Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Patients Included in the Study
2.2. Immunophenotyping Procedure and Instrumentation for Peripheral Blood Analysis
2.3. Assessment of Soluble TLR-2, TLR-3, TLR-4, and TLR-9 Levels in Serum by ELISA
2.4. Statistical Analysis and Data Visualization Methods
3. Results
3.1. Demographic and Clinical Analysis of the Study Patients
3.2. Assessment of Differences in the Percentage of TLR-2, TLR-3, TLR-4, and TLR-9 on DCs and Monocytes and the Concentration of Soluble Forms in Serum between GC Patients and Healthy Volunteers
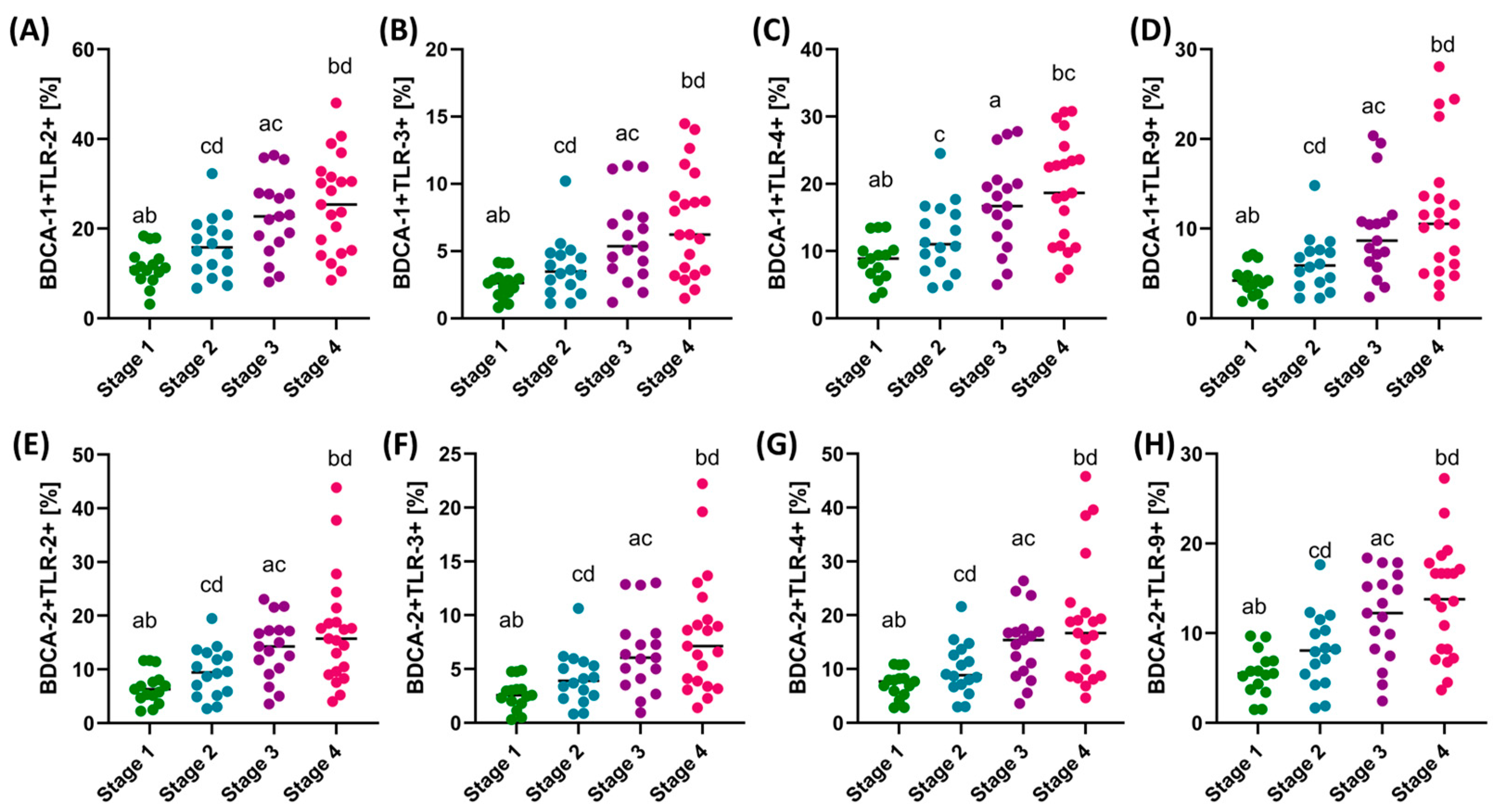
3.3. Assessment of the Differences in the Percentage of TLR-2, TLR-3, TLR-4, and TLR-9 on DCs and Monocytes and the Concentration of Soluble Forms in Serum between Patients with GC Depending on the Type of Cancer and the Stage of the Disease
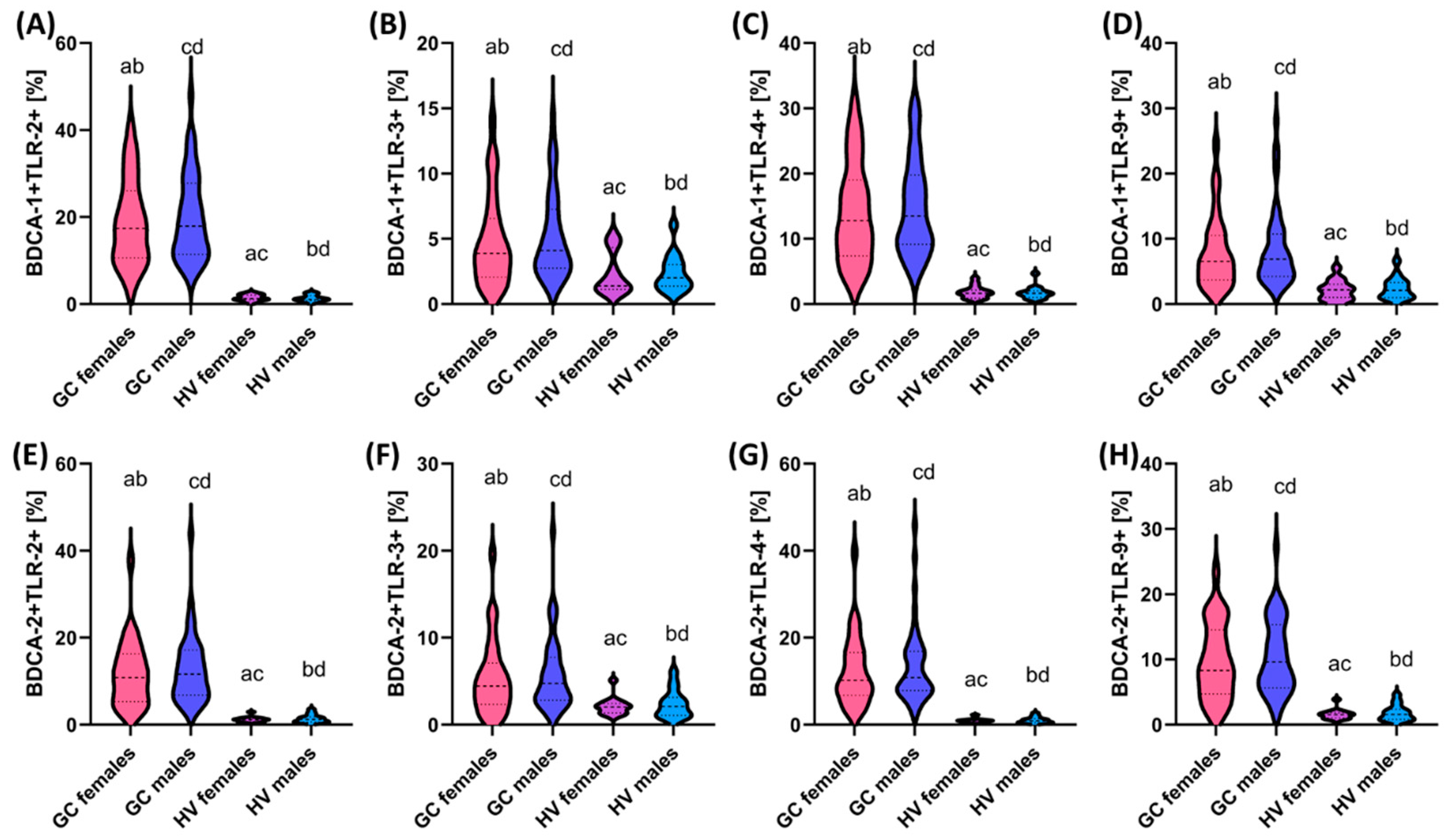
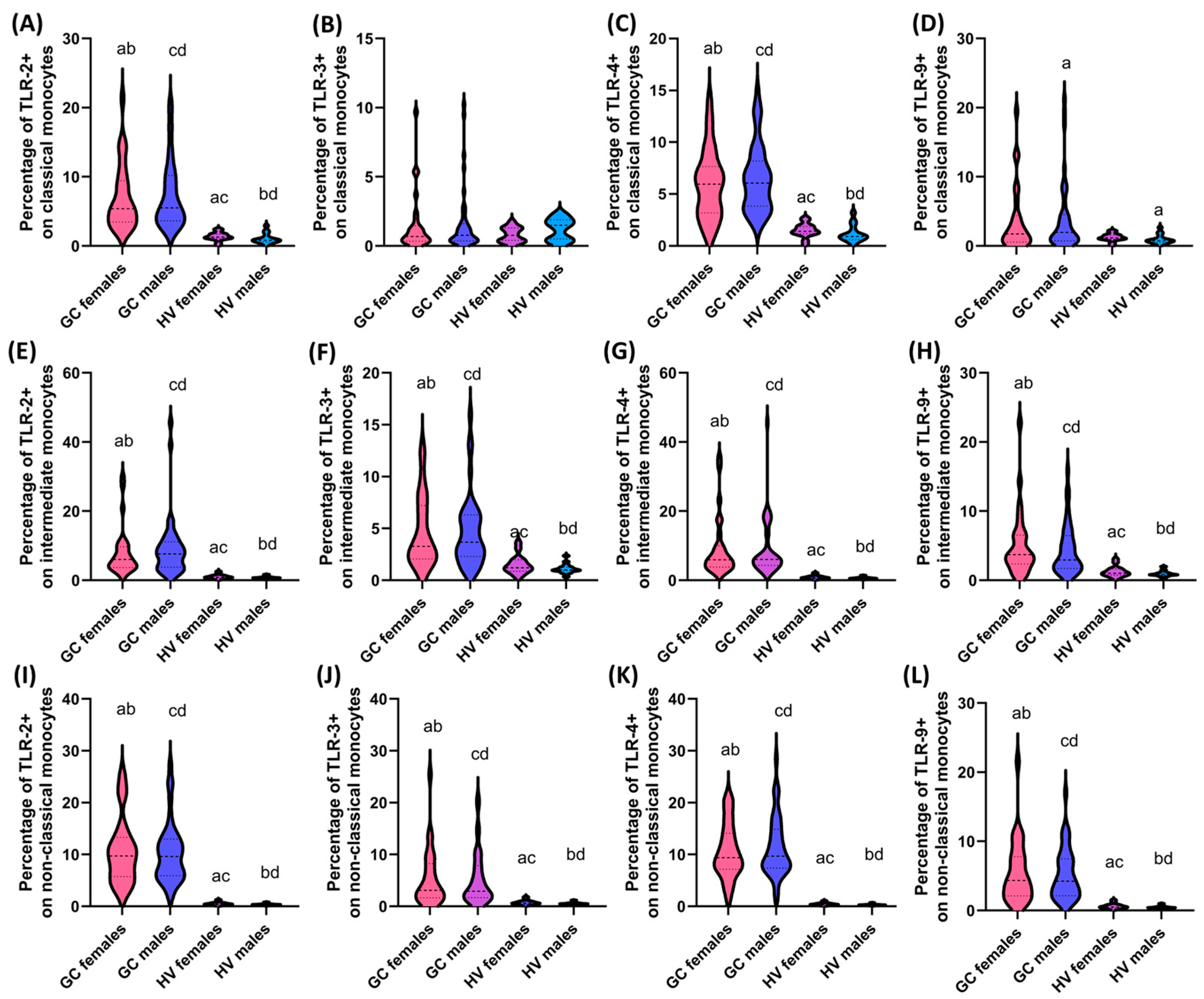
3.4. Assessment of the Differences in the Percentage of TLR-2, TLR-3, TLR-4, and TLR-9 on DCs and Monocytes and the Concentration of Soluble Forms in Serum between Patients with GC Depending on Gender and Age
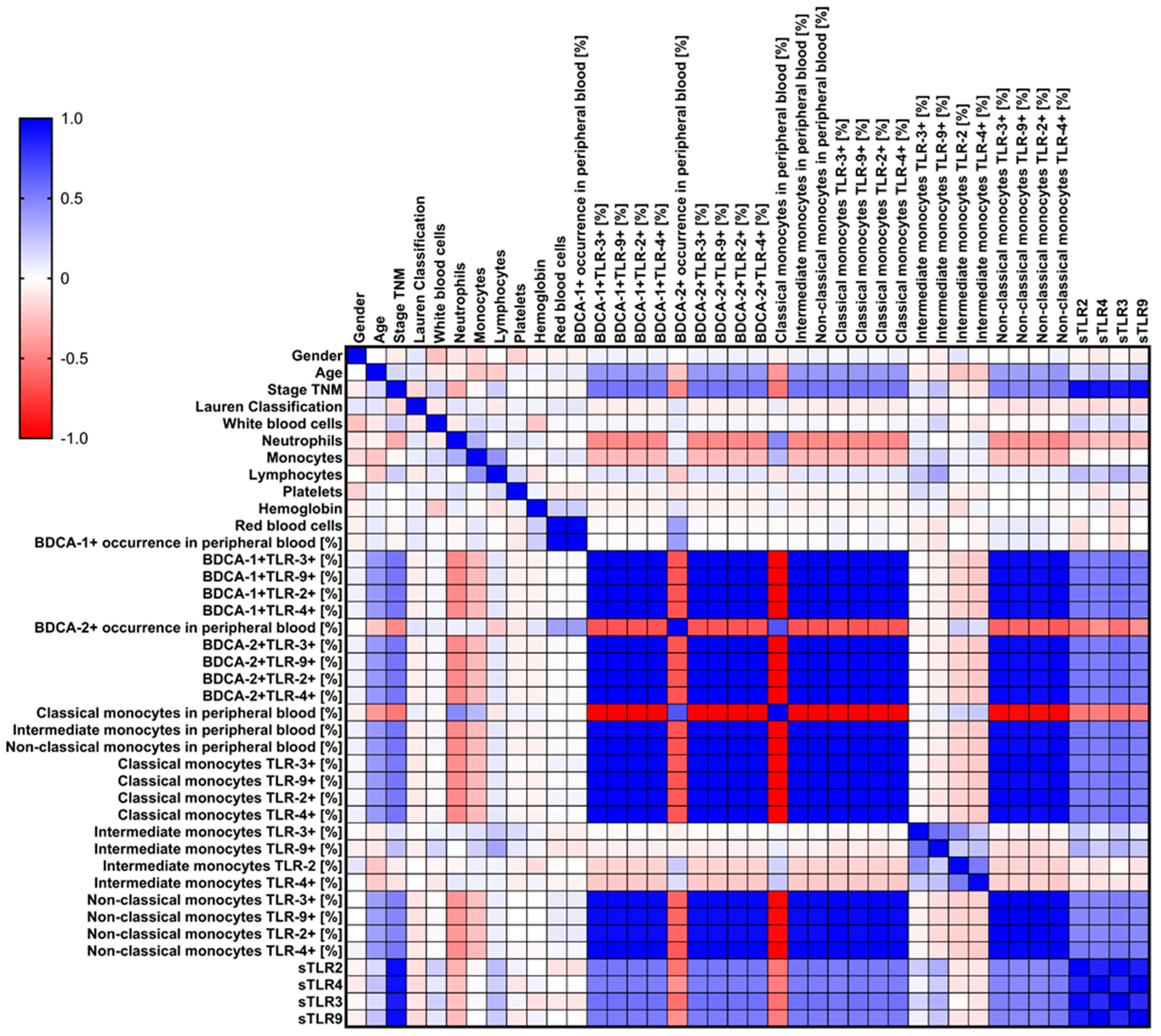
3.5. Analysis of Correlations and ROC Curves of Obtained Test Results with Particular Consideration of Type, Stage, Age, and Gender of Recruited GC Patients
4. Discussion
4.1. Study Limitations
4.2. Could TLRs Be Potential Biomarkers for GC? What Do We Know, and What Should We Investigate?
4.3. Future Perspectives—What Should Be the Subject of Future Research?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stomach Cancer Statistics. WCRF International. Available online: https://www.wcrf.org/cancer-trends/stomach-cancer-statistics/ (accessed on 5 September 2024).
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020–40: A Population-Based Modelling Study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Sukri, A.; Hanafiah, A.; Kosai, N.R. The Roles of Immune Cells in Gastric Cancer: Anti-Cancer or Pro-Cancer? Cancers 2022, 14, 3922. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, R.; Rezaei, N. Role of Monocyte-Derived Dendritic Cells (MoDCs) in Tumor Immune Response. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–18. ISBN 978-3-030-80962-1. [Google Scholar]
- Özdemir, B.H. Role of Immune Cells in the Tumor Microenvironment. In Cancer Research: An Interdisciplinary Approach; Rezaei, N., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 17–47. ISBN 978-3-031-32458-1. [Google Scholar]
- Du, Q.; An, Q.; Zhang, J.; Liu, C.; Hu, Q. Unravelling Immune Microenvironment Features Underlying Tumor Progression in the Single-Cell Era. Cancer Cell Int. 2024, 24, 143. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, Function, and Differentiation Potential of Human Monocyte Subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- Ando, K.; Hamada, K.; Shida, M.; Ohkuma, R.; Kubota, Y.; Horiike, A.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; et al. A High Number of PD-L1+ CD14+ Monocytes in Peripheral Blood Is Correlated with Shorter Survival in Patients Receiving Immune Checkpoint Inhibitors. Cancer Immunol. Immunother. 2021, 70, 337–348. [Google Scholar] [CrossRef]
- Sveen, K.A.; Smith, G.; Goncalves, I.; Edsfeldt, A.; Engstrom, G.; Bjorkbacka, H.; Bengtsson, E. High Levels of Intermediate Cd14++cd16+ Monocytes Predict Incident Atrial Fibrillation in the General Population. Eur. Heart J. 2023, 44, ehad655.440. [Google Scholar] [CrossRef]
- Liu, H.-L.; Feng, X.; Tang, M.-M.; Zhou, H.-Y.; Peng, H.; Ge, J.; Liu, T. Prognostic Significance of Preoperative Lymphocyte to Monocyte Ratio in Patients with Signet Ring Gastric Cancer. World J. Gastrointest. Surg. 2023, 15, 1673–1683. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, D.; Huang, X.; Zhang, M.; Zhu, W.; Xu, C. Significance of Monocyte Infiltration in Patients with Gastric Cancer: A Combined Study Based on Single Cell Sequencing and TCGA. Front. Oncol. 2022, 12, 1001307. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Ohuchida, K.; Katayama, N.; Yamada, Y.; Nakamura, S.; Okuda, S.; Otsubo, Y.; Iwamoto, C.; Torata, N.; Horioka, K.; et al. Tumor-Infiltrating Monocytic Myeloid-Derived Suppressor Cells Contribute to the Development of an Immunosuppressive Tumor Microenvironment in Gastric Cancer. Gastric Cancer 2024, 27, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Wei, S.; Jiang, P.; Xue, L.; Wang, J. Tumor-Associated Macrophages: Potential Therapeutic Strategies and Future Prospects in Cancer. J. Immunother. Cancer 2021, 9, e001341. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Shi, Y.; Yin, B. Macrophage Barrier in the Tumor Microenvironment and Potential Clinical Applications. Cell Commun. Signal. 2024, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Park, R.; Sun, W. The Integration of Immune Checkpoint Inhibitors with VEGF Targeted Agents in Advanced Gastric and Gastroesophageal Adenocarcinoma: A Review on the Rationale and Results of Early Phase Trials. J. Hematol. Oncol. 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.-L.; Kong, B.-H.; Qu, X.; Yang, Q.-F.; Ma, Y.-Y. BDCA-1+, BDCA-2+ and BDCA-3+ Dendritic Cells in Early Human Pregnancy Decidua. Clin. Exp. Immunol. 2008, 151, 399–406. [Google Scholar] [CrossRef]
- Patente, T.A.; Pinho, M.P.; Oliveira, A.A.; Evangelista, G.C.M.; Bergami-Santos, P.C.; Barbuto, J.A.M. Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front. Immunol. 2019, 9, 3176. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Cook, A.; Robinson, B.W.S.; Lake, R.A.; Nowak, A.K. Serial Immunomonitoring of Cancer Patients Receiving Combined Antagonistic Anti-CD40 and Chemotherapy Reveals Consistent and Cyclical Modulation of T Cell and Dendritic Cell Parameters. BMC Cancer 2017, 17, 417. [Google Scholar] [CrossRef]
- Tabarkiewicz, J.; Rybojad, P.; Jablonka, A.; Rolinski, J. CD1c+ and CD303+ Dendritic Cells in Peripheral Blood, Lymph Nodes and Tumor Tissue of Patients with Non-Small Cell Lung Cancer. Oncol. Rep. 2008, 19, 237–243. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Cheng, Y.; Zhang, J.; Ji, F.; Ling, Z. Roles of Plasmacytoid Dendritic Cells in Gastric Cancer. Front. Oncol. 2022, 12, 818314. [Google Scholar] [CrossRef]
- Troise, D.; Infante, B.; Mercuri, S.; Catalano, V.; Ranieri, E.; Stallone, G. Dendritic Cells: A Bridge between Tolerance Induction and Cancer Development in Transplantation Setting. Biomedicines 2024, 12, 1240. [Google Scholar] [CrossRef]
- Hato, L.; Vizcay, A.; Eguren, I.; Pérez-Gracia, J.L.; Rodríguez, J.; Gállego Pérez-Larraya, J.; Sarobe, P.; Inogés, S.; Díaz de Cerio, A.L.; Santisteban, M. Dendritic Cells in Cancer Immunology and Immunotherapy. Cancers 2024, 16, 981. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Qiu, J.; Shi, W.; Zhang, J.; Gao, Q.; Feng, L.; Zhuang, Z. Peripheral CD4+CD25hiCD127low Regulatory T Cells Are Increased in Patients with Gastrointestinal Cancer. BMC Gastroenterol. 2023, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, X.; Zhang, D. TLRs as a Promise Target Along With Immune Checkpoint Against Gastric Cancer. Front. Cell Dev. Biol. 2021, 8, 611444. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, R.; Jia, J.; He, Y.; He, S. Activation of Toll-like Receptor 2 Enhances Peripheral and Tumor-Infiltrating CD8+ T Cell Cytotoxicity in Patients with Gastric Cancer. BMC Immunol. 2021, 22, 67. [Google Scholar] [CrossRef]
- Villarroel-Espindola, F.; Ejsmentewicz, T.; Gonzalez-Stegmaier, R.; Jorquera, R.A.; Salinas, E. Intersections between Innate Immune Response and Gastric Cancer Development. World J. Gastroenterol. 2023, 29, 2222–2240. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Carrillo-Vazquez, D.A.; Padilla-Ortiz, D.M.; Vazquez-Rodriguez, R.; Nuñez-Alvarez, C.; Juarez-Vega, G.; Gomez-Martin, D. TLR Expression in Peripheral Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies: Association with Clinical and Immunological Features. J. Transl. Med. 2020, 18, 125. [Google Scholar] [CrossRef]
- Zargari, S.; Bahari, A.; Goodarzi, M.T.; Mahmoodi, M.; Valadan, R. TLR2 and TLR4 Signaling Pathways and Gastric Cancer: Insights from Transcriptomics and Sample Validation. Iran. Biomed. J. 2022, 26, 36–43. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, Y.; Liu, H.; Yu, Y.; Jiang, Y.; Xue, Y.; Liu, Z.; Wei, M.-X. TLR4 Polymorphisms Associated with Developing Gastric Pre-Cancer Lesions in a Chinese Han Population. Hum. Immunol. 2014, 75, 176–181. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Z.; Wang, C.; Zhang, Y. The TLR/MyD88 Signalling Cascade in Inflammation and Gastric Cancer: The Immune Regulatory Network of Helicobacter Pylori. J. Mol. Med. 2023, 101, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.S.; Zhao, J.; Kazmierski, E.; Kinane, D.F.; Benakanakere, M.R. TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response. Cells 2020, 9, 1910. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, S.; Yang, H. Roles of Toll-Like Receptor 3 in Human Tumors. Front. Immunol. 2021, 12, 667454. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Cai, L.; Xiong, P.; Liu, Z.; Chen, S.; Liu, X.; Lin, R.; Lei, Z.; Tian, D.; Su, M. TLR3 Expression Is a Potential Prognosis Biomarker and Shapes the Immune-Active Tumor Microenvironment in Esophageal Squamous Cell Carcinoma. J. Inflamm. Res. 2022, 15, 1437–1456. [Google Scholar] [CrossRef] [PubMed]
- Dongye, Z.; Li, J.; Wu, Y. Toll-like Receptor 9 Agonists and Combination Therapies: Strategies to Modulate the Tumour Immune Microenvironment for Systemic Anti-Tumour Immunity. Br. J. Cancer 2022, 127, 1584–1594. [Google Scholar] [CrossRef]
- Tang, K.; McLeod, L.; Livis, T.; West, A.C.; Dawson, R.; Yu, L.; Balic, J.J.; Chonwerawong, M.; Wray-McCann, G.; Oshima, H.; et al. Toll-like Receptor 9 Promotes Initiation of Gastric Tumorigenesis by Augmenting Inflammation and Cellular Proliferation. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 567–586. [Google Scholar] [CrossRef]
- Majewski, M.; Mertowska, P.; Mertowski, S.; Torres, K.; Grywalska, E. How Toll-like Receptor 9 Plays a Key Role in the Development of Gastric Cancer and Is Linked to Epstein–Barr Virus Infection. Cancers 2023, 15, 5104. [Google Scholar] [CrossRef]
- Eskuri, M.; Kemi, N.; Helminen, O.; Huhta, H.; Kauppila, J.H. Toll-like Receptors 1, 2, 4, 5, and 6 in Gastric Cancer. Virchows Arch, 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Eskuri, M.; Kemi, N.; Helminen, O.; Huhta, H.; Kauppila, J.H. Toll-like Receptors 3, 7, 8, and 9 in Gastric Cancer. APMIS 2023, 131, 92–99. [Google Scholar] [CrossRef]
- De Oliveira, J.G.; Silva, A.E. Polymorphisms of the TLR2 and TLR4 Genes Are Associated with Risk of Gastric Cancer in a Brazilian Population. World J. Gastroenterol. 2012, 18, 1235–1242. [Google Scholar] [CrossRef]
- Xia, P.; Wu, Y.; Lian, S.; Yan, L.; Meng, X.; Duan, Q.; Zhu, G. Research Progress on Toll-like Receptor Signal Transduction and Its Roles in Antimicrobial Immune Responses. Appl. Microbiol. Biotechnol. 2021, 105, 5341–5355. [Google Scholar] [CrossRef]
- Kos, M.; Bojarski, K.; Mertowska, P.; Mertowski, S.; Tomaka, P.; Dziki, Ł.; Grywalska, E. New Horizons in the Diagnosis of Gastric Cancer: The Importance of Selected Toll-like Receptors in Immunopathogenesis Depending on the Stage, Clinical Subtype, and Gender of Newly Diagnosed Patients. Int. J. Mol. Sci. 2024, 25, 9264. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, B.; Eiró, N.; González-Reyes, S.; González, L.; Aguirre, A.; González, L.O.; Del Casar, J.M.; García-Muñiz, J.L.; Vizoso, F.J. Clinical Significance of Toll-like Receptor 3, 4, and 9 in Gastric Cancer. J. Immunother. 2014, 37, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Nienartowicz, M.; Grabowski, K.; Rosińczuk, J.; Krzystek-Korpacka, M. Toll-like Receptors TLR-2, TLR-4, TLR-7, and TLR-9 in Tumor Tissue and Serum of the Patients with Esophageal Squamous Cell Carcinoma and Gastro-Esophageal Junction Cancer. Adv. Clin. Exp. Med. 2019, 28, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Yu, L.; Ying, L.; Balic, J.; Gao, H.; Deng, N.T.; West, A.; Yan, F.; Ji, C.B.; Gough, D.; et al. Toll-like Receptor 2 Regulates Metabolic Reprogramming in Gastric Cancer via Superoxide Dismutase 2. Int. J. Cancer 2019, 144, 3056–3069. [Google Scholar] [CrossRef]
- Chandrasekar, S.A.; Palaniyandi, T.; Parthasarathy, U.; Surendran, H.; Viswanathan, S.; Wahab, M.R.A.; Baskar, G.; Natarajan, S.; Ranjan, K. Implications of Toll-like Receptors (TLRs) and Their Signaling Mechanisms in Human Cancers. Pathol.-Res. Pract. 2023, 248, 154673. [Google Scholar] [CrossRef]
- Xie, X.-Q.; Zhao, Q.-H.; Wang, H.; Gu, K.-S. Dysregulation of mRNA Profile in Cisplatin-Resistant Gastric Cancer Cell Line SGC7901. World J. Gastroenterol. 2017, 23, 1189–1202. [Google Scholar] [CrossRef]
- Al Othaim, A.; Al-Hawary, S.I.S.; Alsaab, H.O.; Almalki, S.G.; Najm, M.A.A.; Hjazi, A.; Alsalamy, A.; Firras Almulla, A.; Alizadeh, H. Common Variants in Toll-like Receptor Family Genes and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Genet. 2023, 14, 1280051. [Google Scholar] [CrossRef]
- Chuang, H.-C.; Chou, M.-H.; Chien, C.-Y.; Chuang, J.-H.; Liu, Y.-L. Triggering TLR3 Pathway Promotes Tumor Growth and Cisplatin Resistance in Head and Neck Cancer Cells. Oral Oncol. 2018, 86, 141–149. [Google Scholar] [CrossRef]
- Yang, J.-X..; Tseng, J.-C..; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like Receptor (TLR) Agonists as a Driving Force behind next-Generation Vaccine Adjuvants and Cancer Therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef]
- Rolfo, C.; Giovannetti, E.; Martinez, P.; McCue, S.; Naing, A. Applications and Clinical Trial Landscape Using Toll-like Receptor Agonists to Reduce the Toll of Cancer. npj Precis. Oncol. 2023, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Diao, Y.; Gao, N.; Wan, Y.; Zhong, J.; Zheng, H.; Wang, Z.; Jin, G. Gastric Cancer Vaccines Synthesized Using a TLR7 Agonist and Their Synergistic Antitumor Effects with 5-Fluorouracil. J. Transl. Med. 2018, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dong, L.; Shi, H.-T.; Guo, X.-Y.; Qin, B.; Wang, Y.; Li, H. Imiquimod Inhibits the Growth of SGC-7901 Cells in Vitro through Induction of Autophagy and Apoptosis. Mol. Med. Rep. 2016, 13, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ge, X.; Liu, Y.; Li, H.; Zhang, Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics 2022, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Ohadian Moghadam, S.; Nowroozi, M.R. Toll-like Receptors: The Role in Bladder Cancer Development, Progression and Immunotherapy. Scand. J. Immunol. 2019, 90, e12818. [Google Scholar] [CrossRef]
- Kang, T.H.; Mao, C.-P.; Kim, Y.S.; Kim, T.W.; Yang, A.; Lam, B.; Tseng, S.-H.; Farmer, E.; Park, Y.-M.; Hung, C.-F. TLR9 Acts as a Sensor for Tumor-Released DNA to Modulate Anti-Tumor Immunity after Chemotherapy. J. Immunother. Cancer 2019, 7, 260. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Tseng, J.-C.; Huang, L.-R.; Huang, C.-M.; Huang, C.-Y.F.; Chuang, T.-H. Adjuvant Effect of Toll-Like Receptor 9 Activation on Cancer Immunotherapy Using Checkpoint Blockade. Front. Immunol. 2020, 11, 1075. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Shirafkan, F.; Shokri-Shirvani, J.; Morakabati, P.; Alhooei, S.; Pirzadeh, M.; Barari, L.; Hamidian, S.M.T.; Rezaee Cherati, M.; Rajabnia, M.; Nouri, H.R. Expression of TLR1, TLR3 and TLR7 Genes Remarkably down-Regulated from Erosion to Peptic Ulcer and Gastric Cancer Development. Gene Rep. 2021, 24, 101229. [Google Scholar] [CrossRef]
- Kasurinen, A.; Hagström, J.; Laitinen, A.; Kokkola, A.; Böckelman, C.; Haglund, C. Evaluation of Toll-like Receptors as Prognostic Biomarkers in Gastric Cancer: High Tissue TLR5 Predicts a Better Outcome. Sci. Rep. 2019, 9, 12553. [Google Scholar] [CrossRef]
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. NF-κB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Yarmohammadi, A.; Narimani, F.; Wallace, C.E.; Bishayee, A. Modulation of TLR/NF-κB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy. Front. Oncol. 2022, 12, 834072. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.; Naumann, M. NF-κB Signaling in Gastric Cancer. Toxins 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ramnarayanan, K.; Sundar, R.; Padmanabhan, N.; Srivastava, S.; Koiwa, M.; Yasuda, T.; Koh, V.; Huang, K.K.; Tay, S.T.; et al. Single-Cell Atlas of Lineage States, Tumor Microenvironment, and Subtype-Specific Expression Programs in Gastric Cancer. Cancer Discov. 2022, 12, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Xu, R.; Ma, F.; Yang, N.; Li, Y.; Sun, X.; Jin, P.; Kang, W.; Jia, L.; Xiong, J.; et al. scRNA-Seq of Gastric Tumor Shows Complex Intercellular Interaction with an Alternative T Cell Exhaustion Trajectory. Nat. Commun. 2022, 13, 4943. [Google Scholar] [CrossRef]
- Kang, C.; Lee, Y.; Lee, J.E. Recent Advances in Mass Spectrometry-Based Proteomics of Gastric Cancer. World J. Gastroenterol. 2016, 22, 8283–8293. [Google Scholar] [CrossRef]
- Ge, S.; Xia, X.; Ding, C.; Zhen, B.; Zhou, Q.; Feng, J.; Yuan, J.; Chen, R.; Li, Y.; Ge, Z.; et al. A Proteomic Landscape of Diffuse-Type Gastric Cancer. Nat. Commun. 2018, 9, 1012. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Wang, B.; Xu, F.; Ma, F.; Qu, Y.; Jiang, D.; Li, K.; Feng, J.; Tian, S.; et al. Proteomic Characterization of Gastric Cancer Response to Chemotherapy and Targeted Therapy Reveals New Therapeutic Strategies. Nat. Commun. 2022, 13, 5723. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Chong, W.; Shang, L.; Jing, C.; Li, L. Liquid Biopsy in Gastric Cancer: Predictive and Prognostic Biomarkers. Cell Death Dis. 2022, 13, 903. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.-W. Liquid Biopsy: An Emerging Diagnostic, Prognostic, and Predictive Tool in Gastric Cancer. J. Gastric Cancer 2024, 24, 4–28. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, M.; Xu, Y.; Gu, X.; Zou, M.; Abudushalamu, G.; Yao, Y.; Fan, X.; Wu, G. Clinical Application and Detection Techniques of Liquid Biopsy in Gastric Cancer. Mol. Cancer 2023, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.-B.; He, Y.-F.; Li, X.-Q.; Wang, K.; Wang, R.-L. The Role of miRNA and lncRNA in Gastric Cancer. Oncotarget 2017, 8, 81572–81582. [Google Scholar] [CrossRef] [PubMed]
- Gilani, N.; Arabi Belaghi, R.; Aftabi, Y.; Faramarzi, E.; Edgünlü, T.; Somi, M.H. Identifying Potential miRNA Biomarkers for Gastric Cancer Diagnosis Using Machine Learning Variable Selection Approach. Front. Genet. 2022, 12, 779455. [Google Scholar] [CrossRef] [PubMed]
- Azari, H.; Nazari, E.; Mohit, R.; Asadnia, A.; Maftooh, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Shahidsales, S.; Khazaei, M.; et al. Machine Learning Algorithms Reveal Potential miRNAs Biomarkers in Gastric Cancer. Sci. Rep. 2023, 13, 6147. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, H.; Sun, W.; Han, Y.; Li, S.; Qu, Y.; Ying, G.; Ba, Y. MicroRNA-155 Promotes Gastric Cancer Growth and Invasion by Negatively Regulating Transforming Growth Factor-β Receptor 2. Cancer Sci. 2018, 109, 618–628. [Google Scholar] [CrossRef]
- Su, N.; Li, L.; Zhou, E.; Li, H.; Wu, S.; Cao, Z. Resveratrol Downregulates miR-155-5p to Block the Malignant Behavior of Gastric Cancer Cells. BioMed Res. Int. 2022, 2022, 6968641. [Google Scholar] [CrossRef]
- Wan, J.; Xia, L.; Xu, W.; Lu, N. Expression and Function of miR-155 in Diseases of the Gastrointestinal Tract. Int. J. Mol. Sci. 2016, 17, 709. [Google Scholar] [CrossRef]
- Kalajahi, H.G.; Yari, A.; Amini, M.; Catal, T.; Ahmadpour Youshanlui, M.; Pourbagherian, O.; Zhmurov, C.S.; Mokhtarzadeh, A. Therapeutic Effect of microRNA-21 on Differentially Expressed Hub Genes in Gastric Cancer Based on Systems Biology. Sci. Rep. 2023, 13, 21906. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kacimi, S.E.O.; Nguyen, T.L.; Suman, K.H.; Lemus-Martin, R.; Saleem, H.; Do, D.N. MiR-21 in the Cancers of the Digestive System and Its Potential Role as a Diagnostic, Predictive, and Therapeutic Biomarker. Biology 2021, 10, 417. [Google Scholar] [CrossRef]
- Xiong, S.; Hu, M.; Li, C.; Zhou, X.; Chen, H. Role of miR-34 in Gastric Cancer: From Bench to Bedside (Review). Oncol. Rep. 2019, 42, 1635–1646. [Google Scholar] [CrossRef]
- Yong, H.; Fu, J.; Gao, G.; Shi, H.; Zheng, D.; Zhou, X. MiR-34a Suppresses the Proliferation and Invasion of Gastric Cancer by Modulating PDL1 in the Immune Microenvironment. Mol. Cell. Probes 2020, 53, 101601. [Google Scholar] [CrossRef] [PubMed]
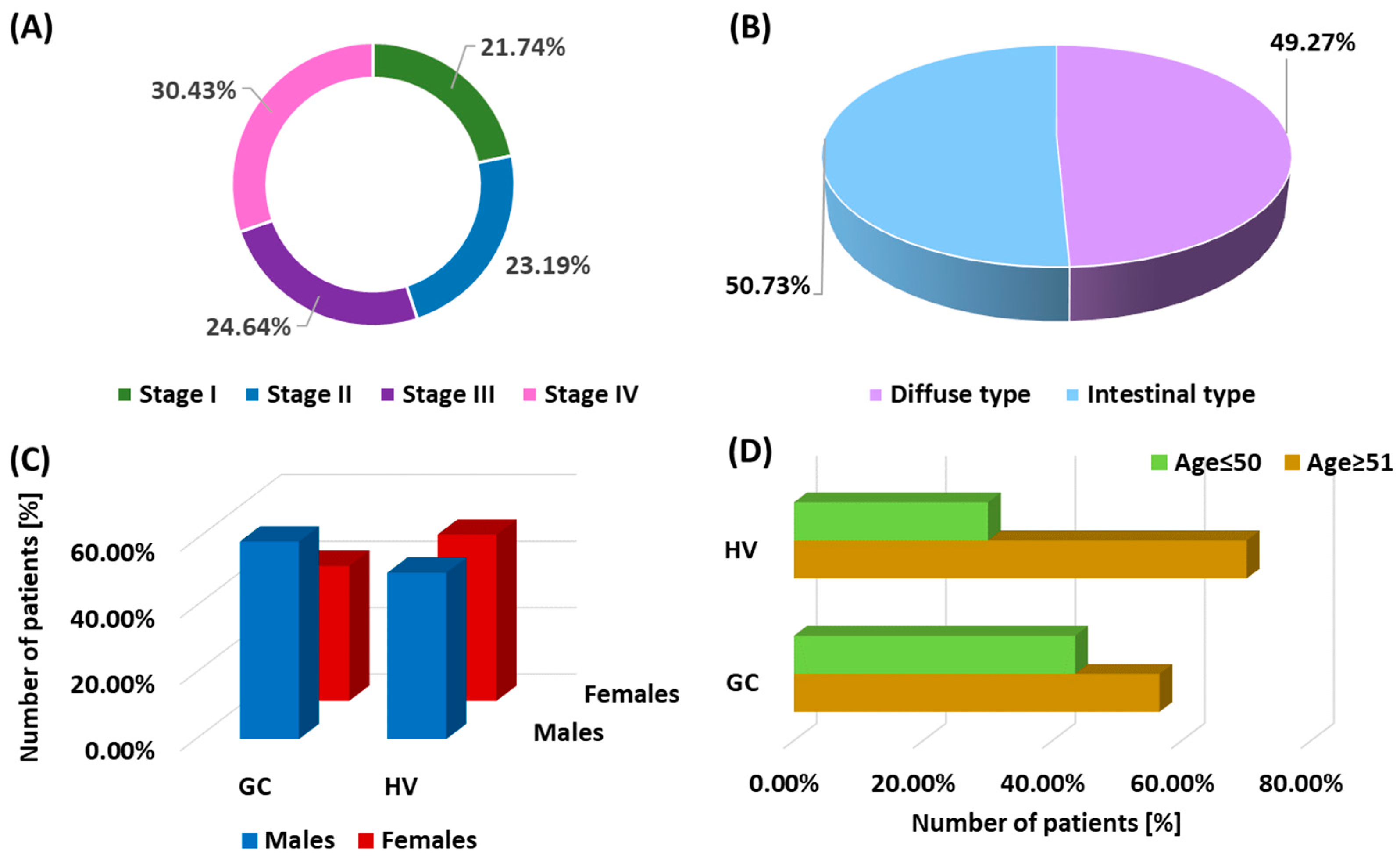






| Parameters | GC Patients | Healthy Volunteers | p-Value | ||
|---|---|---|---|---|---|
| Mean SD | Median (Range) | Mean SD | Median (Range) | ||
| Age | 60.28 ± 14.04 | 58.00 (39.00–86.00) | 58.97 ± 12.65 | 58.50 (37.00–82.00) | 0.669 |
| White blood cells [103/µL] | 7.65 ± 2.08 | 7.63 (4.12–10.88) | 5.90 ± 0.92 | 5.97 (4.13–7.81) | 0.000 * |
| Neutrophils [103/µL] | 4.35 ± 1.06 | 4.13 (2.20–6.10) | 4.91 ± 1.03 | 5.22 (2.77–6.10) | 0.021 * |
| Monocytes [103/µL] | 0.47 ± 0.12 | 0.47 (0.10–0.75) | 0.49 ± 0.11 | 0.52 (0.32–0.68) | 0.398 |
| Lymphocytes [103/µL] | 2.42 ± 0.56 | 2.43 (1.47–4.10) | 2.25 ± 0.50 | 2.27 (1.47–3.14) | 0.244 |
| Platelets [103/µL] | 183.03 ± 50.69 | 182.00 (100.00–268.00) | 263.93 ± 56.46 | 255.50 (190.00–358.00) | 0.000 * |
| Hemoglobin [g/dL] | 12.33 ± 1.56 | 12.12 (10.00–14.90) | 16.13 ± 1.15 | 16.29 (14.32–17.97) | 0.000 * |
| Red blood cells [106/µL] | 3.35 ± 0.85 | 3.27 (2.01–4.99) | 4.03 ± 0.54 | 3.98 (3.14–4.97) | 0.000 * |
| BDCA-1+ occurrence in peripheral blood [%] | 0.07 ± 0.04 | 0.06 (0.01–0.23) | 0.24 ± 0.13 | 0.17 (0.13–0.52) | 0.000 * |
| BDCA-2+ occurrence in peripheral blood [%] | 0.08 ± 0.05 | 0.07 (0.01–0.22) | 0.13 ± 0.07 | 0.12 (0.04–0.33) | 0.000 * |
| Classical monocytes in peripheral blood [%] | 85.70 ± 6.39 | 86.50 (67.47–96.42) | 84.16 ± 8.88 | 80.19 (64.77–96.84) | 0.451 |
| Intermediate monocytes in peripheral blood [%] | 8.19 ± 3.93 | 7.66 (2.14–20.46) | 3.51 ± 0.95 | 3.53 (1.54–5.82) | 0.000 * |
| Non-classical monocytes in peripheral blood [%] | 5.24 ± 2.89 | 5.04 (0.93–15.40) | 2.45 ± 0.96 | 2.52 (0.93–4.28) | 0.000 * |
| Parameters | GC Patients | Healthy Volunteers | p-Value | |||
|---|---|---|---|---|---|---|
| Mean SD | Median (Range) | Mean SD | Median (Range) | |||
| Evaluation of the occurrence of the studied TLRs on DCs [%] | BDCA-1+ TLR-2+ | 19.54 ± 9.77 | 17.78 (3.19–48.00) | 1.41 ± 0.71 | 1.27 (0.28–2.69) | 0.000 * |
| BDCA-1+ TLR-3+ | 5.06 ± 3.37 | 4.09 (0.81–14.47) | 2.34 ± 1.48 | 1.74 (0.73–6.06) | 0.000 * | |
| BDCA-1+ TLR-4+ | 14.56 ± 7.34 | 13.40 (3.06–30.77) | 1.79 ± 0.93 | 1.66 (0.62–4.72) | 0.000 * | |
| BDCA-1+ TLR-9+ | 8.34 ± 5.84 | 6.78 (1.59–28.07) | 2.28 ± 1.55 | 2.15 (0.32–6.61) | 0.000 * | |
| BDCA-2+ TLR-2+ | 12.26 ± 7.72 | 11.45 (2.21–43.86) | 1.30 ± 0.76 | 1.19 (0.34–3.51) | 0.000 * | |
| BDCA-2+ TLR-3+ | 5.63 ± 4.27 | 4.76 (0.30–22.22) | 2.25 ± 1.31 | 2.05 (0.60–6.08) | 0.000 * | |
| BDCA-2+ TLR-4+ | 13.12 ± 8.61 | 10.78 (2.80–45.83) | 0.99 ± 0.58 | 0.90 (0.26–2.67) | 0.000 * | |
| BDCA-2+ TLR-9+ | 10.17 ± 5.68 | 8.42 (1.49–27.27) | 1.71 ± 1.00 | 1.56 (0.45–4.62) | 0.000 * | |
| Evaluation of the occurrence of the tested TLRs on monocytes [%] | Classical monocytes TLR-2+ | 7.25 ± 4.77 | 5.46 (2.01–21.37) | 1.21 ± 0.67 | 1.10 (0.13–2.92) | 0.000 * |
| Classical monocytes TLR-3+ | 1.59 ± 2.22 | 0.71 (0.09–10.22) | 1.01 ± 0.67 | 1.01 (0.13–2.33) | 0.919 | |
| Classical monocytes TLR-4+ | 6.30 ± 3.24 | 6.06 (0.88–14.77) | 1.34 ± 0.74 | 1.21 (0.14–3.21) | 0.000 * | |
| Classical monocytes TLR-9+ | 3.91 ± 4.95 | 1.92 (0.07–21.25) | 1.10 ± 0.61 | 1.00 (0.12–2.65) | 0.004 * | |
| Intermediate monocytes TLR-2 | 8.74 ± 7.98 | 6.84 (0.99–45.59) | 0.93 ± 0.51 | 0.78 (0.21–2.57) | 0.000 * | |
| Intermediate monocytes TLR-3+ | 4.67 ± 3.40 | 3.52 (0.38–15.98) | 1.31 ± 0.72 | 1.11 (0.29–3.64) | 0.000 * | |
| Intermediate monocytes TLR-4+ | 8.99 ± 8.18 | 5.96 (1.50–45.65) | 0.78 ± 0.43 | 0.66 (0.17–2.16) | 0.000 * | |
| Intermediate monocytes TLR-9+ | 4.71 ± 4.07 | 3.45 (0.43–22.76) | 1.10 ± 0.61 | 0.93 (0.25–3.06) | 0.000 * | |
| Non-classical monocytes TLR-2+ | 10.45 ± 5.92 | 9.62 (1.77–27.19) | 0.46 ± 0.25 | 0.39 (0.10–1.28) | 0.000 * | |
| Non-classical monocytes TLR-3+ | 5.31 ± 5.19 | 2.93 (0.24–25.43) | 0.65 ± 0.36 | 0.55 (0.15–1.81) | 0.000 * | |
| Non-classical monocytes TLR-4+ | 11.09 ± 5.47 | 9.66 (1.29–28.44) | 0.39 ± 0.21 | 0.33 (0.09–1.07) | 0.000 * | |
| Non-classical monocytes TLR-9+ | 5.37 ± 3.98 | 4.24 (0.53–21.53) | 0.55 ± 0.30 | 0.46 (0.12–1.52) | 0.000 * | |
| Serum concentration [ng/mL] | sTLR-2 | 20.38 ± 7.20 | 18.91 (9.39–35.58) | 3.78 ± 0.83 | 4.01 (2.09–4.90) | 0.000 * |
| sTLR-3 | 14.23 ± 4.78 | 14.99 (6.85–22.77) | 1.96 ± 0.59 | 1.84 (1.01–2.98) | 0.000 * | |
| sTLR-4 | 20.46 ± 5.94 | 21.43 (10.09–29.89) | 3.15 ± 0.60 | 3.16 (2.04–3.95) | 0.000 * | |
| sTLR-9 | 17.60 ± 5.67 | 18.46 (7.37–26.30) | 1.88 ± 0.49 | 1.95 (1.02–2.96) | 0.000 * | |
| Parameters | GC ≥ 51 Years (Group 1) | GC ≤ 50 Years (Group 2) | HV ≤ 50 Years (Group 3) | HV ≥ 51 Years (Group 4) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | ||
| Evaluation of the occurrence of the studied TLRs on DCs [%] | BDCA-1+ TLR-2+ | 21.07 (10.74–48.00) | 13.84 (3.19–40.63) | 1.29 (0.33–2.69) | 1.25 (0.28–2.69) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.965 |
| BDCA-1+ TLR-3+ | 5.06 (2.38–14.47) | 3.10 (0.81–14.04) | 2.00 (0.87–3.57) | 1.65 (0.73–6.06) | 0.000 * | 0.000 * | 0.000 * | 0.169 | 0.106 | 0.965 | |
| BDCA-1+ TLR-4+ | 16.53 (8.16–30.77) | 10.34 (3.06–30.68) | 1.60 (0.74–3.26) | 1.66 (0.62–4.72) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.929 | |
| BDCA-1+ TLR-9+ | 8.09 (3.85–28.07) | 4.93 (1.59–24.44) | 1.23 (1.00–3.84) | 2.19 (0.32–6.61) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.824 | |
| BDCA-2+ TLR-2+ | 13.40 (5.77–43.86) | 8.20 (2.21–37.78) | 1.22 (0.41–2.84) | 1.17 (0.34–3.51) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.397 | |
| BDCA-2+ TLR-3+ | 5.63 (2.46–22.22) | 3.16 (0.30–19.61) | 2.11 (0.71–4.92) | 2.03 (0.60–6.08) | 0.000 * | 0.000 * | 0.000 * | 0.366 | 0.070 | 0.397 | |
| BDCA-2+ TLR-4+ | 13.92 (6.88–45.83) | 8.31 (2.80–39.60) | 0.93 (0.31–2.16) | 0.89 (0.26–2.67) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.397 | |
| BDCA-2+ TLR-9+ | 11.29 (5.33–27.27) | 6.98 (1.49–23.39) | 1.60 (0.54–3.74) | 1.54 (0.45–4.62) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.397 | |
| Evaluation of the occurrence of the tested TLRs on monocytes [%] | Classical monocytes TLR-2+ | 7.32 (3.36–20.39) | 4.33 (2.01–21.37) | 0.96 (0.46–2.27) | 1.14 (0.13–2.92) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 |
| Classical monocytes TLR-3+ | 1.12 (0.32–10.22) | 0.50 (0.09–9.68) | 1.25 (0.16–2.33) | 0.76 (0.13–1.96) | 0.000 * | 0.585 | 0.089 | 0.093 | 0.047 * | 0.397 | |
| Classical monocytes TLR-4+ | 6.99 (3.35–14.77) | 4.19 (0.88–14.01) | 1.05 (0.51–2.49) | 1.26 (0.14–3.21) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Classical monocytes TLR-9+ | 2.87 (0.63–21.25) | 1.23 (0.07–19.47) | 0.87 (0.42–2.06) | 1.04 (0.12–2.65) | 0.000 * | 0.002 * | 0.000 * | 0.909 | 0.887 | 0.722 | |
| Intermediate monocytes TLR-2 | 6.38 (0.99–39.13) | 7.53 (2.15–45.59) | 0.78 (0.21–1.45) | 0.85 (0.25–2.57) | 0.266 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Intermediate monocytes TLR-3+ | 3.42 (0.63–13.41) | 3.86 (0.38–15.98) | 1.11 (0.29–2.05) | 1.21 (0.35–3.64) | 0.511 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Intermediate monocytes TLR-4+ | 5.61 (1.50–45.65) | 6.61 (1.56–23.15) | 0.66 (0.17–1.22) | 0.72 (0.21–2.16) | 0.457 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Intermediate monocytes TLR-9+ | 3.42 (0.51–15.84) | 3.54 (0.43–22.76) | 0.93 (0.25–1.72) | 1.02 (0.29–3.06) | 0.764 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Non-classical monocytes TLR-2+ | 11.03 (5.40–27.19) | 7.17 (1.77–25.66) | 0.39 (0.10–0.72) | 0.43 (0.12–1.28) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Non-classical monocytes TLR-3+ | 4.64 (1.30–20.77) | 2.15 (0.24–25.43) | 0.55 (0.15–1.02) | 0.60 (0.17–1.81) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Non-classical monocytes TLR-4+ | 11.84 (7.03–28.44) | 8.16 (1.29–20.33) | 0.33 (0.09–0.60) | 0.36 (0.10–1.07) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Non-classical monocytes TLR-9+ | 5.66 (1.90–17.02) | 3.11 (0.53–21.53) | 0.46 (0.12–0.86) | 0.51 (0.15–1.52) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.722 | |
| Serum concentration [ng/mL] | sTLR-2 | 18.21 (10.05–34.11) | 19.03 (9.39–35.58) | 3.58 (2.09–4.70) | 4.08 (2.15–4.90) | 0.430 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.429 |
| sTLR-3 | 11.59 (7.34–21.83) | 15.69 (6.85–22.77) | 2.08 (1.31–2.98) | 1.83 (1.01–2.90) | 0.330 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.324 | |
| sTLR-4 | 19.45 (11.01–27.93) | 23.28 (10.58–29.89) | 2.93 (2.10–3.60) | 3.24 (2.04–3.95) | 0.226 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.184 | |
| sTLR-9 | 17.70 (8.04–24.58) | 20.02 (7.72–26.30) | 1.97 (1.08–2.61) | 1.92 (1.02–2.44) | 0.221 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.324 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, M.; Bojarski, K.; Mertowska, P.; Mertowski, S.; Tomaka, P.; Dziki, Ł.; Grywalska, E. Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors? Cells 2024, 13, 1708. https://doi.org/10.3390/cells13201708
Kos M, Bojarski K, Mertowska P, Mertowski S, Tomaka P, Dziki Ł, Grywalska E. Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors? Cells. 2024; 13(20):1708. https://doi.org/10.3390/cells13201708
Chicago/Turabian StyleKos, Marek, Krzysztof Bojarski, Paulina Mertowska, Sebastian Mertowski, Piotr Tomaka, Łukasz Dziki, and Ewelina Grywalska. 2024. "Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors?" Cells 13, no. 20: 1708. https://doi.org/10.3390/cells13201708
APA StyleKos, M., Bojarski, K., Mertowska, P., Mertowski, S., Tomaka, P., Dziki, Ł., & Grywalska, E. (2024). Immunological Strategies in Gastric Cancer: How Toll-like Receptors 2, -3, -4, and -9 on Monocytes and Dendritic Cells Depend on Patient Factors? Cells, 13(20), 1708. https://doi.org/10.3390/cells13201708







