Gerresheim, G.K.; Roeb, E.; Michel, A.M.; Niepmann, M. Hepatitis C Virus Downregulates Core Subunits of Oxidative Phosphorylation, Reminiscent of the Warburg Effect in Cancer Cells. Cells 2019, 8, 1410. [Google Scholar] [CrossRef]
- Zuo, R.-J.; Gu, X.-W.; Qi, Q.-R.; Wang, T.-S.; Zhao, X.-Y.; Liu, J.-L.; Yang, Z.-M. Warburg-like Glycolysis and Lactate Shuttle in Mouse Decidua during Early Pregnancy. J. Biol. Chem. 2015, 290, 21280–21291. [Google Scholar] [CrossRef]
- Poggel, C.; Adams, T.; Janzen, R.; Hofmann, A.; Hardt, O.; Roeb, E.; Schröder, S.K.; Tag, C.G.; Roderfeld, M.; Weiskirchen, R. Isolation of Hepatocytes from Liver Tissue by a Novel, Semi-Automated Perfusion Technology. Biomedicines 2022, 10, 1410. [Google Scholar] [CrossRef]
- Doncheva, A.I.; Li, Y.; Khanal, P.; Hjorth, M.; Kolset, S.O.; Norheim, F.A.; Kimmel, A.R.; Dalen, K.T. Altered hepatic lipid droplet morphology and lipid metabolism in fasted Plin2-null mice. J. Lipid Res. 2023, 64, 100461. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Fenwick, A.; Savioli, L.; Molyneux, D.H. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 2009, 373, 1570–1575. [Google Scholar] [CrossRef]
- Gomes Casavechia, M.T.; Melo, G.A.N.d.; Da Silva Fernandes, A.C.B.; Castro, K.R.d.; Pedroso, R.B.; Da Silva Santos, T.; Teixeira, J.J.V. Systematic review and meta-analysis on Schistosoma mansoni infection prevalence, and associated risk factors in Brazil. Parasitology 2018, 145, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Woldeyohannes, D.; Sahiledengle, B.; Tekalegn, Y.; Hailemariam, Z. Prevalence of Schistosomiasis (S. mansoni and S. haematobium) and its association with gender of school age children in Ethiopia: A systematic review and meta-analysis. Parasite Epidemiol. Control 2021, 13, e00210. [Google Scholar] [CrossRef] [PubMed]
- Abera, B. The epidemiology of Schistosoma mansoni in the Lake Tana Basin (Ethiopia): Review with retrospective data analyses. Heliyon 2023, 9, e14754. [Google Scholar] [CrossRef] [PubMed]
- Bisetegn, H.; Eshetu, T.; Erkihun, Y. Prevalence of Schistosoma mansoni infection among children in Ethiopia: A systematic review and meta-analysis. Trop. Dis. Travel Med. Vaccines 2021, 7, 30. [Google Scholar] [CrossRef]
- Tazebew, B.; Temesgen, D.; Alehegn, M.; Salew, D.; Tarekegn, M. Prevalence of S. mansoni Infection and Associated Risk Factors among School Children in Guangua District, Northwest Ethiopia. J. Parasitol. Res. 2022, 2022, 1005637. [Google Scholar] [CrossRef]
- Sassa, M.; Chadeka, E.A.; Cheruiyot, N.B.; Tanaka, M.; Moriyasu, T.; Kaneko, S.; Njenga, S.M.; Cox, S.E.; Hamano, S. Prevalence and risk factors of Schistosoma mansoni infection among children under two years of age in Mbita, Western Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0008473. [Google Scholar] [CrossRef]
- Cisse, M.; Sangare, I.; Djibougou, A.D.; Tahita, M.C.; Gnissi, S.; Bassinga, J.K.W.; Konda, S.; Diallo, A.H. Prevalence and risk factors of Schistosoma mansoni infection among preschool-aged children from Panamasso village, Burkina Faso. Parasit. Vectors 2021, 14, 185. [Google Scholar] [CrossRef]
- Lamberti, O.; Kabatereine, N.B.; Tukahebwa, E.M.; Chami, G.F. Schistosoma mansoni infection risk for school-aged children clusters within households and is modified by distance to freshwater bodies. PLoS ONE 2021, 16, e0258915. [Google Scholar] [CrossRef]
- Malta, K.K.; Palazzi, C.; Neves, V.H.; Aguiar, Y.; Silva, T.P.; Melo, R.C.N. Schistosomiasis Mansoni-Recruited Eosinophils: An Overview in the Granuloma Context. Microorganisms 2022, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.A.; Rinaldi, G.; Cortés, A.; Costain, A.; MacDonald, A.S.; Cantacessi, C. The role of the host gut microbiome in the pathophysiology of schistosomiasis. Parasite Immunol. 2023, 45, e12970. [Google Scholar] [CrossRef] [PubMed]
- Pinot de Moira, A.; Fulford, A.J.C.; Kabatereine, N.B.; Ouma, J.H.; Booth, M.; Dunne, D.W. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: The influence of age, sex, ethnicity and IgE. PLoS Negl. Trop. Dis. 2010, 4, e820. [Google Scholar] [CrossRef]
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome egg migration: Mechanisms, pathogenesis and host immune responses. Front. Immunol. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, J.; Sun, L.; Huang, T.; Kong, Y.; Li, L.; Sun, Z.; Yin, M.; Li, X. Mapping Novel Biomarkers of Liver Injury by Tissue Proteomic Analysis. ACS Omega 2021, 6, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Hermiston, M.L.; Xu, Z.; Weiss, A. CD45: A critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef]
- Hoffmann, K.F.; Cheever, A.W.; Wynn, T.A. IL-10 and the dangers of immune polarization: Excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 2000, 164, 6406–6416. [Google Scholar] [CrossRef]
- Wijshake, T.; Rose, J.; Wang, J.; Zielke, J.; Marlar-Pavey, M.; Chen, W.; Collins, J.J.; Agathocleous, M. Schistosome Infection Impacts Hematopoiesis. J. Immunol. 2024, 212, 607–616. [Google Scholar] [CrossRef]
- Friedman, J.F.; Kanzaria, H.K.; McGarvey, S.T. Human schistosomiasis and anemia: The relationship and potential mechanisms. Trends Parasitol. 2005, 21, 386–392. [Google Scholar] [CrossRef]
- Skelly, P.J.; Da’dara, A.A.; Li, X.-H.; Castro-Borges, W.; Wilson, R.A. Schistosome feeding and regurgitation. PLoS Pathog. 2014, 10, e1004246. [Google Scholar] [CrossRef]
- Anthony, B.; Mathieson, W.; Castro-Borges, W.d.; Allen, J. Schistosoma mansoni: Egg-induced downregulation of hepatic stellate cell activation and fibrogenesis. Exp. Parasitol. 2010, 124, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, W.; Feng, J.; Zhu, D.; Chen, J.; Sun, X.; Lyu, L.; Ju, S.; Duan, Y. Recombinant T2 RNase protein of Schistosoma japonicum inhibits expression of α-SMA in LX-2 cells. Parasitol. Res. 2016, 115, 4055–4060. [Google Scholar] [CrossRef] [PubMed]
- Morini, S.; Carotti, S.; Carpino, G.; Franchitto, A.; Corradini, S.G.; Merli, M.; Gaudio, E. GFAP expression in the liver as an early marker of stellate cells activation. Ital. J. Anat. Embryol. 2005, 110, 193–207. [Google Scholar]
- Meurs, L.; Mbow, M.; Vereecken, K.; Menten, J.; Mboup, S.; Polman, K. Bladder morbidity and hepatic fibrosis in mixed Schistosoma haematobium and S. mansoni Infections: A population-wide study in Northern Senegal. PLoS Negl. Trop. Dis. 2012, 6, e1829. [Google Scholar] [CrossRef]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12 (Suppl. S1), S17. [Google Scholar] [CrossRef] [PubMed]
- Straub, A.C.; Clark, K.A.; Ross, M.A.; Chandra, A.G.; Li, S.; Gao, X.; Pagano, P.J.; Stolz, D.B.; Barchowsky, A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J. Clin. Investig. 2008, 118, 3980–3989. [Google Scholar] [CrossRef]
- Inverso, D.; Iannacone, M. Spatiotemporal dynamics of effector CD8+ T cell responses within the liver. J. Leukoc. Biol. 2016, 99, 51–55. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, H.; Straßmann, J.K.; Baier, A.S.; von Bülow, V.; Stettler, F.; Hagen, M.J.; Schmidt, F.P.; Tschuschner, A.; Schmid, A.R.; Zahner, D.; et al. Liver Fibrosis Is Enhanced by a Higher Egg Burden in Younger Mice Infected with S. mansoni. Cells 2024, 13, 1643. https://doi.org/10.3390/cells13191643
Müller H, Straßmann JK, Baier AS, von Bülow V, Stettler F, Hagen MJ, Schmidt FP, Tschuschner A, Schmid AR, Zahner D, et al. Liver Fibrosis Is Enhanced by a Higher Egg Burden in Younger Mice Infected with S. mansoni. Cells. 2024; 13(19):1643. https://doi.org/10.3390/cells13191643
Chicago/Turabian StyleMüller, Heike, Jan K. Straßmann, Anne S. Baier, Verena von Bülow, Frederik Stettler, Maximilian J. Hagen, Fabian P. Schmidt, Annette Tschuschner, Andreas R. Schmid, Daniel Zahner, and et al. 2024. "Liver Fibrosis Is Enhanced by a Higher Egg Burden in Younger Mice Infected with S. mansoni" Cells 13, no. 19: 1643. https://doi.org/10.3390/cells13191643






