Pyridoxamine Attenuates Doxorubicin-Induced Cardiomyopathy without Affecting Its Antitumor Effect on Rat Mammary Tumor Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model of DOX-Induced Cardiomyopathy
2.2. Transthoracic Echocardiography
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Proliferation and Cytotoxicity Assays
2.6. Immunocytochemistry
2.7. Statistical Analysis
3. Results
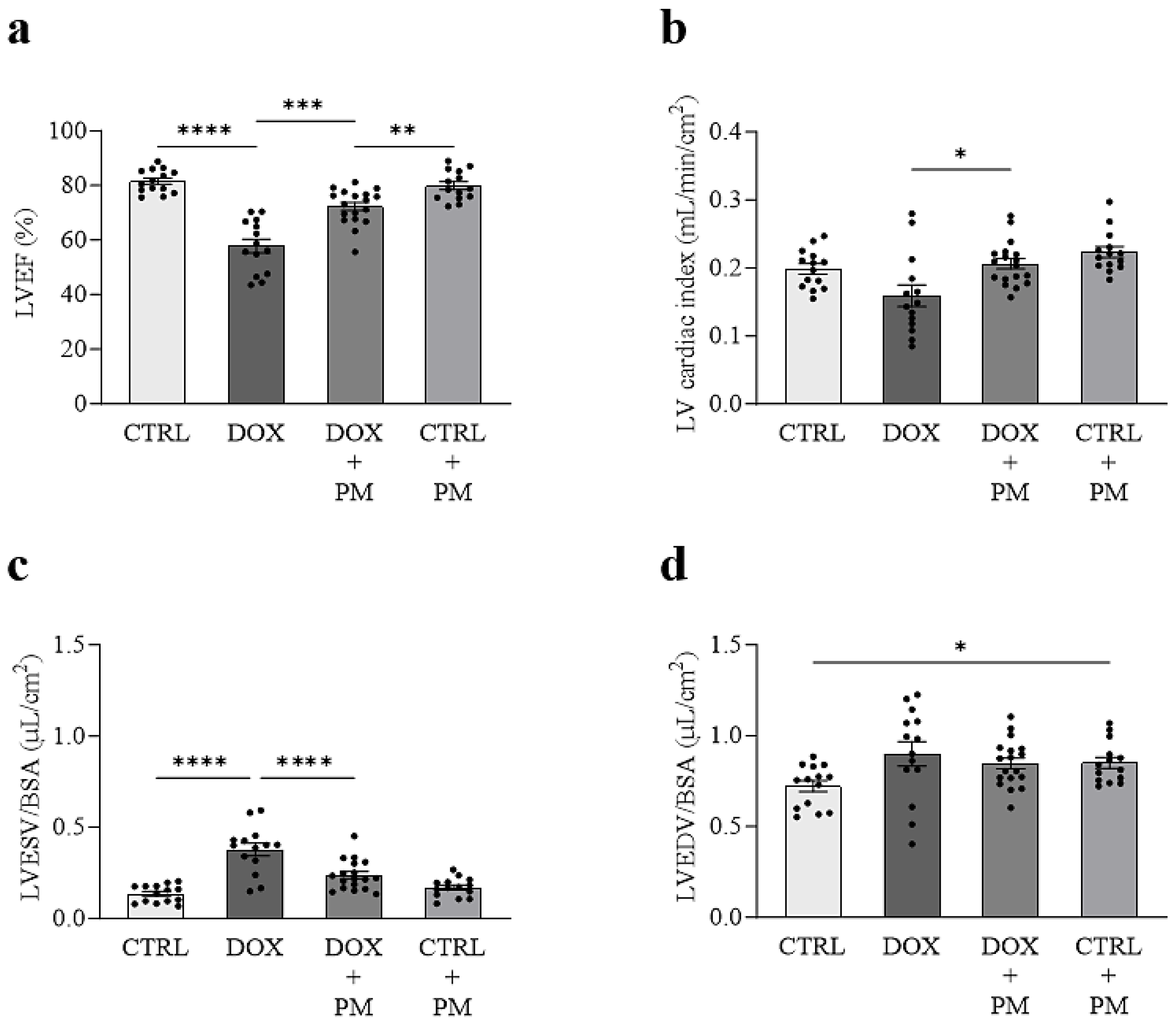
3.1. PM Attenuates DOX-Induced LV Cardiomyopathy In Vivo
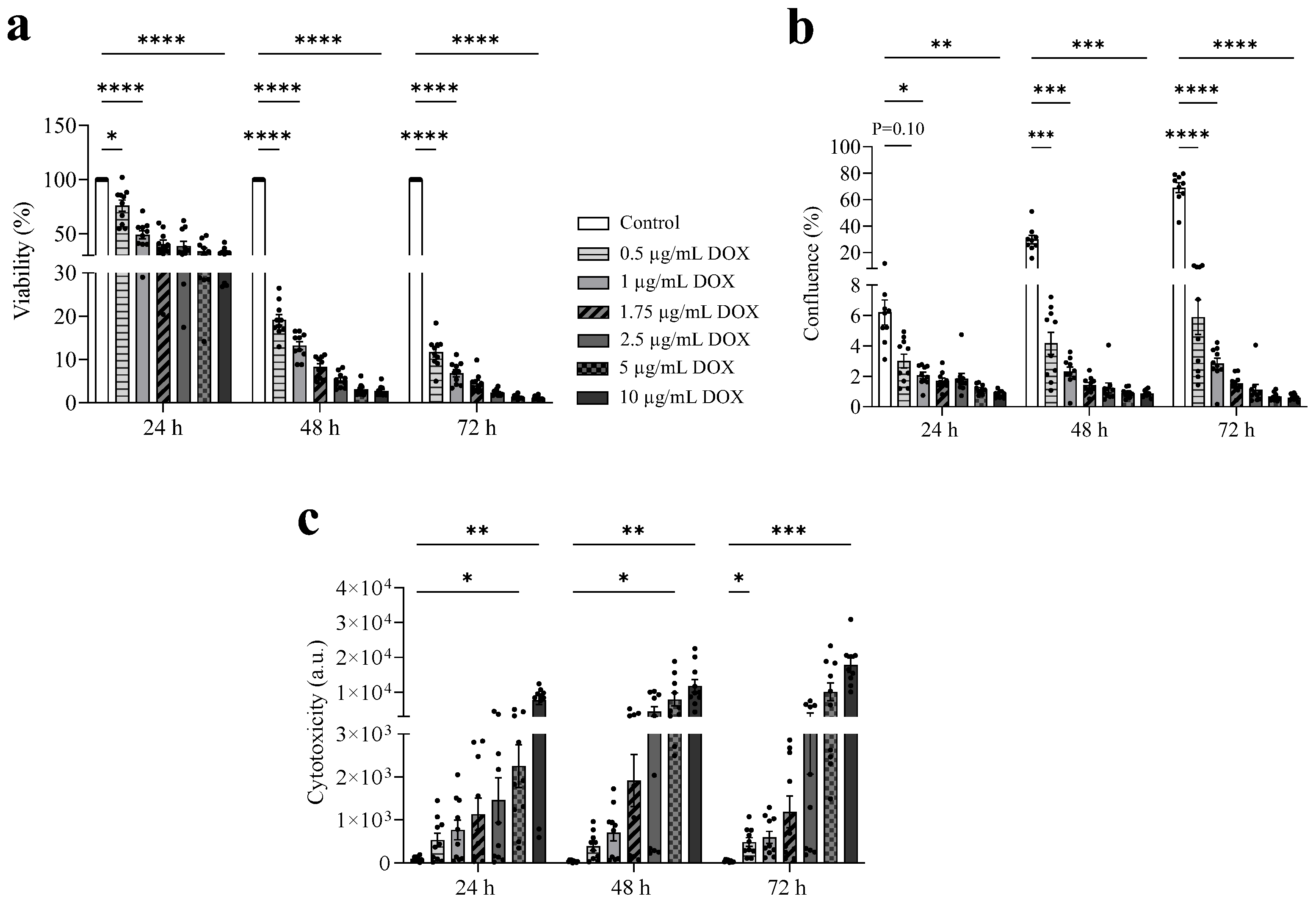
3.2. DOX Exhibits a Dose-Dependent Antitumor Effect
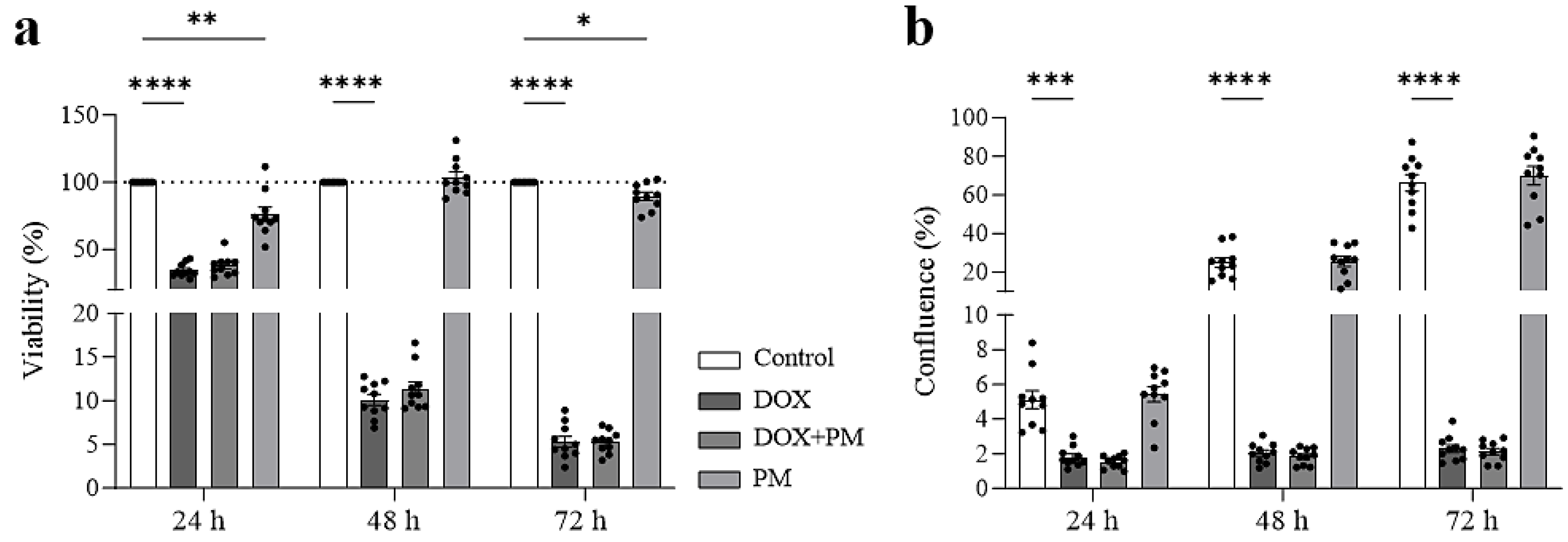
3.3. PM Does Not Interfere with the Antitumor Effect of DOX on Mammary Tumor Cells
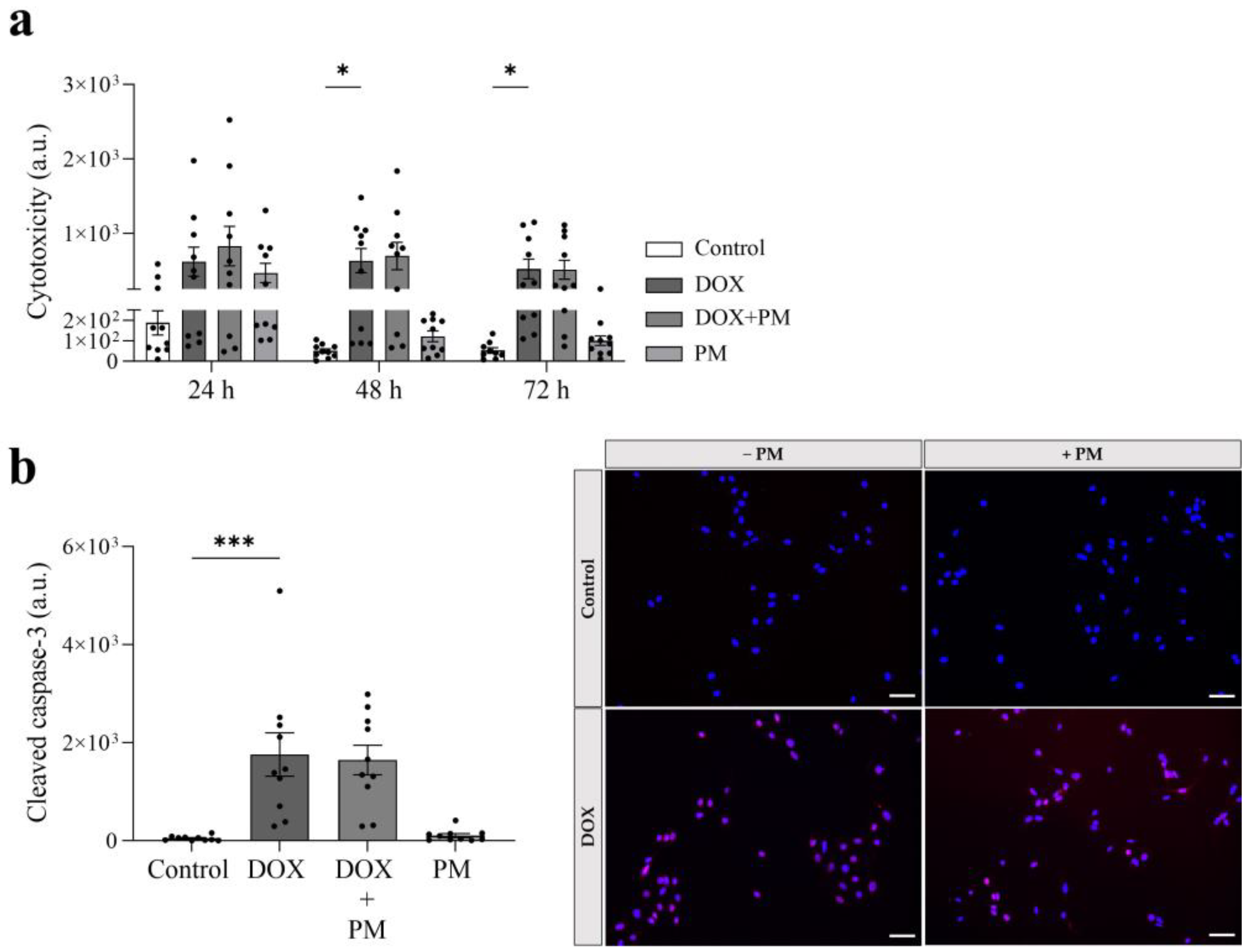
3.4. PM Does Not Affect the Cytotoxic and Apoptotic Effect of DOX
4. Discussion
4.1. PM as a Cardioprotectant during DOX Treatment without Affecting Antitumor Efficacy
4.2. Current Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’Avanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Strongman, H.; Gadd, S.; Matthews, A.A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Does Cardiovascular Mortality Overtake Cancer Mortality during Cancer Survivorship?: An English Retrospective Cohort Study. JACC CardioOncol. 2022, 4, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Garcia-Diez, G.; Monreal-Corona, R.; Mora-Diez, N. Complexes of Copper and Iron with Pyridoxamine, Ascorbic Acid, and a Model Amadori Compound: Exploring Pyridoxamine’s Secondary Antioxidant Activity. Antioxidants 2021, 10, 208. [Google Scholar] [CrossRef]
- Deluyker, D.; Ferferieva, V.; Driesen, R.B.; Verboven, M.; Lambrichts, I.; Bito, V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci. Rep. 2017, 7, 16010. [Google Scholar] [CrossRef]
- Watson, A.M.; Soro-Paavonen, A.; Sheehy, K.; Li, J.; Calkin, A.C.; Koitka, A.; Rajan, S.N.; Brasacchio, D.; Allen, T.J.; Cooper, M.E.; et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia 2011, 54, 681–689. [Google Scholar] [CrossRef]
- Guler, M.N.; Tscheiller, N.M.; Sabater-Molina, M.; Gimeno, J.R.; Nebigil, C.G. Evidence for reciprocal network interactions between injured hearts and cancer. Front. Cardiovasc. Med. 2022, 9, 929259. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; Hulot, J.S.; Tocchetti, C.G.; Aboumsallem, J.P.; Ameri, P.; Anker, S.D.; Bauersachs, J.; Bertero, E.; Coats, A.J.S.; Celutkiene, J.; et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 2272–2289. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Santos-Silva, D.; Rodrigues, T.; Matafome, P.; Crisostomo, J.; Sena, C.; Goncalves, L.; Seica, R. Pyridoxamine reverts methylglyoxal-induced impairment of survival pathways during heart ischemia. Cardiovasc. Ther. 2013, 31, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Asnani, A.; Moslehi, J.J.; Adhikari, B.B.; Baik, A.H.; Beyer, A.M.; de Boer, R.A.; Ghigo, A.; Grumbach, I.M.; Jain, S.; Zhu, H.; et al. Preclinical Models of Cancer Therapy-Associated Cardiovascular Toxicity: A Scientific Statement from the American Heart Association. Circ. Res. 2021, 129, e21–e34. [Google Scholar] [CrossRef]
- Evens, L.; Belien, H.; D’Haese, S.; Haesen, S.; Verboven, M.; Rummens, J.L.; Bronckaers, A.; Hendrikx, M.; Deluyker, D.; Bito, V. Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair? Int. J. Mol. Sci. 2021, 22, 9266. [Google Scholar] [CrossRef]
- Ibiyeye, K.M.; Nordin, N.; Ajat, M.; Zuki, A.B.Z. Ultrastructural Changes and Antitumor Effects of Doxorubicin/Thymoquinone-Loaded CaCO3 Nanoparticles on Breast Cancer Cell Line. Front. Oncol. 2019, 9, 599. [Google Scholar] [CrossRef]
- Sharma, A.; Ozayral, S.; Caserto, J.S.; Ten Cate, R.; Anders, N.M.; Barnett, J.D.; Kandala, S.K.; Henderson, E.; Stewart, J.; Liapi, E.; et al. Increased uptake of doxorubicin by cells undergoing heat stress does not explain its synergistic cytotoxicity with hyperthermia. Int. J. Hyperth. 2019, 36, 712–720. [Google Scholar] [CrossRef]
- Sikora, T.; Morawska, K.; Lisowski, W.; Rytel, P.; Dylong, A. Application of Optical Methods for Determination of Concentration of Doxorubicin in Blood and Plasma. Pharmaceuticals 2022, 15, 112. [Google Scholar] [CrossRef]
- Lupertz, R.; Watjen, W.; Kahl, R.; Chovolou, Y. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology 2010, 271, 115–121. [Google Scholar] [CrossRef]
- Nakada, M.; Niska, J.A.; Tran, N.L.; McDonough, W.S.; Berens, M.E. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am. J. Pathol. 2005, 167, 565–576. [Google Scholar] [CrossRef]
- Herrmann, A.L.; Kuhn, B.J.; Holzer, A.; Krijgsveld, J.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Delineating the Switch between Senescence and Apoptosis in Cervical Cancer Cells under Ciclopirox Treatment. Cancers 2021, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, I.P.; Khumaira, A.; Ilmawati, G.P.N.; Meiyanto, E.; Hermawan, A. Cytotoxic and Antimetastatic Activity of Hesperetin and Doxorubicin Combination Toward Her2 Expressing Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2020, 21, 1259–1267. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Hirji, S.A.; Qamar, A.; Bajaj, N.; Gupta, A.; Zaha, V.; Chandra, A.; Haykowsky, M.; Ky, B.; Moslehi, J.; et al. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients with Cancer Undergoing Chemotherapy. JACC CardioOncol. 2019, 1, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Tabone, M.D.; Mora, J.; Morland, B.; Jones, R.L. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: Re-evaluating the European labeling. Future Oncol. 2018, 14, 2663–2676. [Google Scholar] [CrossRef]
- Chan, B.Y.H.; Roczkowsky, A.; Cho, W.J.; Poirier, M.; Sergi, C.; Keschrumrus, V.; Churko, J.M.; Granzier, H.; Schulz, R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc. Res. 2021, 117, 188–200. [Google Scholar] [CrossRef]
- Hayward, R.; Hydock, D.S. Doxorubicin cardiotoxicity in the rat: An in vivo characterization. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–32. [Google Scholar]
- Haesen, S.; Brillouet, A.; de Laat, I.; Vastmans, L.; Evens, L.; D’Haese, S.; Wolfs, E.; Deluyker, D.; Bito, V. Pyridoxamine Protects against Cardiotoxicity after Doxorubicin Chemotherapy. Circulation 2021, 144, A10507. [Google Scholar] [CrossRef]
- Haesen, S.; Jager, M.M.; Brillouet, A.; de Laat, I.; Vastmans, L.; Verghote, E.; Delaet, A.; D’Haese, S.; Hamad, I.; Kleinewietfeld, M.; et al. Pyridoxamine limits cardiac dysfunction in a rat model of doxorubicin-induced cardiotoxicity. Antioxidants 2023. Paper submitted. [Google Scholar]
- Moriyama, T.; Kemi, M.; Okumura, C.; Yoshihara, K.; Horie, T. Involvement of advanced glycation end-products, pentosidine and N(epsilon)-(carboxymethyl)lysine, in doxorubicin-induced cardiomyopathy in rats. Toxicology 2010, 268, 89–97. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.G.; Houben, A.; Scheijen, J.; Linkens, A.M.A.; Niessen, P.M.; Simons, N.; Hanssen, N.M.J.; Kusters, Y.; Eussen, S.; Miyata, T.; et al. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2023, 25, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; McGill, J.B. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, S.; Hamid, S.A.; Farrag, A.R.; Samir, N.; Hussein, J.S. Cardioprotective effect of date palm against doxorubicin-induced cardiotoxicity. Asian J. Pharm. Clin. Res. 2018, 11, 141–146. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. In vitro combined effect of Doxorubicin and sulfonated zinc Phthalocyanine-mediated photodynamic therapy on MCF-7 breast cancer cells. Tumour Biol. 2017, 39, 1010428317727278. [Google Scholar] [CrossRef] [PubMed]
- Corrie, P.G.; Bulusu, R.; Wilson, C.B.; Armstrong, G.; Bond, S.; Hardy, R.; Lao-Sirieix, S.; Parashar, D.; Ahmad, A.; Daniel, F.; et al. A randomised study evaluating the use of pyridoxine to avoid capecitabine dose modifications. Br. J. Cancer 2012, 107, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Senovilla, L.; Olaussen, K.A.; Pinna, G.; Eisenberg, T.; Goubar, A.; Martins, I.; Michels, J.; Kratassiouk, G.; et al. Prognostic impact of vitamin B6 metabolism in lung cancer. Cell Rep. 2012, 2, 257–269. [Google Scholar] [CrossRef]
- Matsuo, T.; Sadzuka, Y. In Vitro Anticancer Activities of B(6) Vitamers: A Mini-review. Anticancer Res. 2019, 39, 3429–3432. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.; Hageman, G.J.; Bast, A.; Haenen, G. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol. Vitr. 2017, 44, 206–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haesen, S.; Verghote, E.; Heeren, E.; Wolfs, E.; Deluyker, D.; Bito, V. Pyridoxamine Attenuates Doxorubicin-Induced Cardiomyopathy without Affecting Its Antitumor Effect on Rat Mammary Tumor Cells. Cells 2024, 13, 120. https://doi.org/10.3390/cells13020120
Haesen S, Verghote E, Heeren E, Wolfs E, Deluyker D, Bito V. Pyridoxamine Attenuates Doxorubicin-Induced Cardiomyopathy without Affecting Its Antitumor Effect on Rat Mammary Tumor Cells. Cells. 2024; 13(2):120. https://doi.org/10.3390/cells13020120
Chicago/Turabian StyleHaesen, Sibren, Eline Verghote, Ellen Heeren, Esther Wolfs, Dorien Deluyker, and Virginie Bito. 2024. "Pyridoxamine Attenuates Doxorubicin-Induced Cardiomyopathy without Affecting Its Antitumor Effect on Rat Mammary Tumor Cells" Cells 13, no. 2: 120. https://doi.org/10.3390/cells13020120
APA StyleHaesen, S., Verghote, E., Heeren, E., Wolfs, E., Deluyker, D., & Bito, V. (2024). Pyridoxamine Attenuates Doxorubicin-Induced Cardiomyopathy without Affecting Its Antitumor Effect on Rat Mammary Tumor Cells. Cells, 13(2), 120. https://doi.org/10.3390/cells13020120






