Advances in Cancer Therapy: A Comprehensive Review of CDK and EGFR Inhibitors
Abstract
:1. Introduction
2. Cyclin-Dependent Kinases
2.1. FDA-Approved CDK Inhibitors
2.2. Cyclin-Dependent Kinase 2
2.2.1. Pyrazole, Pyrimidine, and Related Derivatives as CDK2 Inhibitors
| Code | Structures | Evaluated Cancer Cell Lines | CDK2 | Ref. | |
|---|---|---|---|---|---|
| Cell lines | IC50/IG50 | IC50 | |||
| St.1 | 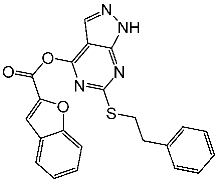 | K-562 MCF-7 | 19.8 µM 18.9 µM | 6.8 µM | [81] |
| St.2 |  | MOLT-4 HL-60 | 0.93 µM 0.80 µM | 22 nM | [82] |
| St.3 | 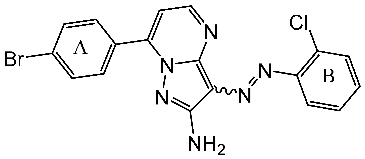 | MOLT-4 HL-60 | 1.28 µM 0.92 µM | 24 nM | |
| St.4 | 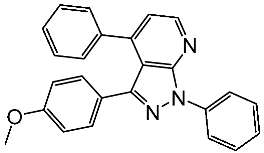 | HeLa | 2.59 μM | 1.63 μM | [83] |
| St.5 | 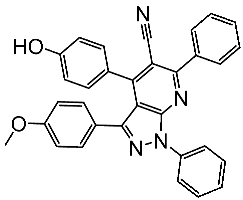 | HCT116 MCF-7 | 4.66 μM 1.98 μM | 0.46 μM | |
| St.6 |  | - | - | 0.36 μM | [84] |
| St.7 | 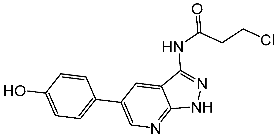 | - | - | 0.66 μM | |
| St.8 | 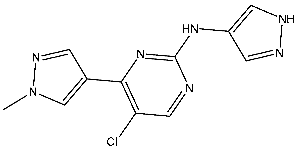 | A2780 MCF-7 | 0.158 μM 0.342 μM | CDK2/E Ki = 0.005 μM | [85] |
| St.9 | 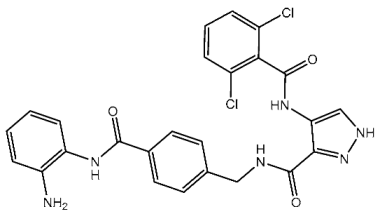 | HCT116 A375 HeLa H460 SMMC77 | 0.71 μM 1.20 μM 1.83 μM 4.19 μM 7.76 μM | 0.30 nM | [86] |
| St.10 | 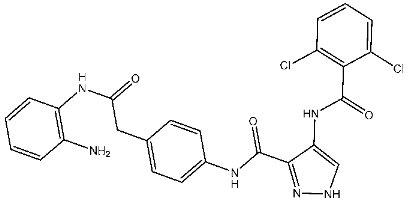 | HCT116 A375 HeLa H460 SMMC77 | 1.45 μM 1.60 μM 3.15 μM 2.63 μM 5.22 μM | 0.56 nM | |
| St.11 | 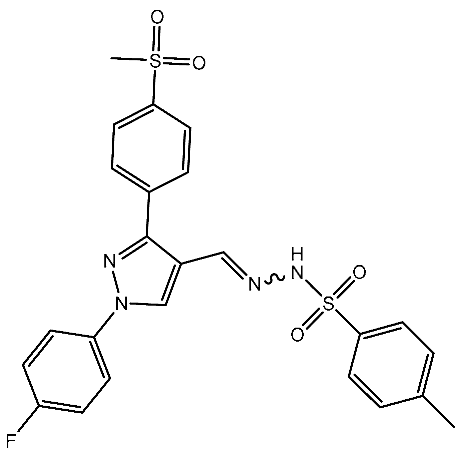 | HepG2 HCT116 MCF-7 | 3.98 μM 8.70 μM 7.85 μM | 0.61 μM | [87] |
| St.12 | 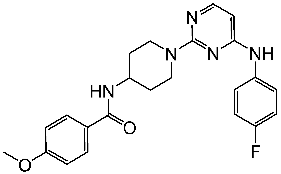 | NA | NA | 45.4 nM | [89] |
| St.13 |  | HT-29 MV4-11 MCF-7 HeLa | 2.12 μM 0.83 μM 3.12 μM 8.61 μM | 64.4 nM | [90] |
| St.14 |  | MCF-7 HCT116 | 1.03 μM 0.9 μM | 0.11 μM | [91] |
| St.15 |  | MCF-7 HCT116 | 1.1 μM 1.4 μM | 0.09 μM | |
| St.16 | 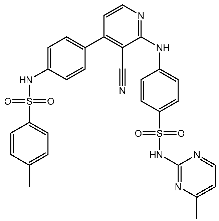 | MCF-7 | 9.10 μM | 1.79 μM | [92] |
| St.17 | 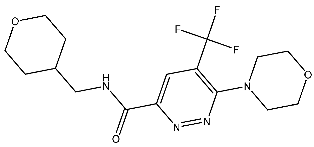 | T-47D MDAMB SKOV3 | 0.43 μM 0.99 μM 15.37 μM | 20.1 nM | [93] |
2.2.2. Thiazole, Thiouracil, and Related Derivatives as CDK2 Inhibitors
| Code | Structure | Evaluated Cancer Cell Lines | CDK2 | Ref. | |
|---|---|---|---|---|---|
| Cell Lines | IC50 or IG50 | IC50 | |||
| St.18 | 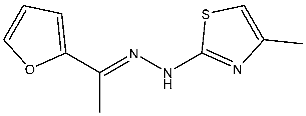 | HepG2 MCF-7 HCT116 WI-38 | 8.49 µM 17.09 µM 22.50 µM 73.45 µM | 0.35 µM | [98] |
| St.19 | 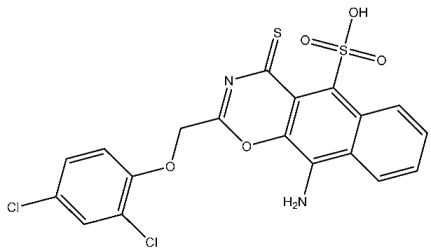 | HCT116 MCF-7 | 3.78 µM 6.41 µM | 0.21 µM | [99] |
| St.20 |  | HCT116 MCF-7 | 5.91 µM 7.39 µM | 0.70 µM | |
| St.21 |  | Panc-1 MCF-7 HT-29 A-549 | 0.80 µM 0.65 µM 0.90 µM 0.95 µM | 18 nM | [100] |
| St.22 | 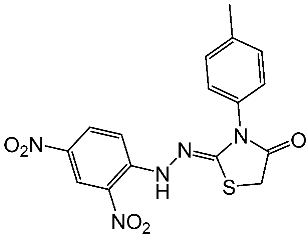 | Panc-1 MCF-7 HT-29 A-549 | 0.70 µM 0.60 µM 0.80 µM 0.80 µM | 14 nM | |
| St.23 | 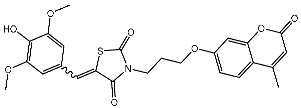 | MCF-7 | 12.15 µg/mL | 7.5 µg/mL | [102] |
| St.24 |  | A2780 HT29 MCF-7 HepG2 | 2.52 µM 1.75 µM 1.67 µM 2.02 µM | 0.41 µM | [103] |
| St.25 | 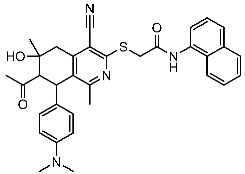 | MCF-7 A459 | 0.21 µM 0.15 µM | 149 nM | [104] |
2.3. Cyclin Dependent Kinase 4/6
2.3.1. Pyrimidine Derivatives as CDK4/6 Inhibitors
2.3.2. Miscellaneous Derivatives CDK4/6 Inhibitors
2.4. Cyclin-Dependent Kinase 9
3. EGFR Inhibitors
3.1. FDA-Approved EGFR-Targeting Drugs
3.2. New EGFR Inhibitors
3.2.1. Quinazoline Derivatives as EGFR Inhibitors
3.2.2. Pyrimidine Derivatives as EGFR Inhibitors
3.2.3. Indole Derivatives as EGFR Inhibitors
| Code | Structure | Evaluated Cancer Cell Lines | EGFR or Related | Ref. | |
|---|---|---|---|---|---|
| Cell Lines | IC50 or Inh.% | IC50 | |||
| St.54 | 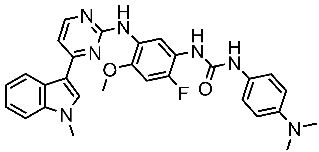 | A549 MCF6 PC3 | >50% | EGFR = 1.026 µM SRC= 2 nM | [174] |
| St.55 |  | A549 MCF-7 Panc-1 HT-29 | 27 nM 30 nM 29 nM 30 nM | EGFR = 68 nM EGFRT790M =8.6 nM BRAFV600E = 35 nM | [175] |
| St.56 | 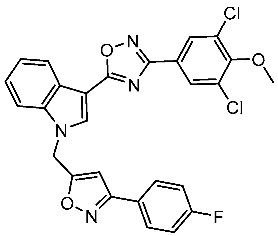 | MCF-7 MDA-MB-231 | 3.12 µM 9.43 µM | EGFR = 311 nM | [176] |
| St.57 |  | MCF-7 MDA-MB-231 | 2.16 µM 8.33 µM | EGFR = 203 nM | [176] |
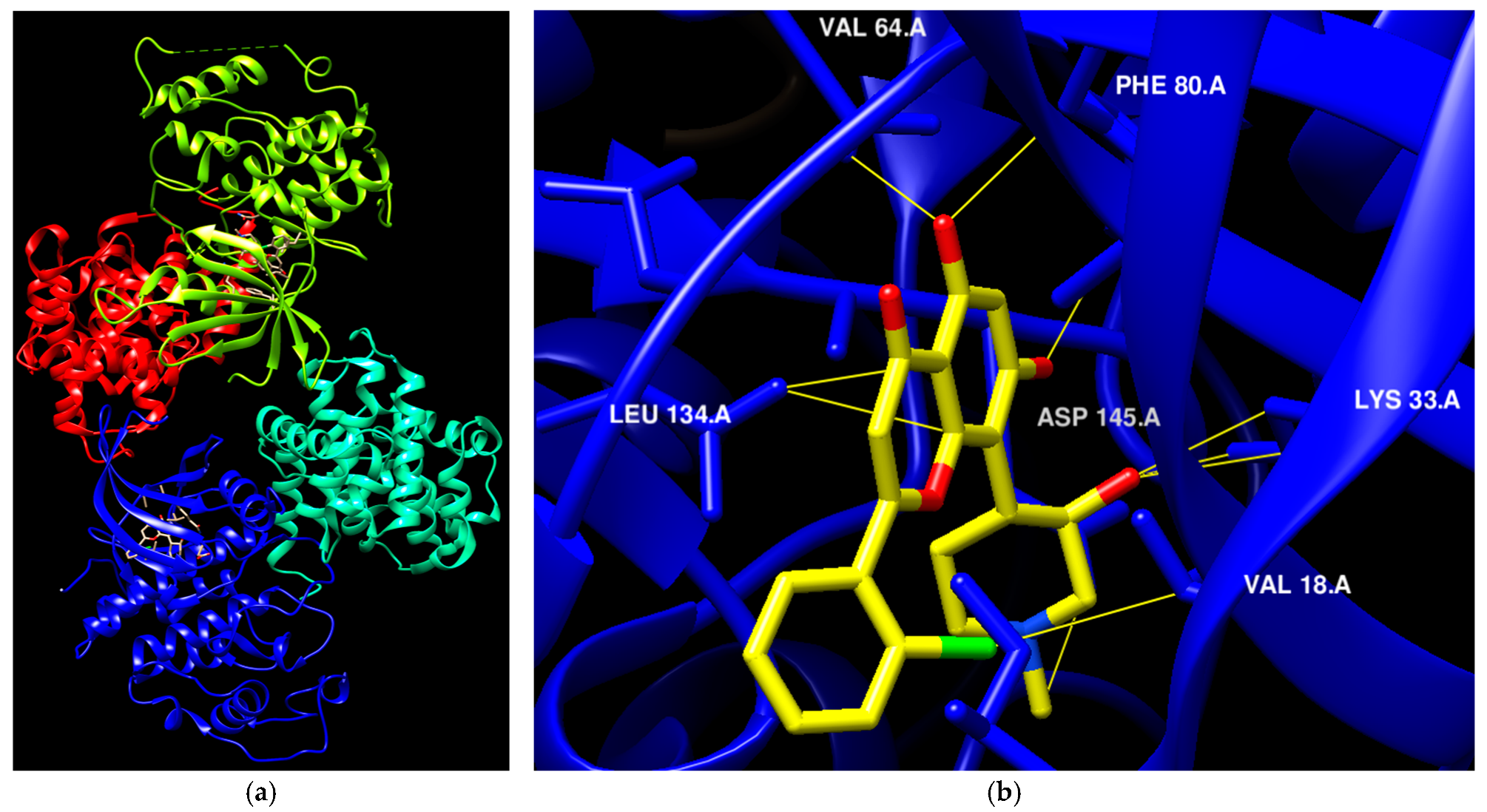
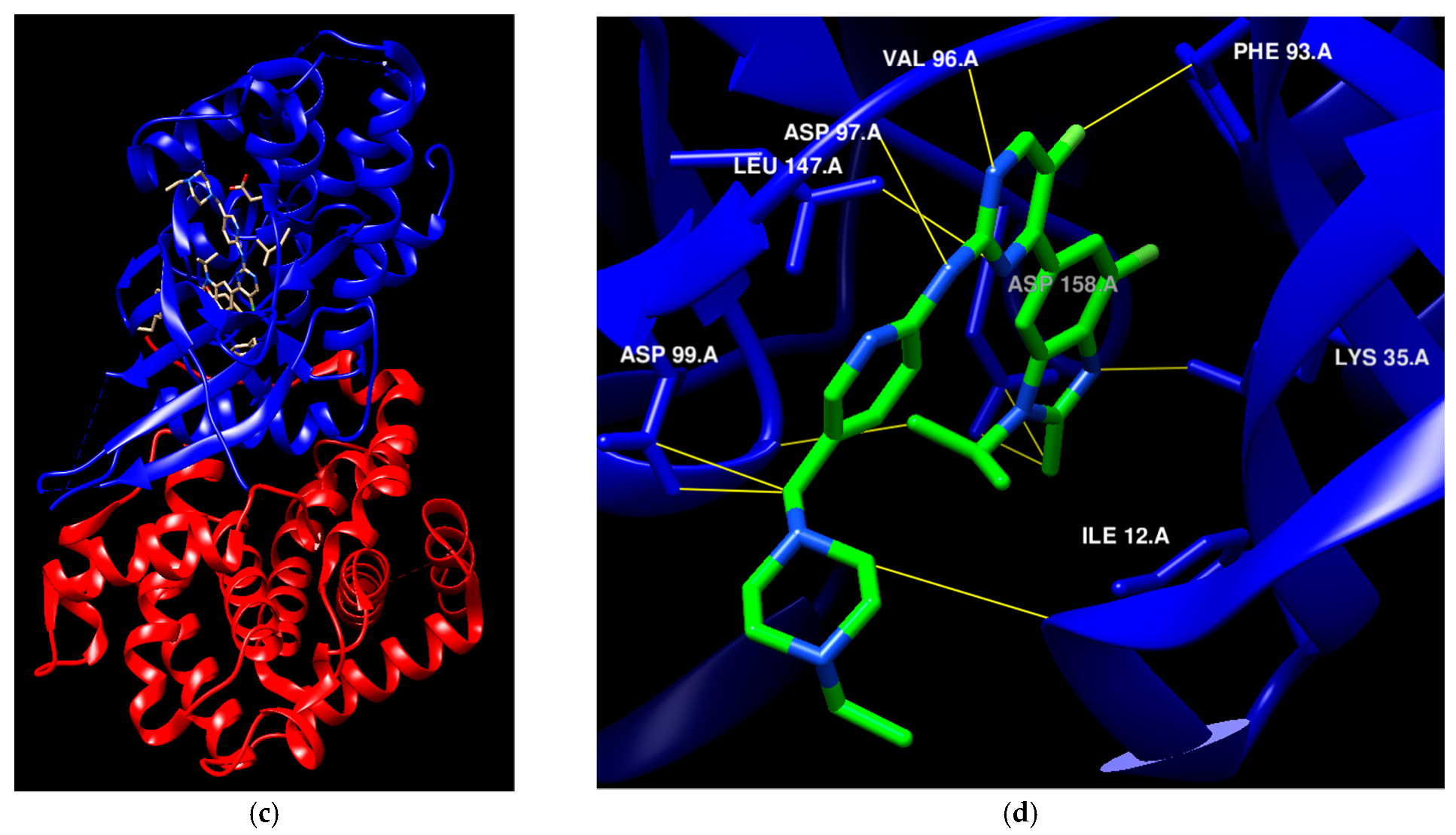
| St.58 | 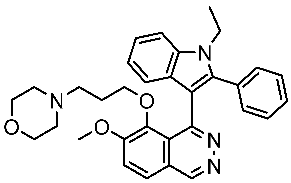 | A549 PC-9 A431 | 4.1 µM 0.5 µM 2.1 µM | EGFRL858R = 1.9 nM EGFRwt = 5.2 nM | [178] |
4. Challenges, Limitations, and Future Directions
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK | cyclin-dependent kinase |
| CDKI | cyclin-dependent kinase inhibitor |
| EGFR | epidermal growth factor receptor |
| VEGFR | vascular endothelial growth factor receptor |
| FDA | the Food and Drug Administration of the United States |
| FGFR | fibroblast growth factor receptor |
| HER2 | human epidermal growth factor receptor-2 |
| NSCLC | non-small cell lung cancer |
| PDGFR | platelet-derived growth factor receptor |
| ATP | adenosine triphosphate |
| HDAC | Histone deacetylase |
| IC50 | inhibitory concentration |
| GI50 | half-maximum growth inhibition |
| PDB | Protein Data Bank |
| SAR | structure-activity relationship |
| WHO | World Health Organization |
| M phase | mitosis phase |
| RTK | receptor tyrosine kinase |
| MOA | Mechanism of action |
| Cancer Cell Lines Explanation | |
| A375 | melanoma |
| A431 | squamous |
| MDA-MB231 | breast |
| Colo205 | colon |
| HCT116 | colon |
| A549 | lung |
| MCF-7 | breast |
| PC3 | prostate |
| HepG2 | liver |
| MDA-MB-468 | breast |
| T-47D | breast |
| HeLa | cervical |
| K-562 | leukemia |
| A2780 | ovarian |
| HL60 | leukemia |
| HT-29 | colon |
| MOLT-4 | lymphoblastic leukemia |
| H460 | lung |
| SMMC77 | liver |
| MV4-11 | leukemia |
| SKOV3 | ovarian |
| WI-38 | lung |
| Panc-1 | pancreas |
| PaCa-2 | pancreas |
| BxPc-3 | pancreas |
| NCIH522 | lung |
| HCT-15 | colon |
| RKO | colon |
| SW480 | colon |
| DLD1 | colon |
| LO2 | liver |
| PC-9 | lung |
| HOP92 | lung |
| SNB-75 | brain |
| DU-145 | brain |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Boyle, P.; Levin, B. World Cancer Report 2008; WHO Report; The International Agency for Research on Cancer: Lyon, France, 2008; pp. 12–510. [Google Scholar]
- Hawash, M. Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef]
- Heptinstall, A.B.; Adiyasa, I.; Cano, C.; Hardcastle, I.R. Recent Advances in CDK Inhibitors for Cancer Therapy. Futur. Med. Chem. 2018, 10, 1369–1388. [Google Scholar] [CrossRef]
- Baytas, S.N.; Inceler, N.; Yılmaz, A. Synthesis, cytotoxicity, and molecular properties prediction of novel 1,3-diarylpyrazole derivatives. Med. Chem. Res. 2013, 22, 4893–4908. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Eid, A.M.; Abubaker, A.; Mufleh, O.; Al-Hroub, Q.; Sobuh, S. Synthesis of novel isoxazole–carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022, 16, 47. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Hawash, M. Highlights on Specific Biological Targets; Cyclin-Dependent Kinases, Epidermal Growth Factor Receptors, Ras Protein, and Cancer Stem Cells in Anticancer Drug Development. Drug Res. 2019, 69, 471–478. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Mahadevan, D. A comprehensive review of protein kinase inhibitors for cancer therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Ficarro, S.B.; McCleland, M.L.; Stukenberg, P.T.; Burke, D.J.; Ross, M.M.; Shabanowitz, J.; Hunt, D.F.; White, F.M. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 2002, 20, 301–305. [Google Scholar] [CrossRef]
- Cohen, P. The regulation of protein function by multisite phosphorylation—A 25 year update. Trends Biochem. Sci. 2000, 25, 596–601. [Google Scholar] [CrossRef]
- Timofeev, O.; Giron, P.; Lawo, S.; Pichler, M.; Noeparast, M. ERK pathway agonism for cancer therapy: Evidence, insights, and a target discovery framework. npj Precis. Oncol. 2024, 8, 70. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Müller, S.; Chaikuad, A.; Gray, N.S.; Knapp, S. The ins and outs of selective kinase inhibitor development. Nat. Chem. Biol. 2015, 11, 818–821. [Google Scholar] [CrossRef]
- Yan, H.; He, L.; Lv, D.; Yang, J.; Yuan, Z. The role of the dysregulated JNK signaling pathway in the pathogenesis of human diseases and its potential therapeutic strategies: A comprehensive review. Biomolecules 2024, 14, 243. [Google Scholar] [CrossRef]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef]
- Iksen; Witayateeraporn, W.; Hardianti, B.; Pongrakhananon, V. Comprehensive review of Bcl-2 family proteins in cancer apoptosis: Therapeutic strategies and promising updates of natural bioactive compounds and small molecules. Phytother. Res. 2024, 38, 2249–2275. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef] [PubMed]
- Carles, F.; Bourg, S.; Meyer, C.; Bonnet, P. PKIDB: A curated, annotated and updated database of protein kinase inhibitors in clinical trials. Molecules 2018, 23, 908. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mursal, M.; Verma, G.; Hasan, S.M.; Khan, M.F. Targeting oncogenic kinases: Insights on FDA approved tyrosine kinase inhibitors. Eur. J. Pharmacol. 2024, 970, 176484. [Google Scholar] [CrossRef]
- Amin, S.M.S.; Patil, P.H.; Desai, M.; Channabasavaiah, J.P. In-silico design, synthesis and biological evaluation of 4-aryl-4H-chromene derivatives as CDK-2 inhibitors: A molecular approach to finding a lead for breast cancer. J. Appl. Pharm. Sci. 2024, 14, 98–111. [Google Scholar] [CrossRef]
- Al Awadh, A.A.; Sakagami, H.; Amano, S.; Sayed, A.M.; Abouelela, M.E.; Alhasaniah, A.H.; Aldabaan, N.; Refaey, M.S.; Abdelhamid, R.A.; Khalil, H.M. In vitro cytotoxicity of Withania somnifera (L.) roots and fruits on oral squamous cell carcinoma cell lines: A study supported by flow cytometry, spectral, and computational investigations. Front. Pharmacol. 2024, 15, 1325272. [Google Scholar] [CrossRef]
- Chung, K.H.; Cho, I.R.; Paik, W.H.; Kim, Y.-T.; Lee, S.H.; Ryu, J.K. Enhanced Anti-tumor Effect of Flavopiridol in Combination with Gemcitabine in Pancreatic Cancer. Anticancer Res. 2024, 44, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Yakan, B.; Hamurcu, Z. Flavopiridol Suppresses Cell Proliferation and Metastasis and Induces Endoplasmic Reticulum Stress of Triple Negative Breast Cancer Cells. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Sarhan, M.O.; Abd El-Karim, S.S.; Anwar, M.M.; Gouda, R.H.; Zaghary, W.A.; Khedr, M.A. Discovery of new coumarin-based lead with potential anticancer, CDK4 inhibition and selective radiotheranostic effect: Synthesis, 2D & 3D QSAR, molecular dynamics, in vitro cytotoxicity, radioiodination, and biodistribution studies. Molecules 2021, 26, 2273. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Jang, H.; Nussinov, R. CDK2 and CDK4: Cell Cycle Functions Evolve Distinct, Catalysis-Competent Conformations, Offering Drug Targets. JACS Au 2024, 4, 1911–1927. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Singh, S. 1,2,3-Triazole Carboxamide Derivatives as Novel Prospective Anticancer Agents: Synthesis, Characterization and In-silico Studies. Int. J. Pharm. Sci. Drug Res. 2024, 16, 213–219. [Google Scholar] [CrossRef]
- Wang, X.-J.; Hou, X.; Zhang, L.-Y.; Wang, B.-Y.; Wu, M.-y.; Chen, H.-J.; Jiang, W.-T.; Qiao, Y.; Lu, M.-x.; Hao, H.-h. Design, synthesis, and antitumor activity of benzimidazole derivatives as CDK4/6 inhibitors. J. Mol. Struct. 2024, 1309, 138189. [Google Scholar] [CrossRef]
- Virgil, H. FDA Expands Abemaciclib Approval to Include HR+ HER2–Node+ High-Risk Breast Cancer. Cancer Network. 2023. Available online: https://www.cancernetwork.com/view/fda-expands-abemaciclib-approval-to-include-hr-her-high-risk-breast-cancer (accessed on 26 June 2024).
- Bournez, C.; Carles, F.; Peyrat, G.; Aci-Sèche, S.; Bourg, S.; Meyer, C.; Bonnet, P. Comparative assessment of protein kinase inhibitors in public databases and in PKIDB. Molecules 2020, 25, 3226. [Google Scholar] [CrossRef] [PubMed]
- Bamborough, P.; Brown, M.J.; Christopher, J.A.; Chung, C.W.; Mellor, G.W. Selectivity of kinase inhibitor fragments. J. Med. Chem. 2011, 54, 5131–5143. [Google Scholar] [CrossRef] [PubMed]
- Stahura, F.L.; Xue, L.; Godden, J.W.; Bajorath, J. Molecular scaffold-based design and comparison of combinatorial libraries focused on the ATP-binding site of protein kinases. J. Mol. Graph. Model. 1999, 17, 1–9, 51–52. [Google Scholar] [CrossRef]
- Marak, B.N.; Dowarah, J.; Khiangte, L.; Singh, V.P. A comprehensive insight on the recent development of Cyclic Dependent Kinase inhibitors as anticancer agents. Eur. J. Med. Chem. 2020, 203, 112571. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, D.; Ortega, S. Cyclins and CDKS in development and cancer: Lessons from genetically modified mice. Front. Biosci. J. Virtual Libr. 2006, 11, 1164–1188. [Google Scholar] [CrossRef] [PubMed]
- Ploumaki, I.; Triantafyllou, E.; Koumprentziotis, I.-A.; Karampinos, K.; Drougkas, K.; Karavolias, I.; Kotteas, E. Cyclin-dependent kinase 4/6 inhibitors as neoadjuvant therapy of hormone receptor-positive/HER2-negative early breast cancer: What do we know so far? Clin. Breast Cancer 2024, 24, e177–e185. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, P.; Baranowska-Bosiacka, I.; Kulczycka, K.; Gutowska, I. Inhibitors of cyclin-dependent kinases: Types and their mechanism of action. Int. J. Mol. Sci. 2021, 22, 2806. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, A.M.; Kaulich, M.; Shapiro, G.S.; Choi, Y.J.; Sicinski, P.; Dowdy, S.F. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife 2014, 3, e02872. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.T.; Rosner, M.R.; Minella, A.C. An integrated view of cyclin E function and regulation. Cell Cycle 2012, 11, 57–64. [Google Scholar] [CrossRef]
- Sánchez-Martínez, C.; Lallena, M.J.; Sanfeliciano, S.G.; de Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorg. Med. Chem. Lett. 2019, 29, 126637. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Orally effective FDA-approved protein kinase targeted covalent inhibitors (TCIs). Pharmacol. Res. 2021, 165, 105422. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J.; Liu, C.; Luo, Y.; Xu, H.; Wang, Y.; Sun, N.; He, J. Making the best use of available weapons for the inevitable rivalry-resistance to EGFR-TKIs. Biomedicines 2023, 11, 1141. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Liu, S.-Y.; Wu, Y.-L. Safety of EGFR-TKIs for EGFR mutation-positive non-small cell lung cancer. Expert Opin. Drug Saf. 2020, 19, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Stoup, N.; Liberelle, M.; Lebègue, N.; Van Seuningen, I. Emerging paradigms and recent progress in targeting ErbB in cancers. Trends Pharmacol. Sci. 2024, 45, 552–576. [Google Scholar] [CrossRef]
- Ramos, R.; Moura, C.S.; Costa, M.; Lamas, N.J.; Correia, R.; Garcez, D.; Pereira, J.M.; Sousa, C.; Vale, N. Enhancing Lung Cancer Care in Portugal: Bridging Gaps for Improved Patient Outcomes. J. Pers. Med. 2024, 14, 446. [Google Scholar] [CrossRef] [PubMed]
- Ricordel, C.; Friboulet, L.; Facchinetti, F.; Soria, J.-C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann. Oncol. 2018, 29, i28–i37. [Google Scholar] [CrossRef] [PubMed]
- Stucchi, E.; Bartolini, M.; Airoldi, M.; Fazio, R.; Daprà, V.; Mondello, G.; Prete, M.G.; Puccini, A.; Santoro, A. Fruquintinib as new treatment option in metastatic colorectal cancer patients: Is there an optimal sequence? Expert Opin. Pharmacother. 2024, 25, 371–382. [Google Scholar] [CrossRef]
- Dhillon, S. Trilaciclib: First approval. Drugs 2021, 81, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R. FDA Approves Quizartinib for Newly Diagnosed FLT3-ITD+ AML. Cancer Network. 2023. Available online: https://www.cancernetwork.com/view/fda-approves-quizartinib-for-newly-diagnosed-flt3-itd-aml (accessed on 28 June 2024).
- Papapetropoulos, A.; Topouzis, S.; Alexander, S.P.; Cortese-Krott, M.; Kendall, D.A.; Martemyanov, K.A.; Mauro, C.; Nagercoil, N.; Panettieri, R.A., Jr.; Patel, H.H. Novel drugs approved by the EMA, the FDA, and the MHRA in 2023: A year in review. Br. J. Pharmacol. 2024, 181, 1553–1575. [Google Scholar] [CrossRef]
- Patrick, G.N.; Zukerberg, L.; Nikolic, M.; de la Monte, S.; Dikkes, P.; Tsai, L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. BioEssays News Rev. Mol. Cell. Dev. Biol. 1995, 17, 471–480. [Google Scholar] [CrossRef]
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259. [Google Scholar] [CrossRef]
- Bilgin, B.; Sendur, M.A.N.; Şener Dede, D.; Akıncı, M.B.; Yalçın, B. A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer. Curr. Med. Res. Opin. 2017, 33, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Poratti, M.; Marzaro, G. Third-generation CDK inhibitors: A review on the synthesis and binding modes of Palbociclib, Ribociclib and Abemaciclib. Eur. J. Med. Chem. 2019, 172, 143–153. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, Q.; Shi, X.; Zhao, Y.; Zou, S. Post-marketing safety concerns with palbociclib: A disproportionality analysis of the FDA adverse event reporting system. Expert Opin. Drug Saf. 2024, 23, 637–648. [Google Scholar]
- Raheem, F.; Ofori, H.; Simpson, L.; Shah, V. Abemaciclib: The first FDA-approved CDK4/6 inhibitor for the adjuvant treatment of HR+ HER2− early breast cancer. Ann. Pharmacother. 2022, 56, 1258–1266. [Google Scholar] [CrossRef]
- Al-Ziftawi, N.H.; Elazzazy, S.; Alam, M.F.; Shafie, A.; Hamad, A.; Bbujassoum, S.; Mohamed Ibrahim, M.I. The effectiveness and safety of palbociclib and ribociclib in stage IV HR+/HER-2 negative breast cancer: A nationwide real world comparative retrospective cohort study. Front. Oncol. 2023, 13, 1203684. [Google Scholar] [CrossRef]
- Young, J.A.; Tan, A.R. Trilaciclib: A First-in-class Therapy to Reduce Chemotherapy-induced Myelosuppression. Oncol. Haematol. 2022, 18, 152–158. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, X.; Han, X.; Xu, N.; Chang, D.C. CDK1 switches mitotic arrest to apoptosis by phosphorylating Bcl-2/Bax family proteins during treatment with microtubule interfering agents. Cell Biol. Int. 2014, 38, 737–746. [Google Scholar] [CrossRef]
- Lee, Y.; Dominy, J.E.; Choi, Y.J.; Jurczak, M.; Tolliday, N.; Camporez, J.P.; Chim, H.; Lim, J.-H.; Ruan, H.-B.; Yang, X. Cyclin D1–Cdk4 controls glucose metabolism independently of cell cycle progression. Nature 2014, 510, 547–551. [Google Scholar] [CrossRef]
- D’costa, M.; Bothe, A.; Das, S.; Kumar, S.U.; Gnanasambandan, R.; Doss, C.G.P. CDK regulators—Cell cycle progression or apoptosis—Scenarios in normal cells and cancerous cells. Adv. Protein Chem. Struct. Biol. 2023, 135, 125–177. [Google Scholar]
- Zabihi, M.; Lotfi, R.; Yousefi, A.-M.; Bashash, D. Cyclins and cyclin-dependent kinases: From biology to tumorigenesis and therapeutic opportunities. J. Cancer Res. Clin. Oncol. 2023, 149, 1585–1606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dagar, G.; Chauhan, R.; Sadida, H.Q.; Almarzooqi, S.K.; Hashem, S.; Uddin, S.; Macha, M.A.; Akil, A.S.A.-S.; Pandita, T.K. Cyclin-dependent kinases in cancer: Role, regulation, and therapeutic targeting. Adv. Protein Chem. Struct. Biol. 2023, 135, 21–55. [Google Scholar]
- Kashyap, D.; Garg, V.K.; Sandberg, E.N.; Goel, N.; Bishayee, A. Oncogenic and tumor suppressive components of the cell cycle in breast cancer progression and prognosis. Pharmaceutics 2021, 13, 569. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalak, M.; Singh, R.; Anwer, M.; Ivanchenko, P.; Randhawa, A.; Ahmed, M.; Ashton, A.W.; Du, Y.; Jiao, X.; Pestell, R. The renaissance of CDK inhibitors in breast cancer therapy: An update on clinical trials and therapy resistance. Cancers 2022, 14, 5388. [Google Scholar] [CrossRef]
- Asghar, A.; Chohan, T.A.; Khurshid, U.; Saleem, H.; Mustafa, M.W.; Khursheed, A.; Alafnan, A.; Batul, R.; Break, M.K.B.; Almansour, K. A systematic review on understanding the mechanistic pathways and clinical aspects of natural CDK inhibitors on cancer progression.: Unlocking cellular and biochemical mechanisms. Chem.-Biol. Interact. 2024, 393, 110940. [Google Scholar] [CrossRef]
- Kuganesan, N. Modulation of Ferroptosis by the Classical p53/p21/CDK/RB/E2F Pathway. Ph.D. Thesis, The University of Toledo, Toledo, OH, USA, 2023. [Google Scholar]
- Shi, S. Targeting Cyclin Dependent Kinase 4/6 for Cancer Therapy. Trans. Cancer 2023, 4, 27–42. [Google Scholar]
- Gallanis, G.T. Effects of CDK4/6 Inhibitor-Induced Stromal Senescence on the Metastatic Niche and Metastasis of Breast Cancer. Ph.D. Thesis, Georgetown University, Washington, DC, USA, 2023. [Google Scholar]
- Liu, J.; Cheng, M.; Xu, J.; Liang, Y.; Yin, B.; Liang, J. Effect of CDK4/6 Inhibitors on Tumor Immune Microenvironment. Immunol. Investig. 2024, 53, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Mandal, R.; Kostova, I.; Raab, M.; Sanhaji, M.; Hehlgans, S.; Diefenhardt, M.; Rödel, C.; Fokas, E.; Strebhardt, K. Prognostic impact of caspase-8, CDK9 and phospho-CDK9 (Thr 186) expression in patients with uterine cervical cancer treated with definitive chemoradiation and brachytherapy. Cancers 2022, 14, 5500. [Google Scholar] [CrossRef]
- Mandal, R.; Becker, S.; Strebhardt, K. Targeting CDK9 for anti-cancer therapeutics. Cancers 2021, 13, 2181. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, R.; De Sanctis, R.; Jacobs, F.; Benvenuti, C.; Gaudio, M.; Saltalamacchia, G.; Torrisi, R.; Masci, G.; Miggiano, C.; Agustoni, F. Cyclin-Dependent Kinase 2 (CDK2) Inhibitors and others novel CDK Inhibitors (CDKi) in Breast Cancer: Clinical Trials, Current Impact, and Future Directions. Crit. Rev. Oncol./Hematol. 2024, 196, 104324. [Google Scholar] [CrossRef] [PubMed]
- Borik, R.M.; Fawzy, N.M.; Abu-Bakr, S.M.; Aly, M.S. Design, synthesis, anticancer evaluation and docking studies of novel heterocyclic derivatives obtained via reactions involving curcumin. Molecules 2018, 23, 1398. [Google Scholar] [CrossRef]
- Sumirtanurdin, R.; Sungkar, S.; Hisprastin, Y.; Sidharta, K.D.; Nurhikmah, D.D. Molecular docking simulation studies of curcumin and its derivatives as cyclin-dependent kinase 2 inhibitors. Turk. J. Pharm. Sci. 2020, 17, 417–423. [Google Scholar] [CrossRef]
- Assali, M.; Abualhasan, M.; Sawaftah, H.; Hawash, M.; Mousa, A. Synthesis, biological activity, and molecular modeling studies of pyrazole and triazole derivatives as selective COX-2 inhibitors. J. Chem. 2020, 2020, 6393428. [Google Scholar] [CrossRef]
- Hawash, M.; Ergun, S.G.; Kahraman, D.C.; Olgac, A.; Hamel, E.; Cetin-Atalay, R.; Baytas, S.N. Novel indole-pyrazole hybrids as potential tubulin-targeting agents; Synthesis, antiproliferative evaluation, and molecular modeling studies. J. Mol. Struct. 2023, 1285, 135477. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M.M.; Kahraman, D.C.; Eren, F.; Cetin Atalay, R.; Baytas, S.N. Synthesis and biological evaluation of novel pyrazolic chalcone derivatives as novel hepatocellular carcinoma therapeutics. Eur. J. Med. Chem. 2017, 129, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Tao, Y.; Meng, Q.; Huang, Y.; Li, S.; Ding, K.; Ma, D.; Ye, Z.; Fan, M. Discovery of novel macrocyclic derivatives as potent and selective cyclin-dependent kinase 2 inhibitors. Bioorg. Med. Chem. 2024, 104, 117711. [Google Scholar] [CrossRef] [PubMed]
- Cherukupalli, S.; Chandrasekaran, B.; Aleti, R.R.; Sayyad, N.; Hampannavar, G.A.; Merugu, S.R.; Rachamalla, H.R.; Banerjee, R.; Karpoormath, R. Synthesis of 4, 6-disubstituted pyrazolo [3,4-d] pyrimidine analogues: Cyclin-dependent kinase 2 (CDK2) inhibition, molecular docking and anticancer evaluation. J. Mol. Struct. 2019, 1176, 538–551. [Google Scholar] [CrossRef]
- Almehmadi, S.J.; Alsaedi, A.M.; Harras, M.F.; Farghaly, T.A. Synthesis of a new series of pyrazolo [1,5-a] pyrimidines as CDK2 inhibitors and anti-leukemia. Bioorg. Chem. 2021, 117, 105431. [Google Scholar] [CrossRef] [PubMed]
- Almansour, B.S.; Binjubair, F.A.; Abdel-Aziz, A.A.-M.; Al-Rashood, S.T. Synthesis and In Vitro Anticancer Activity of Novel 4-Aryl-3-(4-methoxyphenyl)-1-phenyl-1 H-pyrazolo [3,4-b] pyridines Arrest Cell Cycle and Induce Cell Apoptosis by Inhibiting CDK2 and/or CDK9. Molecules 2023, 28, 6428. [Google Scholar] [CrossRef]
- Jing, L.; Tang, Y.; Xiao, Z. Discovery of novel CDK inhibitors via scaffold hopping from CAN508. Bioorg. Med. Chem. Lett. 2018, 28, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Fanta, B.S.; Lenjisa, J.; Teo, T.; Kou, L.; Mekonnen, L.; Yang, Y.; Basnet, S.K.; Hassankhani, R.; Sykes, M.J.; Yu, M. Discovery of N, 4-Di (1 H-pyrazol-4-yl) pyrimidin-2-amine-Derived CDK2 Inhibitors as Potential Anticancer Agents: Design, Synthesis, and Evaluation. Molecules 2023, 28, 2951. [Google Scholar] [CrossRef]
- Cheng, C.; Yun, F.; Ullah, S.; Yuan, Q. Discovery of novel cyclin-dependent kinase (CDK) and histone deacetylase (HDAC) dual inhibitors with potent in vitro and in vivo anticancer activity. Eur. J. Med. Chem. 2020, 189, 112073. [Google Scholar] [CrossRef]
- Shaker, A.M.; Shahin, M.I.; AboulMagd, A.M.; Abdel-Rahman, H.M.; Abou El Ella, D.A. Design, synthesis, and molecular docking of novel 1, 3, 4-triaryl pyrazole derivatives bearing methylsulfonyl moiety with anticancer activity through dual targeting CDK2 and COX-2 enzymes. J. Mol. Struct. 2024, 1301, 137323. [Google Scholar] [CrossRef]
- Hawash, M.; Abdallah, S.; Abudayyak, M.; Melhem, Y.; Shamat, M.A.; Aghbar, M.; Çapan, I.; Abualhasan, M.; Kumar, A.; Kamiński, M. Exploration of isoxazole analogs: Synthesis, COX inhibition, anticancer screening, 3D multicellular tumor spheroids, and molecular modeling. Eur. J. Med. Chem. 2024, 271, 116397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Cheng, X.; Zhang, K.; Wang, H.; Liu, B.; Wang, J. Design, synthesis and biological evaluation of pyrimidine derivatives as novel CDK2 inhibitors that induce apoptosis and cell cycle arrest in breast cancer cells. Bioorg. Med. Chem. 2018, 26, 3491–3501. [Google Scholar] [CrossRef]
- Zeng, W.-B.; Ji, T.-Y.; Zhang, Y.-T.; Ma, Y.-F.; Li, R.; You, W.-W.; Zhao, P.-L. Design, synthesis, and biological evaluation of N-(pyridin-3-yl) pyrimidin-4-amine analogues as novel cyclin-dependent kinase 2 inhibitors for cancer therapy. Bioorg. Chem. 2024, 143, 107019. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H.; Wang, Y.; Liu, Z.; Feng, B.; Tang, C. Synthesis and biological evaluation of novel 5, 6-dihydropyrimido [4,5-f] quinazoline derivatives as potent CDK2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Heiba, H.I.; Elsayed, M.S.; Ghorab, W.M. Design, synthesis and molecular modeling study of certain 4-Methylbenzenesulfonamides with CDK2 inhibitory activity as anticancer and radio-sensitizing agents. Bioorg. Chem. 2018, 80, 276–287. [Google Scholar] [CrossRef]
- Sabt, A.; Eldehna, W.M.; Al-Warhi, T.; Alotaibi, O.J.; Elaasser, M.M.; Suliman, H.; Abdel-Aziz, H.A. Discovery of 3, 6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: Synthesis, biological evaluation and in silico insights. J. Enzym. Inhib. Med. Chem. 2020, 35, 1616–1630. [Google Scholar] [CrossRef]
- Hawash, M.; Baytas, S. Antiproliferative Activities of Some Biologically Important Scaffold. FABAD J. Pharm. Sci 2017, 43, 59–77. [Google Scholar]
- Hawash, M. Thiazole Derivatives as Modulators of GluA2 AMPA Receptors: Potent Allosteric Effects and Neuroprotective Potential. Biomolecules 2023, 13, 1694. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Sabobeh, R.; Abualhasan, M.; Qaoud, M.T. New Thiazole Carboxamide Derivatives as COX Inhibitors: Design, Synthesis, Anticancer Screening, In Silico Molecular Docking, and ADME Profile Studies. ACS Omega 2023, 8, 29512–29526. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Abualhasan, M.; Şüküroğlu, M.K.; Qaoud, M.T.; Kahraman, D.C.; Daraghmeh, H.; Maslamani, L.; Sawafta, M.; Ratrout, A.; et al. Design, synthesis, molecular docking studies and biological evaluation of thiazole carboxamide derivatives as COX inhibitors. BMC Chem. 2023, 17, 11. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; El-Hashash, M.A.; Elkaeed, E.B. Eco-friendly sequential one-pot synthesis, molecular docking, and anticancer evaluation of arylidene-hydrazinyl-thiazole derivatives as CDK2 inhibitors. Bioorg. Chem. 2021, 108, 104615. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Nossier, E.S.; Almehizia, A.A.; Amr, A.E.-G.E. Design, synthesis, anticancer evaluation and molecular docking study of novel 2,4-dichlorophenoxymethyl-based derivatives linked to nitrogenous heterocyclic ring systems as potential CDK-2 inhibitors. J. Mol. Struct. 2022, 1247, 131285. [Google Scholar] [CrossRef]
- Tawfeek, H.N.; Hassan, A.A.; Bräse, S.; Nieger, M.; Mostafa, Y.A.; Gomaa, H.A.; Youssif, B.G.; El-Shreef, E.M. Design, synthesis, crystal structures and biological evaluation of some 1,3-thiazolidin-4-ones as dual CDK2/EGFR potent inhibitors with potential apoptotic antiproliferative effects. Arab. J. Chem. 2022, 15, 104280. [Google Scholar] [CrossRef]
- Abdullah, J.A.; Aldahham, B.J.; Rabeea, M.A.; Asmary, F.A.; Alhajri, H.M.; Islam, M.A. Synthesis, characterization and in-silico assessment of novel thiazolidinone derivatives for cyclin-dependent kinases-2 inhibitors. J. Mol. Struct. 2021, 1223, 129311. [Google Scholar] [CrossRef]
- Manda, R.R.; Nadh, R.V.; Viveka, T.L.; Angajala, G.; Aruna, V. New benzylidene festooned thiazolidinone-coumarin molecular hybrids targeting Human Breast adenocarcinoma cells: Design, synthesis, SAR, molecular Modelling and biological evaluation as CDK2 inhibitors. J. Mol. Struct. 2023, 1285, 135453. [Google Scholar] [CrossRef]
- Fatahala, S.S.; Sayed, A.I.; Mahgoub, S.; Taha, H.; El-Sayed, M.-I.K.; El-Shehry, M.F.; Awad, S.M.; Abd El-Hameed, R.H. Synthesis of novel 2-Thiouracil-5-sulfonamide derivatives as potent inducers of cell cycle arrest and CDK2A inhibition supported by molecular docking. Int. J. Mol. Sci. 2021, 22, 11957. [Google Scholar] [CrossRef]
- Sayed, E.M.; Bakhite, E.A.; Hassanien, R.; Farhan, N.; Aly, H.F.; Morsy, S.G.; Hassan, N.A. Novel tetrahydroisoquinolines as DHFR and CDK2 inhibitors: Synthesis, characterization, anticancer activity and antioxidant properties. BMC Chem. 2024, 18, 34. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.J. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; Karasic, T.B.; DeMichele, A.; Vaughn, D.J.; O’Hara, M.; Perini, R.; Zhang, P.; Lal, P.; Feldman, M.; Gallagher, M.; et al. Palbociclib (PD0332991)—A Selective and Potent Cyclin-Dependent Kinase Inhibitor: A Review of Pharmacodynamics and Clinical Development. JAMA Oncol. 2016, 2, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, H.; Liu, W.; Yao, Z.; Cheng, Z.; Zhang, H.; Zou, H. A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in gliobastoma therapy. Eur. J. Med. Chem. 2018, 144, 1–28. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Q.; Liao, X.; Ge, H.; Huo, G.; Zhang, L.; Chen, N.; Zhai, X.; Hong, Y.; Wang, L. Discovery of 6-(2-(dimethylamino) ethyl)-N-(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo [d] imidazole-6-yl) pyrimidin-2-yl)-5,6,7,8-tetrahydro-1,6-naphthyridin-2-amine as a highly potent cyclin-dependent kinase 4/6 inhibitor for treatment of cancer. Eur. J. Med. Chem. 2019, 178, 352–364. [Google Scholar] [CrossRef]
- Bronner, S.M.; Merrick, K.A.; Murray, J.; Salphati, L.; Moffat, J.G.; Pang, J.; Sneeringer, C.J.; Dompe, N.; Cyr, P.; Purkey, H. Design of a brain-penetrant CDK4/6 inhibitor for glioblastoma. Bioorg. Med. Chem. Lett. 2019, 29, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alsalam, E.; Hafez, H.N.; Assay, M.G.; Ali, A.K.; El-Farargy, A.F.; Abbass, E.M. Exploring the Antiproliferative Potency of Pyrido [2,3-d] Pyrimidine Derivatives: Studies on Design, Synthesis, Anticancer Evaluation, SAR, Docking, and DFT Calculations. Chem. Biodivers. 2024, 21, e202301682. [Google Scholar] [CrossRef]
- Kumar, A.; Bhagat, K.K.; Singh, A.K.; Singh, H.; Angre, T.; Verma, A.; Khalilullah, H.; Jaremko, M.; Emwas, A.-H.; Kumar, P. Medicinal chemistry perspective of pyrido [2,3-d] pyrimidines as anticancer agents. RSC Adv. 2023, 13, 6872–6908. [Google Scholar] [CrossRef]
- Wang, H.; Ba, J.; Kang, Y.; Gong, Z.; Liang, T.; Zhang, Y.; Qi, J.; Wang, J. Recent Progress in CDK4/6 Inhibitors and PROTACs. Molecules 2023, 28, 8060. [Google Scholar] [CrossRef]
- Abbas, S.E.; George, R.F.; Samir, E.M.; Aref, M.M.; Abdel-Aziz, H.A. Synthesis and anticancer activity of some pyrido [2,3-d] pyrimidine derivatives as apoptosis inducers and cyclin-dependent kinase inhibitors. Future Med. Chem. 2019, 11, 2395–2414. [Google Scholar] [CrossRef]
- Al-Attraqchi, O.H.A.; Mordi, M.N. 2D-and 3D-QSAR, molecular docking, and virtual screening of pyrido [2,3-d] pyrimidin-7-one-based CDK4 inhibitors. J. Appl. Pharm. Sci. 2021, 12, 165–175. [Google Scholar]
- Fang, L.; Chu, M.; Yan, C.; Liu, Y.; Zhao, Z. Palbociclib and Michael-acceptor hybrid compounds as CDK4/6 covalent inhibitors: Improved potency, broad anticancer spectrum and overcoming drug resistance. Bioorg. Med. Chem. 2023, 84, 117263. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Quan, Y.; Wang, Y.; Wang, Y.; Li, Y. Design, synthesis, and biological evaluation of 2, 6, 7-substituted pyrrolo [2,3-d] pyrimidines as cyclin dependent kinase inhibitor in pancreatic cancer cells. Bioorg. Med. Chem. Lett. 2021, 33, 127725. [Google Scholar] [CrossRef] [PubMed]
- Sroor, F.M.; Tohamy, W.M.; Zoheir, K.M.; Abdelazeem, N.M.; Mahrous, K.F.; Ibrahim, N.S. Design, synthesis, in vitro anticancer, molecular docking and SAR studies of new series of pyrrolo [2,3-d] pyrimidine derivatives. BMC Chem. 2023, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Y.; Zhang, C.; Huang, Z.; Wang, X.; Nie, Y.; Li, Y.; Liu, Y.; Yang, S.; Xiang, R. Selective and novel cyclin-dependent kinases 4 inhibitor: Synthesis and biological evaluation. Med. Chem. Res. 2018, 27, 1666–1678. [Google Scholar] [CrossRef]
- Divya, V.; Pushpa, V.; Manoj, K. Cyclin dependent kinase 4 inhibitory activity of Thieno [2,3-d] pyrimidin-4-ylhydrazones–Multiple QSAR and docking studies. J. Mol. Struct. 2019, 1183, 263–273. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Ma, Y.-F.; Jian, X.-E.; Ji, J.-H.; You, W.-W.; Zhao, P.-L. Development of pteridin-7 (8H)-one analogues as highly potent cyclin-dependent kinase 4/6 inhibitors: Synthesis, structure-activity relationship, and biological activity. Bioorg. Chem. 2021, 116, 105324. [Google Scholar] [CrossRef]
- Ali, A.M.; Tawfik, S.S.; Bhongade, B.A.; Massoud, M.A.; Mostafa, A.S. Design, synthesis, and in silico insights into dual-inhibition of CDK-6/Aurora A kinase by 2-phenylbenzimidazole-based small molecules. J. Mol. Struct. 2024, 1300, 137215. [Google Scholar] [CrossRef]
- Yousuf, M.; Shamsi, A.; Khan, P.; Shahbaaz, M.; AlAjmi, M.F.; Hussain, A.; Hassan, G.M.; Islam, A.; Rizwanul Haque, Q.M.; Hassan, M.I. Ellagic acid controls cell proliferation and induces apoptosis in breast cancer cells via inhibition of cyclin-dependent kinase 6. Int. J. Mol. Sci. 2020, 21, 3526. [Google Scholar] [CrossRef]
- Fultang, N.; Schwab, A.M.; McAneny-Droz, S.; Heiser, D.; Scherle, P.; Bhagwat, N. PRT2527, a novel highly selective cyclin-dependent kinase 9 (CDK9) inhibitor, has potent antitumor activity in combination with BTK and BCL2 inhibition in various lymphoid malignancies. Cancer Res. 2024, 84 (Suppl. S6), 5966. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, L.; Tang, W.; Ge, Y.; Li, C.; Zhang, J. Recent Discovery and Development of Inhibitors that Target CDK9 and Their Therapeutic Indications. J. Med. Chem. 2024, 67, 5185–5215. [Google Scholar] [CrossRef]
- Walker, R.L.; Hornicek, F.J.; Duan, Z. Transcriptional regulation and therapeutic potential of cyclin-dependent kinase 9 (CDK9) in sarcoma. Biochem. Pharmacol. 2024, 226, 116342. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Y.; Hsieh, M.-S.; Lee, Y.-H.; Hsu, H.-W.; Wo, R.R.; Wang, H.-Y.; Cheng, A.-L.; Hsu, C.-H. Cyclin dependent kinase 9 inhibition reduced programmed death-ligand 1 expression and improved treatment efficacy in hepatocellular carcinoma. Heliyon 2024, 10, e34289. [Google Scholar] [CrossRef]
- Poon, E.; Liang, T.; Jamin, Y.; Walz, S.; Kwok, C.; Hakkert, A.; Barker, K.; Urban, Z.; Thway, K.; Zeid, R.; et al. Orally bioavailable CDK9/2 inhibitor shows mechanism-based therapeutic potential in MYCN-driven neuroblastoma. J. Clin. Investig. 2020, 130, 5875–5892. [Google Scholar] [CrossRef] [PubMed]
- Cidado, J.; Boiko, S.; Proia, T.; Ferguson, D.; Criscione, S.W.; San Martin, M.; Pop-Damkov, P.; Su, N.; Roamio Franklin, V.N.; Sekhar Reddy Chilamakuri, C.; et al. AZD4573 Is a Highly Selective CDK9 Inhibitor That Suppresses MCL-1 and Induces Apoptosis in Hematologic Cancer Cells. Clin. Cancer Res. 2020, 26, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, J.; Zhou, R.; Tang, L.; Ding, S.; Ren, Z.; Song, N.; Hu, B.; Yang, H.; Sun, Y. Identification of a Novel Selective CDK9 Inhibitor for the Treatment of CRC: Design, Synthesis, and Biological Activity Evaluation. J. Med. Chem. 2024, 67, 4739–4756. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, Y.; Chen, H.; Shen, L. Targeting CDK9 with selective inhibitors or degraders in tumor therapy: An overview of recent developments. Cancer Biol. Ther. 2023, 24, 2219470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, C.; Gordon, J.; Yu, S.; Morton, G.; Childers, W.; Abou-Gharbia, M.; Zhang, Y.; Jelinek, J.; Issa, J.-P.J. MC180295 is a highly potent and selective CDK9 inhibitor with preclinical in vitro and in vivo efficacy in cancer. Clin. Epigenetics 2024, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, N.M.; Farouk, F.; George, R.F.; Abbas, S.E.; El-Badry, O.M. Design and synthesis of novel imidazo [4,5-b] pyridine based compounds as potent anticancer agents with CDK9 inhibitory activity. Bioorg. Chem. 2018, 80, 565–576. [Google Scholar] [CrossRef]
- Ihmaid, S.K.; Aljuhani, A.; Alsehli, M.; Rezki, N.; Alawi, A.; Aldhafiri, A.J.; Salama, S.A.; Ahmed, H.E.; Aouad, M.R. Discovery of triaromatic flexible agents bearing 1, 2, 3-Triazole with selective and potent anti-breast cancer activity and CDK9 inhibition supported by molecular dynamics. J. Mol. Struct. 2022, 1249, 131568. [Google Scholar] [CrossRef]
- Yu, Z.; Du, J.; Zhao, Y.; Gao, Y.; Li, Y.; Zhao, K.; Lu, N. A novel kinase inhibitor, LZT-106, downregulates Mcl-1 and sensitizes colorectal cancer cells to BH3 mimetic ABT-199 by targeting CDK9 and GSK-3β signaling. Cancer Lett. 2021, 498, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, B.; Liu, Z.; Gou, S. Design, synthesis and anticancer evaluation of selective 2,4-disubstituted pyrimidine CDK9 inhibitors. Eur. J. Med. Chem. 2022, 244, 114875. [Google Scholar] [CrossRef]
- Gao, G.; Li, J.; Cao, Y.; Li, X.; Qian, Y.; Wang, X.; Li, M.; Qiu, Y.; Wu, T.; Wang, L. Design, synthesis, and biological evaluation of novel 4, 4′-bipyridine derivatives acting as CDK9-Cyclin T1 protein-protein interaction inhibitors against triple-negative breast cancer. Eur. J. Med. Chem. 2023, 261, 115858. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, J.; Ding, S.; Cao, H.; Zhang, Y.; Hu, B.; Han, S.; Yang, H.; Cheng, M.; Li, J. Discovery of novel CDK9 inhibitor with tridentate ligand: Design, synthesis and biological evaluation. Bioorg. Chem. 2024, 150, 107550. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Sorio, C. Current views on the interplay between tyrosine kinases and phosphatases in Chronic Myeloid Leukemia. Cancers 2021, 13, 2311. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Rahman, F.; Rahaman, M.S.; Khan, M.S.; Abrar, S.; Ray, T.K.; Uddin, M.B.; Kali, M.S.K.; Dua, K. Emerging promise of computational techniques in anti-cancer research: At a glance. Bioengineering 2022, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, Y.; Wang, Y.; Zhang, F. IDDF2024-ABS-0250 Washed microbiota transplantation is favorable for relieving the gastrointestinal adverse effects induced by tyrosine kinase inhibitor. Gut 2024, 73, A328–A329. [Google Scholar]
- Song, Z.; Jiang, D.; Yu, L.; Chen, Y.; Zhou, D.; Li, Y.; Wu, D.; Zhang, L.; Miao, L.; Ma, J. Evidence-based expert consensus on clinical management of safety of Bruton’s tyrosine kinase inhibitors (2024). Chin. J. Cancer Res. 2024, 36, 240. [Google Scholar]
- Jha, K.T.; Shome, A.; Chawla, P.A. Recent advances in nitrogen-containing heterocyclic compounds as receptor tyrosine kinase inhibitors for the treatment of cancer: Biological activity and structural activity relationship. Bioorg. Chem. 2023, 138, 106680. [Google Scholar]
- Wirsik, N.M.; Chen, M.; He, L.; Yasar, U.; Gaukel, J.; Quaas, A.; Nienhüser, H.; Leugner, E.; Yuan, S.; Schleussner, N. Targeting the receptor tyrosine kinase MerTK shows therapeutic value in gastric adenocarcinoma. Cancer Med. 2024, 13, e6866. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, H.; Wang, Y.; Yang, J.; Lin, C.; Shi, X.; Zou, Y.; Chen, J.; Jia, X.; Su, L. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer. Oncol. Rep. 2024, 51, 16. [Google Scholar] [CrossRef]
- Gomatou, G.; Syrigos, N.; Kotteas, E. Osimertinib resistance: Molecular mechanisms and emerging treatment options. Cancers 2023, 15, 841. [Google Scholar] [CrossRef]
- Jathal, M.K.; Mudryj, M.M.; Ghosh, P.M. Role of Nuclear ErbB3 localization in regulating ligand-dependent ErbB2-ErbB3 heterodimer activation and downstream targets in prostate cancer. Cancer Res. 2024, 84 (Suppl. S6), 6264. [Google Scholar] [CrossRef]
- Sompallae, R.R.; Dundar, B.; Guseva, N.V.; Bossler, A.D.; Ma, D. EGFR and ERBB2 exon 20 insertion/duplication in advanced non–small cell lung cancer: Genomic profiling and clinicopathologic features. Front. Oncol. 2023, 13, 1163485. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chang, A.Y.-C. Molecular mechanism of action and potential biomarkers of growth inhibition of synergistic combination of afatinib and dasatinib against gefitinib-resistant non-small cell lung cancer cells. Oncotarget 2018, 9, 16533–16546. [Google Scholar] [CrossRef] [PubMed]
- See, H.; Kavanagh, J.J.; Hu, W.; Bast, R. Targeted therapy for epithelial ovarian cancer: Current status and future prospects. Int. J. Gynecol. Cancer 2003, 13, 701–734. [Google Scholar] [CrossRef] [PubMed]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Erlotinib. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 45, pp. 93–117. [Google Scholar]
- Steins, M.; Thomas, M.; Geißler, M. Erlotinib. In Small Molecules in Oncology; Springer: Cham, Switzerland, 2018; pp. 1–17. [Google Scholar]
- Ryan, Q.; Ibrahim, A.; Cohen, M.H.; Johnson, J.; Ko, C.-w.; Sridhara, R.; Justice, R.; Pazdur, R. FDA drug approval summary: Lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist 2008, 13, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.L.; Vellanki, P.J.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Shen, Y.L.; Xia, H.; Li, Y.; Liu, J.; Zirkelbach, J.F. FDA approval summary: Osimertinib for adjuvant treatment of surgically resected non–small cell lung cancer, a Collaborative Project Orbis Review. Clin. Cancer Res. 2021, 27, 6638–6643. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shao, W.; Zhou, C.; Yang, H.; Yang, L.; Cai, Q.; Wang, J.; Shi, Y.; Huang, L.; Zhang, J. Neratinib for HER2-positive breast cancer with an overlooked option. Mol. Med. 2023, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Dacomitinib: First global approval. Drugs 2018, 78, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Horiba, M.N.; Yuan, M.; Cheng, J.; Lemery, S.J.; Shen, Y.L.; Fu, W.; Moore, J.N.; Li, Y.; Bi, Y. FDA approval summary: Tucatinib with trastuzumab for advanced unresectable or metastatic, chemotherapy refractory, HER2-positive RAS wild-type colorectal cancer. Clin. Cancer Res. 2023, 29, 4326–4330. [Google Scholar] [CrossRef]
- Duke, E.S.; Stapleford, L.; Drezner, N.; Amatya, A.K.; Mishra-Kalyani, P.S.; Shen, Y.-L.; Maxfield, K.; Zirkelbach, J.F.; Bi, Y.; Liu, J. FDA approval summary: Mobocertinib for metastatic non–small cell lung cancer with EGFR Exon 20 Insertion Mutations. Clin. Cancer Res. 2023, 29, 508–512. [Google Scholar] [CrossRef]
- Al-Rubaye, I.M.; Mahmood, A.A.R.; Tahtamouni, L.H.; AlSakhen, M.F.; Kanaan, S.I.; Saleh, K.M.; Yasin, S.R. In silico and in vitro evaluation of novel carbothioamide-based and heterocyclic derivatives of 4-(tert-butyl)-3-methoxybenzoic acid as EGFR tyrosine kinase allosteric site inhibitors. Results Chem. 2024, 7, 101329. [Google Scholar] [CrossRef]
- Șandor, A.; Ionuț, I.; Marc, G.; Oniga, I.; Eniu, D.; Oniga, O. Structure–activity relationship studies based on quinazoline derivatives as EGFR kinase inhibitors (2017–present). Pharmaceuticals 2023, 16, 534. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.; Xia, S.; Liu, Q.; Gou, S. Design, synthesis and biological evaluation of sulfamoylphenyl-quinazoline derivatives as potential EGFR/CAIX dual inhibitors. Eur. J. Med. Chem. 2021, 216, 113300. [Google Scholar] [CrossRef] [PubMed]
- Raghu, M.; Swarup, H.; Shamala, T.; Prathibha, B.; Kumar, K.Y.; Alharethy, F.; Prashanth, M.; Jeon, B.-H. Design, synthesis, anticancer activity and docking studies of novel quinazoline-based thiazole derivatives as EGFR kinase inhibitors. Heliyon 2023, 9, e20300. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; El Kerdawy, A.M.; Said, M.A.; Albohy, A.; Elsayed, Z.M.; Aljaeed, N.; Elkaeed, E.B.; Eldehna, W.M.; Abdel-Aziz, H.A.; Abdelmoaz, M.A. Novel 2-(5-Aryl-4,5-dihydropyrazol-1-yl) thiazol-4-one as EGFR inhibitors: Synthesis, biological assessment and molecular docking insights. Drug Des. Dev. Ther. 2023, 16, 1457–1471. [Google Scholar] [CrossRef]
- Tang, S.; Sun, C.; He, X.; Gan, W.; Wang, L.; Qiao, D.; Guan, X.; Xu, S.; Zheng, P.; Zhu, W. Design, synthesis, and biological evaluation of 4-(2-fluorophenoxy)-7-methoxyquinazoline derivatives as dual EGFR/c-Met inhibitors for the treatment of NSCLC. Eur. J. Med. Chem. 2024, 263, 115939. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; El-Hddad, S.S.; Aljohani, A.K.; Khedr, F.; Alatawi, O.M.; Keshek, D.E.; Ahmed, S.; Alsulaimany, M.; Almadani, S.A.; El-Adl, K. Iodoquinazoline-derived VEGFR-2 and EGFRT790M dual inhibitors: Design, synthesis, molecular docking and anticancer evaluations. Bioorg. Chem. 2024, 143, 107062. [Google Scholar] [CrossRef]
- Mohassab, A.M.; Hassan, H.A.; Abou-Zied, H.A.; Fujita, M.; Otsuka, M.; Gomaa, H.A.; Youssif, B.G.; Abdel-Aziz, M. Design and synthesis of new quinoline-ester/-amide derivatives as potent antiproliferative agent targeting EGFR and BRAFV600E kinases. J. Mol. Struct. 2024, 1297, 136953. [Google Scholar] [CrossRef]
- Ibrahim, M.T.; Tabti, K.; Abdulsalam, S.; Tahir, A.S.; Mahmoud, A.; Danmallam, A.M. Discovery of new 2,4-diaminopyrimidines derivatives as EGFRT790M kinase inhibitors: A structure-based approach with DFT calculation, drug-likeness, ADME-toxicity properties evaluation and MD simulation. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 257–273. [Google Scholar] [CrossRef]
- Morjan, R.Y.; El-Hallaq, A.F.; Azarah, J.N.; Almasri, I.M.; Alzaharna, M.M.; Al-Reefi, M.R.; Beadham, I.; Abu-Teim, O.S.; Elmanama, A.A.; Awadallah, A.M. Synthesis, docking and evaluation of novel fused pyrimidine compounds as possible lead compounds with antibacterial and antitumor activities. J. Mol. Struct. 2023, 1288, 135754. [Google Scholar] [CrossRef]
- Ding, S.; Gao, Z.; Hu, Z.; Qi, R.; Zheng, X.; Dong, X.; Zhang, M.; Shen, J.; Long, T.; Zhu, Y. Design, synthesis and biological evaluation of novel osimertinib derivatives as reversible EGFR kinase inhibitors. Eur. J. Med. Chem. 2022, 238, 114492. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.M.; Alanazi, A.S. Novel 7-Deazapurine Incorporating Isatin Hybrid Compounds as Protein Kinase Inhibitors: Design, Synthesis, In Silico Studies, and Antiproliferative Evaluation. Molecules 2023, 28, 5869. [Google Scholar] [CrossRef]
- Elsebaie, H.A.; El-Bastawissy, E.A.; Elberembally, K.M.; Khaleel, E.F.; Badi, R.M.; Shaldam, M.A.; Eldehna, W.M.; Tawfik, H.O.; El-Moselhy, T.F. Novel 4-(2-arylidenehydrazineyl) thienopyrimidine derivatives as anticancer EGFR inhibitors: Design, synthesis, biological evaluation, kinome selectivity and in silico insights. Bioorg. Chem. 2023, 140, 106799. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, N.R.; Sindkhedkar, M.; Joseph, A. Computational Design, Synthesis, and Bioevaluation of 2-(Pyrimidin-4-yl) oxazole-4-carboxamide Derivatives: Dual Inhibition of EGFRWT and EGFRT790M with ADMET Profiling. Bioorg. Chem. 2024, 143, 107027. [Google Scholar]
- Hassan, O.M.; Kubba, A.; Tahtamouni, L.H. Novel 5-bromoindole-2-carboxylic acid derivatives as EGFR inhibitors: Synthesis, docking study, and structure activity relationship. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2023, 23, 1336–1348. [Google Scholar] [CrossRef]
- Mishra, S.; Sahu, A.; Kaur, A.; Kaur, M.; Wal, P. Recent Development in the Search for Epidermal Growth Factor Receptor (EGFR) Inhibitors based on the Indole Pharmacophore. Curr. Top. Med. Chem. 2024, 24, 581–613. [Google Scholar] [CrossRef]
- Olgen, S.; Kaleli, S.N.B.; Karaca, B.T.; Demire, U.U.; Bristow, H.K. Synthesis and anticancer activity of novel indole derivatives as dual EGFR/SRC kinase inhibitors. Curr. Med. Chem. 2024, 31, 3798–3817. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Mohammed, A.F.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, synthesis, and antiproliferative activity of new 5-chloro-indole-2-carboxylate and pyrrolo [3,4-b] indol-3-one derivatives as potent inhibitors of egfrt790m/brafv600e pathways. Molecules 2023, 28, 1269. [Google Scholar] [CrossRef] [PubMed]
- Dubba, A.; Koppula, S.K. Synthesis of Indole-Oxadiazole coupled isoxazole hybrids as potent EGFR targeting anticancer agents. Chem. Biol. Lett. 2024, 11, 651. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, B.; Dai, Q.-Q.; Zhang, X.-N.; Zhang, J.-Y.; Zuo, J.-H.; Liu, H.; Chen, Z.-S.; Li, G.-B.; Wang, S. Discovery of new 4-indolyl quinazoline derivatives as highly potent and orally bioavailable P-glycoprotein inhibitors. J. Med. Chem. 2021, 64, 14895–14911. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Du, L.; Dai, Q.; Li, G.; Yu, B.; Chang, L. Design, synthesis and biological evaluation of structurally new 4-indolyl quinazoline derivatives as highly potent, selective and orally bioavailable EGFR inhibitors. Bioorg. Chem. 2024, 142, 106970. [Google Scholar] [CrossRef] [PubMed]
| Drug Name | Chemical Structure | Year | Therapeutic Indications |
|---|---|---|---|
| Palbociclib | 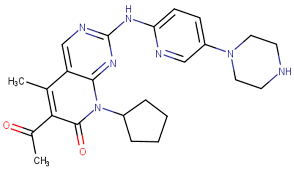 | 2015 | Breast cancer combination therapy [56] |
| Abemaciclib | 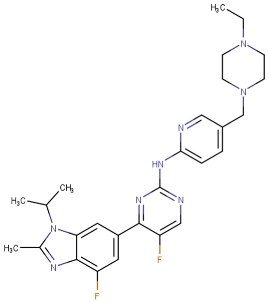 | 2017 | Breast cancer [57] |
| Ribociclib | 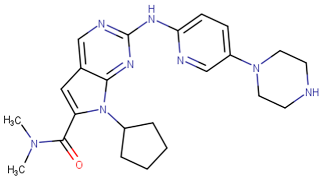 | 2017 | Breast cancer combination therapy [58] |
| Trilaciclib | 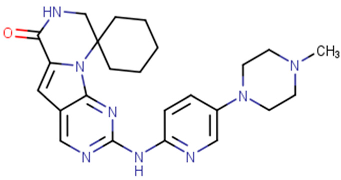 | 2021 | Myeloprotective agent [59] |
| CDKs | Cyclin Partner | Biological Role | Type of Cancer | Ref. |
|---|---|---|---|---|
| CDK1 | B | Regulate M phase | Breast, ovarian, lung | [65,66,67] |
| CDK2 | A | Regulate G1-S phases, Rb-E2F pathway | Breast, ovarian, lung, prostate and many others | [62,68] |
| E | ||||
| CDK4 | D | Regulate G1 phase, Rb-E2F pathway | Skin, breast, bladder, lung | [69,70] |
| CDK6 | D | Regulate G1 phase, Rb-E2F pathway | Bladder, esophageal, gastric, head and neck, pancreatic | [67,71] |
| CDK9 | T | DNA damage repair and RNAPII transcription | Breast, lung, cervical, and many others | [72,73] |
| Code | Structure | Evaluated Cancer Cell Lines | CDK4 | CDK6 | Ref. | |
|---|---|---|---|---|---|---|
| Cell Lines | IC50 | IC50 | ||||
| St.26 | 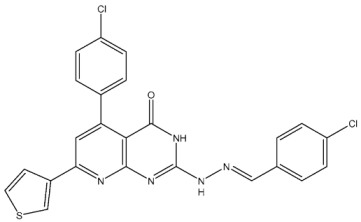 | MCF-7 A549 PC-3 | 1.59 µM 2.48 µM 0.01 µM | - | 115.38 nM | [113] |
| St.27 | 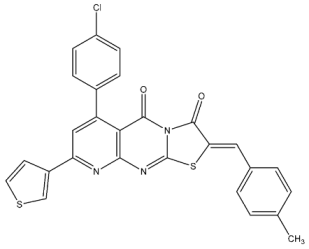 | MCF-7 A549 PC-3 | 0.01 µM 1.69 µM 1.37 µM | - | 726.2 nM | |
| St.28 | 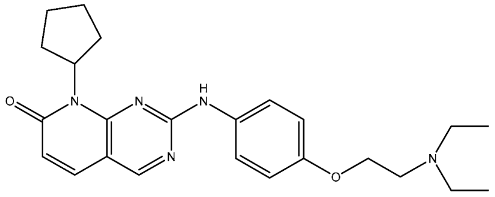 | NA | NA | pIC50 = 9.15 | [114] | |
| St.29 |  | HepG2 A549 MDA-MB-231 MCF-7 | 5.10 µM 1.55 µM 0.51 µM 0.48 µM | 18 nM | 13 nM | [115] |
| St.30 | 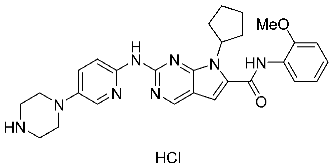 | PaCa-2 BxPc-3 | 1.83 µM 4.12 µM | 20.5 nM | 52.3 nM | [116] |
| St.31 |  | MCF-7 HepG2 | 1.70 µM 37.9 µM | Down-regulation | NA | [117] |
| St.32 | 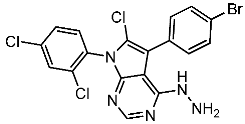 | - | - | 35.0 nM | 318 nM | [118] |
| St.33 | 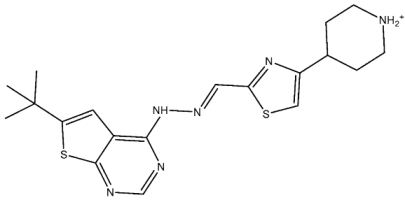 | - | - | 14 nM | - | [119] |
| Code | Structure | Evaluated Cancer Cell Lines | CDK4 | CDK6 | Ref. | |
|---|---|---|---|---|---|---|
| Cell Lines | IC50 or Inh.% | IC50 | ||||
| St.34 | 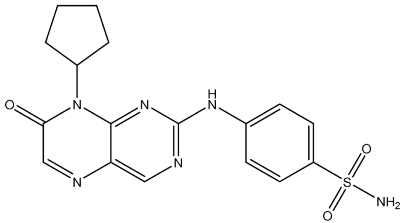 | HCT116 MDA-MB-231 HeLa HT-29 | 0.65 µM 0.39 µM 0.70 µM 2.53 µM | 34 nM | 56.1 nM | [120] |
| St.35 | 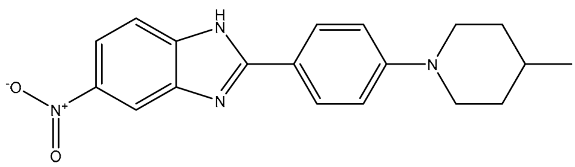 | HL-60 NCIH522 HCT-15 PC-3 MCF-7 | 99.52% 101.83% 93.85% 79.80% 71.81% | - | 172 nM | [121] |
| St.36 | 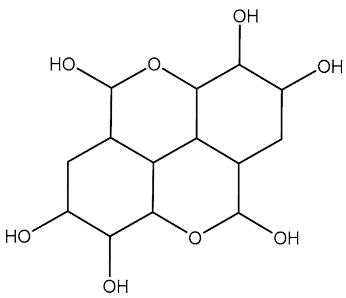 | - | - | 3.05 µM | [122] | |
| Code | Structure | Evaluated Cancer Cell Lines | CDK9 | Ref. | |
|---|---|---|---|---|---|
| Cell Lines | IC50 | IC50 | |||
| St.37 |  | MCF-7 HCT116 | 0.63 µM 5.30 µM | 0.50 µM | [132] |
| St.38 |  | MCF-7 MDA-MB231 HCT116 WI38 | 3.47 µM 1.43 µM 12.56 µM 31.96 µM | 0.38 µM | [133] |
| St.39 |  | HCT116 HT-29 RKO SW480 DLD1 | 0.69 µM 0.88 µM 0.97 µM 0.99 µM 0.71 µM | 30 nM | [134] |
| St.40 |  | A549 H1975 A431 PANC-1 HCT116 LO2 | 0.66 µM 0.43 µM 0.10 µM 0.08 µM 0.09 µM 1.43 µM | 10.4 nM | [135] |
| St.41 | 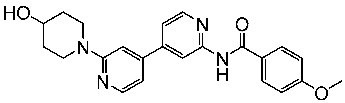 | MDA-MB BT549 HepG2 HeLa A549 | 0.044 µM 0.147 µM 0.392 µM 0.158 µM 2.30 µM | >1 µM | [136] |
| St.42 | 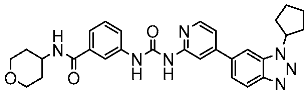 | HCT116 HT-29 | 0.21 nM 0.49 nM | 2.43 nM | [137] |
| Drug Name | Chemical Structure | Year | MOA | Therapeutic Indications |
|---|---|---|---|---|
| Gefitinib | 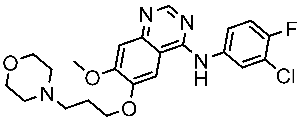 | 2003 | Inhibits intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors | NSCLC [148,149] |
| Erlotinib | 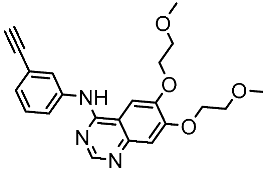 | 2004 | Targets the EGFR tyrosine kinase | NSCLC and pancreatic cancer [150,151] |
| Lapatinib | 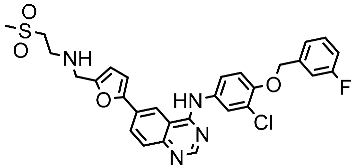 | 2007 | Inhibits intracellular tyrosine kinase domains of both EGFR (ErbB1) and HER2 | Breast cancer [152] |
| Osimertinib | 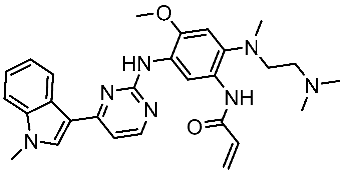 | 2015 | Irreversible inhibits the epidermal growth (ErbB) | NSCLC [153] |
| Neratinib |  | 2017 | Irreversible inhibitors of the receptor tyrosine kinase (RTK), ErbB2, RTK, and EGFR. | Breast cancer [154] |
| Dacomitinib |  | 2018 | Irreversible pan inhibitor of HER tyrosine kinases | Metastatic NSCLC [155] |
| Tucatinib | 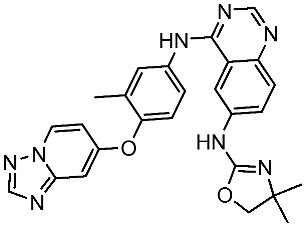 | 2020 | Inhibits ERBB2 (HER2) | Breast cancer and colon cancer [156] |
| Mobocertinib |  | 2021 | Inhibits EGFR/HER2 | NSCLC [157] |
| Code | Structure | Evaluated Cancer Cell Lines | EGFR | Ref. | |
|---|---|---|---|---|---|
| Cell Lines | IC50 | IC50 | |||
| St.43 | 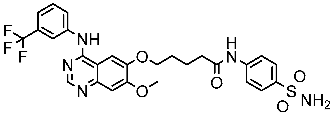 | A549 A431 H1975 | 6.54 µM 4.04 µM 1.94 µM | EGFRWt = 27 nM EGFRT790M = 9.2 nM | [160] |
| St.44 |  | MCF-7 HepG2 A549 | 2.86 µM 5.91 µM 14.79 µM | 2.17–3.62 nM | [161] |
| St.45 | 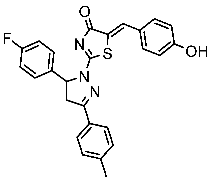 | A549 T-47D | 4.41 µM 1.15 µM | 83 nM | [162] |
| St.46 | 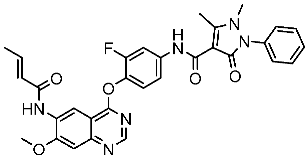 | A549 H1975 PC-9 | 1.06 µM 1.13 µM 1.05 µM | EGFRWt = 99.8nM EGFRL858R = 68.1 nM c-Met = 0.26 nM | [163] |
| St.47 | 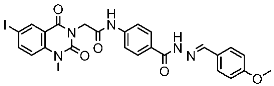 | HepG2 MCF-7 HCT116 A549 | 5.70 µM 7.15 µM 5.76 µM 6.50 µM | EGFRT790M = 0.30 µM VEGFR-2 = 0.90 µM | [164] |
| St.48 | 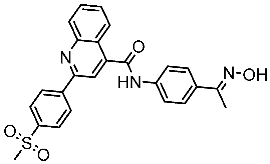 | Panc-1 MCF-7 HT29 A549 | 1.50 µM 1.10 µM 1.60 µM 1.30 µM | EGFR = 105 nM BRAFV600E = 140 nM | [165] |
| Code | Structure | Evaluated Cancer Cell Lines | EGFR or Related | Ref. | |
|---|---|---|---|---|---|
| Cell Lines | IC50 | IC50 | |||
| St.49 | 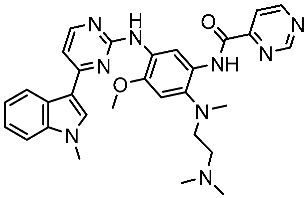 | A549 H1975 | 2.53 µM 1.56 µM | EGFRWt =2 nM EGFR L858R/T790M = 10 nM | [168] |
| St.50 | 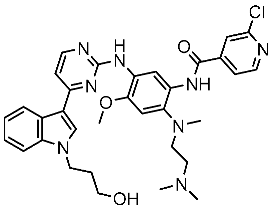 | A549 H1975 | 2.14 µM 1.82 µM | EGFRWt = 743 nM EGFR L858R/T790M = 42 nM | [168] |
| St.51 |  | HeLa MCF-7 HepG2 MDA-MB-231 | 1.98 µM 5.93 µM 6.11 µM 2.48 µM | EGFR = 103 nM VEGFR-2 = 178 nM CDK2 = 131 nM HER2 = 81 nM | [169] |
| St.52 | 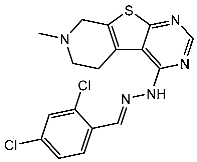 | HL-60 K-562 HOP92 COLO205 SNB-75 LOX MDA-MB-468 | 2.12 µM 2.08 µM 1.95 µM 1.66 µM 1.54 µM 1.49 µM 1.52 µM | 77 nM | [170] |
| St.53 | 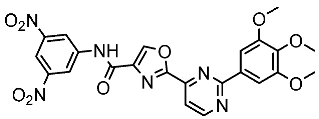 | PC3 A549 HepG2 MDA-MB-468 DU-145 | 0.13 µM 0.15 µM 0.11 µM 0.13 µM 0.10 µM. | EGFRWt = 60 nM EGFRT790M = 72 nM | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawash, M. Advances in Cancer Therapy: A Comprehensive Review of CDK and EGFR Inhibitors. Cells 2024, 13, 1656. https://doi.org/10.3390/cells13191656
Hawash M. Advances in Cancer Therapy: A Comprehensive Review of CDK and EGFR Inhibitors. Cells. 2024; 13(19):1656. https://doi.org/10.3390/cells13191656
Chicago/Turabian StyleHawash, Mohammed. 2024. "Advances in Cancer Therapy: A Comprehensive Review of CDK and EGFR Inhibitors" Cells 13, no. 19: 1656. https://doi.org/10.3390/cells13191656






