Upregulation of Fatty Acid Synthase Increases Activity of β-Catenin and Expression of NOTUM to Enhance Stem-like Properties of Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Studies
2.2. Organoid Culture
2.3. Cell Culture and Transfection
2.4. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
2.5. Protein Extraction and Western Blot Analysis
2.6. T-Cell Factor/Lymphoid Enhancer Factor (TCF/LEF) Reporter Luciferase Assay
2.7. Inhibitors
2.8. Spheroid Formation Assay
2.9. Tumor Organoid Colony Formation Assay
2.10. Click-iT Plus EdU Imaging Analysis
2.11. Human NOTUM ELISA
2.12. Soft Agar Colony Formation Assay
2.13. Cell Viability Assay
2.14. Statistical Analysis
3. Results
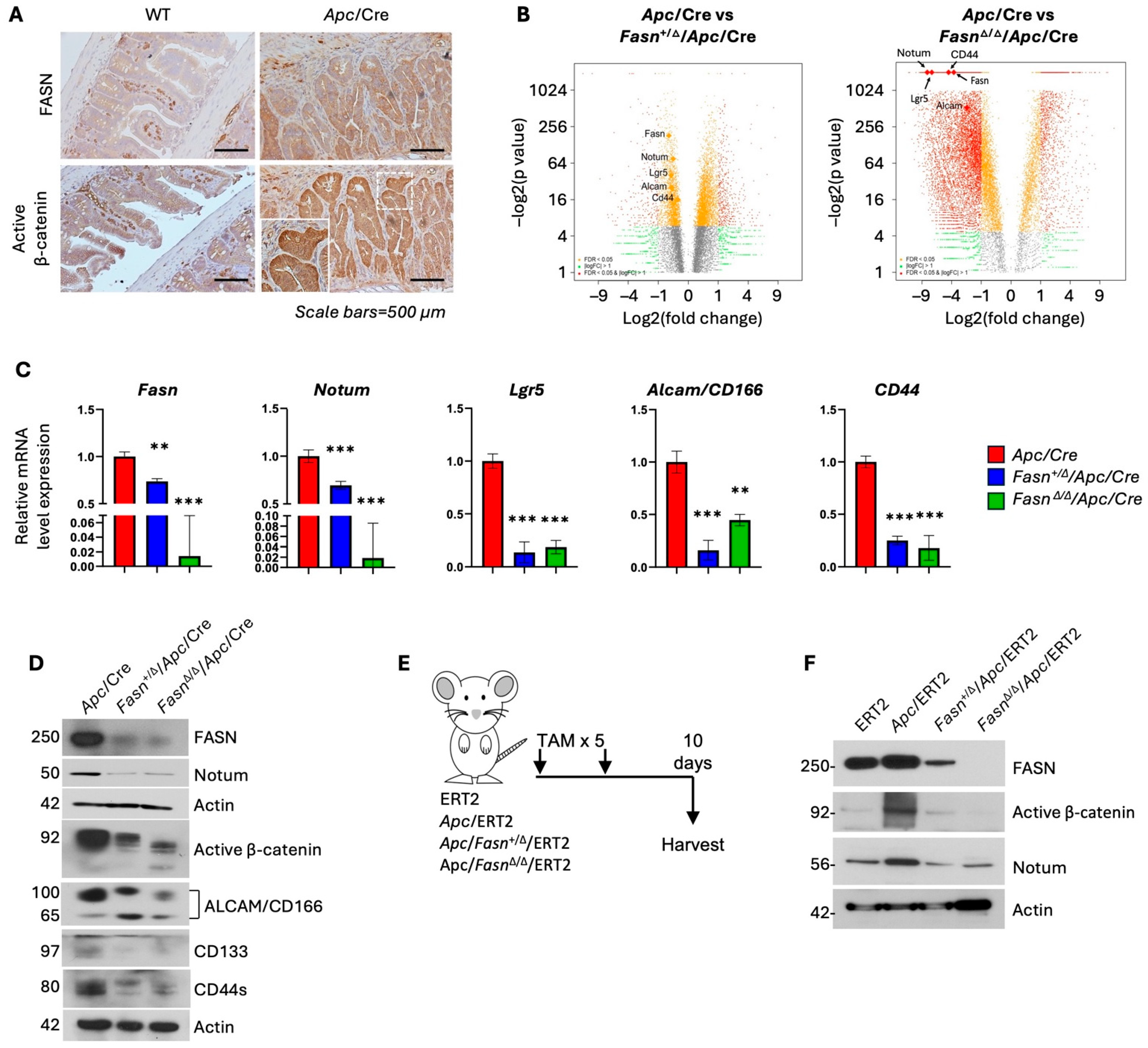
3.1. Hetero- and Homozygous Deletion of Fasn in Apc/VillinCre and Apc/VillinCre-ERT2 Mouse Models Decreases the Expression of Active β-Catenin and Stem Cell Markers
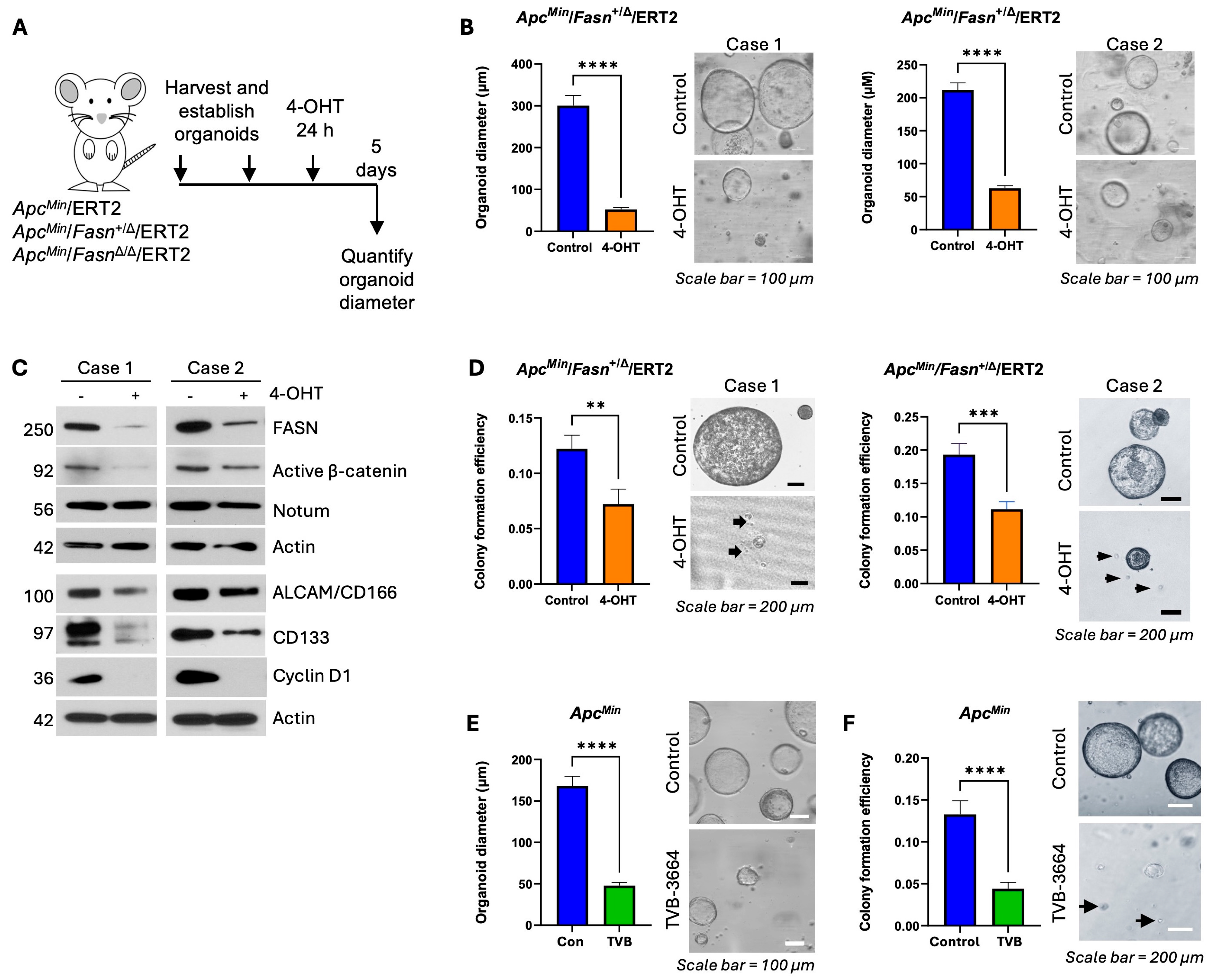
3.2. Downregulation of Fasn Decreases Expression of Active β-Catenin and Notum and Inhibits Organoid Growth and Stemness in ERT2-Inducible ApcMin Organoid Models
3.3. NOTUM Is Overexpressed and Positively Correlates with FASN Expression in Human CRC
3.4. FASN Upregulates β-Catenin Activity and Expression of NOTUM to Promote Stem-like Properties of CRC Cells
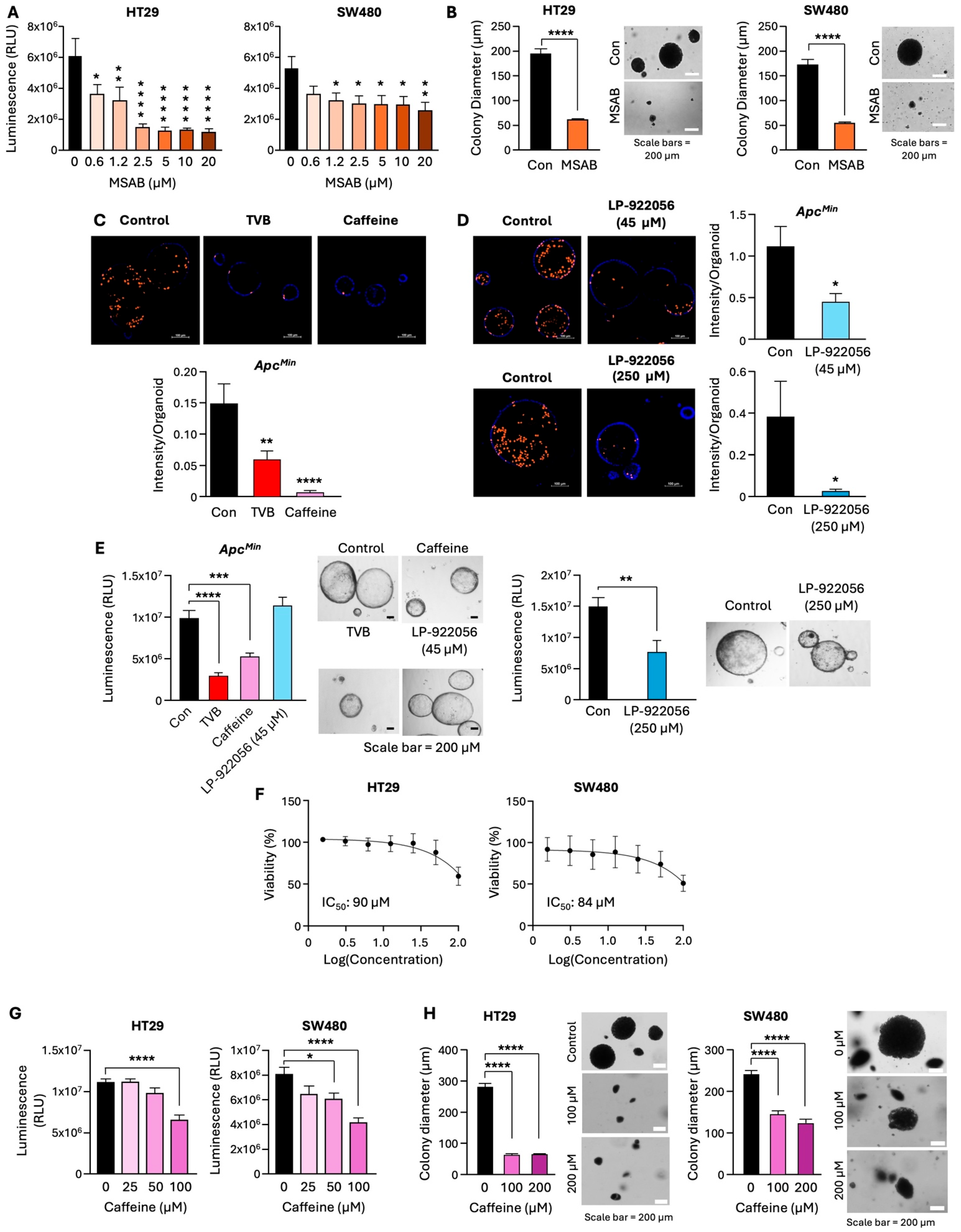
3.5. Pharmacological Inhibition of FASN Decreases β-Catenin Activity, NOTUM Expression, and Stem-like Properties of CRC Cells
3.6. Inhibition of the β-Catenin/NOTUM Axis Leads to a Decrease in Proliferation of Adenoma Organoids and CRC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Harrold, E.; Latham, A.; Pemmaraju, N.; Lieu, C.H. Early-Onset GI Cancers: Rising Trends, Genetic Risks, Novel Strategies, and Special Considerations. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e398068. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Gasnier, M.; Lim, H.Y.G.; Barker, N. Role of Wnt signaling in the maintenance and regeneration of the intestinal epithelium. Curr. Top. Dev. Biol. 2023, 153, 281–326. [Google Scholar]
- Gregorieff, A.; Clevers, H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef]
- Furuta, E.; Okuda, H.; Kobayashi, A.; Watabe, K. Metabolic genes in cancer: Their roles in tumor progression and clinical implications. Biochim. Biophys. Acta 2010, 1805, 141–152. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Rychahou, P.G.; Gulhati, P.; Elliott, V.A.; Mustain, W.C.; O’Connor, K.; Morris, A.J.; Sunkara, M.; Weiss, H.L.; Lee, E.Y.; et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012, 72, 1504–1517. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Zaytseva, Y. Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers 2021, 13, 301. [Google Scholar] [CrossRef]
- Han, L.; Dai, W.; Luo, W.; Ye, L.; Fang, H.; Mo, S.; Li, Q.; Xu, Y.; Wang, R.; Cai, G. Enhanced De Novo Lipid Synthesis Mediated by FASN Induces Chemoresistance in Colorectal Cancer. Cancers 2023, 15, 562. [Google Scholar] [CrossRef]
- Drury, J.; Young, L.E.A.; Scott, T.L.; Kelson, C.O.; He, D.; Liu, J.; Wu, Y.; Wang, C.; Weiss, H.L.; Fan, T.; et al. Tissue-Specific Downregulation of Fatty Acid Synthase Suppresses Intestinal Adenoma Formation via Coordinated Reprograming of Transcriptome and Metabolism in the Mouse Model of Apc-Driven Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, J.; Su, C. Role of Aberrant Lipid Metabolism of Cancer Stem Cells in Cancer Progression. Curr. Cancer Drug Targets 2021, 21, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Kelson, C.O.; Zaytseva, Y.Y. Altered lipid metabolism in APC-driven colorectal cancer: The potential for therapeutic intervention. Front. Oncol. 2024, 14, 1343061. [Google Scholar] [CrossRef] [PubMed]
- Bayle, E.D.; Svensson, F.; Atkinson, B.N.; Steadman, D.; Willis, N.J.; Woodward, H.L.; Whiting, P.; Vincent, J.-P.; Fish, P.V. Carboxylesterase Notum Is a Druggable Target to Modulate Wnt Signaling. J. Med. Chem. 2021, 64, 4289–4311. [Google Scholar] [CrossRef]
- Atkinson, B.N.; Willis, N.J.; Zhao, Y.; Patel, C.; Frew, S.; Costelloe, K.; Magno, L.; Svensson, F.; Jones, E.Y.; Fish, P.V. Designed switch from covalent to non-covalent inhibitors of carboxylesterase Notum activity. Eur. J. Med. Chem. 2023, 251, 115132. [Google Scholar] [CrossRef]
- Flanagan, D.J.; Pentinmikko, N.; Luopajärvi, K.; Willis, N.J.; Gilroy, K.; Raven, A.P.; Mcgarry, L.; Englund, J.I.; Webb, A.T.; Scharaw, S.; et al. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature 2021, 594, 430–435. [Google Scholar] [CrossRef]
- Wang, H.; Gong, P.; Chen, T.; Gao, S.; Wu, Z.; Wang, X.; Li, J.; Marjani, S.L.; Costa, J.; Weissman, S.M.; et al. Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Adv. Sci. 2021, 8, 2004320. [Google Scholar] [CrossRef]
- De Robertis, M.; Arigoni, M.; Loiacono, L.; Riccardo, F.; Calogero, R.A.; Feodorova, Y.; Tashkova, D.; Belovejdov, V.; Sarafian, V.; Cavallo, F.; et al. Novel insights into Notum and glypicans regulation in colorectal cancer. Oncotarget 2015, 6, 41237–41257. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zheng, Y.; Fan, Y.; Yang, Y.; Wang, Y.; Qin, L.; An, Y.; Xu, X.; Zhang, X.; Sun, G.; et al. Enhanced Apc(Min/+) adenoma formation after epithelial CUL4B deletion by recruitment of myeloid-derived suppressor cells. Neoplasia 2024, 53, 101005. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Agro, L.; O’Brien, C. In vitro and in vivo Limiting Dilution Assay for Colorectal Cancer. Bio Protoc. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, C.; Hu, Y.; Mu, L.; Huang, K.; Li, Q.; Li, X.; Tao, D.; Qin, J. Sphere-forming assay vs. organoid culture: Determining long-term stemness and the chemoresistant capacity of primary colorectal cancer cells. Int. J. Oncol. 2019, 54, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Tessmann, J.W.; Deng, P.; Durham, J.; Li, C.; Banerjee, M.; Wang, Q.; Goettl, R.A.; He, D.; Wang, C.; Lee, E.Y.; et al. Perfluorooctanesulfonic acid exposure leads to downregulation of 3-hydroxy-3-methylglutaryl-CoA synthase 2 expression and upregulation of markers associated with intestinal carcinogenesis in mouse intestinal tissues. Chemosphere 2024, 359, 142332. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.J.; Jimenez-Renard, V.; Samino, S.; Capellades, J.; Junza, A.; López-Rodríguez, M.L.; Garcia-Carceles, J.; Lopez-Fabuel, I.; Bolaños, J.P.; Chandel, N.S.; et al. Essentiality of fatty acid synthase in the 2D to anchorage-independent growth transition in transforming cells. Nat. Commun. 2019, 10, 5011. [Google Scholar] [CrossRef]
- Buckley, D.; Duke, G.; Heuer, T.S.; O’Farrell, M.; Wagman, A.S.; McCulloch, W.; Kemble, G. Fatty acid synthase—Modern tumor cell biology insights into a classical oncology target. Pharmacol. Ther. 2017, 177, 23–31. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Rychahou, P.G.; Le, A.-T.; Scott, T.L.; Flight, R.M.; Kim, J.T.; Harris, J.; Liu, J.; Wang, C.; Morris, A.J.; et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget 2018, 9, 24787–24800. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Deng, X.; Byun, S.; Lee, C.; Lee, S.J.; Suh, H.; Zhang, J.; Kang, Q.; Zhang, T.; Westover, K.D.; et al. Direct Targeting of beta-Catenin by a Small Molecule Stimulates Proteasomal Degradation and Suppresses Oncogenic Wnt/beta-Catenin Signaling. Cell Rep. 2016, 16, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, J.; Hillier, J.; Lu, W.; Jones, E.Y. Caffeine inhibits Notum activity by binding at the catalytic pocket. Commun. Biol. 2020, 3, 555. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.S.; Linssen, J.D.; Flanagan, D.J.; Vlahov, N.; Nijman, L.E.; de Groot, N.E.; Elbers, C.C.; Koster, J.; Sansom, O.J.; Vermeulen, L.; et al. Caffeine Limits Expansion of Apc-Deficient Clones in the Intestine by NOTUM Inhibition. Cell. Mol. Gastroenterol. Hepatol. 2023, 16, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.J.; Quintela-Fandino, M. Emerging role of Fatty acid synthase in tumor initiation: Implications for cancer prevention. Mol. Cell. Oncol. 2020, 7, 1709389. [Google Scholar] [CrossRef]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K.; Liu, W.; Kobayashi, A.; Xing, F.; Fukuda, K.; Hirota, S.; Sugai, T.; et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Miyazaki, H.; Vaidyan, L.K.; Kagawa, Y.; Ebrahimi, M.; Yamamoto, Y.; Ogata, M.; Katsuyama, Y.; Sadahiro, H.; Suzuki, M.; et al. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS ONE 2016, 11, e0147717. [Google Scholar] [CrossRef]
- Chen, K.Y.; Liu, X.; Bu, P.; Lin, C.S.; Rakhilin, N.; Locasale, J.W.; Shen, X. A metabolic signature of colon cancer initiating cells. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 4759–4762. [Google Scholar]
- Li, H.; Feng, Z.; He, M.L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics 2020, 10, 7053–7069. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Chen, S.; Cai, J.; Ban, Y.; Peng, Q.; Zhou, Y.; Zeng, Z.; Peng, S.; Li, X.; et al. Correction to: Emerging role of lipid metabolism alterations in Cancer stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 155. [Google Scholar] [CrossRef]
- Linder, M.E.; Deschenes, R.J. Palmitoylation: Policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Zadra, G.; Palescandolo, E.; Fedele, G.; Bailey, D.; Fiore, C.; Nguyen, P.L.; Migita, T.; Zamponi, R.; Di Vizio, D.; et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab. Investig. 2008, 88, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Ackers, I.; Malgor, R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes Vasc. Dis. Res. 2018, 15, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Heuer, T.S.; Ventura, R.; Mordec, K.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G. FASN Inhibition and Taxane Treatment Combine to Enhance Anti-tumor Efficacy in Diverse Xenograft Tumor Models through Disruption of Tubulin Palmitoylation and Microtubule Organization and FASN Inhibition-Mediated Effects on Oncogenic Signaling and Gene Expression. EBioMedicine 2017, 16, 51–62. [Google Scholar] [PubMed]
- Wang, H.; Xi, Q.; Wu, G. Fatty acid synthase regulates invasion and metastasis of colorectal cancer via Wnt signaling pathway. Cancer Med. 2016, 5, 1599–1606. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, D.; Kim, J.; Lee, H.; Ghim, J.; Kang, B.J.; Song, P.; Suh, P.-G.; Ryu, S.H.; Lee, T.G. NOTUM Is Involved in the Progression of Colorectal Cancer. Cancer Genom. Proteom. 2018, 15, 485–497. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Xiao, L.; Dong, P.; Ma, Y.; Zhou, Y.; Yang, J.; Bian, B.; Xie, G.; Chen, L.; et al. Notum enhances gastric cancer stem-like cell properties through upregulation of Sox2 by PI3K/AKT signaling pathway. Cell. Oncol. 2024, 47, 463–480. [Google Scholar] [CrossRef]
- Yeung, T.M.; Gandhi, S.C.; Wilding, J.L.; Muschel, R.; Bodmer, W.F. Cancer stem cells from colorectal cancer-derived cell lines. Proc. Natl. Acad. Sci. USA 2010, 107, 3722–3727. [Google Scholar] [CrossRef]
- Demers, M.; Thibodeau, S.; Noël, D.; Fujita, N.; Tsuruo, T.; Gauthier, R.; Arguin, M.; Vachon, P.H. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J. Cell. Biochem. 2009, 107, 639–654. [Google Scholar] [CrossRef]
- Choi, S.R.; Cho, M.; Kim, H.R.; Ahn, D.H.; Sleisenger, M.H.; Kim, Y.S. Biological properties and expression of mucins in 5-fluorouracil resistant HT29 human colon cancer cells. Int. J. Oncol. 2000, 17, 141–147. [Google Scholar] [CrossRef]
- Chakrabarty, S. Regulation of human colon-carcinoma cell adhesion to extracellular matrix by transforming growth factor beta 1. Int. J. Cancer 1992, 50, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.; Cramer, Z.; Rhoades, J.; Estep, K.N.; Ma, X.; Adams-Tzivelekidis, S.; Katona, B.W.; Johnson, F.B.; Yu, Z.; et al. APC and P53 mutations synergise to create a therapeutic vulnerability to NOTUM inhibition in advanced colorectal cancer. Gut 2023, 72, 2294–2306. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelson, C.O.; Tessmann, J.W.; Geisen, M.E.; He, D.; Wang, C.; Gao, T.; Evers, B.M.; Zaytseva, Y.Y. Upregulation of Fatty Acid Synthase Increases Activity of β-Catenin and Expression of NOTUM to Enhance Stem-like Properties of Colorectal Cancer Cells. Cells 2024, 13, 1663. https://doi.org/10.3390/cells13191663
Kelson CO, Tessmann JW, Geisen ME, He D, Wang C, Gao T, Evers BM, Zaytseva YY. Upregulation of Fatty Acid Synthase Increases Activity of β-Catenin and Expression of NOTUM to Enhance Stem-like Properties of Colorectal Cancer Cells. Cells. 2024; 13(19):1663. https://doi.org/10.3390/cells13191663
Chicago/Turabian StyleKelson, Courtney O., Josiane Weber Tessmann, Mariah E. Geisen, Daheng He, Chi Wang, Tianyan Gao, B. Mark Evers, and Yekaterina Y. Zaytseva. 2024. "Upregulation of Fatty Acid Synthase Increases Activity of β-Catenin and Expression of NOTUM to Enhance Stem-like Properties of Colorectal Cancer Cells" Cells 13, no. 19: 1663. https://doi.org/10.3390/cells13191663
APA StyleKelson, C. O., Tessmann, J. W., Geisen, M. E., He, D., Wang, C., Gao, T., Evers, B. M., & Zaytseva, Y. Y. (2024). Upregulation of Fatty Acid Synthase Increases Activity of β-Catenin and Expression of NOTUM to Enhance Stem-like Properties of Colorectal Cancer Cells. Cells, 13(19), 1663. https://doi.org/10.3390/cells13191663





