Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells
Abstract
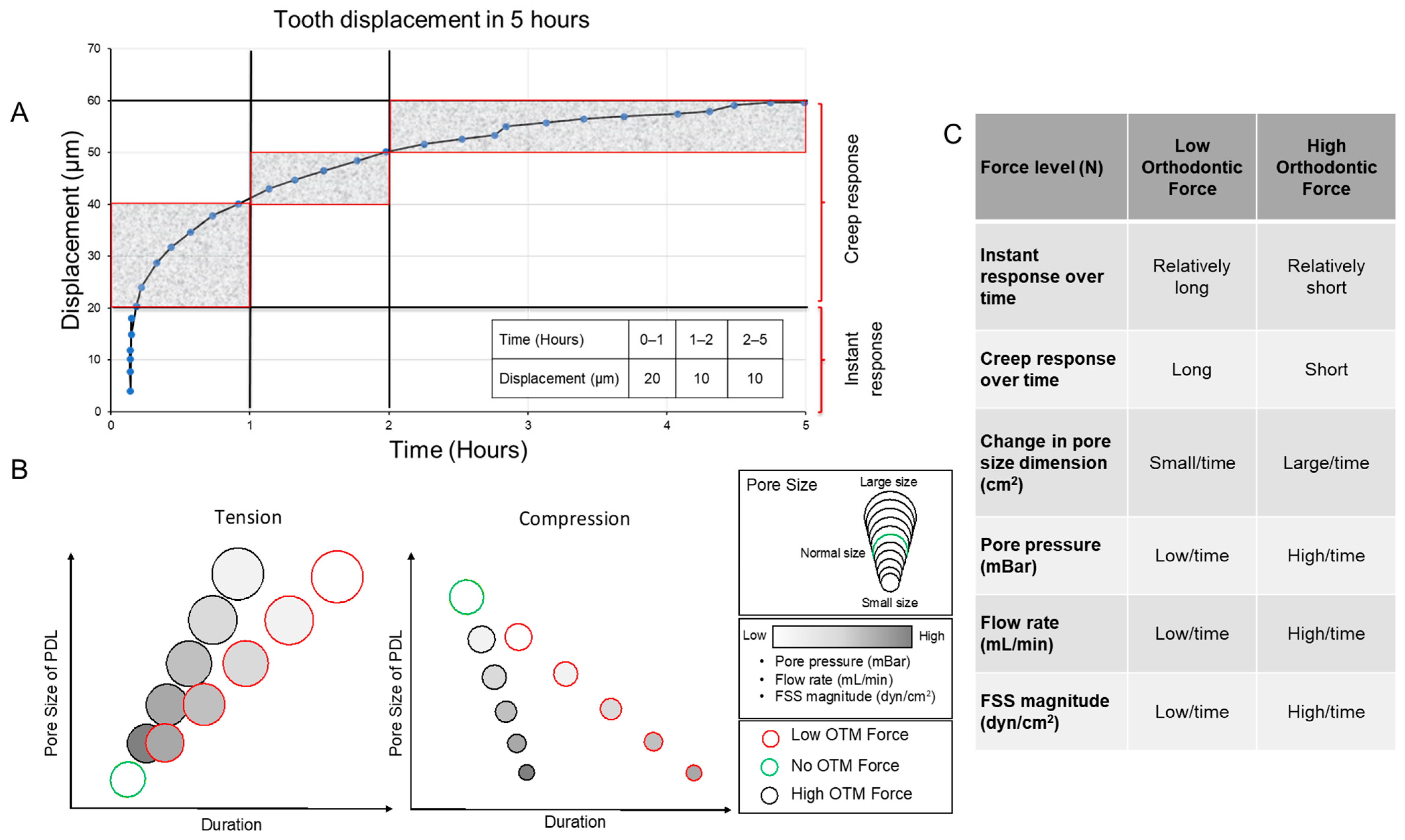
:1. Introduction
2. Materials and Methods
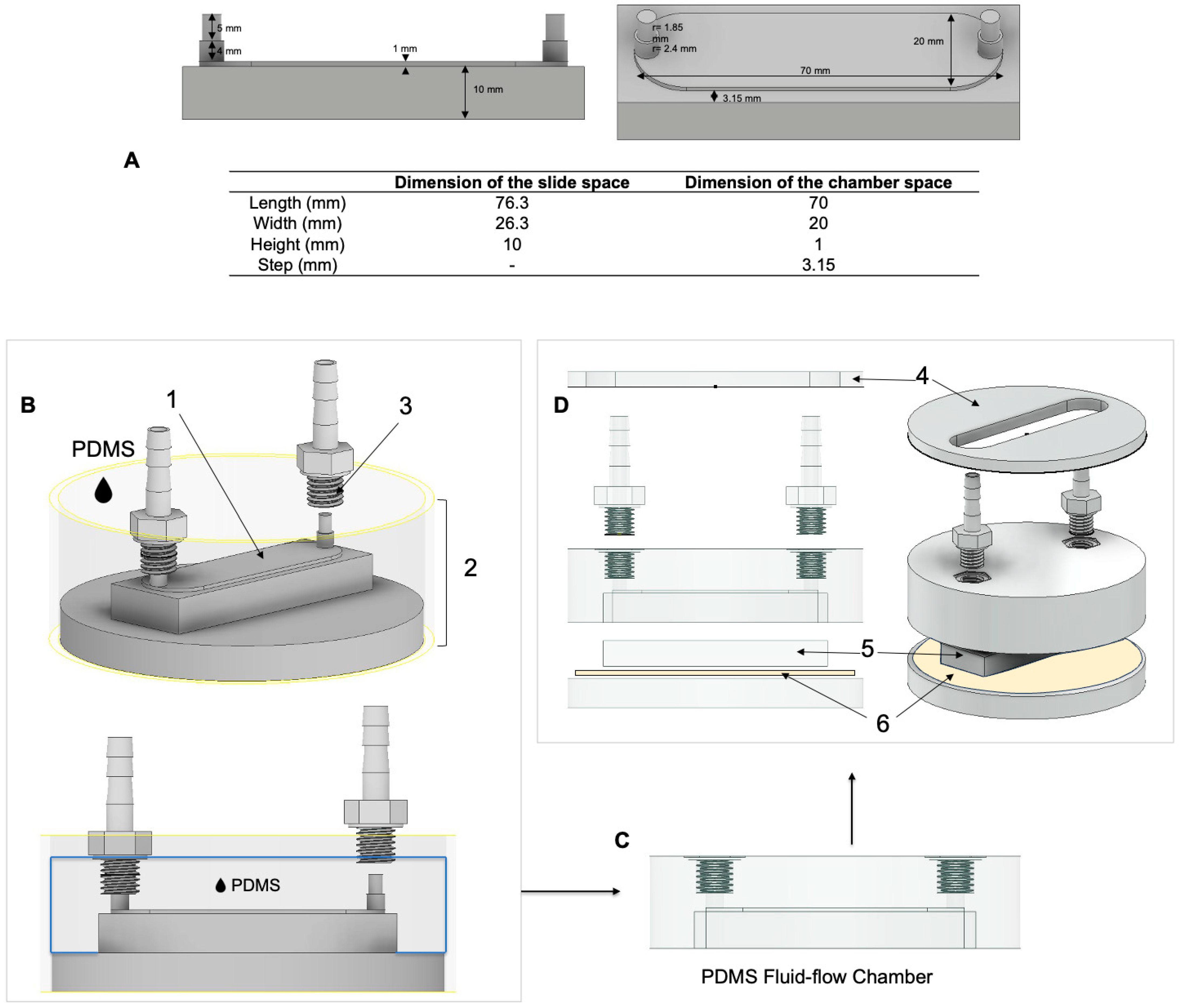
2.1. Design of the Parallel Flow Chamber
2.1.1. Design Considerations
- Biocompatible material not affecting cellular functions, i.e., growth and viability.
- Chamber material compatible with autoclave sterilization.
- The chamber geometry allows for the loading and unloading of a standard microscopic slide (size: 76 × 26 × 1 mm3).
- Easy assembly of the chamber not requiring special tools.
- To ensure precise and robust performance, the design should ease seeding cells in a pre-defined area of uniform fluid flow.
2.1.2. Chamber Design, Computational Fluid Dynamic Simulations, and Construction
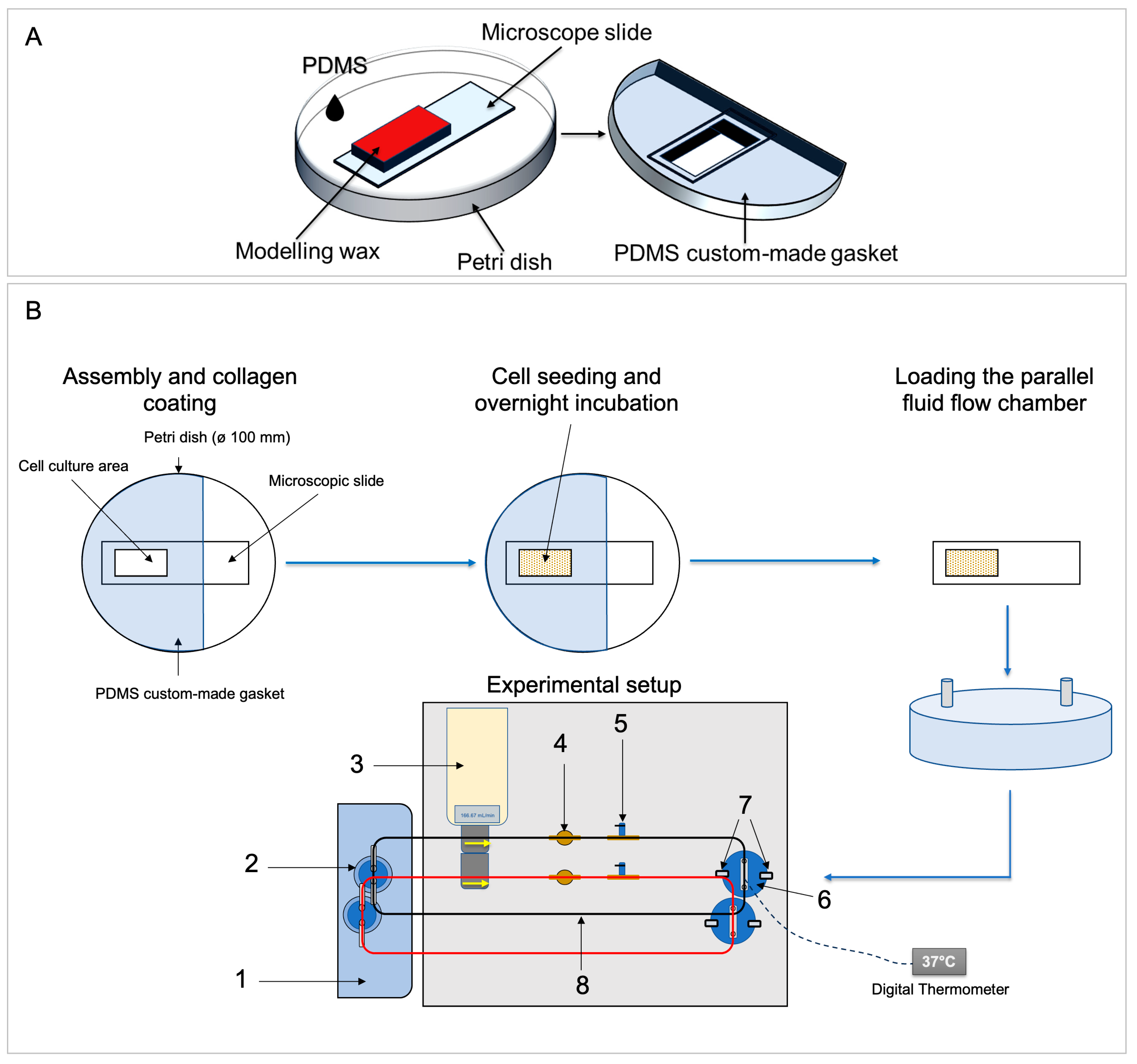
2.1.3. Custom-Made Gasket for Glass Slide Coating and Cell Seeding
2.1.4. Assembly of the FSS System and Temperature Adjustment
2.2. Cell Culture
2.3. Coating and Cell Seeding
2.4. Preparation of a FSS Experiment
2.5. Fluid Flow Shear Stress Application Using a Custom-Made Fluid Flow Apparatus
2.6. Cell Attachment and Cell Viability
2.7. Sample Preparation
2.8. Quantitative Reverse-Transcriptase Polymerase Chain Reaction (RT-qPCR)
2.8.1. Primer Selection
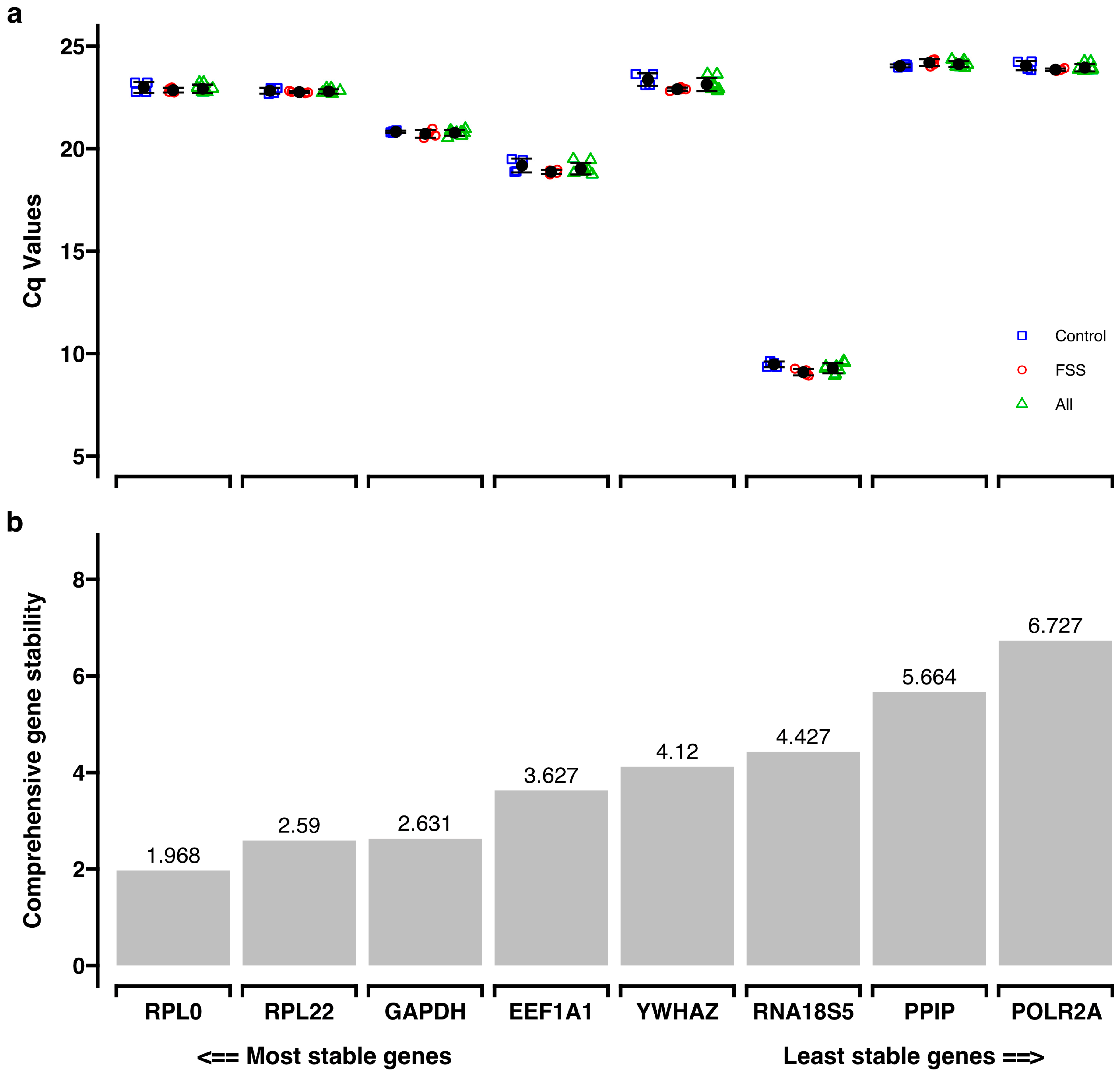
2.8.2. Reference Genes
2.8.3. RT-qPCR Procedure
2.9. Western Blotting
2.10. Statistics
3. Results
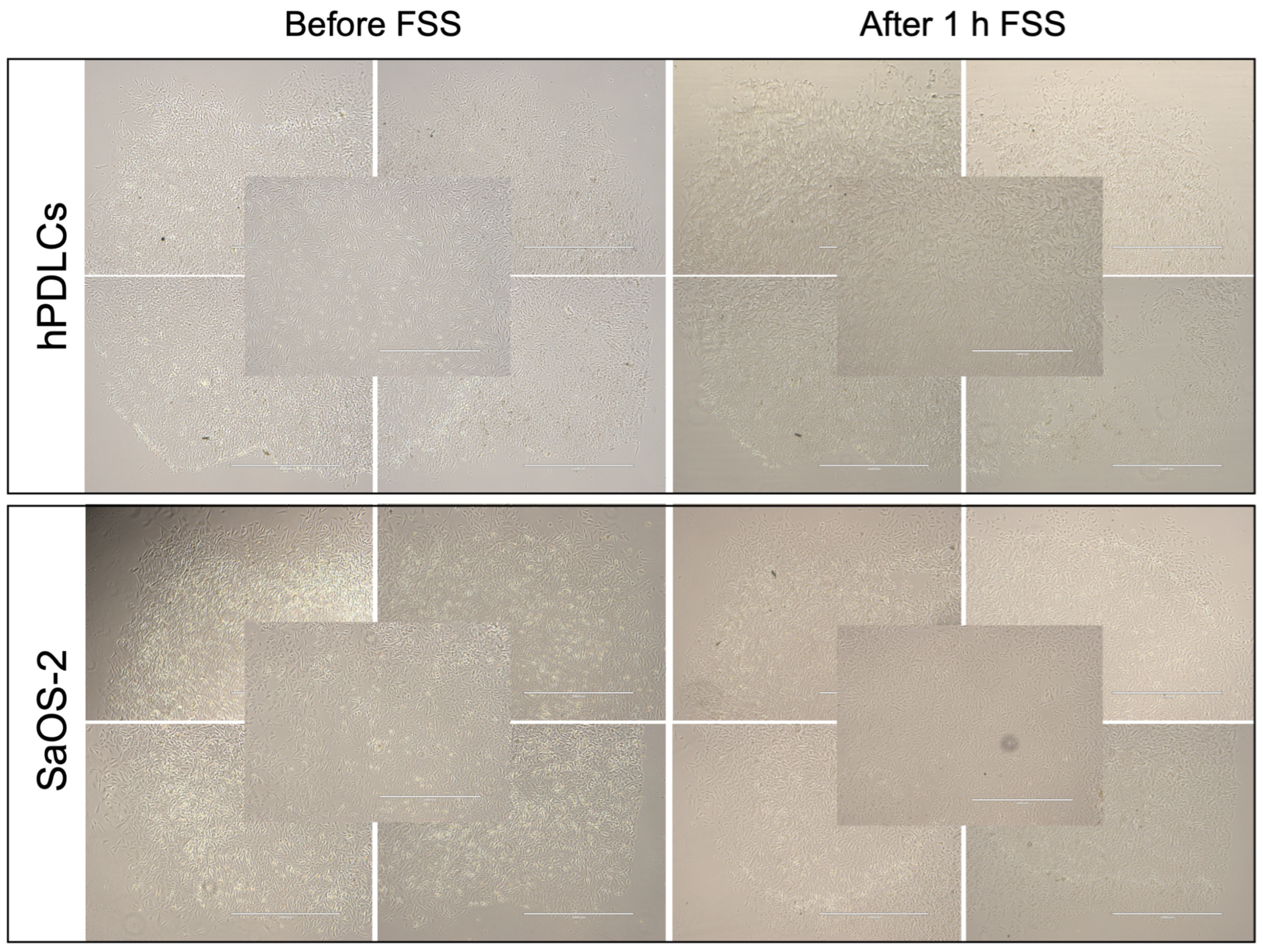
3.1. Cell Attachment
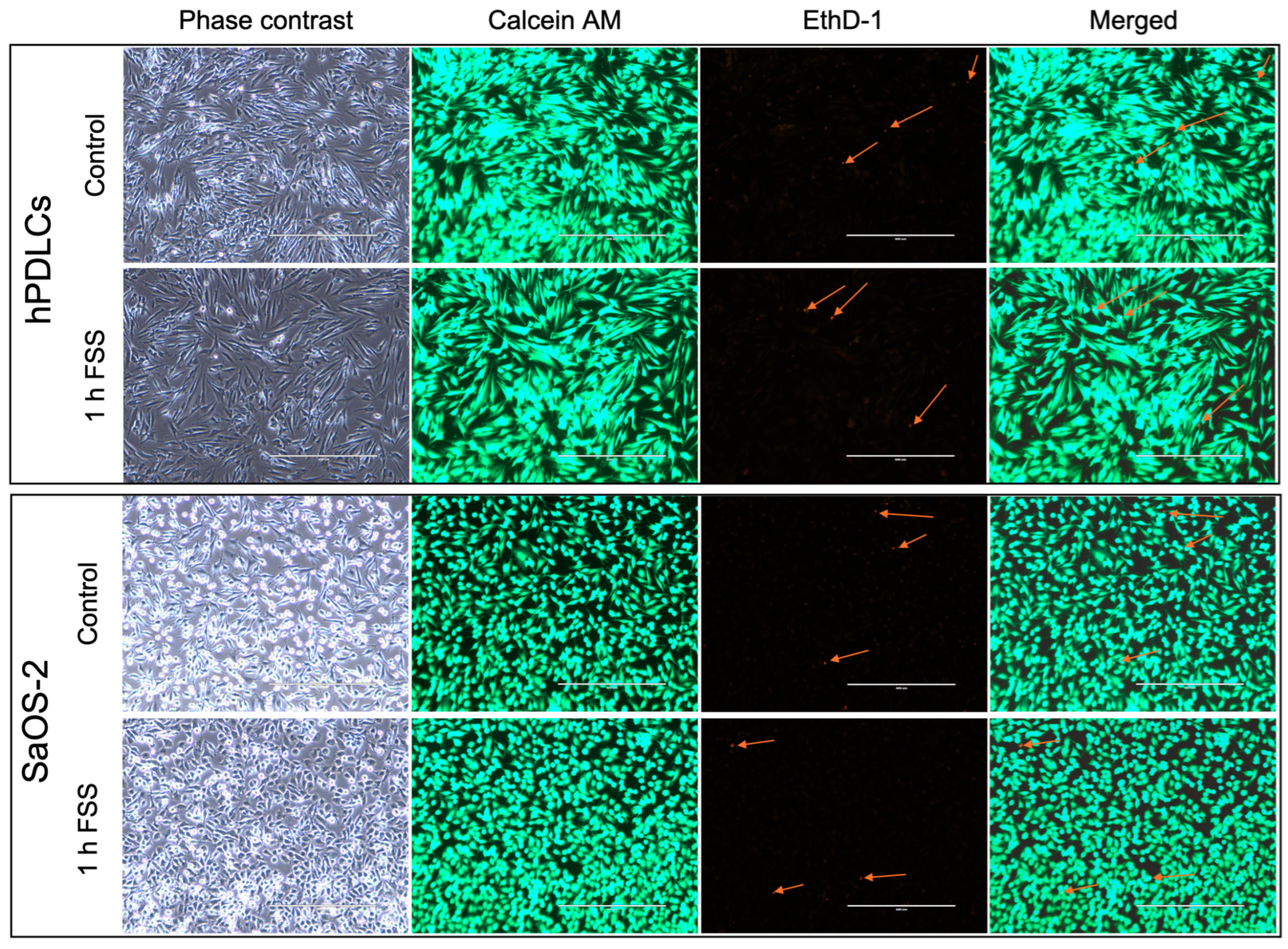
3.2. Cell Viability
3.3. Reference Gene Selection
3.4. Target Gene Expression
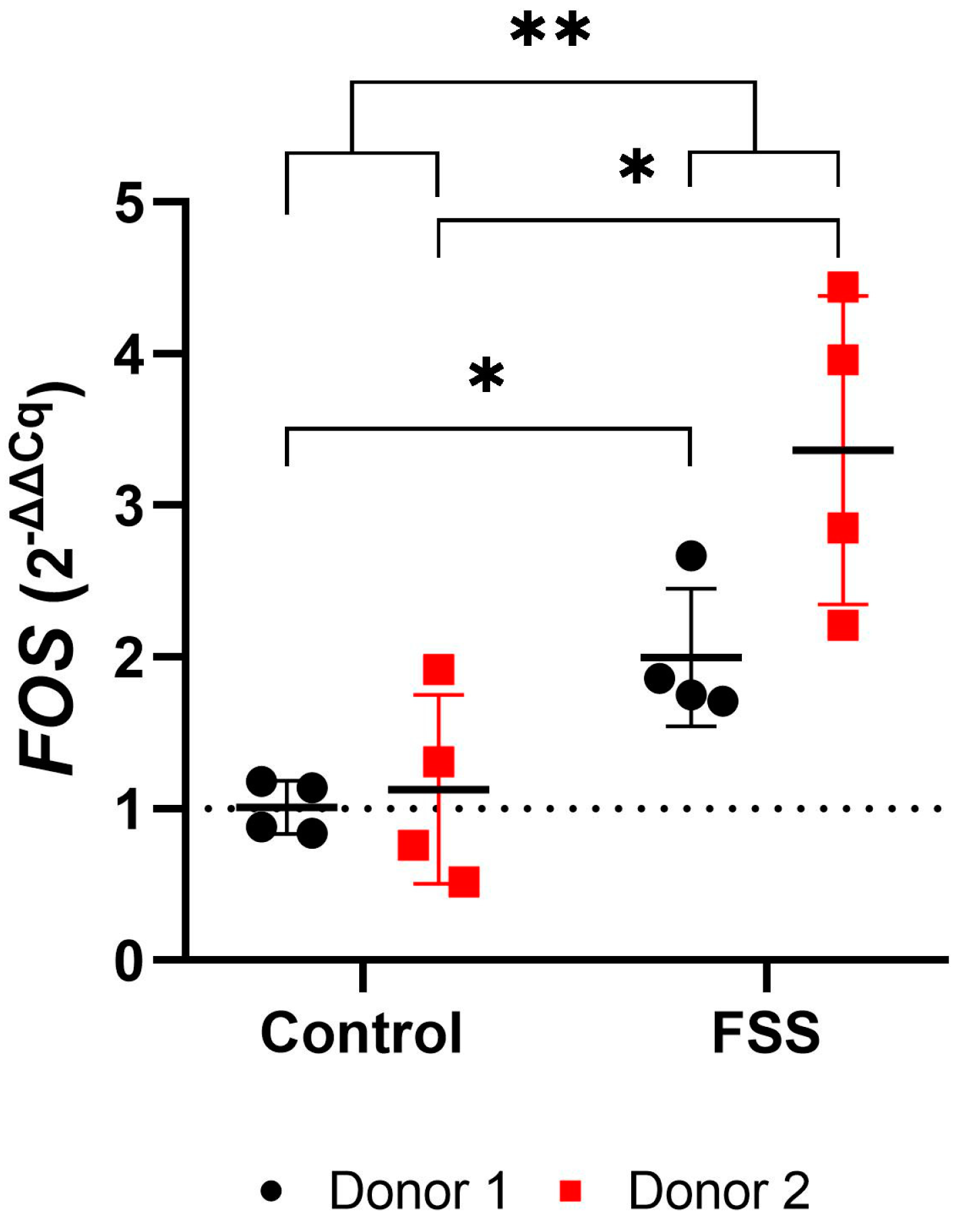
3.4.1. FSS Upregulate the Mechanosensitive FOS Gene
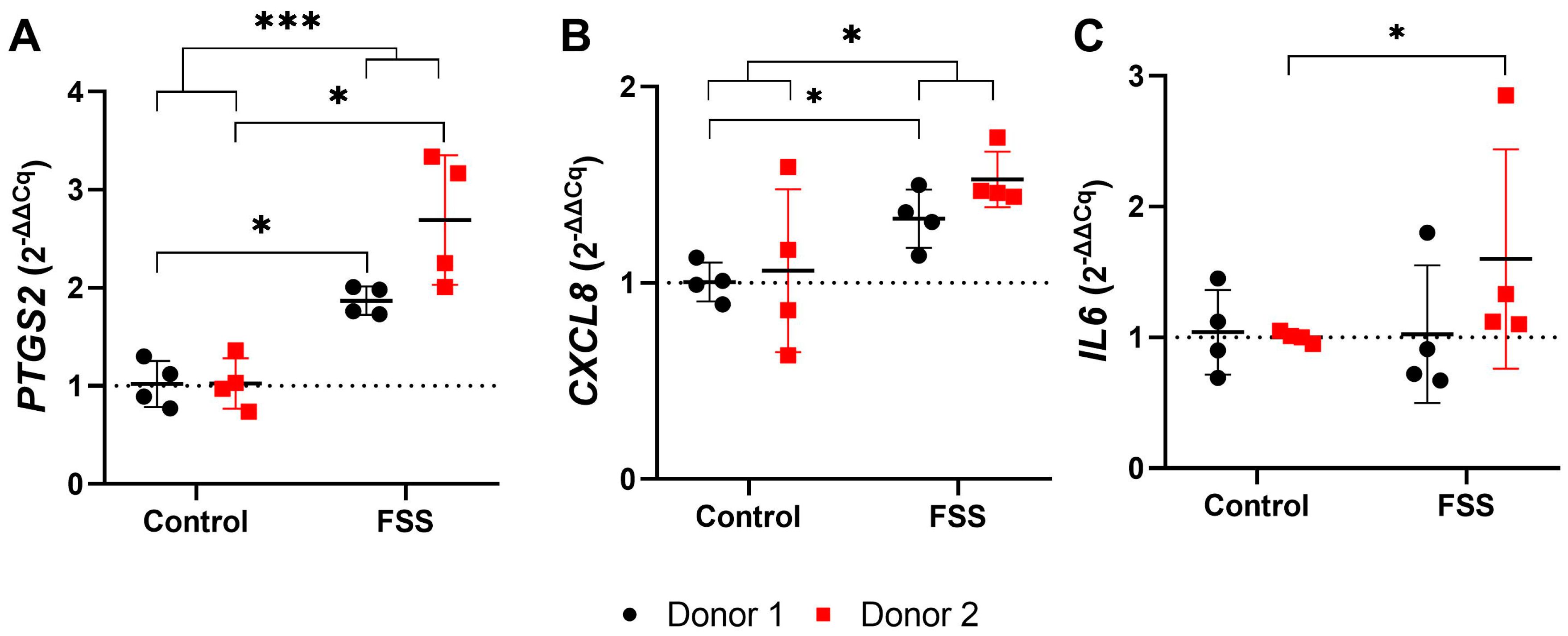
3.4.2. FSS Upregulate Genes Responsible for Inflammation
3.4.3. FSS Upregulate Genes Responsible for Tissue Formation
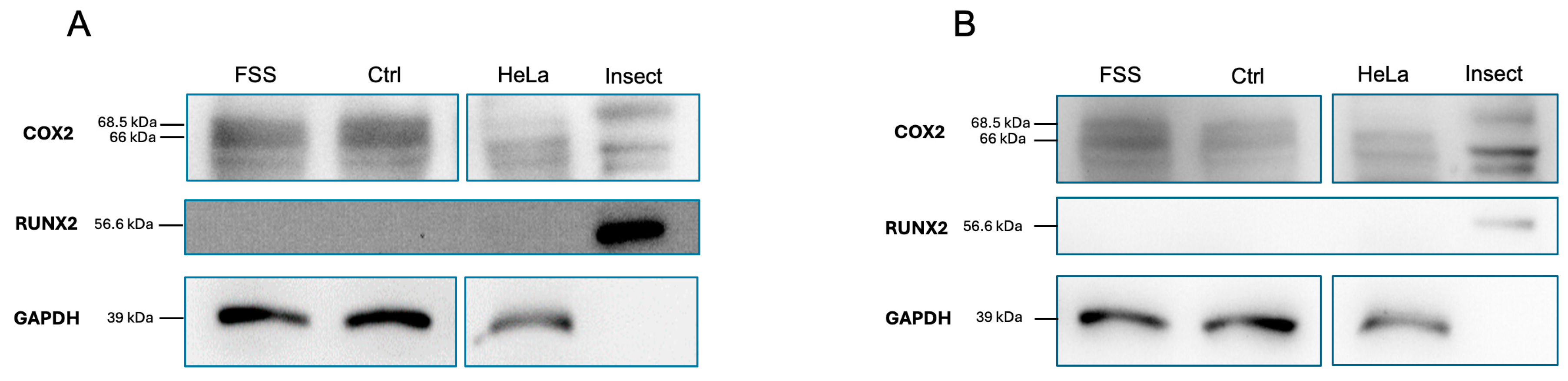
3.4.4. Western Blot Analysis
4. Discussion
4.1. Selection of FSS Parameters and Justification of the Experimental Setting
4.2. Chamber Construction
4.3. Temperature
4.4. Cell Viability and Attachment
4.5. Expression of Target Genes
4.5.1. Effect of FSS on Mechanosensing
4.5.2. Effect of FSS on Osteogenic Differentiation
4.5.3. Effect of FSS on Inflammation
4.5.4. Heterogeneity Between Donors and Fluid Shear Stress
4.6. Strengths and Limitations
4.6.1. Strengths
- Biocompatibility.
- Durability.
- Flexibility (can be adapted to different research questions).
- Decomposability (can be disassembled for sample collection).
- Large sample for further gene/intracellular protein analysis.
- Affordability (cost-effective).
4.6.2. Limitations
- Using a large volume of cell-culturing media (diluted supernatant).
- Technique sensitive during assembly and disassembly
- Reusability requires further steps afterward for disinfection and sterilization.
- The preparation of the experimental setup is completed outside the incubator.
- To account for biological variability, additional donors should be included.
- The flow chamber is not compatible with live microscopy, making it difficult to investigate potential regions of turbulence using a tracer dye or real-time cellular/molecular visualization.
- Only a few genes were included in this study, which may not fully reflect the complete biological picture related to FSS.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| 3D | Three dimensional |
| CAD | Computer-aided design |
| CFD | Computational fluid dynamics |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 (also known as interleukin 8, IL-8) |
| FC | Fold change |
| FOS | Proto-oncogene Fos; subunit of the AP-1 transcription factor |
| FSS | Fluid shear stress |
| hPDLCs | Human periodontal ligament cells |
| IL6 | Interleukin-6 |
| MIQE | “Minimum Information for Publication of Quantitative Real-Time PCR Experiments” |
| OTM | Orthodontic tooth movement |
| PDL | Periodontal ligament |
| PTGS2 | Prostaglandin-endoperoxide synthase (also known as cyclooxygenase-2, COX-2) |
| RUNX2 | Runt-related transcription factor 2 |
| SP7 | Sp7 transcription factor (also known as osterix, OSX) |
| TNFRSF11B | TNF receptor superfamily member 11b (also known as osteoprotegerin, OPG) |
| VEGFA | Vascular endothelial growth factor A |
References
- Feller, L.; Khammissa, R.A.; Schechter, I.; Moodley, A.; Thomadakis, G.; Lemmer, J. Periodontal Biological Events Associated with Orthodontic Tooth Movement: The Biomechanics of the Cytoskeleton and the Extracellular Matrix. Sci. World J. 2015, 2015, 894123. [Google Scholar] [CrossRef] [PubMed]
- Nile, M.; Folwaczny, M.; Wichelhaus, A.; Baumert, U.; Janjic Rankovic, M. Fluid flow shear stress and tissue remodeling-an orthodontic perspective: Evidence synthesis and differential gene expression network analysis. Front. Bioeng. Biotechnol. 2023, 11, 1256825. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Ashrafi, M.; Ghalichi, F.; Mirzakouchaki, B.; Zoljanahi Oskui, I. Numerical simulation of hydro-mechanical coupling of periodontal ligament. Proc. Inst. Mech. Eng. H 2020, 234, 171–178. [Google Scholar] [CrossRef]
- Janjic, M.; Docheva, D.; Trickovic Janjic, O.; Wichelhaus, A.; Baumert, U. In vitro weight-loaded cell models for understanding mechanodependent molecular pathways involved in orthodontic tooth movement: A systematic review. Stem Cells Int. 2018, 2018, 3208285. [Google Scholar] [CrossRef]
- Sun, C.; Janjic Rankovic, M.; Folwaczny, M.; Otto, S.; Wichelhaus, A.; Baumert, U. Effect of Tension on Human Periodontal Ligament Cells: Systematic Review and Network Analysis. Front. Bioeng. Biotechnol. 2021, 9, 695053. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zheng, L.; Na, J.; Li, X.; Yang, Z.; Chen, X.; Song, Y.; Li, C.; Zhou, L.; Fan, Y. Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP. Cell. Mol. Life Sci. 2022, 79, 551. [Google Scholar] [CrossRef]
- Jacobs, C.R.; Yellowley, C.E.; Davis, B.R.; Zhou, Z.; Cimbala, J.M.; Donahue, H.J. Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 1998, 31, 969–976. [Google Scholar] [CrossRef]
- Lu, X.L.; Huo, B.; Park, M.; Guo, X.E. Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone 2012, 51, 466–473. [Google Scholar] [CrossRef]
- Piekarski, K.; Munro, M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature 1977, 269, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Haut, T.R.; Yellowley, C.E.; Donahue, H.J.; Jacobs, C.R. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology 2003, 40, 591–603. [Google Scholar] [PubMed]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; You, L.; Wang, L. Quantifying fluid shear stress in a rocking culture dish. J. Biomech. 2010, 43, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, E.J.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur. J. Oral Sci. 1999, 107, 468–474. [Google Scholar] [CrossRef]
- Klein, Y.; Fleissig, O.; Polak, D.; Barenholz, Y.; Mandelboim, O.; Chaushu, S. Immunorthodontics: In vivo gene expression of orthodontic tooth movement. Sci. Rep. 2020, 10, 8172. [Google Scholar] [CrossRef]
- Bien, S.M. Fluid dynamic mechanisms which regulate tooth movement. Adv. Oral Biol. 1966, 2, 173–201. [Google Scholar]
- van Driel, W.D.; van Leeuwen, E.J.; Von den Hoff, J.W.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Time-dependent mechanical behaviour of the periodontal ligament. Proc. Inst. Mech. Eng. H 2000, 214, 497–504. [Google Scholar] [CrossRef]
- Elveflow Calculator. Available online: https://www.elveflow.com/microfluidic-calculator (accessed on 12 April 2024).
- Janjic Rankovic, M.; Docheva, D.; Wichelhaus, A.; Baumert, U. Effect of static compressive force on in vitro cultured PDL fibroblasts: Monitoring of viability and gene expression over 6 days. Clin. Oral Investig. 2020, 24, 2497–2511. [Google Scholar] [CrossRef]
- Sun, C.; Janjic Rankovic, M.; Folwaczny, M.; Stocker, T.; Otto, S.; Wichelhaus, A.; Baumert, U. Effect of Different Parameters of In Vitro Static Tensile Strain on Human Periodontal Ligament Cells Simulating the Tension Side of Orthodontic Tooth Movement. Int. J. Mol. Sci. 2022, 23, 1525. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 2010, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- RefFinder. Available online: https://www.ciidirsinaloa.com.mx/RefFinder-master/ (accessed on 11 July 2024).
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Chen, C.T.; Malkus, D.S.; Vanderby, R., Jr. A fiber matrix model for interstitial fluid flow and permeability in ligaments and tendons. Biorheology 1998, 35, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, C.I.; Kuijpers-Jagtman, A.M.; Bronkhorst, E.M.; Wagener, F. Optimal force magnitude for bodily orthodontic tooth movement with fixed appliances: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 582–592. [Google Scholar] [CrossRef]
- Kurol, J.; Franke, P.; Lundgren, D.; Owman-Moll, P. Force magnitude applied by orthodontists. An inter- and intra-individual study. Eur. J. Orthod. 1996, 18, 69–75. [Google Scholar] [CrossRef]
- Wills, D.J.; Picton, D.C.; Davies, W.I. An investigation of the viscoelastic properties of the periodontium in monkeys. J. Periodontal Res. 1972, 7, 42–51. [Google Scholar] [CrossRef]
- Romanyk, D.L.; Melenka, G.W.; Carey, J.P. Modeling stress-relaxation behavior of the periodontal ligament during the initial phase of orthodontic treatment. J. Biomech. Eng. 2013, 135, 91007. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Vuuren, L.; Jansen van Vuuren, W.A.; Broadbent, J.M.; Duncan, W.J.; Waddell, J.N. Measurement of tooth displacement. J. Mech. Behav. Biomed. Mater. 2023, 146, 106059. [Google Scholar] [CrossRef] [PubMed]
- Sonam, S.; Sathe, S.R.; Yim, E.K.; Sheetz, M.P.; Lim, C.T. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 2016, 6, 20415. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Sun, Q.; Huo, B. Gradient fluid shear stress regulates migration of osteoclast precursors. Cell Adh. Migr. 2019, 13, 183–191. [Google Scholar] [CrossRef]
- Anderson, E.J.; Falls, T.D.; Sorkin, A.M.; Knothe Tate, M.L. The imperative for controlled mechanical stresses in unraveling cellular mechanisms of mechanotransduction. Biomed. Eng. Online 2006, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- van Midwoud, P.M.; Janse, A.; Merema, M.T.; Groothuis, G.M.; Verpoorte, E. Comparison of biocompatibility and adsorption properties of different plastics for advanced microfluidic cell and tissue culture models. Anal. Chem. 2012, 84, 3938–3944. [Google Scholar] [CrossRef]
- Deng, Y.; Li, G.; Song, W.; Jiang, J. Preparation and properties of pearl powder/polypropylene composites and their biocompatibility. Biomed. Mater. Eng. 2015, 26 (Suppl. S1), S27–S34. [Google Scholar] [CrossRef]
- Huesa, C.; Helfrich, M.H.; Aspden, R.M. Parallel-plate fluid flow systems for bone cell stimulation. J. Biomech. 2010, 43, 1182–1189. [Google Scholar] [CrossRef]
- Yoshino, D.; Sakamoto, N.; Takahashi, K.; Inoue, E.; Sato, M. Development of Novel Flow Chamber to Study Endothelial Cell Morphology: Effects of Shear Flow with Uniform Spatial Gradient on Distribution of Focal Adhesion. J. Biomech. Sci. Eng. 2013, 8, 233–243. [Google Scholar] [CrossRef]
- Kriesi, C.; Steinert, M.; Marmaras, A.; Danzer, C.; Meskenaite, V.; Kurtcuoglu, V. Integrated Flow Chamber System for Live Cell Microscopy. Front. Bioeng. Biotechnol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Karimbux, N.Y.; Rosenblum, N.D.; Nishimura, I. Site-specific expression of collagen I and XII mRNAs in the rat periodontal ligament at two developmental stages. J. Dent. Res. 1992, 71, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.T.; Scanlon, C.S.; Soehren, S.; Matsuo, M.; Kapila, Y.L. Implications of cultured periodontal ligament cells for the clinical and experimental setting: A review. Arch. Oral Biol. 2011, 56, 933–943. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Govey, P.M.; Loiselle, A.E.; Donahue, H.J. Biophysical regulation of stem cell differentiation. Curr. Osteoporos. Rep. 2013, 11, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kefallinou, D.; Grigoriou, M.; Constantoudis, V.; Raptis, I.; Xenogiannopoulou, E.; Dimoulas, A.; Boumpas, D.T.; Tserepi, A. Enhanced and stabilized mesenchymal stem cell growth inside plasma pre-treated and collagen-coated PDMS-based microfluidic chambers. Surf. Coat. Technol. 2023, 466, 129658. [Google Scholar] [CrossRef]
- Nagai, M.; Hayakawa, T.; Fukatsu, A.; Yamamoto, M.; Fukumoto, M.; Nagahama, F.; Mishima, H.; Yoshinari, M.; Nemoto, K.; Kato, T. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent. Mater. J. 2002, 21, 250–260. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Cohn, S.A. Disuse atrophy of the periodontium in mice. Arch. Oral Biol. 1965, 10, 909–919. [Google Scholar] [CrossRef]
- Goto, K.T.; Kajiya, H.; Nemoto, T.; Tsutsumi, T.; Tsuzuki, T.; Sato, H.; Okabe, K. Hyperocclusion stimulates osteoclastogenesis via CCL2 expression. J. Dent. Res. 2011, 90, 793–798. [Google Scholar] [CrossRef]
- Tang, M.; Peng, Z.; Mai, Z.; Chen, L.; Mao, Q.; Chen, Z.; Chen, Q.; Liu, L.; Wang, Y.; Ai, H. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J. Periodontol. 2014, 85, 1806–1813. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, S.K.; Lee, J.W.; Kim, E.C. The role of heme oxygenase-1 in mechanical stress- and lipopolysaccharide-induced osteogenic differentiation in human periodontal ligament cells. Angle Orthod. 2010, 80, 552–559. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R.; Banerjee, C.; Kundu, R.; Harrison, R.J.; Dobner, P.R.; Stein, J.L.; Lian, J.B.; Stein, G.S. Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: Role of Fra-2 and Jun D during differentiation. Endocrinology 1996, 137, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.B.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J. Osteocalcin gene promoter: Unlocking the secrets for regulation of osteoblast growth and differentiation. J. Cell. Biochem. Suppl. 1998, 30–31, 62–72. [Google Scholar] [CrossRef]
- D’Alonzo, R.C.; Selvamurugan, N.; Karsenty, G.; Partridge, N.C. Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation. J. Biol. Chem. 2002, 277, 816–822. [Google Scholar] [CrossRef]
- Ogasawara, A.; Arakawa, T.; Kaneda, T.; Takuma, T.; Sato, T.; Kaneko, H.; Kumegawa, M.; Hakeda, Y. Fluid shear stress-induced cyclooxygenase-2 expression is mediated by C/EBP beta, cAMP-response element-binding protein, and AP-1 in osteoblastic MC3T3-E1 cells. J. Biol. Chem. 2001, 276, 7048–7054. [Google Scholar] [CrossRef]
- Inoue, D.; Kido, S.; Matsumoto, T. Transcriptional induction of FosB/ΔFosB gene by mechanical stress in osteoblasts. J. Biol. Chem. 2004, 279, 49795–49803. [Google Scholar] [CrossRef]
- Raab-Cullen, D.M.; Thiede, M.A.; Petersen, D.N.; Kimmel, D.B.; Recker, R.R. Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif. Tissue Int. 1994, 55, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shao, L.; Li, B.; Zong, C.; Li, J.; Zheng, Q.; Tong, X.; Gao, C.; Wang, J. Extracellular signal-regulated kinase1/2 activated by fluid shear stress promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells through novel signaling pathways. Int. J. Biochem. Cell Biol. 2011, 43, 1591–1601. [Google Scholar] [CrossRef]
- Simmons, C.A.; Matlis, S.; Thornton, A.J.; Chen, S.; Wang, C.Y.; Mooney, D.J. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J. Biomech. 2003, 36, 1087–1096. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Thi, M.M.; Iacobas, D.A.; Iacobas, S.; Spray, D.C. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann. N. Y. Acad. Sci. 2007, 1117, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- van der Meijden, K.; Bakker, A.D.; van Essen, H.W.; Heijboer, A.C.; Schulten, E.A.; Lips, P.; Bravenboer, N. Mechanical loading and the synthesis of 1,25(OH)2D in primary human osteoblasts. J. Steroid Biochem. Mol. Biol. 2016, 156, 32–39. [Google Scholar] [CrossRef]
- Aisha, M.D.; Nor-Ashikin, M.N.; Sharaniza, A.B.; Nawawi, H.; Froemming, G.R. Orbital fluid shear stress promotes osteoblast metabolism, proliferation and alkaline phosphates activity in vitro. Exp. Cell Res. 2015, 337, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tan, S.; Shen, Y.; Chen, R.; Wu, C.; Xu, Y.; Song, Z.; Fu, Q. Inhibition of FSS-induced actin cytoskeleton reorganization by silencing LIMK2 gene increases the mechanosensitivity of primary osteoblasts. Bone 2015, 74, 182–190. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Burger, E.H.; Semeins, C.M.; Raisz, L.G.; Pilbeam, C.C. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J. Bone Miner. Res. 1997, 12, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.D.; Rodan, G.A. Indomethacin inhibition of tenotomy-induced bone resorption in rats. J. Bone Miner. Res. 1988, 3, 409–414. [Google Scholar] [CrossRef]
- Sandy, J.R.; Harris, M. Prostaglandins and tooth movement. Eur. J. Orthod. 1984, 6, 175–182. [Google Scholar] [CrossRef]
- Pavalko, F.M.; Chen, N.X.; Turner, C.H.; Burr, D.B.; Atkinson, S.; Hsieh, Y.F.; Qiu, J.; Duncan, R.L. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. 1998, 275, C1591–C1601. [Google Scholar] [CrossRef]
- Bakker, A.D.; Soejima, K.; Klein-Nulend, J.; Burger, E.H. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J. Biomech. 2001, 34, 671–677. [Google Scholar] [CrossRef]
- Hsu, A.; Zhang, W.; Lee, J.F.; An, J.; Ekambaram, P.; Liu, J.; Honn, K.V.; Klinge, C.M.; Lee, M.J. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int. J. Oncol. 2012, 40, 1619–1626. [Google Scholar] [PubMed]
- Liu, Y.T.; Kardosh, A.; Cooc, J.; Schonthal, A.H. Potential misidentification of cyclooxygenase-2 by Western blot analysis and prevention through the inclusion of appropriate controls. Mol. Biotechnol. 2006, 34, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Soejima, K.; Bandow, K.; Kuroe, K.; Kakimoto, K.; Miyawaki, S.; Okamoto, A.; Matsuguchi, T. Force-induced IL-8 from periodontal ligament cells requires IL-1beta. J. Dent. Res. 2007, 86, 629–634. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, L.; Chen, Y.; Gui, J.; Li, Q.; Huang, Y.; Liu, M.; Jia, X.; Song, W.; Ji, J.; et al. The effects of fluid shear stress on proliferation and osteogenesis of human periodontal ligament cells. J. Biomech. 2016, 49, 572–579. [Google Scholar] [CrossRef]
- Bakker, A.D.; Kulkarni, R.N.; Klein-Nulend, J.; Lems, W.F. IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J. Dent. Res. 2014, 93, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Morita, I.; Maruo, N.; Kubota, T.; Murota, S.; Aso, T. Expression of IL-6 receptor and GP130 in mouse bone marrow cells during osteoclast differentiation. Bone 1998, 22, 487–493. [Google Scholar] [CrossRef]
- Stoddart, M.J.; Richards, R.G.; Alini, M. In vitro experiments with primary mammalian cells: To pool or not to pool? Eur Cell Mater 2012, 24, i. [Google Scholar] [CrossRef]
- Van der Velden, U. Effect of age on the periodontium. J. Clin. Periodontol. 1984, 11, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J. Periodontal Res. 1998, 33, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Watarai, H.; Warita, H.; Soma, K. Effect of nitric oxide on the recovery of the hypofunctional periodontal ligament. J. Dent. Res. 2004, 83, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Ramfjord, S.P. Periodontal effect of nonfunction in monkeys. J. Periodontol. 1971, 42, 748–756. [Google Scholar] [CrossRef] [PubMed]



| Part No. | Part | Type | Source |
|---|---|---|---|
| 1 | Heating water bath | Lauda Aqualine AL5 | Lauda, Lauda-Königshofen, Germany |
| 2 | Reservoir and multiple distributors for bottles GL 45 with connectors | PY86.1 | Carl Roth, Karlsruhe, Germany |
| 3 | Peristaltic pump w/2 pump head (3 rolls) | LabV3 with YZ151x PPS pump heads | Drifton A/S, Hvidovre, Denmark |
| 4 | Constant flow pulse damper | D1606-6B-PSU | SCPOGO LABS, Beijing, China |
| 5a | Bubble trap consisting of barbed T-connector | FESTO T-PK-4, 9585 | Landefeld Druckluft und Hydraulik GmbH, Kassel, Germany |
| 5b | Stainless-steel lever air control valve | L × W × H: 30 × 21 × 8 mm3 | Sourcing map; url: https://sourcingmap.com (accessed on 21 October 2024) |
| 6 | Chamber | See above | |
| 7 | Screw clamps | Wisent Laubsägezwinge | Hornbach, Munich, Germany |
| 8 | Sterile silicone tubing | Longer BioSilicone (WT 1.6 mm, ID 4.8 mm, OD 8.0 mm | Drifton A/S, Hvidovre, Denmark |
| Microscopy slide (with cells seeded in a specified area) | Epredia™ Microscope Slides, Cut, 1mm (AA00000102E01MNZ10) | New Erie Scientific LLC, Portsmouth, NH, USA |
| Gene | GenBank Accession Number | Primer Sequence (f:5-Forward Primer-3; r:5-Reverse Primer-3) | Anneal. Temp. (°C) | Amplicon Length (bp) | Primer Efficiency |
|---|---|---|---|---|---|
| RUNX2 | NM_001015051.4 | f: GCGCATTCCTCATCCCAGTA r: GGCTCAGGTAGGAGGGGTAA | 58 | 176 | 2.033 |
| IL6 | NM_000600.5 | f: TGGCAGAAAACAACCTGAACC r: TGGCTTGTTCCTCACTACTCTC | 58 | 168 | 1.931 |
| PTGS2/COX2 | NM_000963.4 | f: AAGCCTTCTCTAACCTCTCC r: GCCCTCGCTTATGATCTGTC | 58 | 234 | 1.988 |
| FOS | NM_005252.4 | f: GCTTTGCAGACCGAGATTGC r: TTGAGGAGAGGCAGGGTGAA | 58 | 203 | 1.942 |
| SP7 | NM_001173467.3 | f: GGCACAAAGAAGCCGTACTC r: CACTGGGCAGACAGTCAGAA | 61 | 247 | 2.077 |
| TNFRSF11B | NM_002546.4 | f: TCAAGCAGGAGTGCAATCG r: AGAATGCCTCCTCACACAGG | 60 | 342 | 1.972 |
| VEGFA | NM_001317010.2 | f: GCTGTCTTGGGTGCATTGGA r: ATGATTCTGCCCTCCTCCTTCT | 58 | 100 | 2.071 |
| CXCL8/IL8 | NM_001354840.3 | f: CAGAGACAGCAGAGCACACAA r: TTAGCACTCCTTGGCAAAAC | 55 | 170 | 1.948 |
| RPL0 | NM_001002.4 | f: GAAACTCTGCATTCTCGCTTCC r: GACTCGTTTGTACCCGTTGATG | 64 | 120 | 1.988 |
| RPL22 | NM_000983.4 | f: TGATTGCACCCACCCTGTAG r: GGTTCCCAGCTTTTCCGTTC | 61 | 98 | 2.055 |
| Gene | Control | FSS | U-Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Median | Mean | SD | Min | Max | Median | p | Sig.† | ||
| CXCL8 | Donor 1 (N = 4) | 1.00 | 0.10 | 0.89 | 1.13 | 1.00 | 1.33 | 0.15 | 1.14 | 1.50 | 1.33 | 0.029 | * |
| Donor 2 (N = 4) | 1.06 | 0.42 | 0.63 | 1.59 | 1.01 | 1.53 | 0.14 | 1.44 | 1.74 | 1.47 | 0.200 | n.s. | |
| Total (N = 8) | 1.03 | 0.28 | 0.63 | 1.59 | 1.00 | 1.43 | 0.17 | 1.14 | 1.74 | 1.45 | 0.010 | * | |
| FOS | Donor 1 (N = 4) | 1.01 | 0.18 | 0.84 | 1.18 | 1.01 | 2.00 | 0.45 | 1.71 | 2.67 | 1.81 | 0.029 | * |
| Donor 2 (N = 4) | 1.13 | 0.62 | 0.52 | 1.92 | 1.04 | 3.37 | 1.02 | 2.21 | 4.44 | 3.41 | 0.029 | * | |
| Total (N = 8) | 1.07 | 0.43 | 0.52 | 1.92 | 1.01 | 2.68 | 1.03 | 1.71 | 4.44 | 2.44 | 0.001 | ** | |
| IL6 | Donor 1 (N = 4) | 1.04 | 0.32 | 0.69 | 1.45 | 1.01 | 1.03 | 0.53 | 0.67 | 1.80 | 0.82 | 0.886 | n.s. |
| Donor 2 (N = 4) | 1.00 | 0.04 | 0.95 | 1.05 | 1.00 | 1.60 | 0.84 | 1.10 | 2.85 | 1.22 | 0.029 | * | |
| Total (N = 8) | 1.02 | 0.21 | 0.69 | 1.45 | 1.00 | 1.31 | 0.72 | 0.67 | 2.85 | 1.11 | 0.505 | n.s. | |
| PTGS2 | Donor 1 (N = 4) | 1.02 | 0.24 | 0.77 | 1.30 | 1.01 | 1.87 | 0.14 | 1.73 | 2.01 | 1.87 | 0.029 | * |
| Donor 2 (N = 4) | 1.02 | 0.26 | 0.74 | 1.36 | 1.00 | 2.69 | 0.66 | 2.01 | 3.34 | 2.71 | 0.029 | * | |
| Total (N = 8) | 1.02 | 0.23 | 0.74 | 1.36 | 1.00 | 2.28 | 0.62 | 1.73 | 3.34 | 2.01 | <0.001 | *** | |
| RUNX2 | Donor 1 (N = 4) | 1.03 | 0.26 | 0.74 | 1.36 | 1.00 | 1.31 | 0.17 | 1.19 | 1.55 | 1.24 | 0.200 | n.s. |
| Donor 2 (N = 4) | 1.01 | 0.18 | 0.84 | 1.19 | 1.01 | 1.70 | 0.53 | 1.21 | 2.26 | 1.67 | 0.029 | * | |
| Total (N = 8) | 1.02 | 0.21 | 0.74 | 1.36 | 1.00 | 1.50 | 0.42 | 1.19 | 2.26 | 1.28 | 0.005 | ** | |
| SP7 | Donor 1 (N = 4) | 1.01 | 0.15 | 0.85 | 1.18 | 1.00 | 1.47 | 0.67 | 0.80 | 2.32 | 1.38 | 0.486 | n.s. |
| Donor 2 (N = 4) | 1.12 | 0.50 | 0.58 | 1.75 | 1.09 | 15.47 | 24.05 | 0.23 | 51.28 | 5.17 | 0.343 | n.s. | |
| Total (N = 8) | 1.07 | 0.35 | 0.58 | 1.75 | 1.00 | 8.47 | 17.44 | 0.23 | 51.28 | 1.99 | 0.195 | n.s. | |
| TNFRSF11B | Donor 1 (N = 4) | 1.00 | 0.10 | 0.89 | 1.12 | 1.00 | 1.05 | 0.06 | 0.99 | 1.12 | 1.05 | 0.486 | n.s. |
| Donor 2 (N = 4) | 1.02 | 0.22 | 0.77 | 1.30 | 1.01 | 1.09 | 0.26 | 0.73 | 1.35 | 1.13 | 0.686 | n.s. | |
| Total (N = 8) | 1.01 | 0.16 | 0.77 | 1.30 | 1.01 | 1.07 | 0.18 | 0.73 | 1.35 | 1.08 | 0.328 | n.s. | |
| VEGFA | Donor 1 (N = 4) | 1.00 | 0.07 | 0.92 | 1.09 | 1.00 | 1.17 | 0.10 | 1.08 | 1.28 | 1.16 | 0.114 | n.s. |
| Donor 2 (N = 4) | 1.00 | 0.11 | 0.89 | 1.13 | 1.00 | 1.33 | 0.34 | 0.95 | 1.68 | 1.34 | 0.200 | n.s. | |
| Total (N = 8) | 1.00 | 0.08 | 0.89 | 1.13 | 1.00 | 1.25 | 0.25 | 0.95 | 1.68 | 1.19 | 0.021 | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nile, M.; Folwaczny, M.; Kessler, A.; Wichelhaus, A.; Janjic Rankovic, M.; Baumert, U. Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells. Cells 2024, 13, 1751. https://doi.org/10.3390/cells13211751
Nile M, Folwaczny M, Kessler A, Wichelhaus A, Janjic Rankovic M, Baumert U. Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells. Cells. 2024; 13(21):1751. https://doi.org/10.3390/cells13211751
Chicago/Turabian StyleNile, Mustafa, Matthias Folwaczny, Andreas Kessler, Andrea Wichelhaus, Mila Janjic Rankovic, and Uwe Baumert. 2024. "Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells" Cells 13, no. 21: 1751. https://doi.org/10.3390/cells13211751
APA StyleNile, M., Folwaczny, M., Kessler, A., Wichelhaus, A., Janjic Rankovic, M., & Baumert, U. (2024). Development of a Custom Fluid Flow Chamber for Investigating the Effects of Shear Stress on Periodontal Ligament Cells. Cells, 13(21), 1751. https://doi.org/10.3390/cells13211751







