Abstract
CAH3 is the only carbonic anhydrase (CA) present in the thylakoid lumen of the green algae Chlamydomonas reinhardtii. The monomer of the enzyme has a molecular weight of ~29.5 kDa with high CA activity. Through its dehydration activity, CAH3 can be involved either in the carbon-concentrating mechanism supplying CO2 for RuBisCO in the pyrenoid or in supporting the maximal photosynthetic activity of photosystem II (PSII) by accelerating the removal of protons from the active center of the water-oxidizing complex. Both proposed roles are considered in this review, together with a description of the enzymatic parameters of native and recombinant CAH3, the crystal structure of the protein, and the possible use of lumenal CA as a tool for increasing biomass production in higher plants. The identified involvement of lumenal CAH3 in the function of PSII is still unique among green algae and higher plants and can be used to understand the mechanism(s) of the functional interconnection between PSII and the proposed CA(s) of the thylakoid lumen in other organisms.
1. Introduction
Spontaneous conversion between different forms of inorganic carbon (Ci) occurs easily in water solutions. However, the reactions are relatively slow. The rate constants for CO2 hydration and HCO3− dehydration at 25 °C are 0.030–0.037 s−1 and 24–26 s−1, respectively [1,2,3]. Widespread metal-containing enzymes in nature, carbonic anhydrases (CAs, EC 4.2.1.1), catalyze these reactions, i.e., the reversible hydration of CO2 (CO2 + H2O ↔ HCO3− + H+), and human CAII, as the most active CA identified to date, may accelerate the reaction by factor 106 [4].
Many CAs are found to participate in photosynthetic processes in cyanobacteria, algae, and higher plants (see reviews [5,6,7,8]), where they are mainly involved in the rapid conversion of CO2 to HCO3− in the carbon-concentrating mechanism (CCM) or, vice versa, of HCO3− to CO2 to be used by ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO, EC 4.1.1.39).
The need for CA activity near the donor side of photosystem II (PSII) has been proposed for a long time, since the participation of HCO3− (bicarbonate) ions in the operation of the water-oxidizing complex (WOC) of PSII was assumed [9], and since a rapid release of H+ into the thylakoid lumen during the reaction of photosynthetic water oxidation was observed [10]. In addition, the influence of CA inhibitors, as well as the knockout of genes encoding some CAs in higher plants, have changed the activity of PSII, the state of PSII photoprotection mechanisms, and even PSII protein composition [11], despite the fact that the identification of the exact protein(s) in the close vicinity of PSII that have CA activity is still under investigation.
In the green algae Chlamydomonas reinhardtii, thirteen CAs of different families (α, β, γ) have been found to date [6,12], while the limiting CO2-inducible B protein (LCIB) of the chloroplast stroma, which does not have CA activity, was assigned to CAs of the β-family only recently [13]. Many CAs (together with Ci (inorganic carbon) transporters) are involved in the CCM [7,14], whose activity allows for increasing the intracellular pool of Ci (mostly in the form of HCO3−) up to 40–100-fold that of the CO2 level of the aquatic environment [7], to saturate RuBisCO for carboxylation [15]. The expression levels of genes encoding these CAs usually increase significantly in response to CO2 limitation, i.e., CCM activation. For example, the genes encoding CAH1, which is the major periplasmic CA in C. reinhardtii, and mitochondrial CAH4 and CAH5 are strongly up-regulated by low CO2 [16]. The expression level of the gene encoding LCIB is also up-regulated by low CO2 (the same has been observed for the gene encoding LCIC) [17]. However, the genes encoding CAH2 and CAH6 are not up-regulated by low CO2 [16] in spite of the fact that these two CAs are also classified as CCM participants [7,14]. The expression level of the gene encoding CAH2 is even decreased over one hour after the shift of algal cells to low CO2 [16].
Little is known about CAH7, CAH8, and CAH9. The expression levels of the genes encoding CAH7 and CAH8 are not regulated by low CO2 [16], while the gene encoding CAH9 is expressed at a low level [18]. Three mitochondrial γ-CAs (CAG1–3) in C. reinhardtii are probably part of mitochondrial complex I. However, there are no complete data about their roles [6].
A recent study about the localization of CCM components in the cell of C. reinhardtii, with use constructions encoding proteins of interest with a fluorescence tag, clarified the location of CAH2 (plasma membrane and late-secretory pathway), CAH4, CAH5, CAG1–3 (mitochondria), and CAH9 (cytosol) [19]. In addition, this study corrected the localization of β-CA CAH6 found in the flagella, where CAH6, as a CCM participant, can be involved in Ci sensing [19].
CAH3 is the third α-CA (after CAH1 and CAH2) found in C. reinhardtii and is the only CA whose location (and function) is associated with the thylakoid membrane of the algal chloroplast [20,21]. Initially, CAH3 located in the thylakoid lumen was also attributed to participants of the CCM [20], while a high amount of CAH3 protein was found in PSII-enriched membranes, which made it possible to suggest a functional interconnection between CAH3 and PSII [22,23]. A possible dual role of CAH3 in the thylakoid lumen of C. reinhardtii, i.e., participation in CCM and involvement in PSII function, was schematically represented previously [24].
Interestingly, to date, CAH3 from C. reinhardtii has been the only CA clearly observed in PSII preparations [10,23,25,26,27] isolated from cyanobacteria, algae, and higher plants whose activity supports the function of the WOC. This makes CAH3 a unique protein for the study of the involvement of CA(s) in the functioning of the photosynthetic apparatus in general and of PSII in particular.
This review summarizes current knowledge about the structural and functional properties of native and recombinant CAH3 proteins, the proposed roles for CAH3 in photosynthetic processes of the algal chloroplast, and attempts to incorporate CAH3 into the chloroplasts of higher plants in order to increase their bioproductivity.
2. Chloroplast-Located CA Activity
In the early 1990s, the presence of two major periplasmic α-CAs in C. reinhardtii was well known: low-CO2-inducible CAH1 and high-CO2-inducible CAH2, which had tetrameric structures consisting of two large and two small subunits linked by disulfide bonds [28,29]. The sequence homology was high, ~92% [29], making it possible to assume that they were the result of gene duplication [28]. At the same time, the presence of chloroplast-associated CA activity depending on the CO2 level in the growth medium was detected (Table 1).

Table 1.
CA activity (WAU mg Chl−1) of whole C. reinhardtii cells and isolated (solubilized) chloroplasts after growth at 5% (high) and ambient (low) levels of CO2. Note: 137c, wild-type strain; cw15 and CC-503, wall-less mutants; cia5, strain with failed CCM, including periplasmic CAs; cia3, CAH3-deficient mutants (underlined); EZA, CA inhibitor ethoxyzolamide.
The use of a CA-directed photoaffinity reagent (125I-labeled p-aminomethyl-benzenesulfonamide-4-azidosalicylamid) allowed for the detection of a 37 kDa polypeptide in cell lysates associated with a large subunit of periplasmic CA(s), while a 30 kDa photoaffinity-labeled peptide localized predominantly in the membrane fraction was also observed, indicating the presence of intracellular CA [36]. The 30 kDa photoaffinity-labeled peptide was not detected in cell lysates from the cia3 mutant of C. reinhardtii [36], in chloroplasts of which CA activity was almost completely suppressed (Table 1) [32,33] (see also p. 3).
Karlsson and coworkers [21] were able to isolate membrane-associated intracellular CA from the wall-less mutant CC-503 cw92 (mt+) (CC-503), which had a high CA activity of 1260 WAU mg−1 associated with a single polypeptide at around 29.8 kDa. The protein did not show cross-reaction with the antibody against periplasmic CA from C. reinhardtii or pea chloroplastic CA. A 50% inhibition of CA activity was observed with 12 nM acetazolamide (AZA). Based on the sequence of the N-termimi, which has a part identical to C. reinhardtii Fd-NADP+, the target of the precursor should be the chloroplast [21].
It was later shown that thylakoid membranes isolated from CC-503 have ~15 times higher CA activity than thylakoid membranes from the cia3 mutant at the same chlorophyll (Chl) content, while the presence of EZA strongly decreased CA activity in the case of preparations from CC-503 in contrast to preparations isolated from cia3 [23]. The CA activity of membranes enriched by PSII was additionally ~3.4 times higher than that observed for thylakoids from CC-503 [23]. At the same time, the adaptation of CC-503 cells to low-CO2 conditions led to a ~5.5-fold increase in CA activity associated with thylakoids [37].
3. Amino Acid Sequence of the CAH3 Protein and Immuno-Analysis
The amino acid sequence of the full-length chloroplast-targeted polypeptide of CA, named CAH3 (Figure 1), indicates a relation to the α-family with 40% identity to the N-termini of human CAII [20,21]. The sequence of the mature CAH3 protein showed 30–40% identity with α-CAs and up to 90% within the conserved domains, including three His residues functioning as ligands to a Zn atom. The predicted isoelectric point was ~7.78 [20]. The first 72 amino acids of the full-length sequence of the protein include two transport peptides or the stromal-targeting domain formed by 20 amino acids with a cleavage site, Val-Arg-Ala, for stromal peptidase and the lumen-targeting domain of 52 amino acids with a cleavage site, Ala-Lys-Ala, for lumen peptidase following the hydrophobic region [20].

Figure 1.
A full-length amino acid sequence of the CAH3 protein of C. reinhardtii. Two transport peptides are indicated by orange and blue backgrounds and three His of the active center are indicated by green backgrounds. The arrow shows the cleavage site between transport peptides and the mature protein. The cleavage sites for stromal and lumenal peptidases are underlined. The hydrophobic region is shown in italics. The twin Arg motif in the second transport peptide is shown in bold. Two Cys involved in the formation of a disulfide bond are indicated by S–. Two amino acids substituted in the second transport peptide of the cia3 mutant are shown in the gray frame.
A comparison of the sequences for the Cah3 genes obtained from CC-503 and the cia3 mutant C. reinhardtii revealed that two point mutations in the second transit peptides resulted in substitutions of two Leu in a pair into Arg and Met (Figure 1). Importantly, these are placed directly at the beginning of the hydrophobic region and almost immediately after the twin arginine motif, which is critical for thylakoid protein translocase in the ∆pH-driven pathway [38,39]. The same motifs in transit peptides were found in sequences of the PsbP, PsbQ, and PsbT subunits of PSII, as well as sequences of the PsaN subunit of PSI and other proteins of the lumen [38,39]. It has been shown that the amino acid substitution in the twin arginine could strongly reduce (by >100-fold) the translocation rate of the precursor protein or completely block it [38]. Interestingly, the presence of two point changes near the twin arginine motif in the case of CAH3 is enough to completely disrupt the transport of the precursor protein from the stroma to the thylakoid lumen. This explains the absence of the 30 kDa photoaffinity-labeled peptide in cell lysates from cia3 [36], as well as the insignificant CA activity associated with the chloroplast, which is not suppressed by EZA [32,33].
The production of primary antibodies [20,40] made it possible to study the CAH3 content in cells and different preparations obtained from cells to identify the localization of the protein. The association of the CAH3 protein with thylakoid membranes was clearly shown [20,23,40]. Moreover, many signals from the antibodies were observed in membrane preparations enriched by PSII, which directly correlated with the values of CA activity, while photosystem I fragments did not contain CAH3 at all [22,23]. This localization of CA indicated the possible involvement of the CAH3 protein in the function of PSII [20,22,23], in spite of the fact that the participation of CAH3 in CCM was also proposed [20,22].
One of the questionable points of the works presented here was related to the detection of CAH3 in cia3 cells, as well as thylakoids, and the PSII-enriched membranes isolated from this mutant with custom-produced primary antibodies [20,23]. Mitra et al. [40] even compared “Old” (used in [20,23]) and “New” antibodies, produced by their group, and showed the presence of an additional strong unspecific signal from the protein close to CAH3 in molecular weight when “Old” antibodies were used. The “New” antibody indeed did not detect CAH3 in cia3 cells, in contrast to the wild-type (WT) 137c strain. In agreement, the commercially available primary antibody against CAH3 (Agrisera, Sweden) does not detect CAH3 in PSII preparations from the cia3 mutant, while a significant amount of CAH3 is observed in PSII-enriched membranes from CC-503 (wall-deficient mutant) [10,25,26]. Based on the fact that the Cah3 gene is constitutively expressed [14,16] and only slightly (or even not [14]) regulated by CO2 level, the absence of CAH3 in cia3 cells indicates the fast proteolysis of the precursor CAH3 protein in chloroplast stroma.
A study performed by Mitra et al. [40] of the immunolocalization of CAH3 with transmission electron microscopy using the “New” antibodies and protein A conjugated to colloidal gold (the secondary antibodies) showed a high distribution of CAH3 throughout the thylakoids of the chloroplast in the cells of the WT 137c strain. In addition, the signal from gold particles related to CAH3 proteins was clearly detected in association with thylakoids penetrating the pyrenoid matrix (tubules). The cells of the cia3 and ca-1 (has a stop codon in the Cah3 gene) mutants contained extremely low counts of gold particles, in agreement with Western blot results for CAH3 [40].
In contrast, other groups using the “Old” antibodies showed a much higher concentration of gold particles inside the pyrenoid of WT 137c cells in association with tubules [24,41]. The gold particles were also observed in the pyrenoid area of cia3 cells without a clear association with tubules, which was in agreement with the Western blot-detected CAH3 protein in cia3 cells [41]. Taking into account the above-mentioned possible unspecific detection of a protein close to CAH3 in molecular weight using the “Old” antibodies [40], the obtained results need to be clarified. The “Old” antibodies were also used in the work, which showed a relocation of CAH3 proteins (identified using transmission electron microscopy) phosphorylated at a low CO2 level from the stroma thylakoid to the tubules of the pyrenoid. The authors of [37] noted that the maximum amount of CAH3 (gold particles) in the pyrenoid could reach ~37% at low CO2, but at high CO2 the value was only ~19%. At the same time, Western-blot showed a similar content of CAH3 in fractions of extrinsic proteins obtained from thylakoids of cells grown at high CO2 and adapted to low CO2. Using transmission electron microscopy, Mitchell et al. [42] showed a light-induced increase in CAH3 (gold particles) content in the pyrenoid from ~22% up to ~40% (by ~45%) with the use of a commercially available primary antibody. Interestingly, the pyrenoid area was also increased by ~37% in such cells (from 1.4 µm2 to 2.2 µm2).
In spite of the early obtained data about the constitutive expression of the Cah3 gene [14,16], which was independent of a low CO2 level as well as CIA5 regulation (the same was shown for the Cah6 gene), Tirumani et al. [43,44] showed an almost complete decrease in Cah3 transcripts upon adaptation of cells to light or high CO2 in a synchronized culture grown in a 12 h light/12 h dark regime. The CAH3 protein level was also changed, with the maximum observed at 6–12 h in the light and the minimum at 9–12 h in the dark [43]. The use of the immuno-fluorescence approach and antibodies against CAH3 indicated a diffuse pattern of the protein distribution among the thylakoids in the dark, with the maximum concentration of CAH3 in the pyrenoid area in the light, which partially correlated with data obtained by Mitchell et al. [42]. However, in another work, Mitchell et al. [45] showed no significant differences in Cah3 gene expression levels under cell (WT 2137) adaptation to low CO2 in agreement with previously obtained data [14,16]. In addition, the in vivo detection of the signal from constructs encoding the CAH3 protein fused to a fluorescent tag showed a uniform distribution of CAH3 throughout the chloroplast (thylakoids), including the pyrenoid area in C. reinhardtii cells (CC-4533) grown under light and adapted to an ambient CO2 level for 24 h [19,46].
4. Production of the Recombinant Protein and Its Crystal Structure
There have been only two attempts to produce a recombinant protein of CAH3 (rCAH3) to date [25,40]. However, the same 349-amino-acid-long pET32-Xa-CAH3 plasmid was used in both studies. The construction contained a mature part of CAH3 and a 156-amino-acid N-terminal extension including His6-tag thioredoxin and a Factor Xa cleavage site (trx-His6-Xa-CAH3 fusion protein). Purified rCAH3 showed a high CA activity comparable with that of native CAH3, CAH1, and rCAH6, while it was significantly higher than the activity observed for rCAH7 and rCAH8 (Table 2). Interestingly, there were no differences in CA activity between the Factor X-cleaved rCAH3 protein and the uncleaved fusion protein [40].
The sensitivity of rCAH3 to sulfonamide inhibitors was extremely high with IC50 in nM concentrations, which was similar to that of native CAH3 (Table 2). Close values of IC50 were obtained for two other α CAs of C. reinhardtii, CAH1 and CAH2. The monovalent anions cyanide and azide suppressed the activity of rCAH3 in the μM range. The activity of rCAH3 was sensitive to sulfhydryl-reducing agents; thus, the incubation of rCAH3 with 10 mM of Cys, β-mercaptoethanol, or dithiothreitol decreased CA activity by ~47%, ~60%, and ~80%, respectively [40]. The same was observed for CAH1 and CAH2 (Table 2) [29,47]. However, 2 mM of dithiothreitol led to a complete inhibition of rCAH3 in [48]. The suppression of the CA activity of rCAH3 using dithiothreitol was completely reversible upon the oxidation of the SH groups of Cys90 and Cys258 (Figure 1), although the process required much longer times and a full restoration of CA activity was observed after 4 h [48]. The incubation of the rCAH3 protein for 15 min at different temperatures showed the optimum to be around 32–33 °C and a loss of a half of the activity above 43 °C [40].
The use of membrane inlet mass spectrometry (MIMS) based on monitoring the 18O exchange rate between C18O2 isotopes (dissolved in added air-saturated H218O) and water allowed the pH dependence of rCAH3 CA activity to be investigated [48], while most of the commonly used methods based on time-resolved changes in pH are not suitable for this. The obtained results indicated that rCAH3 had an optimum CA activity near pH 6.5, with a residual CA activity of ~60% at pH 7.0 and of ~80% and ~45% at pH 6.0 and pH 5.5, respectively. At the same time, bovine CAII showed maximal CA activity at pH 7.0 with small residual CA activity at pH below 6.0.
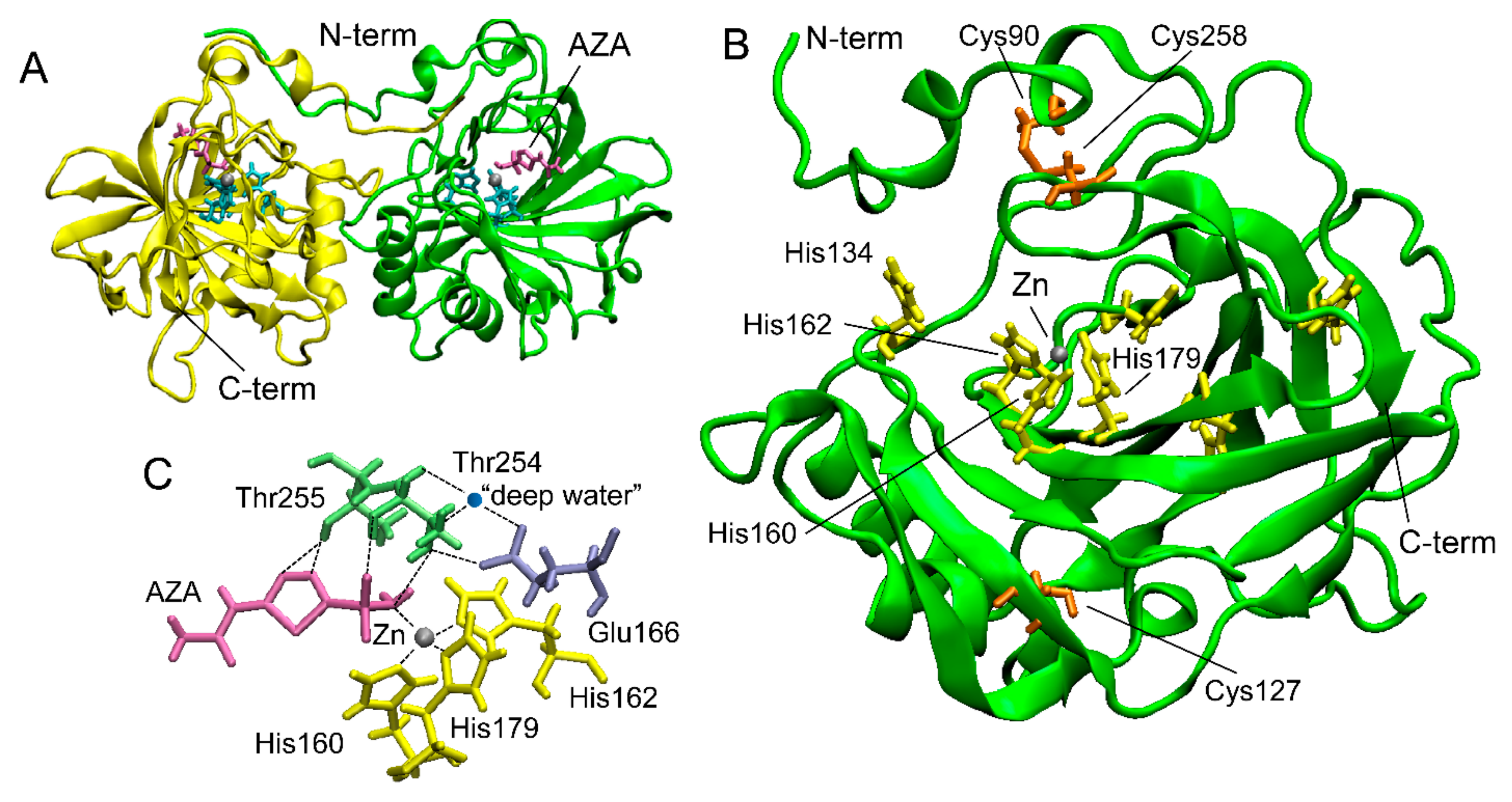
Two crystal structures of rCAH3 in complex with AZA and phosphate ions were determined with 2.6 Å and 2.7 Å, respectively [48]. In both cases, each unit cell contained four dimers of rCAH3 (Figure 2A). Such packing is unusual for α-CAs. In accordance with known structures of other α-CAs, a monomer of rCAH3 is formed around a central core by 10-stranded β-sheets with two α-helices near one side of the sheet (Figure 2B). The full length of the molecule is ~45 Å. The active site is represented by an 8–9 Å-deep hydrophobic cavity where the catalytic Zn ion is coordinated by the residues of three His (160, 162, 179). The water molecule (“deep water”) structurally conserved among α-CAs has hydrogen bonds with Thr254, Glu166, and Tyr77 (Figure 2C). The monomer of rCAH3 has three Cys (90, 127, 258) with the formation of a disulfide bond between Cys90 and Cys258. As mentioned above, this disulfide bond is important for the CA activity of the enzyme.
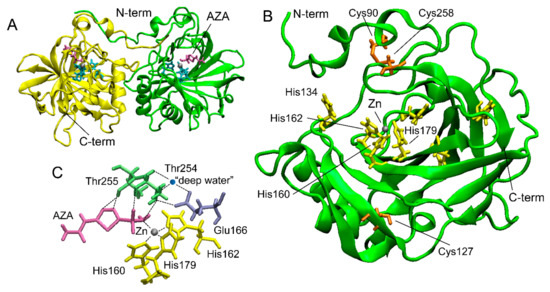
Figure 2.
The crystal structure of rCAH3 obtained at pH 4.1 in a complex with AZA (PDB ID: 4xiw, https://www.wwpdb.org/pdb?id=pdb_00004xiw (accessed on 22 November 2023)). (A) A typical dimer of two rCAH3 molecules formed under crystallization. Four such dimers form each unit cell [48]. (B) The structure of an rCAH3 monomer. (C) Relative positions of amino acid residues, Zn, “deep water”, and AZA in the active center of rCAH3. His are shown in cyan (in A) or in yellow. Cys are shown in orange. Thr and Glu are shown in green and in violet, respectively. Zn and water are represented by gray and blue spheres, respectively. A molecule of AZA is shown in magenta. The positions of N- and C-terms are indicated.

Table 2.
Maximal CA activities (WAU mg−1) and influences of inhibitors and reductants of the disulfide bond obtained for some recombinant (r) and native (n) proteins of C. reinhardtii as well as bovine CAII. IC50—half-maximal inhibitory concentration (M); DDT—dithiothreitol; ME—β-mercaptoethanol; ↓ or ↑—decrease or increase in CA activity; α and β indicate the CA family.
Table 2.
Maximal CA activities (WAU mg−1) and influences of inhibitors and reductants of the disulfide bond obtained for some recombinant (r) and native (n) proteins of C. reinhardtii as well as bovine CAII. IC50—half-maximal inhibitory concentration (M); DDT—dithiothreitol; ME—β-mercaptoethanol; ↓ or ↑—decrease or increase in CA activity; α and β indicate the CA family.
| Protein | WAU | IC50 | DTT/β-ME | Ref. |
|---|---|---|---|---|
| rCAH3 (α) | 750 (1600) | AZA, 8 × 10−9 EZA, 6 × 10−9 Azide, 3.2 × 10−5 Cyanide, 5.9 × 10−5 | ↓ | [25] (Shutova et al., 2008, unpublished data) |
| rCAH6 (β) | 940 | EZA, 2 × 10−6 Azide, 1.5 × 10−5 Cyanide, 5 × 10−5 | no | [25] |
| rCAH7 (β) | 3.1 | AZA, 4.4 × 10−5 EZA, 4.4 × 10−4 Azide, 3.2 × 10−5 Cyanide, 6.1 × 10−4 | ↑ | [16] |
| rCAH8 (β) | 4.2 | AZA, 1.7 × 10−7 EZA, 4.1 × 10−4 Azide, 1.9 × 10−3 Cyanide, 1.5 × 10−5 | no | [16] |
| rLCIC, rLCIB, rLCIC-LCIB (β) | no | – | – | [13] |
| nCAH3 (α) | 2040 | AZA, 12 × 10−9 | – | [20] |
| nCAH1 (α) | 2580 | AZA, 3 × 10−9 EZA, 2 × 10−9 | ↓ | [29,47,49] |
| nCAH2 (α) | 3300 | AZA, 3 × 10−9 EZA, 2 × 10−9 | ↓ | [29] |
| r-Bovine CAII (α) | 9333 | AZA, 1.4 × 10−8 EZA, 1.2 × 10−9 Azide, 1.1 × 10−3 Cyanide, 4.9 × 10−5 | no | [40,50] |
In addition to the three His of the active center, the monomer of rCAH3 has His134 on the bottom edge of the cavity (Figure 2). As is known, a similar His (His64) plays a role as a proton shuttle in human CAII between a bulk solution and a Zn-bound solvent molecule with a rate of more than 105 s−1 [51]. The same His64 is also present in bovine CAII (https://www.wwpdb.org/pdb?id=pdb_00001v9e (accessed on 22 November 2023)). A structural analysis of human CAII at different pH values showed no major pH-induced conformational changes in the active site [52], while His64 could be found in the “out” (at pH 5.7) or “in” (at pH 8.5) conformation oriented away or toward the Zn ion, respectively [51,52]. Whether the unusual pH optimum of rCAH3 is caused by His134 is not yet known. In addition, rCAH3 has no His at the N-term (Figure 2), in contrast to human and bovine CAIIs that have 5 His among the first 20 amino acids of the N-term oriented to the cavity. The monomer of rCAH3 does not contain the three loop regions present in human and bovine CAIIs, which can explain its shallow and broader cavity with a more hydrophobic entrance as compared to human and bovine CAIIs, which are probably needed for the interaction of the protein with the thylakoid membrane [48].
5. Involvement CAH3 in CCM
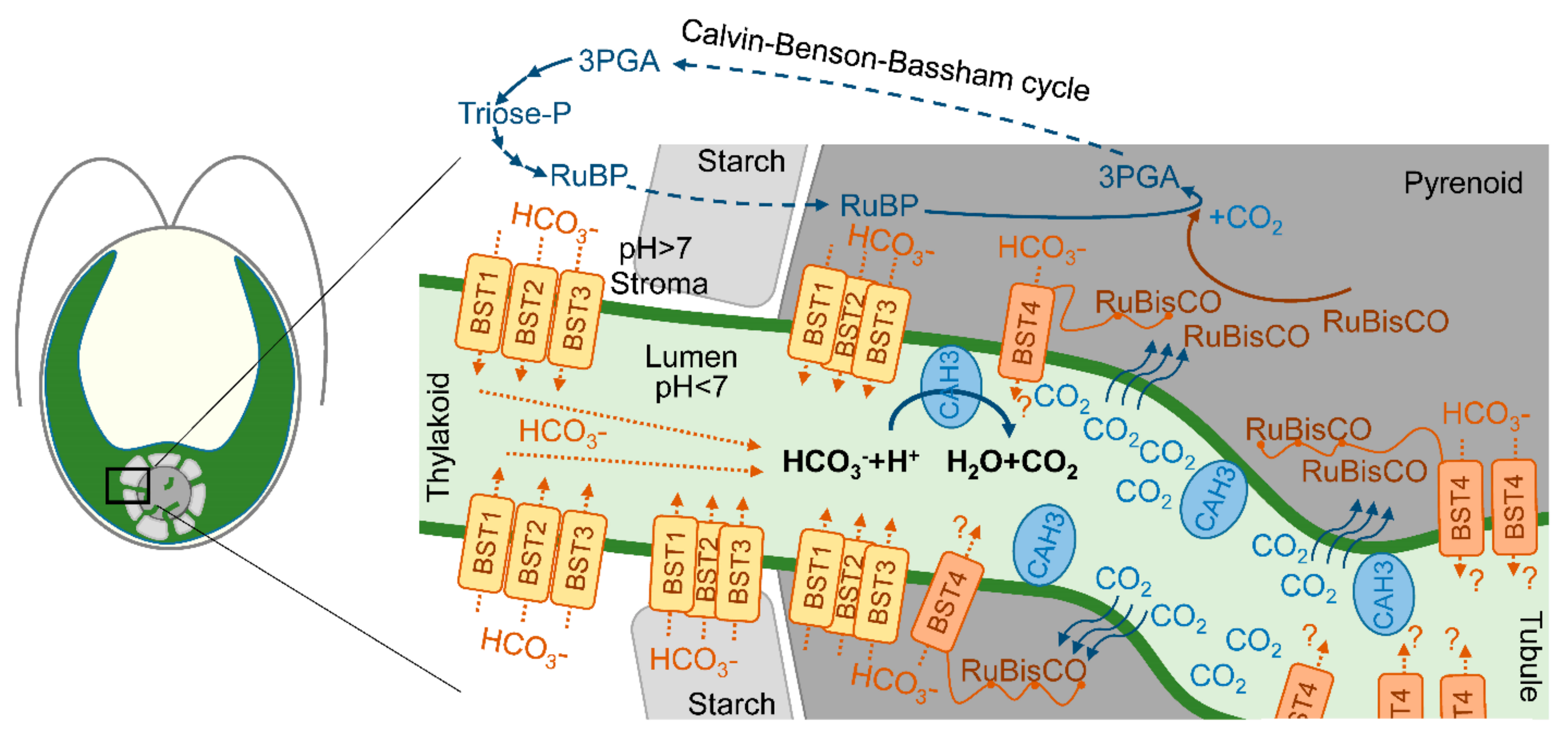
It was clearly shown that the cia3 (CAH3-deficient) mutant requires a high CO2 level for its growth [20,53,54]. Thus, the direct or indirect participation of CAH3 in the CCM of C. reinhardtii is often proposed. At the same time, the authors of [20,22] suggested that PSII can drive the lumenal part of CCM via the CA activity of PSII-associated CAH3, providing CO2 for carboxylation byRuBisCO. However, using transmission electron microscopy, Mitra et al. [40] showed the presence of CAH3 not only in association with stromal thylakoids but with thylakoids (tubules) penetrating the pyrenoid where PSII complexes are exactly absent. Thus, CAH3 can be involved in CCM independently of PSII as single molecules. The proposed role of CAH3 was in the acceleration of the HCO3− dehydration reaction (HCO3− + H2O → H+ + CO2) in the lumen of tubules located inside the pyrenoid matrix. Produced CO2 easily passes through the thylakoid membrane and can be used by RuBisCO (Figure 3) [24,40,55]. The presence of CAH3 in the pyrenoid area was also shown by other groups using transmission electron microscopy [24,37,41,42], as discussed above (Section 3).
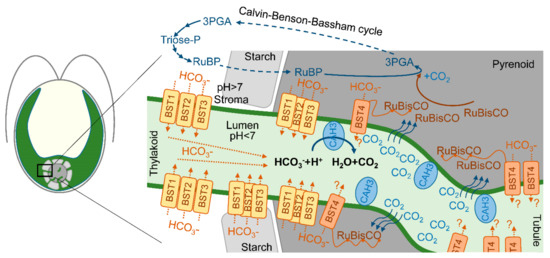
Figure 3.
A scheme of proposed localization of CAH3 in the lumen of intrapyrenoid thylakoids (tubules) and a possible role of CAH3 in CO2 production, which passes the thylakoid membrane to the pyrenoid matrix where it can be captured by RuBisCO in the Calvin–Benson–Bassham cycle. A simplified Calvin–Benson–Bassham cycle is adapted from [56]; RuBP—ribulose-1,5-biphosphate; 3PGA—3-phosphoglyceric acid. Putative localization and roles of four bestrophin-like proteins (BST1-3 and BST4 (RuBisCO binding membrane protein)) as HCO3− transporters of the thylakoid membrane are adapted from [53,57].
The in vivo monitoring of 16O2, 18O2, and CO2 fluxes using MIMS indicated an absence of limitations in the electron transport in PSII of the cia3 mutant under low-CO2 conditions compared to WT (CC-400) [58], while net CO2 uptake was greater in the mutant [58], which was a result of the known higher Ci accumulation inside cells without CAH3 (Table 3) [34,55]. Nevertheless, if WT cell was able to consume all Ci of the solution, the cia3 mutant cells could only draw down up to ~35 μM of Ci [58].
RuBisCO in the cia3 mutant showed extra sensitivity to O2, with a higher CO2 compensation point that was strongly influenced by the O2 concentration. A study of metabolites indicated a much higher ribulose-1,5-biphosphate (RuBP) pool size in the cia3 mutant at 200–600 μM of Ci, while the pool size of 3-phosphoglyceric acid (3PGA) showed an inverse pattern [58]. In C. reinhardtii, both of the intermediates are mostly localized in the pyrenoid matrix (when the pyrenoid is formed), where PuBP is converted to 3PGA by RuBusCO with CO2 assimilation during the Calvin–Benson–Bassham cycle (Figure 3). Thus, the absence of CAH3 in the lumen of tubules can suppress the supply of CO2 for fixation by RuBisCO impairing the CCM. It should be noted that the authors of [58] used relatively distinct strains (CC-400, cia3) compared, for example, with the works by [10,23,25,26,27], where closely related strains (CC-503, cia3) obtained from WT CC-137c [59] were used. Cells were not preadapted to low-CO2 conditions, and measurements required a long assay period to complete each data set.

Table 3.
Intracellular Ci accumulation after 80 s (* 60 s) by WT and different CCM mutants of C. reinhardtii acclimated to low CO2. Mutants ca-1-12-1C, WT-su6, and ad1-su6 are deficient (def.) in terms of CAH3 production (underlined).
Table 3.
Intracellular Ci accumulation after 80 s (* 60 s) by WT and different CCM mutants of C. reinhardtii acclimated to low CO2. Mutants ca-1-12-1C, WT-su6, and ad1-su6 are deficient (def.) in terms of CAH3 production (underlined).
| Strains | Initial External Ci, μM | Internal Ci, mM | Ref. |
|---|---|---|---|
| WT 2137 | 80 | ~1.0 | |
| +50 µM EZA | 80 | ~10.0 | [34,35] |
| ca-1-12-1C | 80 | ~13.2 | |
| Strain 90 | 20 | ~0.25 * | |
| +50 µM EZA | 20 | ~1.10 * | [60] |
| +50 µM AZA | 20 | ~0.25 * | |
| WT CC-125 | 50 | 1.80 ± 0.33 | |
| ad1 (LCIB–def.) | 50 | 0.26 ± 0.08 | [55] |
| WT-su6 | 50 | 7.60 ± 1.25 | |
| ad1-su6 | 50 | 6.15 ± 1.15 | |
| cia5 | 50 | 0.19 ± 0.05 | |
| WT CC-125 | 50 | ~1.25 | |
| ad1 (LCIB–def.) | 50 | ~0.20 | [61] |
| WT D66 | 50 | ~0.95 | |
| bsti-1 (BST1–def.) | 50 | ~0.33 | [53] |
| bsti-2 (BST2–def.) | 50 | ~0.50 | |
| bsti-3 (BST3–def.) | 50 | ~0.51 | |
| WT D66 | 25 | ~0.38 | |
| cah4/5-1 (CAH4/5–def.) | 25 | ~0.10 | [54] |
| cah4/5-2 (CAH4/5–def.) | 25 | ~0.20 |
The LCIB (together with LCIC) protein of the chloroplast stroma is known as a participant of the CCM in C. reinhardtii [13]. A study with an LCIB-deficient mutant ad1 clearly shows an “air dier” phenotype when cells are unable to grow under low CO2 [55,61]. To reach the maximum photosynthetic O2 evolution, the ad1 mutant acclimated to low CO2 required an addition of more than 400 μM NaHCO3 (~100 μM for WT), but even in this case the obtained value was lower by ~40% compared to the WT [55,61]. In agreement, the intracellular accumulation of Ci by the ad1 mutant is ~6 times lower compared to that of the WT (Table 3). Two transformants of the ad1 mutant, ad-su6 and ad-su7, with deletions in the Cah3 gene that stop its expression, were surprisingly able to grow under low CO2 [55]. They accumulated 3.5–4 times more intracellular Ci compared to that of the WT or 24–29 times more compared to that of the ad1 mutant, which results were consistent with that observed for the CAH3-deficient mutant ca-1-12-1C (or with experiments with the addition of EZA, which is able to penetrate through bilipid membranes and inhibit CAH3 in contrast to AZA) (Table 3). Such a high level of Ci may be needed to sufficiently provide CO2 to RuBisCO in the absence (or inhibition) of CAH3, as mentioned above; however, this suggestion requires further investigation. The authors of [55] noted that CAH3 mutations are epistatic to LCIB mutations and that LCIB activity should be downstream of CAH3.
One of the suggested problems associated with the involvement of CAH3 in CCM in tubules is the need to supply a lot of HCO3− ions for the dehydration activity of CA. While most of the Ci in the stroma are represented as HCO3− ions due to the alkaline pH, these are not able to pass the thylakoid membrane, unlike CO2 molecules. In addition, the acidic pH of the lumen favors the conversion of Ci into CO2 [10]. Recently, three similar bestrophin-like (BST1-3) proteins of the thylakoid membrane were suggested as HCO3− transporters [53]. The visualization of BST1-3 localization via protein-linking Venus fluorescence showed a distribution of the signal among the thylakoids of the chloroplast concentrated around the pyrenoid. Moreover, the presence of BST1-3 extended into the tubules. This localization of transporters can explain the supply of HCO3− for CAH3 in the tubules for CCM operation (Figure 3). Low CO2 induced an increase in the transcript levels of all three genes encoding BST1-3. At the same time, the knockout mutants (bsti-1, -2, -3) accumulated 47–63% less intracellular Ci compared to the WT (D66) (Table 3), while they were also able to grow at a low CO2 in contrast to the cia3 mutant. Another BST4 protein (also known as RuBisCO-binding membrane protein) exclusively localizes in the tubules of the pyrenoid (Figure 3). BST4 has a conserved bestrophin domain similar to that observed for BST1-3; therefore, its role in HCO3− ions for CAH3 activity in CCM was proposed [57]. However, the data obtained so far show that BST4 is not a component of CCM, but it may be involved in the pH regulation of the lumen in addition to the main function of BST4 of binding RuBisCO to tubules [62].
Interestingly, the knockout of two mitochondrial Cas, CAH4 and CAH5, which are known as CCM participants, also led to a decrease in the accumulation of intracellular Ci by up to 74% as compared to the WT (D66) (Table 3), while the mutants cah4/5-1 and cah4/5-1 were able to grow at low CO2 [54]. Thus, CAH3 mutation leads to an overaccumulation of intracellular Ci in contrast to mutations of other CCM participants, but the molecular mechanism is still unknown. The inability of the CAH3-deficient mutants cia3 and ca-1-12-1C to grow at low CO2 [20,53,54] makes this question more complicated.
The role of CAH3 in CCM was also studied using mutants with a blocked formation of the pyrenoid (pyr−). As shown in [45], the absence of the pyrenoid did not influence the expression level of the Cah3 gene. The same was observed even after the cells were adapted to low CO2. Mass spectrometry analysis showed that many proteins related to CCM were less abundant in pyr− cells adapted to low CO2 compared to pyr+ cells, while the difference observed for CAH3 content was not statistically significant. In spite of the fact that pyr− mutants are limited in terms of the enzymatic activity of RuBisCO at low CO2, starch granules, as well as a complex of modified thylakoids (knitted tubules), can be observed in the usual area of the pyrenoid formation [63]. It is likely that part of the CAH3 molecules can be localized in those tubules with residual CCM function.
6. Functional and Structural Interconnection between CAH3 and PSII
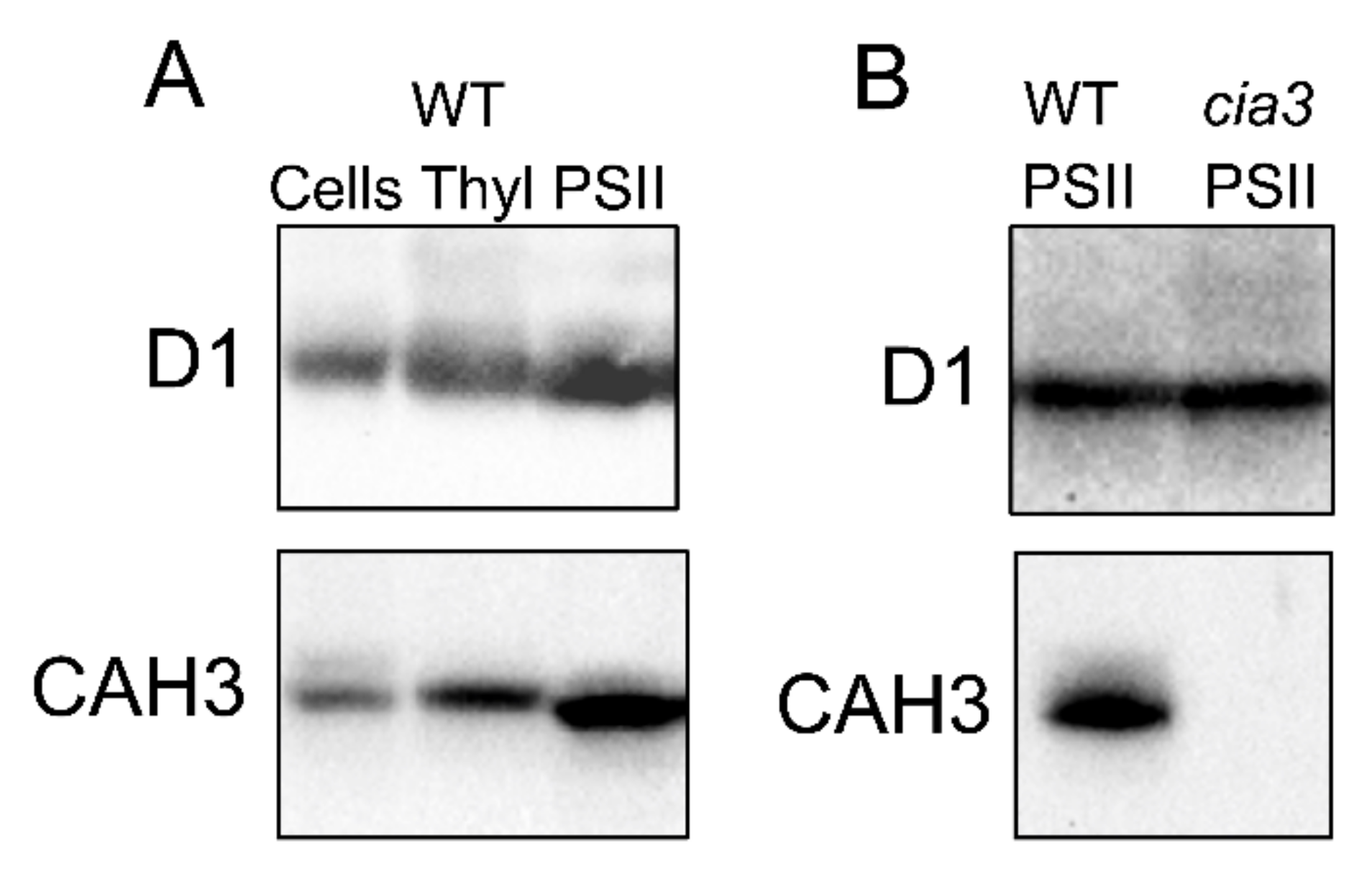
The role of CAH3 CA activity in supporting of the optimal PSII photochemistry was proposed by Karlsson et al. [20] together with the first description of the novel α-CA (CAH3) of the thylakoid membrane. Later, the results of Western blot analyses performed in many studies clearly showed a high abundance of CAH3 in PSII-enriched preparations (Figure 4A; Section 3), indicating an interconnection between the activities of CAH3 and PSII.
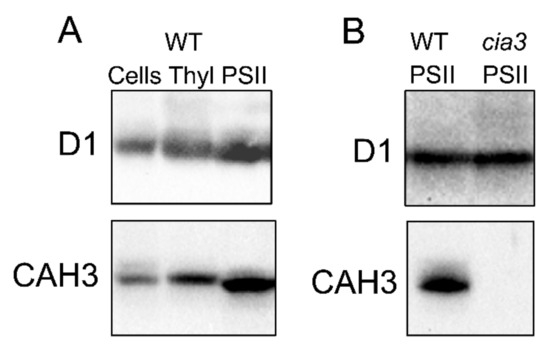
Figure 4.
Results of Western blot analysis with use of primary antibodies against D1 and CAH3 proteins of (A) whole cells, thylakoids (Thyl), and PSII-enriched membranes (PSII) isolated from WT (CC-503), as well as of (B) PSII-enriched membranes (PSII) isolated from WT (CC-503) and a cia3 mutant. Samples were loaded at 1.5 μg Chl per lane. For a complete description of the analysis, see [10,26].
The use of artificial acceptors, such as 2,6-dichloro-1,4-benzoquinone (DCBQ) paired with potassium ferricyanide (FeCy), which are capable of directly taking electrons from the PSII acceptor side even in the case of whole cells [64], allows for PSII function to be studied independently of other participants of the electron transport chain, as well as independently of the carboxylation status of RuBisCO. This approach clearly demonstrates the dependence of the stability of PSII function, and especially of WOC activity, on the presence of CAH3. It was shown that PSII in thylakoids from the cia3 mutant had much stronger photoinhibition (at 600 μmol photons m−1 s−1, 10 min) compared to that from WT (CC-503), and the presence of EZA during illumination decreased the functional stability of PSII from WT to the level of cia3 [23]. On the contrary, the addition of an excess of HCO3−, which may imitate dehydratase CAH3 activity (HCO3− + H2O → H+ + CO2), protected the PSII of cia3 from photoinhibition. The same stronger suppression of PSII in the cia3 mutant compared to the WT (CC-400) by the light of >400 μmol photons m−1 s−1 was observed in whole cells using MIMS, while the authors of [58] concluded that the presence of RuBisCO inhibition caused by O2 accumulated in a closed chamber. Nevertheless, the study of chloroplast morphology in vivo under continuous illumination using moderate-intensity light (200 μmol photons m−1 s−1) indicated the development of an unusual morphology in the case of the cia3 mutant in contrast to the WT [65]. The clear separation of the chloroplast into lobes and the basal region was diminished with the formation of a net-like structure inside the cell. The main Chl fluorescence intensity was shifted from the edges, demonstrating a relocation of a major part of PSII. Since the WT and the cia3 mutant cells had the same photosynthetic activity of PSII, as well as the same content of D1 protein, the author of [65] proposed an initiation of a peculiar protection mechanism in cia3 cells against the photoinhibition of PSII. This consists of an absorption of excess light by PSII remaining near the edges of the chloroplast to provide optimal light conditions for the main part of PSII located under them. Thus, PSII of cia3 mutant cells is indeed more sensitive to light.
The study of PSII-enriched membranes isolated from WT (CC-503) and cia3 mutant cells grown under optimal conditions including a supply of 5% CO2 surprisingly showed no significant differences in their main structural and functional futures [26]. The preparations contained a similar amount of PSII per the same amount of Chl, as can be seen from the Western blot analysis against D1 (PsbA) (Figure 4B) and PsbO proteins [10,25,26], as well as from the cytochrome b559 and Mn atom contents [10]. This is in spite of the fact that PSII from the cia3 mutant did not contain any CAH3 (Figure 4B). The curves of the pH dependence of O2-evolving activity showed the same shape below 6.5. However, differences can be seen at pH above 6.5, where the WOC activity in PSII from the cia3 mutant was more suppressed compared to preparations from WT cells (maximum difference ~20% observed at pH 7.0) [10]. The addition of AZA or EZA decreased the photosynthetic activity of PSII from WT cells to that detected for PSII from the cia3 mutant, indicating the role of the CA activity of CAH3 in this effect. On the other hand, the addition of 0.5 мM HCO3− completely restored the O2-evolving activity of PSII from the cia3 mutant up to the level of PSII from WT cells, which shows the importance of catalyzing the HCO3− dehydration reaction in agreement with that mentioned above. The exact influence of the CA activity of CAH3 on the function of the WOC was clearly shown in the study with 2,6-dichlorophenolindophenol. The rate of its photoreduction by PSII from H2O (the WOC function) at pH 7.0 was significantly lower in PSII from the cia3 mutant compared to PSII from WT, while the presence of 1,5-diphenylcarbazide, which is able to effectively donate electrons to PSII separately from the WOC, completely removed this difference. In addition, the CA activity of CAH3 also protected the function of the WOC from irreversible inactivation under pH higher than 7.0 [10].
The depletion of CO2/HCO3− from the medium (Ci-free) allowed a similar effect at pH 5.5 to be identified [25]. The O2-evolving activity of PSII from WT under these conditions could be increased by ~12% through the addition of 2 mM HCO3− and by ~36% in the case of PSII from the cia3 mutant, i.e., the difference reached ~24%. The measurement of flash-induced O2 evolution patterns showed a decrease in the turnover efficiency of the WOC in PSII from both the WT and cia3 mutant in the Ci-free medium. The increase in the miss parameter was higher in PSII from the cia3 mutant (~7%) compared to PSII from the WT (~4%), and the difference should be obviously higher under continuous illumination and in vivo [48]. The reconstruction of PSII from the cia3 mutant by the addition of rCAH3 in Ci-free medium resulted in a much stronger stimulation (~70%) of O2 evolution when HCO3− was added [25]. Moreover, this high activity was stable even under continuous illumination (at 200 μmol photons m−1 s−1, 180 s) of the samples. The dependence of the O2-evolving activity of PSII on rCAH3 content showed the maximal value at a molecular stoichiometric ratio of 1:1. The results of the Western blot analysis indicated a complete binding of rCAH3 molecules with the membrane fraction of PSII-enriched membranes [25].
The inhibition of CAH3 activity and/or addition of HCO3− to PSII-enriched membranes, imitating dehydratase activity, had no influence on WOC activity at pH 6.5, which is optimal for the operation of the WOC. Moreover, there were no differences in the rates of 2,6-dichlorophenolindophenol photoreduction by PSII isolated from both the WT and cia3 mutant, nor was there an influence of 1,5-diphenylcarbazide [10]. All of these results indicated that PSII from the cia3 mutant functioned at its maximum possible values, equal to those of PSII from WT, and that CAH3 CA activity had no influence on the function of PSII from the WT. Therefore, the functional interconnection between PSII and CAH3 becomes more pronounced at nonoptimal conditions for the WOC. In different studies [10,25], the same role of CAH3 for PSII was hypothesized, which is the acceleration of proton removal from the active center of the WOC to the thylakoid lumen (Figure 5).
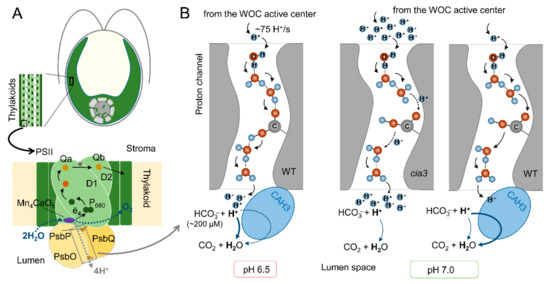
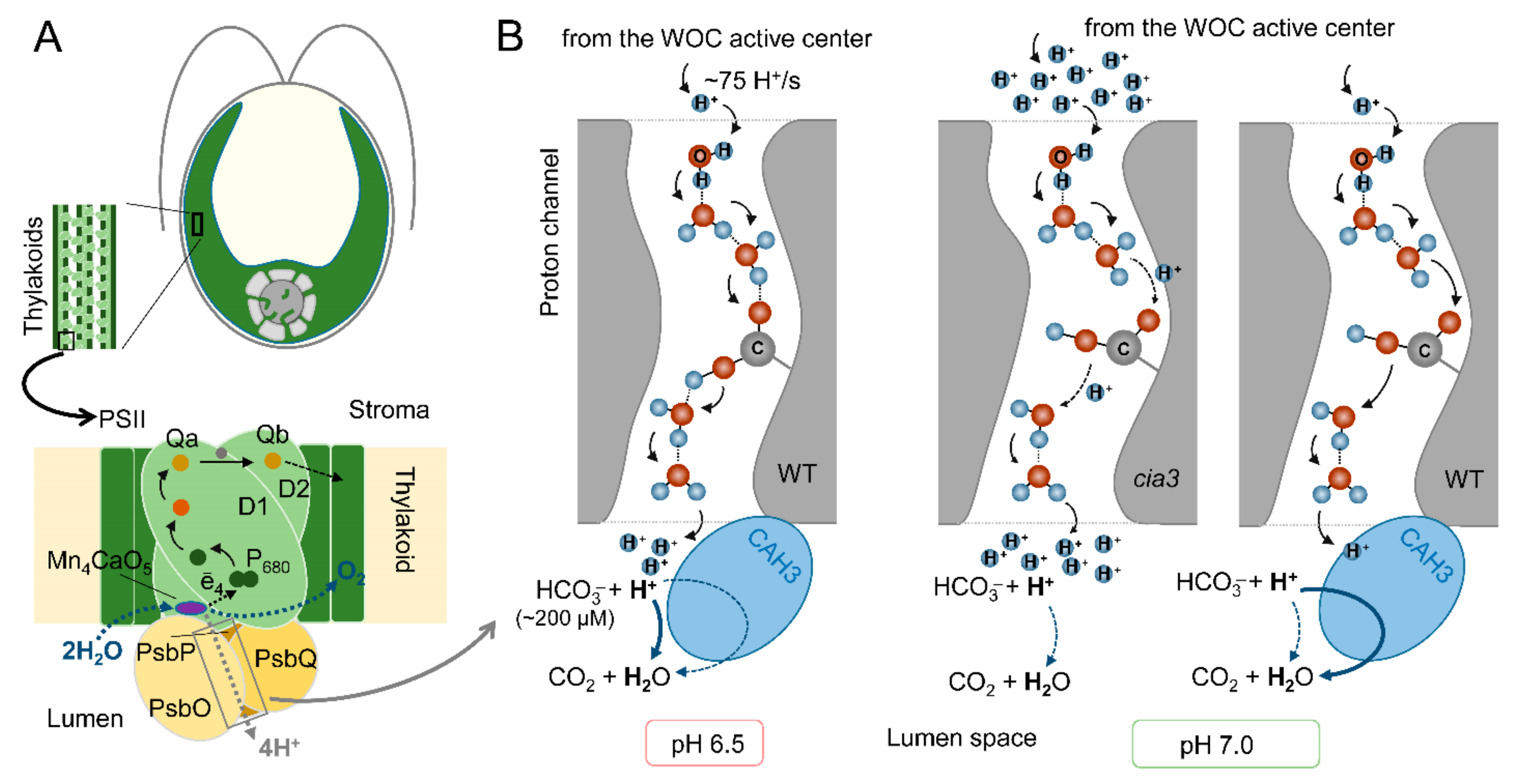
Figure 5.
The proposed role of CAH3 activity in the acceleration of proton removal from the active center of the WOC of PSII from the WT and cia3 mutant at optimal pH 6.5 and nonoptimal pH 7.0: (A) A schematic representation of the PSII complex including a dimer of D1 and D2 proteins with cofactors of the electron transfer chain, proteins of the WOC (PsbO/P/Q), and Mn4CaO5 cluster, which is the active center of the WOC (for more information, see [66]). CAH3 protein is not indicated because its exact location near the WOC has not yet been established. (B) A possible involvement of CAH3 in the H+ “neutralization” near outlets of the proton channels in the lumen. A proton channel scheme was created based on the data from [67], where H+, water molecules, and an amino acid residue are presented. The thickness of the arrows indicates the effectiveness of proton transfer or of the dehydration reaction. For details, see the main text.
Photosynthetic water oxidation results in the production of 4H+ per O2 (2H2O → 4ē + O2 + 4H+) (Figure 5A). A calculation based on the O2 evolution activity of PSII preparations (280 μmol O2 (mg Chl h)−1) indicated a H+ production rate of ~75 H+/s per PSII [10]. This requires a rapid removal of H+ from the active center of the WOC (to maintain its optimal activity) to the lumen via the proton channels including Cl− atoms, water molecules, and amino acid residues of WOC proteins [67]. At an optimal pH for the WOC of 6.5, the effectiveness of H+ transfer via the channels is maximal and fully covers the proton production (Figure 5B). In addition, the Ci content in the medium at this pH was estimated as ~380 μM, with part of HCO3− equal to 200 μM (~53%) [10], which is involved in a spontaneous dehydration reaction near outlets of the proton channels even in the absence of CAH3. An increase (or decrease) in pH induces conformational perturbations in proteins of PSII including the WOC [66], which is followed by changes in the spatial position of amino acids. This can result in increasing the distances between water molecules and amino acid residues involved in the formation of the proton channel and consequently in the suppression of H+ transfer (Figure 5B). At pH 7.0, the medium contains ~460 μM Ci, and the spontaneous dehydration reaction is unfavorable (~380 μM HCO3−, (~83%)) [10]. In the absence (cia3) or under the inhibition of CAH3, this leads to an accumulation of H+ near the outlets of the proton channels, which can be an additional suppressive factor for effective H+ transfer from the active center of the WOC, where local acidification inhibits its function. As shown, only a three- to fourfold increase in HCO3− content can stimulate the photosynthetic activity of PSII, while the presence of active CAH3 (WT) accelerates the interconnection of H+ with HCO3− of the lumen, supporting a high proton gradient in the channel, rapid H+ removal, and, thus, the optimal operation of the active center of the WOC. At pH 5.5, in addition to the consequences of conformational changes in PSII, the content of Ci is lower (~250 μM) with a very small part of HCO3− (~40 μM, ~16%) [10]. However, a favorable spontaneous dehydration reaction (more favorable than that at pH 6.5, Figure 5B), even in the presence of only ~40 μM HCO3− in the medium [10], was able to support a high level of H+ removal from the active center of the WOC through their “neutralization” near the outlets of the proton channels. The inhibition of WOC activity due to the suppression of H+ transfer to the lumen was observed under the depletion of CO2/HCO3− from the medium [25].
In addition to the clear influence of the CA activity of CAH3 on PSII function and its stability, evidence was obtained about the presence of some conformational changes in PSII complexes assembled in the absence of the CAH3 protein, for example, in the case of the cia3 mutant. The shape of the fast Chl fluorescence rise kinetic (OJ(I)P) measured for PSII-enriched membranes isolated from the cia3 mutant had a more straightened J–P phase compared to the PSII from WT, resulting in a ~33% reduction in the Area parameter [26]. This indicated the suppression in the electron transfer between Qa and Qb on the PSII acceptor side (Figure 5A). It was proposed that this could be a result of some conformational changes in the native structure of the WOC induced by the loss of the CAH3 protein and transmission from the donor to the acceptor sides of PSII through the subunits of cytochrome b559 connecting with PsbP. (Other ways have been reviewed recently [66].) This is supported by the increased portion of the low-potential form of the cytochrome b559 in PSII from the cia3 mutant [26]. Later, it was shown that the WOC in PSII from the cia3 mutant is indeed characterized by lower functional stability under increased Cl− content with a simultaneously stronger binding of the PsbP protein to the WOC [27]. Interestingly, the inhibition of CA activity had no influence on the obtained results, directly indicating the structural role of the CAH3 protein in the PSII complex of C. reinhardtii. This structural role of an active CA protein in a multiprotein complex has not been identified previously.
7. Incorporation of CAH3 into Higher Plants
The components of the CCM of algae are often considered to be a tool for improving the biomass production of higher plants due to chloroplasts saturation by Ci. The possibility of the CAH3 protein (or a ~37 kDa construct containing the catalytic part of CAH3 [68]) to localize in the thylakoids of higher plants [46,68] and increase CO2 assimilation was shown, which resulted in a larger size of plants and number of leaves and an acceleration of flowering [68]. The CAH3-containing mutants of tobacco plants showed an ~18% increase in both the maximal RuBisCO carboxylation rate and the maximal rate of linear electron transport, as well as higher levels of the effective quantum yield of PSII and the photochemical quenching of Chl fluorescence (qP) of PSII. The authors of [68] largely attributed this to a higher concentration of CO2 in the vicinity of RuBisCO. However, they also mentioned the possibility of CAH3 involvement in the acceleration of H+ removal from the active center of the WOC through catalyzing the dehydration reaction, as was proposed for C. reinhardtii PSII. On the one hand, the obtained results require further study to identify whether CAH3 is associated with PSII, which enhances linear electron transport resulting in an increase in the activity of RuBisCO, or, in contrast, whether it operates independently by removing the CO2 limitation on the carboxylation rate of RuBisCO. On the other hand, the results clearly show the possibility of using CAH3 as an effective tool for increasing the biomass production of higher plants, which can be used in agriculture in the future.
Author Contributions
Conceptualization, V.V.T.; validation, V.V.T. and A.K.S.; formal analysis, V.V.T. and A.K.S.; writing—original draft preparation, V.V.T. and A.K.S.; writing—review and editing, V.V.T. and A.K.S.; visualization, V.V.T.; supervision, V.V.T.; project administration, V.V.T. and A.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Russian Science Foundation (project no. 23-24-00550).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.; Conway, W.; Burns, R.; McCann, N.; Maeder, M. Comprehensive Study of the Hydration and Dehydration Reactions of Carbon Dioxide in Aqueous Solution. J. Phys. Chem. A 2010, 114, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
- Soli, A.L.; Byrne, R.H. CO2 System Hydration and Dehydration Kinetics and the Equilibrium CO2/H2CO3 Ratio in Aqueous NaCl Solution. Mar. Chem. 2002, 78, 65–73. [Google Scholar] [CrossRef]
- Johnson, K.S. Carbon Dioxide Hydration and Dehydration Kinetics in Seawater1. Limnol. Oceanogr. 1982, 27, 849–855. [Google Scholar] [CrossRef]
- Hilvo, M.; Baranauskiene, L.; Salzano, A.M.; Scaloni, A.; Matulis, D.; Innocenti, A.; Scozzafava, A.; Monti, S.M.; Di Fiore, A.; De Simone, G.; et al. Biochemical Characterization of CA IX, One of the Most Active Carbonic Anhydrase Isozymes. J. Biol. Chem. 2008, 283, 27799–27809. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.N.; Ignatova, L.K.; Nadeeva-Zhurikova, E.M.; Fedorchuk, T.P.; Ivanov, B.N.; Borisova-Mubarakshina, M.M. Advances in Understanding the Physiological Role and Locations of Carbonic Anhydrases in C3 Plant Cells. Protoplasma 2021, 258, 249–262. [Google Scholar] [CrossRef]
- Aspatwar, A.; Haapanen, S.; Parkkila, S. An Update on the Metabolic Roles of Carbonic Anhydrases in the Model Alga Chlamydomonas reinhardtii. Metabolites 2018, 8, 22. [Google Scholar] [CrossRef]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 Concentrating Mechanism and Photosynthetic Carbon Assimilation in Limiting CO2: How Chlamydomonas Works against the Gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The Many Types of Carbonic Anhydrases in Photosynthetic Organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Klimov, V.; Baranov, S. Bicarbonate Requirement for the Water-Oxidizing Complex of Photosystem II. Biochim. Biophys. Acta—Bioenerg. 2001, 1503, 187–196. [Google Scholar] [CrossRef]
- Terentyev, V.V.; Shukshina, A.K.; Shitov, A.V. Carbonic Anhydrase CAH3 Supports the Activity of Photosystem II under Increased PH. Biochim. Biophys. Acta—Bioenerg. 2019, 1860, 582–590. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Fedorchuk, T.P.; Terentyev, V.V.; Dymova, O.V.; Naydov, I.A.; Golovko, T.K.; Borisova-Mubarakshina, M.M.; Ivanov, B.N. The Role of Carbonic Anhydrase α-CA4 in the Adaptive Reactions of Photosynthetic Apparatus: The Study with α-CA4 Knockout Plants. Protoplasma 2020, 257, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Moroney, J.V.; Ma, Y.; Frey, W.D.; Fusilier, K.A.; Pham, T.T.; Simms, T.A.; DiMario, R.J.; Yang, J.; Mukherjee, B. The Carbonic Anhydrase Isoforms of Chlamydomonas reinhardtii: Intracellular Location, Expression, and Physiological Roles. Photosynth. Res. 2011, 109, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Sun, J.; Wunder, T.; Tang, D.; Cousins, A.B.; Sze, S.K.; Mueller-Cajar, O.; Gao, Y.-G. Structural Insights into the LCIB Protein Family Reveals a New Group of β-Carbonic Anhydrases. Proc. Natl. Acad. Sci. USA 2016, 113, 14716–14721. [Google Scholar] [CrossRef] [PubMed]
- Jungnick, N.; Ma, Y.; Mukherjee, B.; Cronan, J.C.; Speed, D.J.; Laborde, S.M.; Longstreth, D.J.; Moroney, J.V. The Carbon Concentrating Mechanism in Chlamydomonas reinhardtii: Finding the Missing Pieces. Photosynth. Res. 2014, 121, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the Functions and Ecophysiological Relevance of the Diverse Carbonic Anhydrases in Microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef] [PubMed]
- Ynalvez, R.A.; Xiao, Y.; Ward, A.S.; Cunnusamy, K.; Moroney, J.V. Identification and Characterization of Two Closely Related Β-carbonic Anhydrases from Chlamydomonas reinhardtii. Physiol. Plant. 2008, 133, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Miura, K.; Fukuzawa, H. Expression Analysis of Genes Associated with the Induction of the Carbon-Concentrating Mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2008, 147, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Cardol, P.; Gonzaález-Halphen, D.; Reyes-Prieto, A.; Baurain, D.; Matagne, R.F.; Remacle, C. The Mitochondrial Oxidative Phosphorylation Proteome of Chlamydomonas reinhardtii Deduced from the Genome Sequencing Project. Plant Physiol. 2005, 137, 447–459. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A Spatial Interactome Reveals the Protein Organization of the Algal CO2-Concentrating Mechanism. Cell 2017, 171, 133–147.e14. [Google Scholar] [CrossRef]
- Karlsson, J.; Ciarke, A.K.; Chen, Z.Y.; Hugghins, S.Y.; Park, Y.I.; Husic, H.D.; Moroney, J.V.; Samuelsson, G. A Novel α-Type Carbonic Anhydrase Associated with the Thylakoid Membrane in Chlamydomonas reinhardtii Is Required for Growth at Ambient CO2. EMBO J. 1998, 17, 1208–1216. [Google Scholar] [CrossRef]
- Karlsson, J.; Hiltonen, T.; Husic, H.D.; Ramazanov, Z.; Samuelsson, G. Intracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1995, 109, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Karlsson, J.; Rojdestvenski, I.; Pronina, N.; Klimov, V.; Öquist, G.; Samuelsson, G. Role of a Novel Photosystem II-Associated Carbonic Anhydrase in Photosynthetic Carbon Assimilation in Chlamydomonas reinhardtii. FEBS Lett. 1999, 444, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.; Shutova, T.; Moskvin, O.; Forssén, M.; Klimov, V.V.; Samuelsson, G. A Photosystem II-Associated Carbonic Anhydrase Regulates the Efficiency of Photosynthetic Oxygen Evolution. EMBO J. 2002, 21, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Markelova, A.G.; Sinetova, M.P.; Kupriyanova, E.V.; Pronina, N.A. Distribution and Functional Role of Carbonic Anhydrase Cah3 Associated with Thylakoid Membranes in the Chloroplast and Pyrenoid of Chlamydomonas reinhardtii. Russ. J. Plant Physiol. 2009, 56, 761–768. [Google Scholar] [CrossRef]
- Shutova, T.; Kenneweg, H.; Buchta, J.; Nikitina, J.; Terentyev, V.; Chernyshov, S.; Andersson, B.; Allakhverdiev, S.I.; Klimov, V.V.; Dau, H.; et al. The Photosystem II-Associated Cah3 in Chlamydomonas Enhances the O2 Evolution Rate by Proton Removal. EMBO J. 2008, 27, 782–791. [Google Scholar] [CrossRef]
- Terentyev, V.V.; Shukshina, A.K.; Ashikhmin, A.A.; Tikhonov, K.G.; Shitov, A.V. The Main Structural and Functional Characteristics of Photosystem-II-Enriched Membranes Isolated from Wild Type and Cia3 Mutant Chlamydomonas reinhardtii. Life 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Shukshina, A.K.; Terentyev, V.V. Involvement of Carbonic Anhydrase CAH3 in the Structural and Functional Stabilization of the Water-Oxidizing Complex of Photosystem II from Chlamydomonas reinhardtii. Biochemistry 2021, 86, 867–877. [Google Scholar] [CrossRef]
- Rawat, M.; Moroney, J.V. Partial Characterization of a New Isoenzyme of Carbonic Anhydrase Isolated from Chlamydomonas reinhardtii. J. Biol. Chem. 1991, 266, 9719–9723. [Google Scholar] [CrossRef]
- Tachiki, A.; Fukuzawa, H.; Miyachi, S. Characterization of Carbonic Anhydrase Isozyme CA2, Which Is the CAH2 Gene Product, in Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 1992, 56, 794–798. [Google Scholar] [CrossRef]
- Sültemeyer, D.F.; Fock, H.P.; Canvin, D.T. Mass Spectrometric Measurement of Intracellular Carbonic Anhydrase Activity in High and Low Ci Cells of Chlamydomonas. Plant Physiol. 1990, 94, 1250–1257. [Google Scholar] [CrossRef][Green Version]
- Karlsson, J.; Ramazanov, Z.; Hiltonen, T.; Gardeström, P.; Samuelsson, G. Effect of Vanadate on Photosynthesis and the ATP/ADP Ratio in Low-CO2-Adapted Chlamydomonas reinhardtii Cells. Planta 1994, 192, 46–51. [Google Scholar] [CrossRef]
- Katzman, G.L.; Carlson, S.J.; Marcus, Y.; Moroney, J.V.; Togasaki, R.K. Carbonic Anhydrase Activity in Isolated Chloroplasts of Wild-Type and High-CO2-Dependent Mutants of Chlamydomonas reinhardtii as Studied by a New Assay. Plant Physiol. 1994, 105, 1197–1202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramazanov, Z.; Cardenas, J. Piotorespiratory Ammonium Assimilation in Chloroplasts of Chlamydomonas reinhardtii. Physiol. Plant. 1994, 91, 495–502. [Google Scholar] [CrossRef]
- Spalding, M.H.; Spreitzer, R.J.; Ogren, W.L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardtii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983, 73, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Funke, R.P.; Kovar, J.L.; Weeks, D.P. Lntracellular Carbonic Anhydrase Is Essential to Photosynthesis in Chlamydomonas reinhardtii at Atmospheric LeveIs of CO2. Plant Physiol. 1997, 114, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Husic, H.D.; Marcus, C.A. Identification of Lntracellular Carbonic Anhydrase in Chlamydomonas reinhardii with a Carbonic Anhydrase-Directed Photoaff Inity Label. Plant Physiol. 1994, 105, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rivero, A.; Shutova, T.; Román, M.J.; Villarejo, A.; Martinez, F. Phosphorylation Controls the Localization and Activation of the Lumenal Carbonic Anhydrase in Chlamydomonas reinhardtii. PLoS ONE 2012, 7, e49063. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, A.M.; Mant, A.; Karnauchov, I.; Brink, S.; Herrmann, R.G.; Klösgen, R.B.; Robinson, C. A New Type of Signal Peptide: Central Role of a Twin-Arginine Motif in Transfer Signals for the Delta PH-Dependent Thylakoidal Protein Translocase. EMBO J. 1995, 14, 2715–2722. [Google Scholar] [CrossRef]
- Mori, H.; Cline, K. Post-Translational Protein Translocation into Thylakoids by the Sec and ΔpH-Dependent Pathways. Biochim. Biophys. Acta—Mol. Cell Res. 2001, 1541, 80–90. [Google Scholar] [CrossRef]
- Mitra, M.; Mason, C.B.; Xiao, Y.; Ynalvez, R.A.; Lato, S.M.; Moroney, J.V. The Carbonic Anhydrase Gene Families of Chlamydomonas reinhardtii. Can. J. Bot. 2005, 83, 780–795. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Kupriyanova, E.V.; Markelova, A.G.; Allakhverdiev, S.I.; Pronina, N.A. Identification and Functional Role of the Carbonic Anhydrase Cah3 in Thylakoid Membranes of Pyrenoid of Chlamydomonas reinhardtii. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.C.; Meyer, M.T.; Griffiths, H. Dynamics of Carbon-Concentrating Mechanism Induction and Protein Relocalization during the Dark-to-Light Transition in Synchronized Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Tirumani, S.; Kokkanti, M.; Chaudhari, V.; Shukla, M.; Rao, B.J. Regulation of CCM Genes in chlamydomonas reinhardtii during Conditions of Light–Dark Cycles in Synchronous Cultures. Plant Mol. Biol. 2014, 85, 277–286. [Google Scholar] [CrossRef]
- Tirumani, S.; Gothandam, K.M.; J Rao, B. Coordination between Photorespiration and Carbon Concentrating Mechanism in Chlamydomonas reinhardtii: Transcript and Protein Changes during Light-Dark Diurnal Cycles and Mixotrophy Conditions. Protoplasma 2019, 256, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.C.; Metodieva, G.; Metodiev, M.V.; Griffiths, H.; Meyer, M.T. Pyrenoid Loss Impairs Carbon-Concentrating Mechanism Induction and Alters Primary Metabolism in Chlamydomonas reinhardtii. J. Exp. Bot. 2017, 68, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.; Feike, D.; Mackinder, L.C.M.; Meyer, M.T.; Griffiths, H.; Jonikas, M.C.; Smith, A.M.; McCormick, A.J. Introducing an Algal Carbon-concentrating Mechanism into Higher Plants: Location and Incorporation of Key Components. Plant Biotechnol. J. 2016, 14, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Muto, S.; Miyachi, S. Structural Analysis of Periplasmic Carbonic Anhydrase 1 of Chlamydomonas reinhardtii. Eur. J. Biochem. 1993, 214, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, R.; Shevela, D.; Hainzl, T.; Grundström, C.; Shutova, T.; Messinger, J.; Samuelsson, G.; Sauer-Eriksson, A.E. Crystal Structure and Functional Characterization of Photosystem II-Associated Carbonic Anhydrase CAH3 in Chlamydomonas reinhardtii. Plant Physiol. 2015, 167, 950–962. [Google Scholar] [CrossRef]
- Yang, S.Y.; Tsuzuki, M.; Miyachi, S. Carbonic Anhydrase of Chlamydomonas: Purification and Studies on Its Induction Using Antisemm against Chlamydomonas Carbonic Anhydrase. Plant Cell Physiol 1985, 26, 25–34. [Google Scholar]
- Lee, C.H.; Jang, E.K.; Yeon, Y.J.; Pack, S.P. Stabilization of Bovine Carbonic Anhydrase II through Rational Site-Specific Immobilization. Biochem. Eng. J. 2018, 138, 29–36. [Google Scholar] [CrossRef]
- Fisher, Z.; Hernandez Prada, J.A.; Tu, C.; Duda, D.; Yoshioka, C.; An, H.; Govindasamy, L.; Silverman, D.N.; McKenna, R. Structural and Kinetic Characterization of Active-Site Histidine as a Proton Shuttle in Catalysis by Human Carbonic Anhydrase II. Biochemistry 2005, 44, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Christianson, D.W. Unexpected PH-Dependent Conformation of His-64, the Proton Shuttle of Carbonic Anhydrase II. J. Am. Chem. Soc. 1991, 113, 9455–9458. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lau, C.S.; Walker, C.E.; Rai, A.K.; Prejean, C.I.; Yates, G.; Emrich-Mills, T.; Lemoine, S.G.; Vinyard, D.J.; Mackinder, L.C.M.; et al. Thylakoid Localized Bestrophin-like Proteins Are Essential for the CO2 Concentrating Mechanism of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 16915–16920. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Chen, T.; Moroney, J.V. Mitochondrial Carbonic Anhydrases Are Needed for Optimal Photosynthesis at Low CO2 levels in Chlamydomonas. Plant Physiol. 2021, 187, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, D.; Wang, Y.; Spalding, M.H. Thylakoid Lumen Carbonic Anhydrase (CAH3) Mutation Suppresses Air-Dier Phenotype of LCIB Mutant in Chlamydomonas reinhardtii. Plant Physiol. 2009, 149, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Machingura, M.C.; Moroney, J.V. Closing the Circle. Elife 2018, 7, e42507. [Google Scholar] [CrossRef]
- Adler, L.; Lau, C.S.; Shaikh, K.M.; van Maldegem, K.A.; Payne-Dwyer, A.L.; Lefoulon, C.; Girr, P.; Atkinson, N.; Barrett, J.; Emrich-Mills, T.Z.; et al. The Role of BST4 in the Pyrenoid of Chlamydomonas reinhardtii. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hanson, D.T.; Franklin, L.A.; Samuelsson, G.; Badger, M.R. The Chlamydomonas reinhardtii Cia3 Mutant Lacking a Thylakoid Lumen-Localized Carbonic Anhydrase Is Limited by CO2 Supply to Rubisco and Not Photosystem II Function in Vivo. Plant Physiol. 2003, 132, 2267–2275. [Google Scholar] [CrossRef]
- Moroney, J.V.; Tolbert, N.E.; Sears, B.B. Complementation Analysis of the Inorganic Carbon Concentrating Mechanism of Chlamydomonas reinhardtii. Mol. Gen. Genet. MGG 1986, 204, 199–203. [Google Scholar] [CrossRef]
- Moroney, J.V.; Husic, H.D.; Tolbert, N.E. Effect of Carbonic Anhydrase Inhibitors on Inorganic Carbon Accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985, 79, 177–183. [Google Scholar] [CrossRef]
- Wang, Y.; Spalding, M.H. An Inorganic Carbon Transport System Responsible for Acclimation Specific to Air Levels of CO2 in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2006, 103, 10110–10115. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Itakura, A.K.; Patena, W.; Wang, L.; He, S.; Emrich-Mills, T.; Lau, C.S.; Yates, G.; Mackinder, L.C.M.; Jonikas, M.C. Assembly of the Algal CO2-Fixing Organelle, the Pyrenoid, Is Guided by a Rubisco-Binding Motif. Sci. Adv. 2020, 6, eabd2408. [Google Scholar] [CrossRef] [PubMed]
- Caspari, O.D.; Meyer, M.T.; Tolleter, D.; Wittkopp, T.M.; Cunniffe, N.J.; Lawson, T.; Grossman, A.R.; Griffiths, H. Pyrenoid Loss in Chlamydomonas reinhardtii Causes Limitations in CO2 Supply, but Not Thylakoid Operating Efficiency. J. Exp. Bot. 2017, 68, 3903–3913. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, V.V.; Shukshina, A.K.; Chetverkina, A.A. Action of 2,6-Dichloro-1,4-Benzoquinone on the O2-Evolving Activity of Photosystem II in Chlamydomonas reinhardtii Cells with and without Cell Wall: Inhibitory Effect of Its Oxidized Form. Cells 2023, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, V.V. Loss of Carbonic Anhydrase in the Thylakoid Lumen Causes Unusual Moderate-Light-Induced Rearrangement of the Chloroplast in Chlamydomonas reinhardtii as a Way of Photosystem II Photoprotection. Plant Physiol. Biochem. 2021, 168, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, V.V. Macromolecular Conformational Changes in Photosystem II: Interaction between Structure and Function. Biophys. Rev. 2022, 14, 871–886. [Google Scholar] [CrossRef]
- Gabdulkhakov, A.; Guskov, A.; Broser, M.; Kern, J.; Müh, F.; Saenger, W.; Zouni, A. Probing the Accessibility of the Mn4Ca Cluster in Photosystem II: Channels Calculation, Noble Gas Derivatization, and Cocrystallization with DMSO. Structure 2009, 17, 1223–1234. [Google Scholar] [CrossRef]
- Nölke, G.; Barsoum, M.; Houdelet, M.; Arcalís, E.; Kreuzaler, F.; Fischer, R.; Schillberg, S. The Integration of Algal Carbon Concentration Mechanism Components into Tobacco Chloroplasts Increases Photosynthetic Efficiency and Biomass. Biotechnol. J. 2019, 14, 1800170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).