The Dual Role of Chemerin in Lung Diseases
Abstract
:1. Introduction
2. Chemerin and Its Receptors
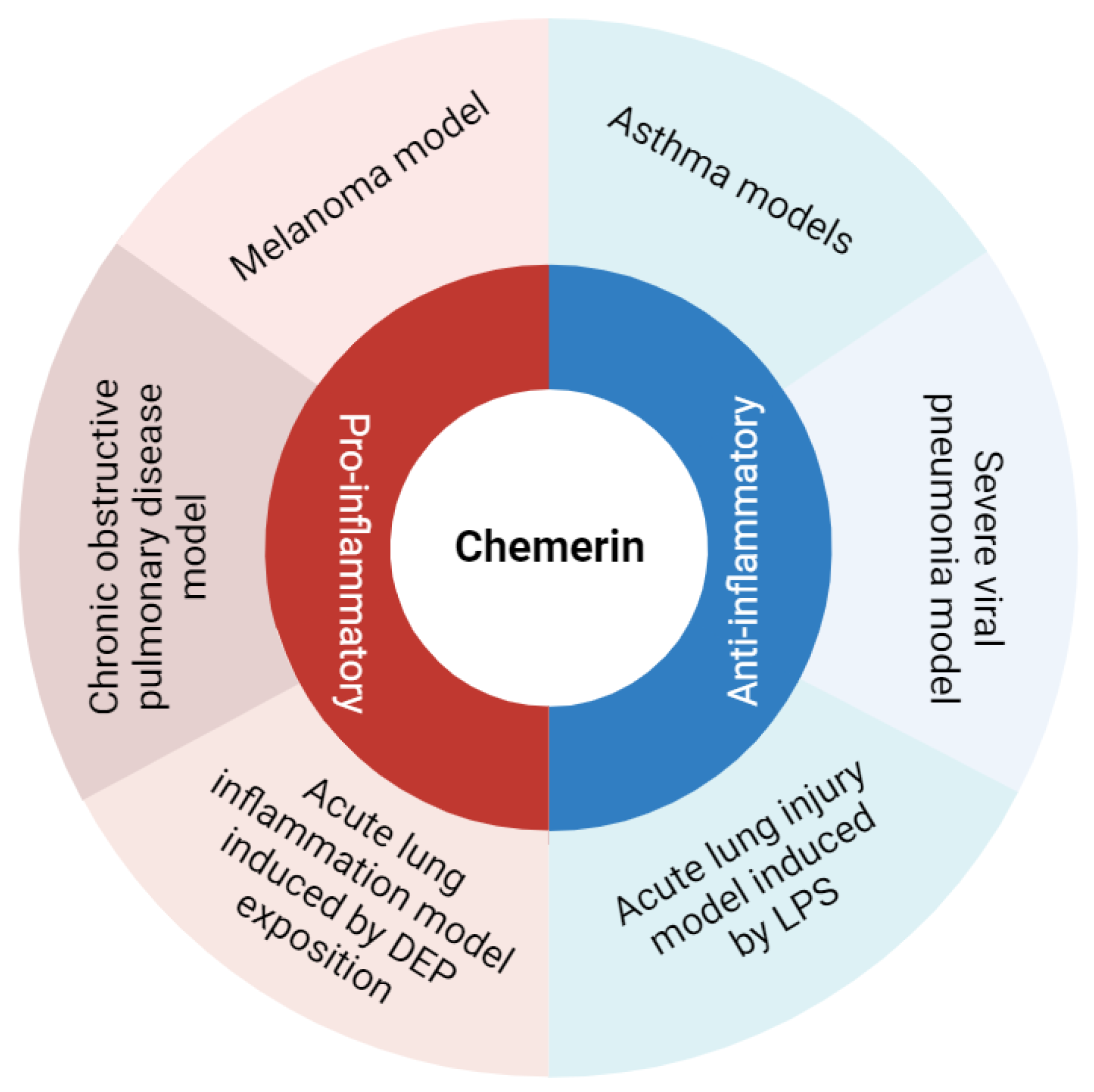
3. Chemerin and Lung Inflammation
3.1. Chemerin and Acute Respiratory Distress Syndrome
3.2. Chemerin, Lung Infection and Sepsis
| Diseases | Human Study | In Vivo Study | In Vitro Study |
|---|---|---|---|
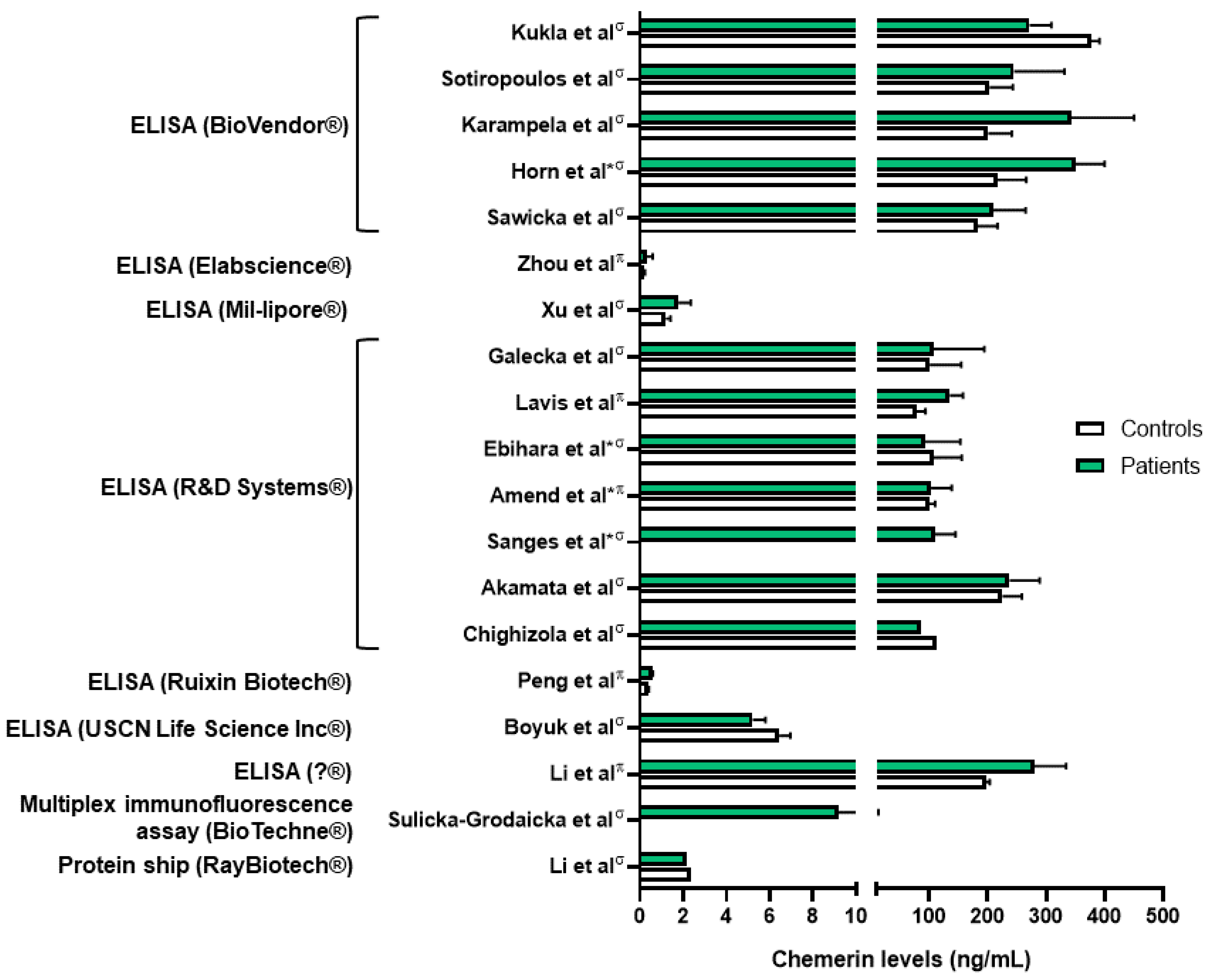
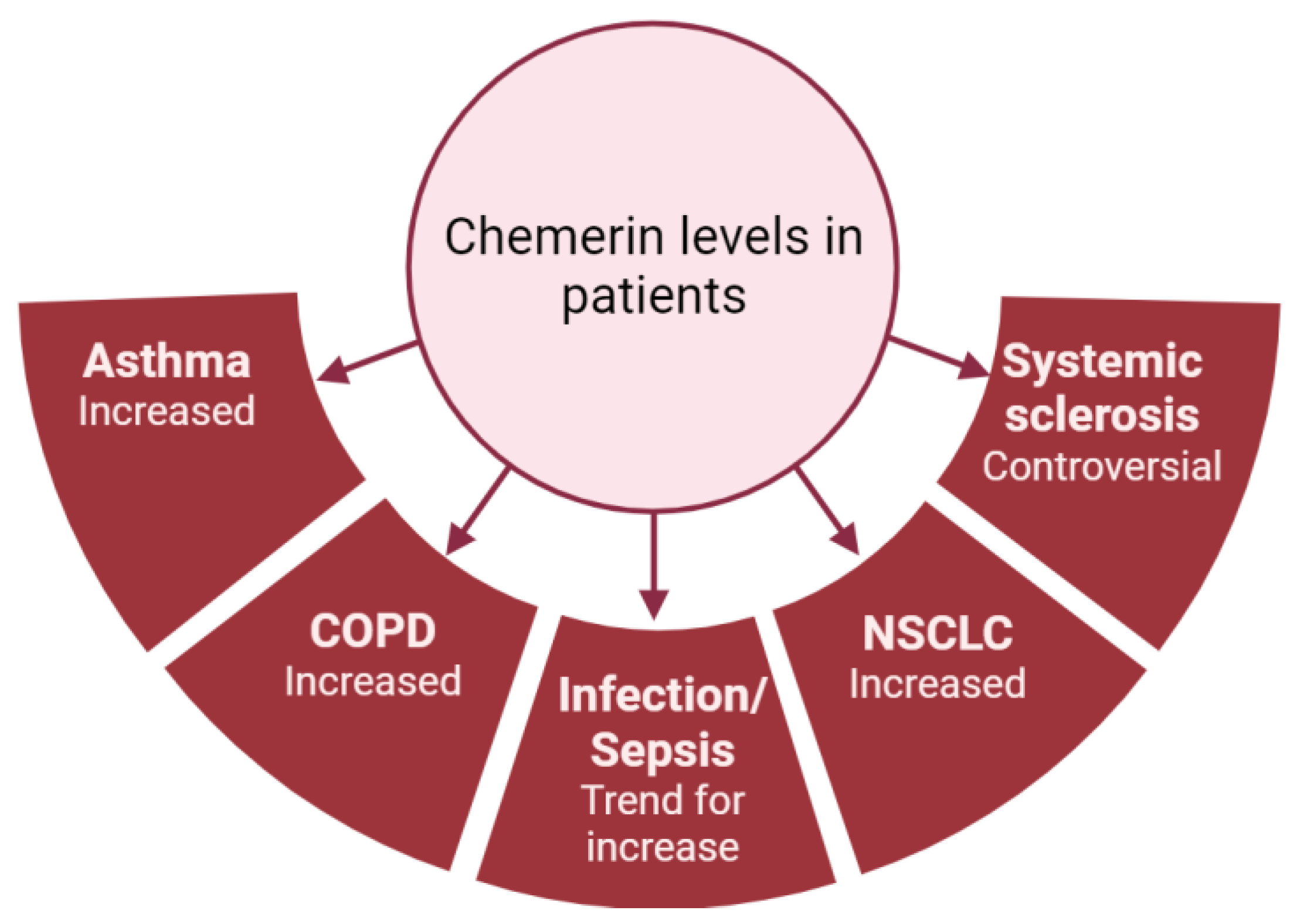
| Acute lung inflammation and sepsis | Amend et al., Karampela et al., Horn et al.: higher chemerin levels (plasma and serum) in septic patients compared to controls [48,49,50]. Karampela et al., Horn et al.: association between chemerin levels and severity of the sepsis [49,50]. Ebihara et al.: no difference in chemerin levels between septic patients and controls [51]. | Luangsay et al.: anti-inflammatory properties of the chemerin/CMKLR1 axis in the LPS model [35]. Provoost et al.: pro-inflammatory properties in the DEP model [37]. Luangsay et al., Malik et al.: effects of chemerin mediated only by CMKLR1 and not by CCRL2 [35,40]. Luangsay et al., Provoost et al., Razvi et al., Malik et al., Zou et al.: elevation of chemerin (BALF and lung) in all models of acute lung inflammation [35,37,39,40,41]. Horn et al.: higher blood chemerin concentrations in mice with severe septic shock [50]. | Bondue et al.: no synergistic effect of chemerin on stimulation of pro- or anti-inflammatory cytokines’ secretion by activated macrophages [52]. Cash et al.: inhibition of secretion of pro-inflammatory cytokines and stimulation of secretion of anti-inflammatory cytokines by macrophages induced by picomolar concentrations of chemerin [53]. |
| Lung infection | Kukla et al.: lower serum chemerin levels in COVID-19 patients compared to controls [45]. Lavis et al., Amend et al.: higher plasma chemerin levels in severe COVID-19 patients compared to controls [48,54]. Sulicka-Grodzicka et al.: no difference in serum chemerin levels between severe and non-severe patients [46]. Esendagli et al.: higher serum chemerin levels in COVID-19 patients with good prognosis compared to patients with bad prognosis [47]. Lavis et al.: higher plasma chemerin levels in deceased COVID-19 patients compared to recovered, and independent risk factor for mortality [54]. | Bondue et al.: anti-inflammatory properties of the chemerin/CMKLR1 axis in a model of severe lung pneumonia, mediated by non-leucocytic cells [55]. | Shirato et al.: decreased viral replication in A549 cells inactivated for RARRES2 [56]. |
| Asthma | Zhou et al.: higher plasma chemerin levels in asthmatic patients compared to controls [57]. | Provoost et al., Zhao L. et al.: anti-inflammatory properties of chemerin in asthmatic mouse models induced by house dust mite and DEP and by ovalbumin [37,58]. | Zhao L. et al.: inhibition of CCL2 secretion by primary lung epithelial cells if exposed to chemerin [58]. |
| Chronic obstructive pulmonary disease (COPD) | Boyuk et al., Li C. et al.: higher chemerin levels (serum and plasma) in COPD patients [59,60]. Li C. et al.: association between plasma chemerin levels and disease severity (hospitalizations) [60]. Galecka et al.: no significant difference in serum chemerin levels between COPD patients and controls [61]. | Demoor et al.: pro-inflammatory properties of the chemerin/CMKLR1 axis in COPD mouse model induced by the subacute and chronic exposure to tobacco smoke [62]. | Absence of in vitro study regarding chemerin and COPD. |
| Systemic sclerosis (SSc) | Sawicka et al.: higher serum chemerin concentrations in SSc patients compared to controls [63]. Akamata et al.: no difference in serum chemerin concentrations between SSc patients and controls [64]. Chighizola et al.: lower serum chemerin concentrations in SSc patients compared to controls [65]. Sanges et al.: higher serum chemerin concentrations in SSc patients with PAH compared to SSc patients without PAH. Upregulation of the chemerin/CMKLR1 axis in lung vessels from PAH-SSc patients [66]. Peng et al.: higher plasma chemerin levels in PAH patients compared to controls [67]. Saygin et al.: upregulation of chemerin in fibroblasts from patients with idiopathic PAH [68]. | Omori et al., Peng et al.: upregulation of CMKLR1 expression in lungs and of chemerin expression in plasma and lungs in PAH rats [67,69]. | Omori et al.: contraction of isolated pulmonary arteries induced by chemerin and greater effect on arteries isolated from PAH rats [69]. Hanthazi et al.: potentiation of vasoconstrictor effects and antagonization of vasodilatator effects by chemerin [70]. Peng et al.: upregulation of chemerin and CMKLR1 expression by isolated smooth muscle cells if exposed to recombinant chemerin or hypoxia. Migration and proliferation of these cells by chemerin [67]. |
| Lung cancer (NSCLC) | Sotiropoulos et al., Xu et al.: higher serum chemerin levels in patients with NSCLC compared to controls but controversy over the association between chemerin levels, lymph node involvement and tumoral stage [71,72]. Li F. et al.: no difference in serum chemerin levels between NSCLC patients and controls [73]. Zhao S. et al., Cai et al.: association between increased chemerin expression by tumor cells in lung slides from patients with NSCLC and good prognosis [74,75]. Zhao H. et al.: association between higher expression of RARRES2 in patients with NSCLC and good prognosis [76]. | No mouse model studying chemerin and lung cancer but other studies showing anti-tumoral properties of chemerin. Pachynski et al.: increased recruitment of NK and T cells in a mouse model of melanoma tumor cells overexpressing chemerin leading to smaller tumors [77]. Al Delbany et al.: decreased neoangiogenesis in a chemical model of mouse skin carcinogenesis leading to smaller tumors [27]. Dubois-Vedrenne et al.: involvement of chemerin only in latter stages of tumorigenesis [78]. | Controversy over the anti- or pro-tumoral role of chemerin. |
3.3. Chemerin and Obstructive Pulmonary Diseases
3.4. Chemerin and Autoimmune Diseases
3.5. Chemerin and Lung Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory Mechanisms in the Lung. J. Inflamm. Res. 2008, 2, 1–11. [Google Scholar] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-Induced Gene 2 (TIG2), a Novel Retinoid-Responsive Gene in Skin. J. Investig. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.-D.; Vulcano, M.; Mirjolet, J.-F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific Recruitment of Antigen-Presenting Cells by Chemerin, a Novel Processed Ligand from Human Inflammatory Fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Krautbauer, S.; Wanninger, J.; Eisinger, K.; Hader, Y.; Beck, M.; Kopp, A.; Schmid, A.; Weiss, T.S.; Dorn, C.; Buechler, C. Chemerin Is Highly Expressed in Hepatocytes and Is Induced in Non-Alcoholic Steatohepatitis Liver. Exp. Mol. Pathol. 2013, 95, 199–205. [Google Scholar] [CrossRef]
- Roh, S.; Song, S.-H.; Choi, K.-C.; Katoh, K.; Wittamer, V.; Parmentier, M.; Sasaki, S. Chemerin--a New Adipokine That Modulates Adipogenesis via Its Own Receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1013–1018. [Google Scholar] [CrossRef]
- Weigert, J.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Wiest, R.; Farkas, S.; Scherer, M.N.; Schäffler, A.; Aslanidis, C.; et al. Systemic Chemerin Is Related to Inflammation Rather than Obesity in Type 2 Diabetes. Clin. Endocrinol. 2010, 72, 342–348. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin Is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Wittamer, V.; Grégoire, F.; Robberecht, P.; Vassart, G.; Communi, D.; Parmentier, M. The C-Terminal Nonapeptide of Mature Chemerin Activates the Chemerin Receptor with Low Nanomolar Potency. J. Biol. Chem. 2004, 279, 9956–9962. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Du, X.-Y.; Zhao, L.; Morser, J.; Leung, L.L.K. Proteolytic Cleavage of Chemerin Protein Is Necessary for Activation to the Active Form, Chem157S, Which Functions as a Signaling Molecule in Glioblastoma. J. Biol. Chem. 2011, 286, 39510–39519. [Google Scholar] [CrossRef]
- Wittamer, V.; Bondue, B.; Guillabert, A.; Vassart, G.; Parmentier, M.; Communi, D. Neutrophil-Mediated Maturation of Chemerin: A Link between Innate and Adaptive Immunity. J. Immunol. 2005, 175, 487–493. [Google Scholar] [CrossRef]
- Kulig, P.; Kantyka, T.; Zabel, B.A.; Banaś, M.; Chyra, A.; Stefańska, A.; Tu, H.; Allen, S.J.; Handel, T.M.; Kozik, A.; et al. Regulation of Chemerin Chemoattractant and Anti-Bacterial Activity by Human Cysteine Cathepsins. J. Immunol. 2011, 187, 1403–1410. [Google Scholar] [CrossRef]
- Du, X.-Y.; Zabel, B.A.; Myles, T.; Allen, S.J.; Handel, T.M.; Lee, P.P.; Butcher, E.C.; Leung, L.L. Regulation of Chemerin Bioactivity by Plasma Carboxypeptidase N, Carboxypeptidase B (Activated Thrombin-Activable Fibrinolysis Inhibitor), and Platelets. J. Biol. Chem. 2009, 284, 751–758. [Google Scholar] [CrossRef]
- Guillabert, A.; Wittamer, V.; Bondue, B.; Godot, V.; Imbault, V.; Parmentier, M.; Communi, D. Role of Neutrophil Proteinase 3 and Mast Cell Chymase in Chemerin Proteolytic Regulation. J. Leucoc. Biol. 2008, 84, 1530–1538. [Google Scholar] [CrossRef]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin Activation by Serine Proteases of the Coagulation, Fibrinolytic, and Inflammatory Cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Guo, B.; Chang, L.; Li, Y. Chemerin Induces Insulin Resistance in Rat Cardiomyocytes in Part through the ERK1/2 Signaling Pathway. Pharmacology 2014, 94, 259–264. [Google Scholar] [CrossRef]
- De Henau, O.; Degroot, G.-N.; Imbault, V.; Robert, V.; De Poorter, C.; Mcheik, S.; Galés, C.; Parmentier, M.; Springael, J.-Y. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 2016, 11, e0164179. [Google Scholar] [CrossRef]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a Putative Chemoattractant Receptor, Is Expressed in Monocyte-Derived Dendritic Cells and Macrophages and Is a Coreceptor for SIV and Some Primary HIV-1 Strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The Role of Chemerin in the Colocalization of NK and Dendritic Cell Subsets into Inflamed Tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-like Receptor 1 Expression and Chemerin-Directed Chemotaxis Distinguish Plasmacytoid from Myeloid Dendritic Cells in Human Blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef]
- Vermi, W.; Riboldi, E.; Wittamer, V.; Gentili, F.; Luini, W.; Marrelli, S.; Vecchi, A.; Franssen, J.-D.; Communi, D.; Massardi, L.; et al. Role of ChemR23 in Directing the Migration of Myeloid and Plasmacytoid Dendritic Cells to Lymphoid Organs and Inflamed Skin. J. Exp. Med. 2005, 201, 509–515. [Google Scholar] [CrossRef]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and Its Receptors in Leukocyte Trafficking, Inflammation and Metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of Chemerin Receptor (ChemR23) in Human Endothelial Cells: Chemerin-Induced Endothelial Angiogenesis. Int. J. Mol. Sci. 2010, 391, 1762–1768. [Google Scholar] [CrossRef]
- Rourke, J.L.; Muruganandan, S.; Dranse, H.J.; McMullen, N.M.; Sinal, C.J. Gpr1 Is an Active Chemerin Receptor Influencing Glucose Homeostasis in Obese Mice. J. Endocrinol. 2014, 222, 201–215. [Google Scholar] [CrossRef]
- Al Delbany, D.; Robert, V.; Dubois-Vedrenne, I.; Del Prete, A.; Vernimmen, M.; Radi, A.; Lefort, A.; Libert, F.; Wittamer, V.; Sozzani, S.; et al. Expression of CCRL2 Inhibits Tumor Growth by Concentrating Chemerin and Inhibiting Neoangiogenesis. Cancers 2021, 13, 5000. [Google Scholar] [CrossRef]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.-Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast Cell-Expressed Orphan Receptor CCRL2 Binds Chemerin and Is Required for Optimal Induction of IgE-Mediated Passive Cutaneous Anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute Respiratory Distress Syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Domscheit, H.; Hegeman, M.A.; Carvalho, N.; Spieth, P.M. Molecular Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents. Front. Physiol. 2020, 11, 36. [Google Scholar] [CrossRef]
- Tsikis, S.T.; Fligor, S.C.; Hirsch, T.I.; Pan, A.; Yu, L.J.; Kishikawa, H.; Joiner, M.M.; Mitchell, P.D.; Puder, M. Lipopolysaccharide-Induced Murine Lung Injury Results in Long-Term Pulmonary Changes and Downregulation of Angiogenic Pathways. Sci. Rep. 2022, 12, 10245. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Day, D.E.; Nadeau, B.A.; McGuire, S.R.; Hoesel, L.M.; Ipaktchi, K.; Zetoune, F.S.; Sarma, J.V.; Leng, L.; et al. Acute Lung Injury Induced by Lipopolysaccharide Is Independent of Complement Activation. J. Immunol. 2008, 180, 7664–7672. [Google Scholar] [CrossRef]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.-D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 Is Expressed in Dendritic Cell Subsets and Macrophages, and Mediates an Anti-Inflammatory Activity of Chemerin in a Lung Disease Model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef]
- Mannes, P.Z.; Barnes, C.E.; Biermann, J.; Latoche, J.D.; Day, K.E.; Zhu, Q.; Tabary, M.; Xiong, Z.; Nedrow, J.R.; Izar, B.; et al. Molecular Imaging of Chemokine-like Receptor 1 (CMKLR1) in Experimental Acute Lung Injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2216458120. [Google Scholar] [CrossRef]
- Provoost, S.; De Grove, K.C.; Fraser, G.L.; Lannoy, V.J.; Tournoy, K.G.; Brusselle, G.G.; Maes, T.; Joos, G.F. Pro- and Anti-Inflammatory Role of ChemR23 Signaling in Pollutant-Induced Inflammatory Lung Responses. J. Immunol. 2016, 196, 1882–1890. [Google Scholar] [CrossRef]
- Sokolowska, M.; Quesniaux, V.F.J.; Akdis, C.A.; Chung, K.F.; Ryffel, B.; Togbe, D. Acute Respiratory Barrier Disruption by Ozone Exposure in Mice. Front. Immunol. 2019, 10, 2169. [Google Scholar] [CrossRef]
- Razvi, S.S.; Richards, J.B.; Malik, F.; Cromar, K.R.; Price, R.E.; Bell, C.S.; Weng, T.; Atkins, C.L.; Spencer, C.Y.; Cockerill, K.J.; et al. Resistin Deficiency in Mice Has No Effect on Pulmonary Responses Induced by Acute Ozone Exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1174–L1185. [Google Scholar] [CrossRef]
- Malik, F.; Cromar, K.R.; Atkins, C.L.; Price, R.E.; Jackson, W.T.; Siddiqui, S.R.; Spencer, C.Y.; Mitchell, N.C.; Haque, I.U.; Johnston, R.A. Chemokine (C-C Motif) Receptor-Like 2 Is Not Essential for Lung Injury, Lung Inflammation, or Airway Hyperresponsiveness Induced by Acute Exposure to Ozone. Physiol. Rep. 2017, 5, e13545. [Google Scholar] [CrossRef]
- Zou, R.; Wang, M.-H.; Chen, Y.; Fan, X.; Yang, B.; Du, J.; Wang, X.-B.; Liu, K.-X.; Zhou, J. Hydrogen-Rich Saline Attenuates Acute Lung Injury Induced by Limb Ischemia/Reperfusion via Down-Regulating Chemerin and NLRP3 in Rats. Shock 2019, 52, 134–141. [Google Scholar] [CrossRef]
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Hirano, S.; Ichinose, T.; Shimada, A.; Yoshikawa, T. The Role of Toll-like Receptor 4 in Airway Inflammation Induced by Diesel Exhaust Particles. Arch. Toxicol. 2006, 80, 275–279. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Lee, J.W.; Falsey, A.R.; Demont, C.; Nyawanda, B.O.; Cai, B.; Fuentes, R.; Stoszek, S.K.; et al. Global and Regional Burden of Hospital Admissions for Pneumonia in Older Adults: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222 (Suppl. S7), S570–S576. [Google Scholar] [CrossRef]
- Kukla, M.; Menżyk, T.; Dembiński, M.; Winiarski, M.; Garlicki, A.; Bociąga-Jasik, M.; Skonieczna, M.; Hudy, D.; Maziarz, B.; Kusnierz-Cabala, B.; et al. Anti-Inflammatory Adipokines: Chemerin, Vaspin, Omentin Concentrations and SARS-CoV-2 Outcomes. Sci. Rep. 2021, 11, 21514. [Google Scholar] [CrossRef]
- Sulicka-Grodzicka, J.; Surdacki, A.; Surmiak, M.; Sanak, M.; Wizner, B.; Sydor, W.; Bociąga-Jasik, M.; Strach, M.; Korkosz, M.; Skladany, L.; et al. Chemerin as a Potential Marker of Resolution of Inflammation in COVID-19 Infection. Biomedicines 2022, 10, 2462. [Google Scholar] [CrossRef]
- Esendagli, D.; Topcu, D.; Gul, E.; Alperen, C.; Sezer, R.; Erol, C.; Akcay, S. Can Adipokines Predict Clinical Prognosis and Post-COVID Lung Sequelae? Respir. Investig. 2023, 61, 618–624. [Google Scholar] [CrossRef]
- Amend, P.; Mester, P.; Schmid, S.; Müller, M.; Buechler, C.; Pavel, V. Plasma Chemerin Is Induced in Critically Ill Patients with Gram-Positive Infections. Biomedicines 2023, 11, 1779. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating Chemerin and Its Kinetics May Be a Useful Diagnostic and Prognostic Biomarker in Critically Ill Patients with Sepsis: A Prospective Study. Biomolecules 2022, 12, 301. [Google Scholar] [CrossRef]
- Horn, P.; Metzing, U.B.; Steidl, R.; Romeike, B.; Rauchfuß, F.; Sponholz, C.; Thomas-Rüddel, D.; Ludewig, K.; Birkenfeld, A.L.; Settmacher, U.; et al. Chemerin in Peritoneal Sepsis and Its Associations with Glucose Metabolism and Prognosis: A Translational Cross-Sectional Study. Crit. Care 2016, 20, 39. [Google Scholar] [CrossRef]
- Ebihara, T.; Matsumoto, H.; Matsubara, T.; Matsuura, H.; Hirose, T.; Shimizu, K.; Ogura, H.; Kang, S.; Tanaka, T.; Shimazu, T. Adipocytokine Profile Reveals Resistin Forming a Prognostic-Related Cytokine Network in the Acute Phase of Sepsis. Shock 2021, 56, 718–726. [Google Scholar] [CrossRef]
- Bondue, B.; Henau, O.D.; Luangsay, S.; Devosse, T.; de Nadaï, P.; Springael, J.-Y.; Parmentier, M.; Vosters, O. The Chemerin/ChemR23 System Does Not Affect the Pro-Inflammatory Response of Mouse and Human Macrophages Ex Vivo. PLoS ONE 2012, 7, e40043. [Google Scholar] [CrossRef]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.C.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.L.; Greaves, D.R. Synthetic Chemerin-Derived Peptides Suppress Inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef]
- Lavis, P.; Morra, S.; Orte Cano, C.; Albayrak, N.; Corbière, V.; Olislagers, V.; Dauby, N.; Del Marmol, V.; Marchant, A.; Decaestecker, C.; et al. Chemerin Plasma Levels Are Increased in COVID-19 Patients and Are an Independent Risk Factor of Mortality. Front. Immunol. 2022, 13, 941663. [Google Scholar] [CrossRef]
- Bondue, B.; Vosters, O.; de Nadai, P.; Glineur, S.; Henau, O.D.; Luangsay, S.; Gool, F.V.; Communi, D.; Vuyst, P.D.; Desmecht, D.; et al. ChemR23 Dampens Lung Inflammation and Enhances Anti-Viral Immunity in a Mouse Model of Acute Viral Pneumonia. PLoS Pathog. 2011, 7, e1002358. [Google Scholar] [CrossRef]
- Shirato, K.; Ujike, M.; Kawase, M.; Matsuyama, S. Identification of CCL2, RARRES2 and EFNB2 as Host Cell Factors That Influence the Multistep Replication of Respiratory Syncytial Virus. Virus Res. 2015, 210, 213–226. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, Y.; Hu, L.; Li, Q.; Jin, M.; Jiang, E. Relationship of Circulating Chemerin and Omentin Levels with Th17 and Th9 Cell Immune Responses in Patients with Asthma. J. Asthma 2018, 55, 579–587. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, W.; Yang, X.; Lin, Y.; Lv, J.; Dou, X.; Luo, Q.; Dong, J.; Chen, Z.; Chu, Y.; et al. Chemerin Suppresses Murine Allergic Asthma by Inhibiting CCL2 Production and Subsequent Airway Recruitment of Inflammatory Dendritic Cells. Allergy 2014, 69, 763–774. [Google Scholar] [CrossRef]
- Boyuk, B.; Guzel, E.C.; Atalay, H.; Guzel, S.; Mutlu, L.C.; Kucukyalçin, V. Relationship between Plasma Chemerin Levels and Disease Severity in COPD Patients. Clin. Respir. J. 2015, 9, 468–474. [Google Scholar] [CrossRef]
- Li, C.; Yan, L.; Song, J. Plasma level of chemerin in COPD patients and the relationship between chemerin and lipid metabolism. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016, 41, 676–683. [Google Scholar] [CrossRef]
- Gałecka, E.; Kumor-Kisielewska, A.; Górski, P. Association of Serum Deiodinase Type 2 Level with Chronic Obstructive Pulmonary Disease in the Polish Population. Acta Biochim. Pol. 2019, 66, 177–182. [Google Scholar] [CrossRef]
- Demoor, T.; Bracke, K.R.; Dupont, L.L.; Plantinga, M.; Bondue, B.; Roy, M.-O.; Lannoy, V.; Lambrecht, B.N.; Brusselle, G.G.; Joos, G.F. The Role of ChemR23 in the Induction and Resolution of Cigarette Smoke-Induced Inflammation. J. Immunol. 2011, 186, 5457–5467. [Google Scholar] [CrossRef]
- Sawicka, K.; Michalska-Jakubus, M.; Potembska, E.; Kowal, M.; Pietrzak, A.; Krasowska, D. Visfatin and Chemerin Levels Correspond with Inflammation and Might Reflect the Bridge between Metabolism, Inflammation and Fibrosis in Patients with Systemic Sclerosis. Postepy Dermatol. Alergol. 2019, 36, 551–565. [Google Scholar] [CrossRef]
- Akamata, K.; Asano, Y.; Taniguchi, T.; Yamashita, T.; Saigusa, R.; Nakamura, K.; Noda, S.; Aozasa, N.; Toyama, T.; Takahashi, T.; et al. Increased Expression of Chemerin in Endothelial Cells Due to Fli1 Deficiency May Contribute to the Development of Digital Ulcers in Systemic Sclerosis. Rheumatology 2015, 54, 1308–1316. [Google Scholar] [CrossRef]
- Chighizola, C.B.; Raschi, E.; Privitera, D.; Luppino, A.F.; Artusi, C.; Schioppo, T.; Mastaglio, C.; Ingegnoli, F.; Borghi, M.O.; Meroni, P.L. Serum Chemerin in Systemic Sclerosis: A Novel Marker of Early Diffuse Disease? Clin. Exp. Rheumatol. 2017, 35 (Suppl. S106), 223–224. [Google Scholar]
- Sanges, S.; Rice, L.; Tu, L.; Valenzi, E.; Cracowski, J.-L.; Montani, D.; Mantero, J.C.; Ternynck, C.; Marot, G.; Bujor, A.M.; et al. Biomarkers of Haemodynamic Severity of Systemic Sclerosis-Associated Pulmonary Arterial Hypertension by Serum Proteome Analysis. Ann. Rheum. Dis. 2023, 82, 365–373. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y.; Li, Y.; Feng, P.; Zheng, Y.; Dong, Y.; Yang, Y.; Wang, R.; Li, A.; Yan, J.; et al. Chemerin Regulates the Proliferation and Migration of Pulmonary Arterial Smooth Muscle Cells via the ERK1/2 Signaling Pathway. Front. Pharmacol. 2022, 13, 767705. [Google Scholar] [CrossRef]
- Saygin, D.; Tabib, T.; Bittar, H.E.T.; Valenzi, E.; Sembrat, J.; Chan, S.Y.; Rojas, M.; Lafyatis, R. Transcriptional Profiling of Lung Cell Populations in Idiopathic Pulmonary Arterial Hypertension. Pulm. Circ. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Omori, A.; Goshima, M.; Kakuda, C.; Kodama, T.; Otani, K.; Okada, M.; Yamawaki, H. Chemerin-9-Induced Contraction Was Enhanced through the Upregulation of Smooth Muscle Chemokine-like Receptor 1 in Isolated Pulmonary Artery of Pulmonary Arterial Hypertensive Rats. Pflugers Arch. 2020, 472, 335–342. [Google Scholar] [CrossRef]
- Hanthazi, A.; Jespers, P.; Vegh, G.; Degroot, G.-N.; Springael, J.-Y.; Lybaert, P.; Dewachter, L.; Mc Entee, K. Chemerin Influences Endothelin- and Serotonin-Induced Pulmonary Artery Vasoconstriction in Rats. Life Sci. 2019, 231, 116580. [Google Scholar] [CrossRef]
- Sotiropoulos, G.P.; Dalamaga, M.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Kotopouli, M.; Karampela, I.; Christodoulatos, G.S.; Lekka, A.; Papavassiliou, A.G. Chemerin as a Biomarker at the Intersection of Inflammation, Chemotaxis, Coagulation, Fibrinolysis and Metabolism in Resectable Non-Small Cell Lung Cancer. Lung Cancer 2018, 125, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Yang, Y.; Wang, Y.-C.; Yan, J.; Qian, L.-H. Prognostic Significance of Serum Chemerin Levels in Patients with Non-Small Cell Lung Cancer. Oncotarget 2017, 8, 22483–22489. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, Y.; Li, J.; Gao, C.; Dong, X.; Ren, P.; Meng, C.; Chen, C. The Clinical Significance of Serum Adipocytokines Level in Patients with Lung Cancer. J. Thorac. Dis. 2019, 11, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, C.; Ye, Y.; Peng, F.; Chen, Q. Expression of Chemerin Correlates with a Favorable Prognosis in Patients With Non-Small Cell Lung Cancer. Lab. Med. 2011, 42, 553–557. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, Z.; Qi, L.; Wang, T.; Shen, Y.; Huang, J. Tazarotene-Induced Gene 2 Is Associated with Poor Survival in Non-Small Cell Lung Cancer. Oncol. Lett. 2016, 12, 2680–2685. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Guo, L.; Shi, S.; Lu, C. A Robust Seven-Gene Signature Associated with Tumor Microenvironment to Predict Survival Outcomes of Patients with Stage III-IV Lung Adenocarcinoma. Front. Genet. 2021, 12, 684281. [Google Scholar] [CrossRef] [PubMed]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The Chemoattractant Chemerin Suppresses Melanoma by Recruiting Natural Killer Cell Antitumor Defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [CrossRef]
- Dubois-Vedrenne, I.; De Henau, O.; Robert, V.; Langa, F.; Javary, J.; Al Delbany, D.; Vosters, O.; Angelats-Canals, E.; Vernimmen, M.; Luangsay, S.; et al. Expression of Bioactive Chemerin by Keratinocytes Inhibits Late Stages of Tumor Development in a Chemical Model of Skin Carcinogenesis. Front. Oncol. 2019, 9, 1253. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Li, S. Association between Serum Chemerin Concentrations and Clinical Indices in Obesity or Metabolic Syndrome: A Meta-Analysis. PLoS ONE 2014, 9, e113915. [Google Scholar] [CrossRef]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and Attributable Health Burden of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Melén, E.; Lehtimäki, L.; Shaw, D. Asthma. Lancet 2023, 401, 858–873. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.L.; Veeranki, S.P.; Ameredes, B.T.; Calhoun, W.J. Diagnosis and Management of Asthma in Adults: A Review. JAMA 2017, 318, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic Obstructive Pulmonary Disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Li, N.; Li, P.; Wang, Z.; Ting, W.; Liu, X.; Wu, W. Chemerin: A Potential Regulator of Inflammation and Metabolism for Chronic Obstructive Pulmonary Disease and Pulmonary Rehabilitation. Biomed. Res. Int. 2020, 2020, 4574509. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic Sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.J.; Olson, A.L.; Fischer, A.; Bull, T.; Brown, K.K.; Raghu, G. Scleroderma Lung Disease. Eur. Respir. Rev. 2013, 22, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Miyabe, Y.; Takayasu, A.; Fukuda, S.; Miyabe, C.; Ebisawa, M.; Yokoyama, W.; Watanabe, K.; Imai, T.; Muramoto, K.; et al. Chemerin Activates Fibroblast-like Synoviocytes in Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2011, 13, R158. [Google Scholar] [CrossRef]
- Shin, W.J.; Zabel, B.A.; Pachynski, R.K. Mechanisms and Functions of Chemerin in Cancer: Potential Roles in Therapeutic Intervention. Front. Immunol. 2018, 9, 2772. [Google Scholar] [CrossRef]
- Kumar, J.D.; Kandola, S.; Tiszlavicz, L.; Reisz, Z.; Dockray, G.J.; Varro, A. The Role of Chemerin and ChemR23 in Stimulating the Invasion of Squamous Oesophageal Cancer Cells. Br. J. Cancer 2016, 114, 1152–1159. [Google Scholar] [CrossRef]
- Farsam, V.; Basu, A.; Gatzka, M.; Treiber, N.; Schneider, L.A.; Mulaw, M.A.; Lucas, T.; Kochanek, S.; Dummer, R.; Levesque, M.P.; et al. Senescent Fibroblast-Derived Chemerin Promotes Squamous Cell Carcinoma Migration. Oncotarget 2016, 7, 83554–83569. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavis, P.; Bondue, B.; Cardozo, A.K. The Dual Role of Chemerin in Lung Diseases. Cells 2024, 13, 171. https://doi.org/10.3390/cells13020171
Lavis P, Bondue B, Cardozo AK. The Dual Role of Chemerin in Lung Diseases. Cells. 2024; 13(2):171. https://doi.org/10.3390/cells13020171
Chicago/Turabian StyleLavis, Philomène, Benjamin Bondue, and Alessandra Kupper Cardozo. 2024. "The Dual Role of Chemerin in Lung Diseases" Cells 13, no. 2: 171. https://doi.org/10.3390/cells13020171




