The Role of Progranulin (PGRN) in the Pathogenesis of Glioblastoma Multiforme
Abstract
:1. Introduction
2. Biological Functioning and Molecular Aspects of PGRN
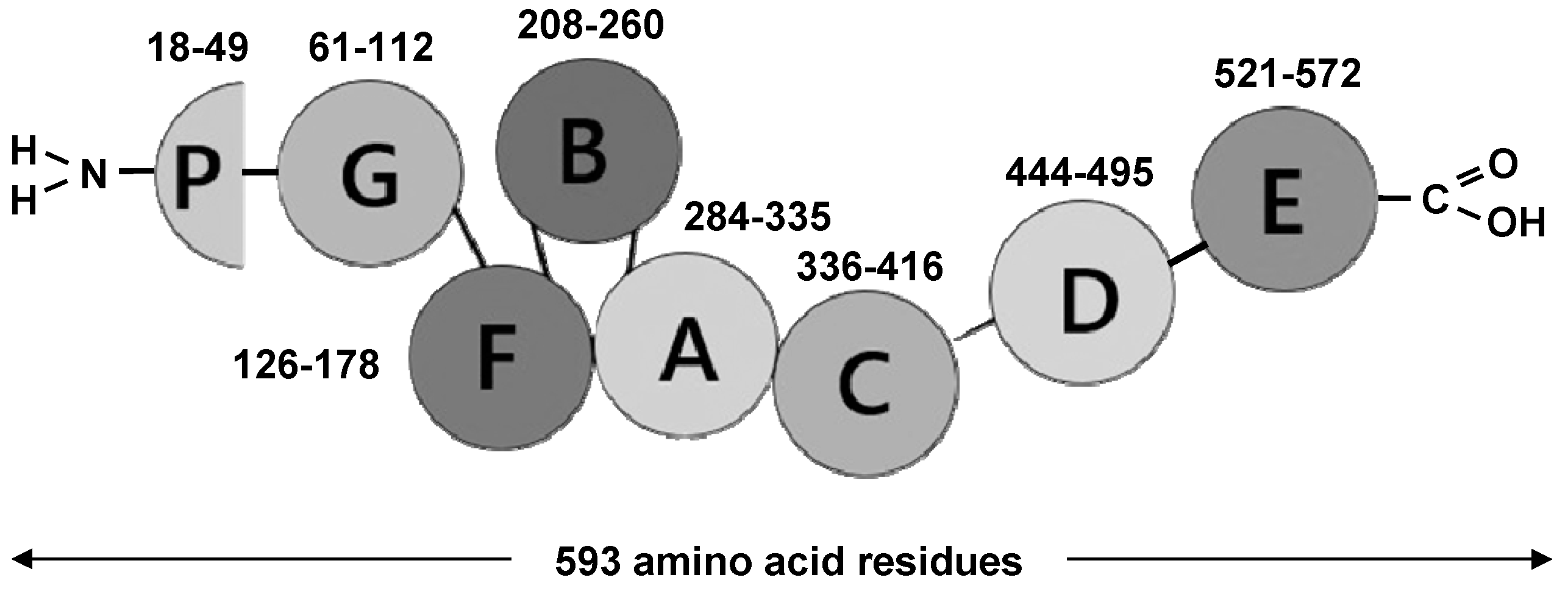
2.1. PGRN as Unique Multifunctional Growth Factor
2.2. Overview of PGRN Associated Receptors and Signalling Pathways
3. Neurobiology and Executive Functional Aspects of PGRN
4. Role and Function of PGRN in Oncogenesis and Cancer Development
5. Integrative Survey of the Role of PGRN in the Pathogenesis of GBM
6. PGRN-Related Receptors and Signalling Pathways in the Context of GBM
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.J.; Ryken, T.C. Congress of neurological surgeons systematic review and evidence-based clinical practice parameter guidelines for the treatment of adults with newly diagnosed glioblastoma: Introduction and Methods. J. Neuro-Oncol. 2020, 150, 87–93. [Google Scholar] [CrossRef]
- Goel, N.J.; Bird, C.E.; Hicks, W.H.; Abdullah, K.G. Economic implications of the modern treatment paradigm of glioblastoma: An analysis of global cost estimates and their utility for cost assessment. J. Med Econ. 2021, 24, 1018–1024. [Google Scholar] [CrossRef]
- Rodríguez-Camacho, A.; Flores-Vázquez, J.G.; Moscardini-Martelli, J.; Torres-Ríos, J.A.; Olmos-Guzmán, A.; Ortiz-Arce, C.S.; Cid-Sánchez, D.R.; Pérez, S.R.; Macías-González, M.D.S.; Hernández-Sánchez, L.C.; et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7207. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and immunoregulation in glioblastoma and brain metastases: Recent developments in imaging approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef]
- Brat, D.J.; Castellano-Sanchez, A.A.; Hunter, S.B.; Pecot, M.; Cohen, C.; Hammond, E.H.; Devi, S.N.; Kaur, B.; Van Meir, E.G. Pseudopalisades in Glioblastoma Are Hypoxic, Express Extracellular Matrix Proteases, and Are Formed by an Actively Migrating Cell Population. Cancer Res. 2004, 64, 920–927. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Albulescu, R.; Codrici, E.; Popescu, I.D.; Mihai, S.; Necula, L.G.; Petrescu, D.; Teodoru, M.; Tanase, C.P. Cytokine Patterns in Brain Tumour Progression. Mediat. Inflamm. 2013, 2013, 979748. [Google Scholar] [CrossRef]
- Yeo, E.C.F.; Brown, M.P.; Gargett, T.; Ebert, L.M. The Role of Cytokines and Chemokines in Shaping the Immune Microenvironment of Glioblastoma: Implications for Immunotherapy. Cells 2021, 10, 607. [Google Scholar] [CrossRef]
- Ventura, E.; Ducci, G.; Dominguez, R.B.; Ruggiero, V.; Belfiore, A.; Sacco, E.; Vanoni, M.; Iozzo, R.V.; Giordano, A.; Morrione, A. Progranulin Oncogenic Network in Solid Tumors. Cancers 2023, 15, 1706. [Google Scholar] [CrossRef]
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Cruts, M.; Gijselinck, I.; van der Zee, J.; Engelborghs, S.; Wils, H.; Pirici, D.; Rademakers, R.; Vandenberghe, R.; Dermaut, B.; Martin, J.-J.; et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442, 920–924. [Google Scholar] [CrossRef]
- He, Z.; Bateman, A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J. Mol. Med. 2003, 81, 600–612. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Shi, W.; Zhang, L.; Liu, S.; Lian, Y.; Liang, S.; Wang, H. Progranulin Regulates Inflammation and Tumor. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 88–102. [Google Scholar] [CrossRef]
- Daniel, R.; He, Z.; Carmichael, K.P.; Halper, J.; Bateman, A. Cellular Localization of Gene Expression for Progranulin. J. Histochem. Cytochem. 2000, 48, 999–1009. [Google Scholar] [CrossRef]
- Daniel, R.; Daniels, E.; He, Z.; Bateman, A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev. Dyn. 2003, 227, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Bennett, H.P.J. The granulin gene family: From cancer to dementia. BioEssays 2009, 31, 1245–1254. [Google Scholar] [CrossRef]
- De Muynck, L.; Van Damme, P. Cellular Effects of Progranulin in Health and Disease. J. Mol. Neurosci. 2011, 45, 549–560. [Google Scholar] [CrossRef]
- Koo, D.H.; Park, C.-Y.; Lee, E.S.; Ro, J.; Oh, S.W. Progranulin as a Prognostic Biomarker for Breast Cancer Recurrence in Patients Who Had Hormone Receptor-Positive Tumors: A Cohort Study. PLoS ONE 2012, 7, e39880. [Google Scholar] [CrossRef]
- Serrero, G. Progranulin/GP88, A Complex and Multifaceted Player of Tumor Growth by Direct Action and via the Tumor Mi-croenvironment. Adv. Exp. Med. Biol. 2021, 1329, 475–498. [Google Scholar] [CrossRef]
- Buraschi, S.; Xu, S.-Q.; Stefanello, M.; Moskalev, I.; Morcavallo, A.; Genua, M.; Tanimoto, R.; Birbe, R.; Peiper, S.C.; Gomella, L.G.; et al. Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin. Oncotarget 2016, 7, 39980–39995. [Google Scholar] [CrossRef]
- Liau, L.M.; Lallone, R.L.; Seitz, R.S.; Buznikov, A.; Gregg, J.P.; Kornblum, H.I.; Nelson, S.F.; Bronstein, J.M. Identification of a human glio-ma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res. 2000, 60, 1353–1360. [Google Scholar]
- Shoyab, M.; McDonald, V.L.; Byles, C.; Todaro, G.J.; Plowman, G.D. Epithelins 1 and 2: Isolation and characterization of two cysteine-rich growth-modulating proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 7912–7916. [Google Scholar] [CrossRef]
- Bateman, A.; Belcourt, D.; Bennett, H.; Lazure, C.; Solomon, S. Granulins, a novel class of peptide from leukocytes. Biochem. Biophys. Res. Commun. 1990, 173, 1161–1168. [Google Scholar] [CrossRef]
- Bhandari, V.; Palfree, R.G.; Bateman, A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc. Natl. Acad. Sci. USA 1992, 89, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Plowman, G.; Green, J.; Neubauer, M.; Buckley, S.; McDonald, V.; Todaro, G.; Shoyab, M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J. Biol. Chem. 1992, 267, 13073–13078. [Google Scholar] [CrossRef] [PubMed]
- Anakwe, O.O.; Anakwe, O.O.; Gerton, G.L. Acrosome biogenesis begins during meiosis: Evidence from the synthesis and distribution of an acrosomal glycoprotein, acrogranin, during guinea pig spermatogenesis. Biol. Reprod. 1990, 42, 317–328. [Google Scholar] [CrossRef]
- Baba, T.; Hoff, H.B.; Nemoto, H.; Lee, H.; Orth, J.; Arai, Y.; Gerton, G.L. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol. Reprod. Dev. 1993, 34, 233–243. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, G.; Crabb, J.; Serrero, G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J. Biol. Chem. 1993, 268, 10863–10869. [Google Scholar] [CrossRef]
- Zanocco-Marani, T.; Bateman, A.; Romano, G.; Valentinis, B.; He, Z.H.; Baserga, R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999, 59, 5331–5340. [Google Scholar]
- Serrero, G. Autocrine growth factor revisited: PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem. Biophys. Res. Commun. 2003, 308, 409–413. [Google Scholar] [CrossRef]
- Tangkeangsirisin, W.; Serrero, G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis 2004, 25, 1587–1592. [Google Scholar] [CrossRef]
- Parnell, P.G.; Wunderlich, J.; Carter, B.; Halper, J. Purification of transforming growth factor type e. J. Cell. Biochem. 1990, 42, 111–116. [Google Scholar] [CrossRef]
- Palfree, R.G.E.; Bennett, H.P.J.; Bateman, A. The Evolution of the Secreted Regulatory Protein Progranulin. PLoS ONE 2015, 10, e0133749. [Google Scholar] [CrossRef]
- Bateman, A.; Bennett, H. Granulins: The structure and function of an emerging family of growth factors. J. Endocrinol. 1998, 158, 145–151. [Google Scholar] [CrossRef]
- Hrabal, R.; Chen, Z.; James, S.; Bennett, H.P.; Ni, F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat. Struct. Mol. Biol. 1996, 3, 747–752. [Google Scholar] [CrossRef]
- Zhu, J.; Nathan, C.; Jin, W.; Sim, D.; Ashcroft, G.S.; Wahl, S.M.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Wright, C.D.; et al. Conversion of Proepithelin to Epithelins: Roles of SLPI and Elastase in Host Defense and Wound Repair. Cell 2002, 111, 867–878. [Google Scholar] [CrossRef]
- Xu, D.; Suenaga, N.; Edelmann, M.J.; Fridman, R.; Muschel, R.J.; Kessler, B.M. Novel MMP-9 Substrates in Cancer Cells Revealed by a Label-free Quantitative Proteomics Approach. Mol. Cell. Proteom. 2008, 7, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.-S.; Choi, N.; Tarassishin, L.; Lee, S.C. Regulation of Progranulin Expression in Human Microglia and Proteolysis of Progranulin by Matrix Metalloproteinase-12 (MMP-12). PLoS ONE 2012, 7, e35115. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-H.; Wang, D.-W.; Kong, L.; Zhang, Y.; Luan, Y.; Kobayashi, T.; Kronenberg, H.M.; Yu, X.-P.; Liu, C.-J. ADAMTS-7, a Direct Target of PTHrP, Adversely Regulates Endochondral Bone Growth by Associating with and Inactivating GEP Growth Factor. Mol. Cell. Biol. 2009, 29, 4201–4219. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Fröhlich, L.; Sixt, M.; Lämmermann, T.; Pfister, H.; Bateman, A.; Belaaouaj, A.; Ring, J.; Ollert, M.; Fässler, R.; et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Investig. 2008, 118, 2438–2447. [Google Scholar] [CrossRef]
- Devoogdt, N.; Rasool, N.; Hoskins, E.; Simpkins, F.; Tchabo, N.; Kohn, E.C. Overexpression of protease inhibitor-dead secretory leukocyte protease inhibitor causes more aggressive ovarian cancer in vitro and in vivo. Cancer Sci. 2009, 100, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Yamashita, S.; Ohama, T.; Saga, A.; Yamamoto-Kakuta, A.; Hamada, Y.; Sougawa, N.; Ohyama, R.; Sawa, Y.; Matsuyama, A. HDL/Apolipoprotein A-I Binds to Macrophage-Derived Progranulin and Suppresses its Conversion into Proinflammatory Granulins. J. Atheroscler. Thromb. 2010, 17, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Willnow, T.E.; Petersen, C.M.; Nykjaer, A. VPS10P-domain receptors—Regulators of neuronal viability and function. Nat. Rev. Neurosci. 2008, 9, 899–909. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.-X.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin Controls Intracellular Sorting of Brain-Derived Neurotrophic Factor to the Regulated Secretory Pathway. J. Neurosci. 2005, 25, 6156–6166. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Jacobsen, C.; Olivecrona, G.; Gliemann, J.; Petersen, C.M. Sortilin/Neurotensin Receptor-3 Binds and Mediates Degradation of Lipoprotein Lipase. J. Biol. Chem. 1999, 274, 8832–8836. [Google Scholar] [CrossRef]
- Tang, W.; Lu, Y.; Tian, Q.-Y.; Zhang, Y.; Guo, F.-J.; Liu, G.-Y.; Syed, N.M.; Lai, Y.; Lin, E.A.; Kong, L.; et al. The Growth Factor Progranulin Binds to TNF Receptors and Is Therapeutic Against Inflammatory Arthritis in Mice. Science 2011, 332, 478–484. [Google Scholar] [CrossRef]
- Liu, C.-J. Progranulin: A promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011, 585, 3675–3680. [Google Scholar] [CrossRef]
- Tian, Q.; Zhao, Y.; Mundra, J.J.; Gonzalez-Gugel, E.; Jian, J.; Uddin, S.M.Z.; Liu, C. Three TNFR-binding domains of PGRN act independently in inhibition of TNF-alpha binding and activity. Front. Biosci. 2014, 19, 1176–1185. [Google Scholar] [CrossRef]
- Feng, J.Q.; Guo, F.; Jiang, B.; Zhang, Y.; Frenkel, S.; Wang, D.; Tang, W.; Xie, Y.; Liu, C. Granulin epithelin precursor: A bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J. 2010, 24, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Jung, T.W.; Hong, H.C.; Choi, H.Y.; Seo, J.-A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H.; et al. Progranulin Protects Vascular Endothelium against Atherosclerotic Inflammatory Reaction via Akt/eNOS and Nuclear Factor-κB Pathways. PLoS ONE 2013, 8, e76679. [Google Scholar] [CrossRef]
- Monami, G.; Gonzalez, E.M.; Hellman, M.; Gomella, L.G.; Baffa, R.; Iozzo, R.V.; Morrione, A. Proepithelin Promotes Migration and Invasion of 5637 Bladder Cancer Cells through the Activation of ERK1/2 and the Formation of a Paxillin/FAK/ERK Complex. Cancer Res. 2006, 66, 7103–7110. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.C.; Liu, H.; Talwar, A.; Jian, J. New discovery rarely runs smooth: An update on progranulin/TNFR interactions. Protein Cell 2015, 6, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.-X.; Gao, W.; Liu, W.; Liu, D.-S. Progranulin-Derived Atsttrin Directly Binds to TNFRSF25 (DR3) and Inhibits TNF-Like Ligand 1A (TL1A) Activity. PLoS ONE 2014, 9, e92743. [Google Scholar] [CrossRef]
- Bittner, S.; Ehrenschwender, M. Multifaceted death receptor 3 signaling—Promoting survival and triggering death. FEBS Lett. 2017, 591, 2543–2555. [Google Scholar] [CrossRef]
- Bittner, S.; Knoll, G.; Füllsack, S.; Kurz, M.; Wajant, H.; Ehrenschwender, M. Soluble TL1A is sufficient for activation of death receptor 3. FEBS J. 2015, 283, 323–336. [Google Scholar] [CrossRef]
- Aiba, Y.; Nakamura, M. The Role of TL1A and DR3 in Autoimmune and Inflammatory Diseases. Mediat. Inflamm. 2013, 2013, 258164. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Buraschi, S.; Goyal, A.; Sharpe, C.; Natkanski, E.; Schaefer, L.; Morrione, A.; Iozzo, R.V. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 2016, 215, 687–703. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Pasquale, E.B. Eph receptors in the adult brain. Curr. Opin. Neurobiol. 2004, 14, 288–296. [Google Scholar] [CrossRef]
- Murai, K.K.; Pasquale, E.B. Eph Receptors, Ephrins, and Synaptic Function. Neuroscientist 2004, 10, 304–314. [Google Scholar] [CrossRef]
- Tandon, M.; Vemula, S.V.; Mittal, S.K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets 2010, 15, 31–51. [Google Scholar] [CrossRef]
- Chitramuthu, B.; Bateman, A. Progranulin and the receptor tyrosine kinase EphA2, partners in crime? J. Cell Biol. 2016, 215, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Buti, L.; Lee, S.; Matsuwaki, T.; Spooner, E.; Brinkmann, M.M.; Nishihara, M.; Ploegh, H.L. Granulin Is a Soluble Cofactor for Toll-like Receptor 9 Signaling. Immunity 2011, 34, 505–513. [Google Scholar] [CrossRef]
- Vollmer, J. TLR9 in Health and Disease. Int. Rev. Immunol. 2006, 25, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Y. Targeting the TLR9–MyD88 pathway in the regulation of adaptive immune responses. Expert Opin. Ther. Targets 2010, 14, 787–796. [Google Scholar] [CrossRef]
- Petkau, T.L.; Neal, S.; Orban, P.; MacDonald, J.; Hill, A.; Lu, G.; Feldman, H.; Mackenzie, I.; Leavitt, B. Progranulin expression in the developing and adult murine brain. J. Comp. Neurol. 2010, 518, 3931–3947. [Google Scholar] [CrossRef]
- Bhandari, V.; Giaid, A.; Bateman, A. The complementary deoxyribonucleic acid sequence, tissue distribution, and cellular localization of the rat granulin precursor. Endocrinology 1993, 133, 2682–2689. [Google Scholar] [CrossRef]
- Matsuwaki, T.; Asakura, R.; Suzuki, M.; Yamanouchi, K.; Nishihara, M. Age-Dependent Changes in Progranulin Expression in the Mouse Brain. J. Reprod. Dev. 2011, 57, 113–119. [Google Scholar] [CrossRef]
- Petoukhov, E.; Fernando, S.; Mills, F.; Shivji, F.; Hunter, D.; Krieger, C.; Silverman, M.A.; Bamji, S.X. Activity-dependent secretion of progranulin from synapses. J. Cell Sci. 2013, 126, 5412–5421. [Google Scholar] [CrossRef]
- Townley, R.A.; Boeve, B.F.; Benarroch, E.E. Progranulin. Neurology 2018, 90, 118–125. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Wu, J.; Wei, Y.; Chen, X.; Lin, Z.; Nie, S. A narrative review of multiple mechanisms of progranulin in cancer: A potential target for anti-cancer therapy. Transl. Cancer Res. 2021, 10, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Minami, K.; Otagaki, S.; Tsujiuchi, T. Rapid establishment of highly migratory cells from cancer cells for investigating cellular functions. J. Recept. Signal Transduct. 2019, 39, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Daya, M.; Loilome, W.; Techasen, A.; Thanee, M.; Sa-Ngiamwibool, P.; Titapun, A.; Yongvanit, P.; Namwat, N. Progranulin modulates cholangiocarcinoma cell proliferation, apoptosis, and motility via the PI3K/pAkt pathway. OncoTargets Ther. 2018, ume 11, 395–408. [Google Scholar] [CrossRef]
- Guha, R.; Yue, B.; Dong, J.; Banerjee, A.; Serrero, G. Anti-progranulin/GP88 antibody AG01 inhibits triple negative breast cancer cell proliferation and migration. Breast Cancer Res. Treat. 2021, 186, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Arechavaleta-Velasco, F.; Perez-Juarez, C.E.; Gerton, G.L.; Diaz-Cueto, L. Progranulin and its biological effects in cancer. Med. Oncol. 2017, 34, 194. [Google Scholar] [CrossRef]
- Yabe, K.; Yamamoto, Y.; Takemura, M.; Hara, T.; Tsurumi, H.; Serrero, G.; Nabeshima, T.; Saito, K. Progranulin depletion inhibits proliferation via the transforming growth factor beta/SMAD family member 2 signaling axis in Kasumi-1 cells. Heliyon 2021, 7, e05849. [Google Scholar] [CrossRef]
- Ventura, E.; Xie, C.; Buraschi, S.; Belfiore, A.; Iozzo, R.V.; Giordano, A.; Morrione, A. Complexity of progranulin mechanisms of action in mesothelioma. J. Exp. Clin. Cancer Res. 2022, 41, 333. [Google Scholar] [CrossRef]
- Xu, S.-Q.; Buraschi, S.; Morcavallo, A.; Genua, M.; Shirao, T.; Peiper, S.C.; Gomella, L.G.; Birbe, R.; Belfiore, A.; Iozzo, R.V.; et al. A novel role for drebrin in regulating progranulin bioactivity in bladder cancer. Oncotarget 2015, 6, 10825–10839. [Google Scholar] [CrossRef]
- Kamrava, M.; Simpkins, F.; Alejandro, E.; Michener, C.; Meltzer, E.; Kohn, E.C. Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin–epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene 2005, 24, 7084–7093. [Google Scholar] [CrossRef] [PubMed]
- Kuşoğlu, A.; Avcı, B. Cancer stem cells: A brief review of the current status. Gene 2018, 681, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.; Pauwels, E.; Parkinson, G.; Landberg, G.; Le, T.; Demillo, V.G.; Lumangtad, L.A.; Jones, D.E.; Islam, A.; Olsen, R.; et al. Reduction of Progranulin-Induced Breast Cancer Stem Cell Propagation by Sortilin-Targeting Cyclotriazadisulfonamide (CADA) Compounds. J. Med. Chem. 2021, 64, 12865–12876. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Lan, Y.-J.; Sam, N.B.; Cheng, M.-H.; Pan, H.-F.; Gao, J. Progranulin as a Potential Therapeutic Target in Immune-Mediated Diseases. J. Inflamm. Res. 2021, ume 14, 6543–6556. [Google Scholar] [CrossRef]
- Liu, Y.; Xi, L.; Liao, G.; Wang, W.; Tian, X.; Wang, B.; Chen, G.; Han, Z.; Wu, M.; Wang, S.; et al. Inhibition of PC cell-derived growth factor (PCDGF)/granulin-epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK 4 and inactivation of MMP-2. BMC Cancer 2007, 7, 22. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Toh, H.; Cao, M.; Daniels, E.; Bateman, A. Expression of the Growth Factor Progranulin in Endothelial Cells Influences Growth and Development of Blood Vessels: A Novel Mouse Model. PLoS ONE 2013, 8, e64989. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Li, J.-S.; Liang, Q.-P.; He, D.-Z.; Zhao, J. Expression of PC cell-derived growth factor and vascular endothelial growth factor in esophageal squamous cell carcinoma and their clinicopathologic significance. Chin. Med. J. 2008, 121, 881–886. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.L.; Dong, T.T.; Shen, Y.H.; Guo, X.S.; Liu, C.Y.; Liu, J.; Zhang, P.; Li, J.; Sun, Y.P. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am. J. Cancer Res. 2015, 5, 3085–3097. [Google Scholar]
- Huang, H.; Li, J.; Lu, Y.; Min, L.; Li, D.; Dai, L. Role of midkine-progranulin interaction during angiogenesis of hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 8809–8820. [Google Scholar]
- Binișor, I.; Baniță, I.M.; Alexandru, D.; Mehedinți, M.C.; Jurja, S.; Andrei, A.-M.; Pisoschi, C.G. Progranulin: A proangiogenic factor in visceral adipose tissue in tumoral and non-tumoral visceral pathology. Exp. Ther. Med. 2021, 22, 1337. [Google Scholar] [CrossRef]
- Li, G.; Dong, T.; Yang, D.; Gao, A.; Luo, J.; Yang, H.; Wang, L. Progranulin promotes lymphangiogenesis through VEGF-C and is an independent risk factor in human esophageal cancers. Hum. Pathol. 2018, 75, 116–124. [Google Scholar] [CrossRef]
- Kwack, K.H.; Lee, H.-W. Progranulin Inhibits Human T Lymphocyte Proliferation by Inducing the Formation of Regulatory T Lymphocytes. Mediat. Inflamm. 2017, 2017, 7682083. [Google Scholar] [CrossRef]
- Cheung, P.F.; Yip, C.W.; Wong, N.C.; Fong, D.Y.; Ng, L.W.; Wan, A.M.; Wong, C.K.; Cheung, T.T.; Ng, I.O.; Poon, R.T.; et al. Granulin–Epithelin Precursor Renders Hepatocellular Carcinoma Cells Resistant to Natural Killer Cytotoxicity. Cancer Immunol. Res. 2014, 2, 1209–1219. [Google Scholar] [CrossRef]
- Voshtani, R.; Song, M.; Wang, H.; Li, X.; Zhang, W.; Tavallaie, M.S.; Yan, W.; Sun, J.; Wei, F.; Ma, X. Progranulin promotes melanoma progression by inhibiting natural killer cell recruitment to the tumor microenvironment. Cancer Lett. 2019, 465, 24–35. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, T.; Shi, H.; Yao, M.; Zhang, D.; Qian, H.; Zeng, Q.; Wang, Y.; Jin, F.; Chai, C.; et al. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8+ T cell exclusion. J. Exp. Clin. Cancer Res. 2021, 40, 4. [Google Scholar] [CrossRef]
- Cheung, P.F.; Yang, J.; Fang, R.; Borgers, A.; Krengel, K.; Stoffel, A.; Althoff, K.; Yip, C.W.; Siu, E.H.L.; Ng, L.W.C.; et al. Progranulin mediates immune evasion of pancreatic ductal adenocarcinoma through regulation of MHCI expression. Nat. Commun. 2022, 13, 156. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Yin, J.; Lin, T.; Zhang, J. Progranulin overexpression predicts overall survival in patients with glioblastoma. Med Oncol. 2011, 29, 2423–2431. [Google Scholar] [CrossRef]
- Bandey, I.; Chiou, S.-H.; Huang, A.-P.; Tsai, J.-C.; Tu, P.-H. Progranulin promotes Temozolomide resistance of glioblastoma by orchestrating DNA repair and tumor stemness. Oncogene 2014, 34, 1853–1864. [Google Scholar] [CrossRef]
- Vachher, M.; Arora, K.; Burman, A.; Kumar, B. NAMPT, GRN, and SERPINE1 signature as predictor of disease progression and survival in gliomas. J. Cell. Biochem. 2019, 121, 3010–3023. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, L.; Yang, M.; Lim, Y.; Zhu, Y.-H.; Fu, D.-L.; Li, Z.-W.; Zhong, J.-H.; Xiao, Z.-C.; Zhou, X.-F. ProBDNF and its receptors are upregulated in glioma and inhibit the growth of glioma cells in vitro. Neuro-Oncol. 2013, 15, 990–1007. [Google Scholar] [CrossRef]
- Marsland, M.; Dowdell, A.; Faulkner, S.; Gedye, C.; Lynam, J.; Griffin, C.P.; Marsland, J.; Jiang, C.C.; Hondermarck, H. The Membrane Protein Sortilin Is a Potential Biomarker and Target for Glioblastoma. Cancers 2023, 15, 2514. [Google Scholar] [CrossRef]
- Yang, W.; Wu, P.-F.; Ma, J.-X.; Liao, M.-J.; Wang, X.-H.; Xu, L.-S.; Xu, M.-H.; Yi, L. Sortilin promotes glioblastoma invasion and mesenchymal transition through GSK-3β/β-catenin/twist pathway. Cell Death Dis. 2019, 10, 208. [Google Scholar] [CrossRef]
- Yang, W.; Xiang, Y.; Liao, M.-J.; Wu, P.-F.; Yang, L.; Huang, G.-H.; Shi, B.-Z.; Yi, L.; Lv, S.-Q. Presenilin1 inhibits glioblastoma cell invasiveness via promoting Sortilin cleavage. Cell Commun. Signal. 2021, 19, 112. [Google Scholar] [CrossRef]
- Kato, T.; Sawamura, Y.; Tada, M.; Sakuma, S.; Sudo, M.; Abe, H. p55 and p 75 Tumor Necrosis Factor Receptor Expression on Human Glioblastoma Cells. Neurol. Med.-Chir. 1995, 35, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Li, L.; Tang, H.; Xie, Y.; Puliyappadamba, V.T.; Raisanen, J.; Burma, S.; Boothman, D.A.; Cochran, B.; Wu, J.; et al. Cytoplasmic TRADD Confers a Worse Prognosis in Glioblastoma. Neoplasia 2013, 15, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.E.; Morshed, R.A.; Yamini, B. Nuclear factor-κB in glioblastoma: Insights into regulators and targeted therapy. Neuro-Oncology 2015, 18, 329–339. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Cassar, E.; Razqan, M.A.M.; Szydzik, C.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Elevation of circulating TNF receptor 2 in cancer: A systematic meta-analysis for its potential as a diagnostic cancer biomarker. Front. Immunol. 2022, 13, 918254. [Google Scholar] [CrossRef]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a Novel Molecular Marker and Target in Glioblastoma Multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef]
- Ferluga, S.; Tomé, C.M.L.; Herpai, D.M.; D’Agostino, R.; Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 2016, 7, 59860–59876. [Google Scholar] [CrossRef]
- Baharuddin, W.N.A.; Yusoff, A.A.M.; Abdullah, J.M.; Osman, Z.F.; Ahmad, F. Roles of EphA2 Receptor in Angiogenesis Signaling Pathway of Glioblastoma Multiforme. Malays. J. Med. Sci. 2018, 25, 22–27. [Google Scholar] [CrossRef]
- Miao, H.; Gale, N.W.; Guo, H.; Qian, J.; Petty, A.; Kaspar, J.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.; Hambardzumyan, D.; et al. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene 2014, 34, 558–567. [Google Scholar] [CrossRef]
- Das, S.; Marsden, P.A. Angiogenesis in Glioblastoma. New Engl. J. Med. 2013, 369, 1561–1563. [Google Scholar] [CrossRef]
- Shen, L.; Sun, R.; Kan, S.; Wang, Z.; Yu, Z. EphA2, vascular endothelial growth factor, and vascular endothelial growth factor correlate with adverse outcomes and poor survival in patients with glioma. Medicine 2021, 100, e23985. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. et Biophys. Acta (BBA) Rev. Cancer 2010, 1806, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.K.; Naik, S.; Kakarla, S.; Brawley, V.S.; Shaffer, D.R.; Yi, Z.; Rainusso, N.; Wu, M.-F.; Liu, H.; Kew, Y.; et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol. Ther. 2013, 21, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Herpai, D.; Quigley, M.; Cecere, T.E.; Robertson, J.L.; D’agostino, R.B.; Hinckley, J.; Tatter, S.B.; Dickinson, P.J.; Debinski, W. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro-Oncology 2020, 23, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Fehri, E.; Ennaifer, E.; Rhouma, R.B.H.; Ardhaoui, M.; Boubaker, S. TLR9 and Glioma: Friends or Foes? Cells 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Jiang, T.; Zhang, Y. TLR9 expression is associated with prognosis in patients with glioblastoma multiforme. J. Clin. Neurosci. 2012, 19, 75–80. [Google Scholar] [CrossRef]
- Meng, Y.; Kujas, M.; Marie, Y.; Paris, S.; Thillet, J.; Delattre, J.-Y.; Carpentier, A.F. Expression of TLR9 within human glioblastoma. J. Neuro-Oncol. 2008, 88, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Miyar, A.; Habibi, I.; Ebrahimi, A.; Mansourpour, D.; Mokarizadeh, A.; Rajabi, A.; Farshgar, R.; Eshaghzadeh, M.; Zamani-Ahmadmahmudi, M.; Nodushan, S.M.H.T. Predictive and prognostic value of TLR9 and NFKBIA gene expression as potential biomarkers for human glioma diagnosis. J. Neurol. Sci. 2016, 368, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Merrell, M.A.; Ilvesaro, J.M.; Lehtonen, N.; Sorsa, T.; Gehrs, B.; Rosenthal, E.; Chen, D.; Shackley, B.; Harris, K.W.; Selander, K.S. Toll-Like Receptor 9 Agonists Promote Cellular Invasion by Increasing Matrix Metalloproteinase Activity. Mol. Cancer Res. 2006, 4, 437–447. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Yan, Y.; Ying, Q.; Jiang, T.; Xu, K.; Wu, A. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer 2010, 10, 415. [Google Scholar] [CrossRef]
- Sandholm, J.; Tuomela, J.; Kauppila, J.H.; Harris, K.W.; Graves, D.; Selander, K.S. Hypoxia regulates Toll-like receptor-9 expression and invasive function in human brain cancer cells in vitro. Oncol. Lett. 2014, 8, 266–274. [Google Scholar] [CrossRef]
- Herrmann, A.; Cherryholmes, G.; Schroeder, A.; Phallen, J.; Alizadeh, D.; Xin, H.; Wang, T.; Lee, H.; Lahtz, C.; Swiderski, P.; et al. TLR9 Is Critical for Glioma Stem Cell Maintenance and Targeting. Cancer Res 2014, 74, 5218–5228. [Google Scholar] [CrossRef]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The multifactorial roles of microglia and macrophages in the maintenance and progression of glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef] [PubMed]
- Karachi, A.; Dastmalchi, F.; Mitchell, D.A.; Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro-Oncology 2018, 20, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Shakeel, F.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Thermodynamic Solubility Profile of Temozolomide in Different Commonly Used Pharmaceutical Solvents. Molecules 2022, 27, 1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Sher, D.J.; Henson, J.W.; Avutu, B.; Hochberg, F.H.; Batchelor, T.T.; Martuza, R.L.; Barker, F.G.; Loeffler, J.S.; Chakravarti, A. The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma. J. Neuro-Oncol. 2008, 88, 43–50. [Google Scholar] [CrossRef]
- Back, M.F.; Ang, E.L.; Ng, W.-H.; See, S.-J.; Lim, C.T.; Chan, S.; Yeo, T.-T. Improved Median Survival for Glioblastoma Multiforme Following Introduction of Adjuvant Temozolomide Chemotherapy. Ann. Acad. Med. Singap. 2007, 36, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Gander, M.; Leyvraz, S.; Newlands, E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001, 2, 552–560. [Google Scholar] [CrossRef]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jiménez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med.-Chir. 2018, 58, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Mizoguchi, M.; Hata, N.; Murata, H.; Hatae, R.; Amano, T.; Nakamizo, A.; Sasaki, T. Complex DNA repair pathways as possible therapeutic targets to overcome temozolomide resistance in glioblastoma. Front. Oncol. 2012, 2, 186. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, R.; Cuomo, M.; Buonaiuto, M.; Costabile, D.; Franca, R.A.; Caro, M.D.B.D.; Catapano, G.; Chiariotti, L.; Visconti, R. MGMT and Whole-Genome DNA Methylation Impacts on Diagnosis, Prognosis and Therapy of Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 7148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Salehi, F.; Scheithauer, B.W.; Rotondo, F.; Syro, L.V.; Kovacs, K. Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res. 2009, 29, 3759–3768. [Google Scholar] [PubMed]
- Cenciarini, M.; Valentino, M.; Belia, S.; Sforna, L.; Rosa, P.; Ronchetti, S.; D’adamo, M.C.; Pessia, M. Dexamethasone in Glioblastoma Multiforme Therapy: Mechanisms and Controversies. Front. Mol. Neurosci. 2019, 12, 65. [Google Scholar] [CrossRef]
- Kostaras, X.; Cusano, F.; Kline, G.; Roa, W.; Easaw, J. Use of Dexamethasone in Patients with High-Grade Glioma: A Clinical Practice Guideline. Curr. Oncol. 2014, 21, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Carminucci, A.; Tejero, R.; Huang, Y.; Danish, S.; Friedel, R.H.; Foty, R. Teaching an Old Drug New Tricks: Dexamethasone as an In Vivo Inhibitor of Glioblastoma Dispersal. Cureus 2020, 12, e7749. [Google Scholar] [CrossRef]
- Wang, W.; Hayashi, J.; Serrero, G. PC Cell–Derived Growth Factor Confers Resistance to Dexamethasone and Promotes Tumorigenesis in Human Multiple Myeloma. Clin. Cancer Res. 2006, 12, 49–56. [Google Scholar] [CrossRef]
- Lo, H.-C.; Hsu, J.-H.; Lai, L.-C.; Tsai, M.-H.; Chuang, E.Y. MicroRNA-107 enhances radiosensitivity by suppressing granulin in PC-3 prostate cancer cells. Sci. Rep. 2020, 10, 14584. [Google Scholar] [CrossRef]
- Greither, T.; Steiner, T.; Bache, M.; Serrero, G.; Otto, S.; Taubert, H.; Eckert, A.W.; Kappler, M. GP88/PGRN Serum Levels Are Associated with Prognosis for Oral Squamous Cell Carcinoma Patients. Biology 2021, 10, 400. [Google Scholar] [CrossRef]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Immunological Aspects of Malignant Gliomas. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2016, 43, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Pino, J.; Scotece, M.; Conde, J.; Lago, F.; Gonzalez-Gay, M.A.; Mera, A.; Gómez, R.; Mobasheri, A.; Gualillo, O. Progranulin as a biomarker and potential therapeutic agent. Drug Discov. Today 2017, 22, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Cheung, S.T.; Bennett, H.P.J. A Brief Overview of Progranulin in Health and Disease. Methods Mol. Biol. 2018, 1806, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Senhaji, N.; Houssaini, A.S.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Jobling, P.; Rowe, C.W.; Oliveira, S.R.; Roselli, S.; Thorne, R.F.; Oldmeadow, C.; Attia, J.; Jiang, C.C.; Zhang, X.D.; et al. Neurotrophin Receptors TrkA, p75NTR, and Sortilin Are Increased and Targetable in Thyroid Cancer. Am. J. Pathol. 2018, 188, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Roselli, S.; Pundavela, J.; Demont, Y.; Faulkner, S.; Keene, S.; Attia, J.; Jiang, C.C.; Zhang, X.D.; Walker, M.M.; Hondermarck, H. Sortilin is associated with breast cancer aggressiveness and contributes to tumor cell adhesion and invasion. Oncotarget 2015, 6, 10473–10486. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.-C.; Demeule, M.; Charfi, C.; Zgheib, A.; Larocque, A.; Danalache, B.A.; Ouanouki, A.; Béliveau, R.; Marsolais, C.; Annabi, B. The Peptide-Drug Conjugate TH1902: A New Sortilin Receptor-Mediated Cancer Therapeutic against Ovarian and Endometrial Cancers. Cancers 2022, 14, 1877. [Google Scholar] [CrossRef]
- Demeule, M.; Charfi, C.; Currie, J.-C.; Zgheib, A.; Danalache, B.A.; Béliveau, R.; Marsolais, C.; Annabi, B. The TH1902 Docetaxel Peptide-Drug Conjugate Inhibits Xenografts Growth of Human SORT1-Positive Ovarian and Triple-Negative Breast Cancer Stem-like Cells. Pharmaceutics 2022, 14, 1910. [Google Scholar] [CrossRef]
- Guo, G.; Gong, K.; Puliyappadamba, V.T.; Panchani, N.; Pan, E.; Mukherjee, B.; Damanwalla, Z.; Bharia, S.; Hatanpaa, K.J.; Gerber, D.E.; et al. Efficacy of EGFR plus TNF inhibition in a preclinical model of temozolomide-resistant glioblastoma. Neuro-Oncology 2019, 21, 1529–1539. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, B.; Liu, H.; Shi, L. TNF Inhibitor Pomalidomide Sensitizes Glioblastoma Cells to EGFR Inhibition. Ann. Clin. Lab. Sci. 2020, 50, 474–480. [Google Scholar] [PubMed]
- Day, B.W.; Stringer, B.W.; Al-Ejeh, F.; Ting, M.J.; Wilson, J.; Ensbey, K.S.; Jamieson, P.R.; Bruce, Z.C.; Lim, Y.C.; Offenhäuser, C.; et al. EphA3 Maintains Tumorigenicity and Is a Therapeutic Target in Glioblastoma Multiforme. Cancer Cell 2013, 23, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, S.; Yu, Y.; Lv, Y.; Wang, A.; Yan, X.; Li, N.; Sha, C.; Sun, K.; Li, Y. Intranasal Delivery of Temozolomide-Conjugated Gold Nanoparticles Functionalized with Anti-EphA3 for Glioblastoma Targeting. Mol. Pharm. 2021, 18, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, M.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. CpG-Based Nanovaccines for Cancer Immunotherapy. Int. J. Nanomed. 2021, ume 16, 5281–5299. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- Xu, J.; Xilouri, M.; Bruban, J.; Shioi, J.; Shao, Z.; Papazoglou, I.; Vekrellis, K.; Robakis, N.K. Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiol. Aging 2011, 32, 2326.e5–2326.e16. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniatowski, Ł.A.; Woźnica, M.; Wojdasiewicz, P.; Mela-Kalicka, A.; Romanowska-Próchnicka, K.; Purrahman, D.; Żurek, G.; Krawczyk, M.; Nameh Goshay Fard, N.; Furtak-Niczyporuk, M.; et al. The Role of Progranulin (PGRN) in the Pathogenesis of Glioblastoma Multiforme. Cells 2024, 13, 124. https://doi.org/10.3390/cells13020124
Poniatowski ŁA, Woźnica M, Wojdasiewicz P, Mela-Kalicka A, Romanowska-Próchnicka K, Purrahman D, Żurek G, Krawczyk M, Nameh Goshay Fard N, Furtak-Niczyporuk M, et al. The Role of Progranulin (PGRN) in the Pathogenesis of Glioblastoma Multiforme. Cells. 2024; 13(2):124. https://doi.org/10.3390/cells13020124
Chicago/Turabian StylePoniatowski, Łukasz A., Michał Woźnica, Piotr Wojdasiewicz, Aneta Mela-Kalicka, Katarzyna Romanowska-Próchnicka, Daryush Purrahman, Grzegorz Żurek, Maciej Krawczyk, Najmeh Nameh Goshay Fard, Marzena Furtak-Niczyporuk, and et al. 2024. "The Role of Progranulin (PGRN) in the Pathogenesis of Glioblastoma Multiforme" Cells 13, no. 2: 124. https://doi.org/10.3390/cells13020124





