Does Incretin Agonism Have Sustainable Efficacy?
Abstract
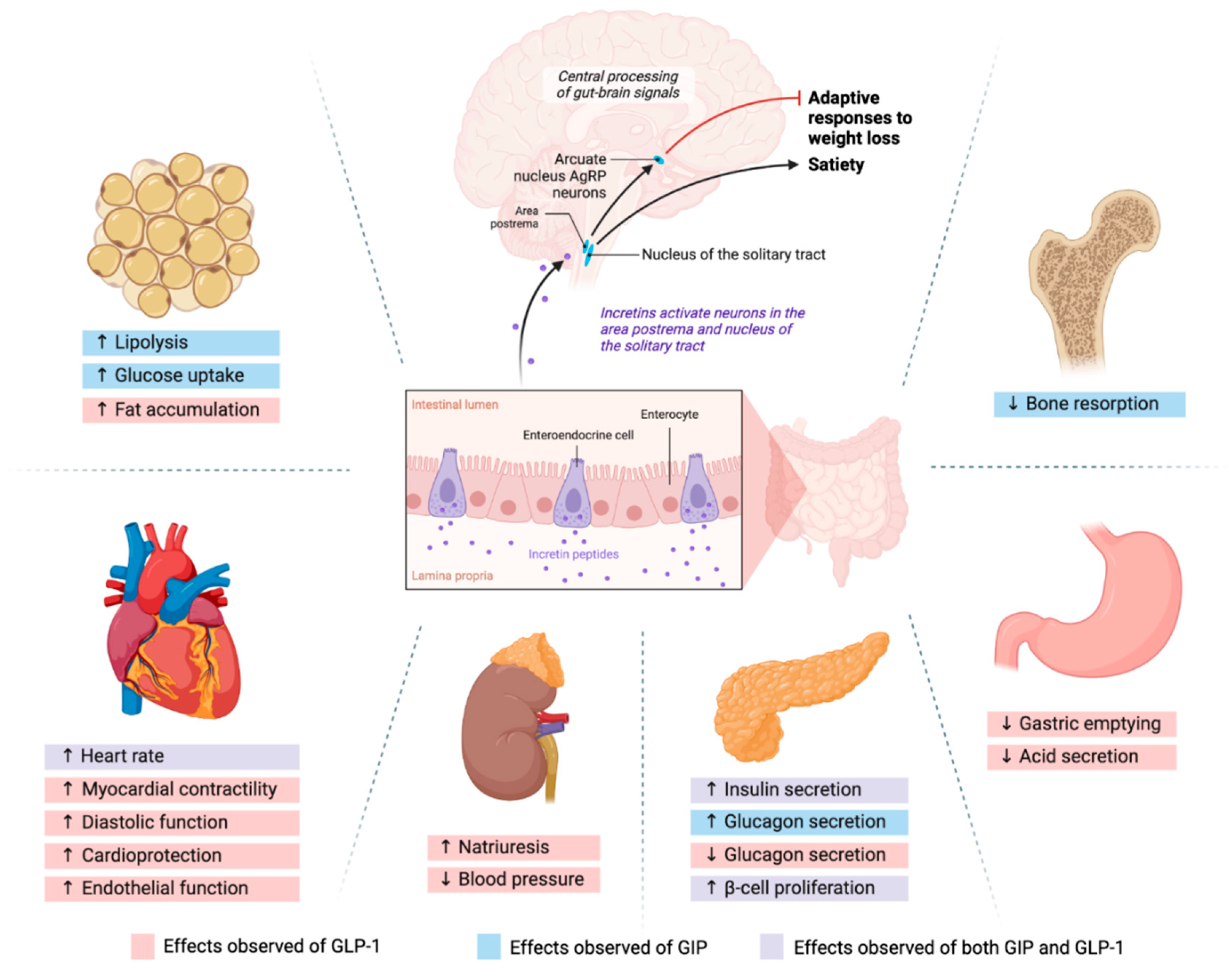
1. Introduction
1.1. GLP-1 and GIP Mechanism of Action
1.2. Amylin Mechanism of Action
1.3. Glucagon Mechanism of Action
- (a)
- (b)
- Suppress food intake by slowing gastric emptying and increasing satiety, which lead to weight loss [11]. Interestingly, semaglutide did not delay gastric emptying (GE) when assessed using paracetamol absorption in a recent trial [47]. The reason for the conflicting results may be that older studies used the dye dilution method to quantify GE [48]. The dye dilution method is not as precise as the validated GE assessment method, scintigraphy, while recent studies used paracetamol which is known to have comparable precision to scintigraphy [49]. Although the recent study reporting the null effects of semaglutide on GE used a more precise method, it should be noted that the authors were employees of Novo Nordisk, the maker of semaglutide. Murine models of GLP-1 did not slow effects on gastric emptying [50]. These reports indirectly suggest that manipulating the neurohormonal axis using incretins may be the cause of weight loss. The same pathway also reduces the craving for alcohol intake. Unfortunately, when incretin’s manipulation of neuronal pathways is terminated, approximately 2/3 of the lost weight was regained [25,51].
- (c)
- (d)
1.4. Long-Term Efficacy of Incretin Agonism
2. Role of Incretins in the Neurohormonal Axis of Appetite Control
3. Challenges in Incretin Agonism
3.1. Involvement of β-Arrestins
3.2. β-Cell Exhaustion and Failure
4. Potential Adverse Effects of Incretin Agonism
5. Future Directions
Funding
Conflicts of Interest
References
- Barre, L. Sur les possibilities d’un traitement du diabete par. L’incretine. Bull. Acad. R. Med. Belg. 1932, 12, 620–634. [Google Scholar]
- Gupta, K.; Raja, A. Physiology, Gastric Inhibitory Peptide; StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, B.; Pais, R.; Engelstoft, M.S.; Milev, N.B.; Richards, P.; Christiansen, C.B.; Egerod, K.L.; Jensen, S.M.; Habib, A.M.; Gribble, F.M.; et al. GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J. Endocrinol. 2016, 228, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Winther, J.B.; Holst, J.J. Glucagon agonism in the treatment of metabolic diseases including type 2 diabetes mellitus and obesity. Diabetes Obes. Metab. 2024, 26, 3501–3512. [Google Scholar] [CrossRef]
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake. Cell Metab. 2019, 30, 987–996.e986. [Google Scholar] [CrossRef]
- Liskiewicz, A.; Khalil, A.; Liskiewicz, D.; Novikoff, A.; Grandl, G.; Maity-Kumar, G.; Gutgesell, R.M.; Bakhti, M.; Bastidas-Ponce, A.; Czarnecki, O.; et al. Glucose-dependent insulinotropic polypeptide regulates body weight and food intake via GABAergic neurons in mice. Nat. Metab. 2023, 5, 2075–2085. [Google Scholar] [CrossRef]
- Martinez de Morentin, P.B.; Gonzalez, J.A.; Dowsett, G.K.C.; Martynova, Y.; Yeo, G.S.H.; Sylantyev, S.; Heisler, L.K. A brainstem to hypothalamic arcuate nucleus GABAergic circuit drives feeding. Curr. Biol. 2024, 34, 1646–1656.e1644. [Google Scholar] [CrossRef]
- Asmar, M.; Tangaa, W.; Madsbad, S.; Hare, K.; Astrup, A.; Flint, A.; Bülow, J.; Holst, J.J. On the role of glucose-dependent insulintropic polypeptide in postprandial metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E614–E621. [Google Scholar] [CrossRef]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E621–E625. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.C.E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs. Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Bonora, E.; Nevarez Ruiz, L.; Li, Y.G.; Yu, Z.; Milicevic, Z.; Malik, R.; Bethel, M.A.; Cox, D.A. Efficacy and Safety of Dulaglutide 3.0 mg and 4.5 mg Versus Dulaglutide 1.5 mg in Metformin-Treated Patients With Type 2 Diabetes in a Randomized Controlled Trial (AWARD-11). Diabetes Care 2021, 44, 765–773. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.V.; Wilding, J.P.H.; Skjøth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Sattar, N.; Pavo, I.; Haupt, A.; Duffin, K.L.; Yang, Z.; Wiese, R.J.; Tuttle, K.R.; Cherney, D.Z.I. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: Post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022, 10, 774–785. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Triple-Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): A randomised, open-label, parallel-group, phase 3 trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Bedossa, P.; Fraessdorf, M.; Neff, G.W.; Lawitz, E.; Bugianesi, E.; Anstee, Q.M.; Hussain, S.A.; Newsome, P.N.; Ratziu, V.; et al. A Phase 2 Randomized Trial of Survodutide in MASH and Fibrosis. N. Engl. J. Med. 2024, 391, 311–319. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: A randomized phase 2a trial. Nat. Med. 2024, 30, 2037–2048. [Google Scholar] [CrossRef]
- Piccini, S.; Favacchio, G.; Panico, C.; Morenghi, E.; Folli, F.; Mazziotti, G.; Lania, A.G.; Mirani, M. Time-dependent effect of GLP-1 receptor agonists on cardiovascular benefits: A real-world study. Cardiovasc. Diabetol. 2023, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. GIP and GLP-1: Stepsiblings Rather Than Monozygotic Twins Within the Incretin Family. Diabetes 2019, 68, 897–900. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef] [PubMed]
- El, K.; Campbell, J.E. The role of GIP in α-cells and glucagon secretion. Peptides 2020, 125, 170213. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Incretin action in the pancreas: Potential promise, possible perils, and pathological pitfalls. Diabetes 2013, 62, 3316–3323. [Google Scholar] [CrossRef]
- Urva, S.; Coskun, T.; Loghin, C.; Cui, X.; Beebe, E.; O’Farrell, L.; Briere, D.A.; Benson, C.; Nauck, M.A.; Haupt, A. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP-1 receptor agonists. Diabetes Obes. Metab. 2020, 22, 1886–1891. [Google Scholar] [CrossRef]
- Hayes, M.R.; Borner, T.; De Jonghe, B.C. The Role of GIP in the Regulation of GLP-1 Satiety and Nausea. Diabetes 2021, 70, 1956–1961. [Google Scholar] [CrossRef]
- Hayes, M.R.; Mietlicki-Baase, E.G.; Kanoski, S.E.; De Jonghe, B.C. Incretins and amylin: Neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 2014, 34, 237–260. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. Role and Cytotoxicity of Amylin and Protection of Pancreatic Islet β-Cells from Amylin Cytotoxicity. Cells 2018, 7, 95. [Google Scholar] [CrossRef]
- Kosinski, J.R.; Hubert, J.; Carrington, P.E.; Chicchi, G.G.; Mu, J.; Miller, C.; Cao, J.; Bianchi, E.; Pessi, A.; Sinharoy, R.; et al. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity 2012, 20, 1566–1571. [Google Scholar] [CrossRef]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, M.; Sud, N.; Christian, P.; Shen, J.; Song, Y.; Pashaj, A.; Zhang, K.; Carr, T.; Su, Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: Implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci. Rep. 2016, 6, 32246. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef]
- Janket, S.J.; Javaheri, H.; Ackerson, L.K.; Ayilavarapu, S.; Meurman, J.H. Oral Infections, Metabolic Inflammation, Genetics, and Cardiometabolic Diseases. J. Dent. Res. 2015, 94, 119s–127s. [Google Scholar] [CrossRef] [PubMed]
- Oussaada, S.M.; Kilicarslan, M.; de Weijer, B.A.; Gilijamse, P.W.; Şekercan, A.; Virtue, S.; Janssen, I.M.C.; van de Laar, A.; Demirkiran, A.; van Wagensveld, B.A.; et al. Tissue-specific inflammation and insulin sensitivity in subjects with obesity. Diabetes Res. Clin. Pract. 2024, 211, 111663. [Google Scholar] [CrossRef]
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 2021, 23, 754–762. [Google Scholar] [CrossRef]
- Willms, B.; Werner, J.; Holst, J.J.; Orskov, C.; Creutzfeldt, W.; Nauck, M.A. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: Effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J. Clin. Endocrinol. Metab. 1996, 81, 327–332. [Google Scholar] [CrossRef]
- Tong, J.; D’Alessio, D. Give the receptor a brake: Slowing gastric emptying by GLP-1. Diabetes 2014, 63, 407–409. [Google Scholar] [CrossRef]
- Maida, A.; Lovshin, J.A.; Baggio, L.L.; Drucker, D.J. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology 2008, 149, 5670–5678. [Google Scholar] [CrossRef]
- Rosenstock, J.; Lee, C.J.; Fernández Landó, L.; Liu, M.; Karanikas, C.A.; Thieu, V.T. Impact on glycated haemoglobin and body weight changes after stopping tirzepatide for 4 weeks in the SURPASS-1 monotherapy trial. Diabetes Obes. Metab. 2024, 26, 396–399. [Google Scholar] [CrossRef]
- Ackeifi, C.; Wang, P.; Karakose, E.; Manning Fox, J.E.; González, B.J.; Liu, H.; Wilson, J.; Swartz, E.; Berrouet, C.; Li, Y.; et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β cell regeneration. Sci. Transl. Med. 2020, 12, eaaw9996. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hu, Y.; Li, Y.Y.; Cao, X.; Bai, N.; Lu, T.T.; Li, G.Q.; Li, N.; Wang, A.N.; Mao, X.M. Glucagon-like peptide-1 receptor agonists and risk of bone fracture in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2019, 35, e3168. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.K.; Sørensen, V.; Sandsdal, R.M.; Lehmann, E.W.; Lundgren, J.R.; Juhl, C.R.; Janus, C.; Ternhamar, T.; Stallknecht, B.M.; Holst, J.J.; et al. Bone Health After Exercise Alone, GLP-1 Receptor Agonist Treatment, or Combination Treatment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open. 2024, 7, e2416775. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.G.; Jensen, M.T.; Mensberg, P.; Storgaard, H.; Nyby, S.; Jensen, J.S.; Knop, F.K.; Vilsbøll, T. Effect of exercise combined with glucagon-like peptide-1 receptor agonist treatment on cardiac function: A randomized double-blind placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 1040–1044. [Google Scholar] [CrossRef]
- Dunning, B.E.; Gerich, J.E. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 2007, 28, 253–283. [Google Scholar] [CrossRef]
- Hernández-Cascales, J. Does glucagon have a positive inotropic effect in the human heart? Cardiovasc. Diabetol. 2018, 17, 148. [Google Scholar] [CrossRef]
- Kaur, S.; Rose, R.A. New insights into the effects of glucagon-like peptide-1 on heart rate and sinoatrial node function. Cardiovasc. Res. 2024, 120, 1367–1368. [Google Scholar] [CrossRef]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J.; et al. GLP-1 increases heart rate by a direct action on the sinus node. Cardiovasc. Res. 2024, 120, 1427–1441. [Google Scholar] [CrossRef]
- Petersen, K.M.; Bøgevig, S.; Riis, T.; Andersson, N.W.; Dalhoff, K.P.; Holst, J.J.; Knop, F.K.; Faber, J.; Petersen, T.S.; Christensen, M.B. High-Dose Glucagon Has Hemodynamic Effects Regardless of Cardiac Beta-Adrenoceptor Blockade: A Randomized Clinical Trial. J. Am. Heart Assoc. 2020, 9, e016828. [Google Scholar] [CrossRef]
- Alexander, J.T.; Staab, E.M.; Wan, W.; Franco, M.; Knitter, A.; Skandari, M.R.; Bolen, S.; Maruthur, N.M.; Huang, E.S.; Philipson, L.H.; et al. The Longer-Term Benefits and Harms of Glucagon-Like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2022, 37, 415–438. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Wysham, C.H.; Hardy, E.; Han, J.; Iqbal, N. Efficacy and tolerability of exenatide once weekly over 7 years in patients with type 2 diabetes: An open-label extension of the DURATION-1 study. J. Diabetes Complicat. 2019, 33, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Kenward, M.G.; Fairclough, D.L.; Horton, N.J. Differential dropout and bias in randomised controlled trials: When it matters and when it may not. BMJ 2013, 346, e8668. [Google Scholar] [CrossRef] [PubMed]
- Moll, H.; Frey, E.; Gerber, P.; Geidl, B.; Kaufmann, M.; Braun, J.; Beuschlein, F.; Puhan, M.A.; Yebyo, H.G. GLP-1 receptor agonists for weight reduction in people living with obesity but without diabetes: A living benefit-harm modelling study. EClinicalMedicine 2024, 73, 102661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Tan, Y.; Chen, Y.; Zhou, X.; Liu, S.; Yu, J. GLP-1RAs caused gastrointestinal adverse reactions of drug withdrawal: A system review and network meta-analysis. Front. Endocrinol. 2023, 14, 1149328. [Google Scholar] [CrossRef]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Delgado, T.C. Glutamate and GABA in Appetite Regulation. Front. Endocrinol. 2013, 4, 103. [Google Scholar] [CrossRef]
- Rau, A.R.; Hentges, S.T. GABAergic Inputs to POMC Neurons Originating from the Dorsomedial Hypothalamus Are Regulated by Energy State. J. Neurosci. 2019, 39, 6449–6459. [Google Scholar] [CrossRef]
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC Neurons: From Birth to Death. Annu. Rev. Physiol. 2017, 79, 209–236. [Google Scholar] [CrossRef]
- Sohn, J.W.; Xu, Y.; Jones, J.E.; Wickman, K.; Williams, K.W.; Elmquist, J.K. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 2011, 71, 488–497. [Google Scholar] [CrossRef]
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010, 30, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef] [PubMed]
- Beck, B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1159–1185. [Google Scholar] [CrossRef]
- Williams, D.L. The diverse effects of brain glucagon-like peptide 1 receptors on ingestive behaviour. Br. J. Pharmacol. 2022, 179, 571–583. [Google Scholar] [CrossRef]
- Jones, B.; McGlone, E.R.; Fang, Z.; Pickford, P.; Corrêa, I.R., Jr.; Oishi, A.; Jockers, R.; Inoue, A.; Kumar, S.; Görlitz, F.; et al. Genetic and biased agonist-mediated reductions in β-arrestin recruitment prolong cAMP signaling at glucagon family receptors. J. Biol. Chem. 2021, 296, 100133. [Google Scholar] [CrossRef]
- Bitsi, S.; El Eid, L.; Manchanda, Y.; Oqua, A.I.; Mohamed, N.; Hansen, B.; Suba, K.; Rutter, G.A.; Salem, V.; Jones, B.; et al. Divergent acute versus prolonged pharmacological GLP-1R responses in adult β cell-specific β-arrestin 2 knockout mice. Sci. Adv. 2023, 9, eadf7737. [Google Scholar] [CrossRef]
- Zaïmia, N.; Obeid, J.; Varrault, A.; Sabatier, J.; Broca, C.; Gilon, P.; Costes, S.; Bertrand, G.; Ravier, M.A. GLP-1 and GIP receptors signal through distinct β-arrestin 2-dependent pathways to regulate pancreatic β cell function. Cell Rep. 2023, 42, 113326. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. G Protein-Coupled Receptor Signaling Through β-Arrestin-Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef]
- Peterson, Y.K.; Luttrell, L.M. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol. Rev. 2017, 69, 256–297. [Google Scholar] [CrossRef]
- Willard, F.S.; Douros, J.D.; Gabe, M.B.; Showalter, A.D.; Wainscott, D.B.; Suter, T.M.; Capozzi, M.E.; van der Velden, W.J.; Stutsman, C.; Cardona, G.R.; et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020, 5, e140532. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Nelson, C.D.; Garrison, T.R.; Miller, W.E.; Lefkowitz, R.J. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. USA 2003, 100, 1740–1744. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, H.W.; Chen, K.H.; Chen, Y.S.; Sheen-Chen, S.M.; Lin, C.R. Antinociceptive potentiation and attenuation of tolerance by intrathecal β-arrestin 2 small interfering RNA in rats. Br. J. Anaesth. 2011, 107, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Beautrait, A.; Paradis, J.S.; Zimmerman, B.; Giubilaro, J.; Nikolajev, L.; Armando, S.; Kobayashi, H.; Yamani, L.; Namkung, Y.; Heydenreich, F.M.; et al. A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat. Commun. 2017, 8, 15054. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, M.; Eichel, K.; Avino, S.; Zhao, X.; Steffen, D.J.; Feng, X.; Kawakami, K.; Aoki, J.; Messer, K.; Sunahara, R.; et al. Genetic evidence that β-arrestins are dispensable for the initiation of β(2)-adrenergic receptor signaling to ERK. Sci. Signal. 2017, 10, eaal3395. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Aston-Mourney, K.; Proietto, J.; Morahan, G.; Andrikopoulos, S. Too much of a good thing: Why it is bad to stimulate the beta cell to secrete insulin. Diabetologia 2008, 51, 540–545. [Google Scholar] [CrossRef]
- Killion, E.A.; Chen, M.; Falsey, J.R.; Sivits, G.; Hager, T.; Atangan, L.; Helmering, J.; Lee, J.; Li, H.; Wu, B.; et al. Chronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nat. Commun. 2020, 11, 4981. [Google Scholar] [CrossRef]
- Campbell, J.E. Targeting the GIPR for obesity: To agonize or antagonize? Potential mechanisms. Mol. Metab. 2021, 46, 101139. [Google Scholar] [CrossRef]
- Grill, V.; Björklund, A. Overstimulation and beta-cell function. Diabetes 2001, 50 (Suppl. S1), S122–S124. [Google Scholar] [CrossRef]
- Swisa, A.; Glaser, B.; Dor, Y. Metabolic Stress and Compromised Identity of Pancreatic Beta Cells. Front. Genet. 2017, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Insulin: Potential negative consequences of early routine use in patients with type 2 diabetes. Diabetes Care. 2011, 34 (Suppl. S2), S225–S230. [Google Scholar] [CrossRef] [PubMed]
- Labuzek, K.; Kozłowski, M.; Szkudłapski, D.; Sikorska, P.; Kozłowska, M.; Okopień, B. Incretin-based therapies in the treatment of type 2 diabetes--more than meets the eye? Eur. J. Intern. Med. 2013, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Whitticar, N.B.; Nunemaker, C.S. Reducing Glucokinase Activity to Enhance Insulin Secretion: A Counterintuitive Theory to Preserve Cellular Function and Glucose Homeostasis. Front. Endocrinol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Page, K.A.; Reisman, T. Interventions to preserve beta-cell function in the management and prevention of type 2 diabetes. Curr. Diab Rep. 2013, 13, 252–260. [Google Scholar] [CrossRef]
- Singh, S.; Chang, H.Y.; Richards, T.M.; Weiner, J.P.; Clark, J.M.; Segal, J.B. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: A population-based matched case-control study. JAMA Intern. Med. 2013, 173, 534–539. [Google Scholar] [CrossRef]
- Gudin, B.; Ladhari, C.; Robin, P.; Laroche, M.L.; Babai, S.; Hillaire-Buys, D.; Faillie, J.L. Incretin-based drugs and intestinal obstruction: A pharmacovigilance study. Therapie 2020, 75, 641–647. [Google Scholar] [CrossRef]
- Kalas, M.A.; Galura, G.M.; McCallum, R.W. Medication-Induced Gastroparesis: A Case Report. J. Investig. Med. High. Impact Case Rep. 2021, 9, 23247096211051919. [Google Scholar] [CrossRef]
- Monami, M.; Nreu, B.; Scatena, A.; Cresci, B.; Andreozzi, F.; Sesti, G.; Mannucci, E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes. Metab. 2017, 19, 1233–1241. [Google Scholar] [CrossRef]
- Silverii, G.A.; Monami, M.; Gallo, M.; Ragni, A.; Prattichizzo, F.; Renzelli, V.; Ceriello, A.; Mannucci, E. Glucagon-like peptide-1 receptor agonists and risk of thyroid cancer: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef] [PubMed]
- Wettergren, A.; Wøjdemann, M.; Holst, J.J. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am. J. Physiol. 1998, 275, G984–G992. [Google Scholar] [CrossRef] [PubMed]
- Patel, F.; Gan, A.; Chang, K.; Vega, K.J. Acute Pancreatitis in a Patient Taking Semaglutide. Cureus 2023, 15, e43773. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, K.A.; Alsuhaibani, D.; Batarfi, B.; Alshammari, T.M. Pancreatitis with use of new diabetic medications: A real-world data study using the post-marketing FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 2024, 15, 1364110. [Google Scholar] [CrossRef]
- Koehler, J.A.; Baggio, L.L.; Lamont, B.J.; Ali, S.; Drucker, D.J. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009, 58, 2148–2161. [Google Scholar] [CrossRef]
- Valente, R.; Coppola, A.; Scandavini, C.M.; Halimi, A.; Magnusson, A.; Lauro, A.; Sotirova, I.; Arnelo, U.; Franklin, O. Interactions between the Exocrine and the Endocrine Pancreas. J. Clin. Med. 2024, 13, 1179. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Zhou, Q.; Wang, M.W.; Li, Z. A potentially serious adverse effect of GLP-1 receptor agonists. Acta Pharm. Sin. B 2023, 13, 2291–2293. [Google Scholar] [CrossRef]
- Grennan, K.; Meneley, A.; Shuman, M.; Borg, C.; Janitz, T.; Brahmbhatt, P.; Venegas, C. A Case of Fatal Fulminant Necrotizing Pancreatitis in a Patient with Recent Tirzepatide Initiation. Endocr. Pract. 2024, 30, S68. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; de Souza, L.G.; Nunes, G.P.; Martin, I.F.; de Oliveira, Y.U.; Pinheiro, F.M.M.; Costa, L.N.; Caprio, M.; Della-Morte, D.; Infante, M. The first report of leukocytoclastic vasculitis induced by once-weekly subcutaneous semaglutide. Curr. Med. Res. Opin. 2024, 40, 1525–1531. [Google Scholar] [CrossRef]
- Lorenz, M.; Lawson, F.; Owens, D.; Raccah, D.; Roy-Duval, C.; Lehmann, A.; Perfetti, R.; Blonde, L. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc. Diabetol. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kawabe, A.; Matsumura, M.; Aso, Y.; Yasu, T.; Banba, N.; Nakamoto, T. Effects of GLP-1 Receptor Agonists on Heart Rate and the Autonomic Nervous System Using Holter Electrocardiography and Power Spectrum Analysis of Heart Rate Variability. Diabetes Care. 2016, 39, e22–e23. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Veith, R.C.; Taborsky, G.J., Jr. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology 1987, 121, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Amir, S. Central glucagon-induced hyperglycemia is mediated by combined activation of the adrenal medulla and sympathetic nerve endings. Physiol. Behav. 1986, 37, 563–566. [Google Scholar] [CrossRef]
- Routsolias, J.C.; Berg, S.E.; Paloucek, F.P. Does Glucagon Really Work for Beta Blocker Overdose? J. Med. Toxicol. 2023, 19, 7–8. [Google Scholar] [CrossRef]
- Holt, M.K.; Trapp, S. The physiological role of the brain GLP-1 system in stress. Cogent Biol. 2016, 2, 1229086. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J.; Marina, N.; Manton, R.N.; Reimann, F.; Gribble, F.M.; Trapp, S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience 2015, 284, 872–887. [Google Scholar] [CrossRef]
- Xu, X.Y.; Wang, J.X.; Chen, J.L.; Dai, M.; Wang, Y.M.; Chen, Q.; Li, Y.H.; Zhu, G.Q.; Chen, A.D. GLP-1 in the Hypothalamic Paraventricular Nucleus Promotes Sympathetic Activation and Hypertension. J. Neurosci. 2024, 44, e2032232024. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Gil-Lozano, M.; Toba, L.; Fandiño, J.; Ogando, H.; González-Matías, L.C.; Mallo, F. Stressing diabetes? The hidden links between insulinotropic peptides and the HPA axis. J. Endocrinol. 2016, 230, R77–R94. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Gil-Lozano, M.; Pérez-Tilve, D.; Alvarez-Crespo, M.; Martís, A.; Fernandez, A.M.; Catalina, P.A.; Gonzalez-Matias, L.C.; Mallo, F. GLP-1(7-36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology 2010, 151, 2629–2640. [Google Scholar] [CrossRef]
- Kuckuck, S.; van der Valk, E.S.; Scheurink, A.J.W.; van der Voorn, B.; Iyer, A.M.; Visser, J.A.; Delhanty, P.J.D.; van den Berg, S.A.A.; van Rossum, E.F.C. Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones. Obes. Rev. 2023, 24, e13539. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.; Mastitskaya, S.; Hosford, P.S.; Basalay, M.; Specterman, M.; Aziz, Q.; Li, Y.; Orini, M.; Taggart, P.; Lambiase, P.D.; et al. Modulation of Cardiac Ventricular Excitability by GLP-1 (Glucagon-Like Peptide-1). Circ. Arrhythm. Electrophysiol. 2018, 11, e006740. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Phizackerley, D. Semaglutide reduces the absolute risk of major cardiovascular events by 1.5. BMJ 2024, 384, q53. [Google Scholar] [CrossRef]
- Heile, M.; Wyne, K.; Billings, L.K.; Cannon, A.; Handelsman, Y.; Shannon, M. Cardiovascular Outcomes with Once-Weekly GLP-1 RAs: Clinical and Economic Implications. J. Manag. Care Spec. Pharm. 2018, 24, S42–S52. [Google Scholar] [CrossRef]
- Mozaffarian, D. GLP-1 Agonists for Obesity-A New Recipe for Success? JAMA 2024, 331, 1007–1008. [Google Scholar] [CrossRef]
- O’Neil, P.M. Long-term maintenance of weight loss. Lancet Public Health 2022, 7, e806–e807. [Google Scholar] [CrossRef]
- O’Neil, P.; Adams, H. (Eds.) The-Upsides-and-Downsides-of-Blockbuster-Weight-Loss-Drugs; Medical University of South Carolina: Charleston, SC, USA, 2023. [Google Scholar]
- Rebello, C.; Greenway, F.L.; Dhurandhar, N.V. Functional foods to promote weight loss and satiety. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 596–604. [Google Scholar] [CrossRef]
- Seier, S.; Larsen, K.S.; Pedersen, J.; Biccler, J.; Gudbergsen, H. Tapering semaglutide to the most effective dose: Real-world evidence from a digital weight management programme (TAILGATE). In Proceedings of the 31st European Congress on Obesity, Venice, Italy, 12–15 May 2024; p. 449. [Google Scholar]
| First Author, Trial Name/ID, Year, Phase | Sample Size, Duration | Target Population, Methods | Objectives, Results (s), and Comments |
|---|---|---|---|
| Le Roux, [NCT01272219], 2017, Phase 3 [15] | N = 2254, 68 weeks or 160 weeks | Prediabetic obese and overweight with co-morbidities cohort Drug: Lira 3.0 mg or placebo SC injection once daily | Objectives: Weight reduction/maintenance, T2D onset delay. Results: By 160 weeks, DM Dx given to (2%) of 1472 in Lira vs. (6%) of 738 in the placebo. Time to DM diagnosis was 99 wks in Lira vs. 87 wks in placebo. |
| Husain M, PIONEER 6 [NCT02692716], 2019, phase 3 [16] | N = 3183, Median 62 weeks | T2D with high cardiovascular risk cohort Drug: Sema or placebo Oral administration once daily | Objectives: Cardiovascular safety of oral Sema, QD in T2D patients. Primary outcome: incidence of MACE. Results: MACE occurred in 3.8% in Sema vs. 4.8% in placebo including 15 CVD mort. in Sema vs. 30 in placebo. |
| Frías JP, AWARD-11 [NCT03495102], 2021, phase 3 [14] | N = 1842, 52 weeks (36 weeks primary endpoint) | T2D Patients inadequately controlled with metformin Drug: Dula 1.5 mg, 3.0 mg, or 4.5 mg SC injection once weekly | Objectives: Change in HbA1c by week 36 from baseline. Results: At 36 wks, Dula 4.5 mg superior to 1.5 mg with [ETD] −0.24% but Tx estimand of 3.0 mg was not significant (p = 0.096). However, vomiting nearly doubled in 4.5 mg level. (5.6% vs. 9.3%) |
| Rubino D, STEP-4 [NCT03548987], 2021, phase 3a [12] | N = 803, 68 weeks | Obese or overweight cohort without T2D Drug: Sema 2.4 mg or placebo SC injection once weekly Primary outcome: Change in body weight (%) | Objectives: Comparison of SC Sema continued or switch to placebo, both with lifestyle intervention. Wt. change week 20–68: Sema −7.9% vs. placebo +6.9%. G-I adverse events: Sema 49.1% vs. placebo 26.1% (1.88 times more in Sema group). |
| Frías JP, SURPASS-2 [NCT03987919], 2021, phase 3 [18] | N = 1879, 40 weeks | Metformin-treated T2D cohort Drug: Sema 1 mg or Tirzep 5 mg, 10 mg, 15 mg SC injection once weekly | Objectives: Compare effect of Sema and Tirzep on blood sugar levels. Outcome = Change in HbA1c by week 40. The diff. between groups Tirzep 5-mg, 10-mg, and 15-mg, and Sema were −0.15%, −0.39%, and −0.45%, respectively. Serious adverse events: 5–7% in Tirzep vs. 3% in Sema. |
| Ludvik B, SURPASS-3 [NCT03882970], 2021, phase 3 [23] | N = 1444, 52 weeks | Metformin-treated or metformin with SGLT2i-treated T2D cohort Drug: Tirzep 5 mg, 10 mg, 15 mg, or insulin degludec 100 U/mL (titrated) SC injection once weekly (Tirzep), SC injection once daily (insulin degludec) | Objectives: Assess safety and efficacy of Tirzep versus insulin degludec on blood sugar levels. Results: Non-inferiority of Tirzep to insulin. HbA1c change in Tirzep 5, 10, 15 mg at week 52 were −1·93%, −2·20%, and −2·37%, respectively, and −1.34% in insulin. G-I adverse events: 7% in Tirzep vs. 1% in insulin group. Hypoglycemia: 4% in Tirzep vs. 7% in insulin gr. |
| Del Prato S, SURPASS-4 [NCT03730662], 2021, phase 3 [24] | N = 2002, 52 weeks (treatment continued until maximum 104 weeks) | Metformin-treated, sulfonylurea-treated, SGLT2i-treated T2D cohort Drug: Tirzep 5 mg, 10 mg, 15 mg, or glargine 100 U/mL (titrated) SC once weekly (Tirzep), SC once daily (glargine) | Objective: Assess efficacy and safety of Tirzep versus insulin glargine in adults with high CVD risk and T2D. Primary outcome: Non-inferiority of Tirzep 10 mg or/and 15 mg versus glargine. Mean HbA1c change at week 52: −2·43% and −2·58%, with Tirzep 10, 15 mg, respectively, vs. −1·44% with glargine. |
| Rubino DM, STEP-8 [NCT04074161], 2022, phase 3b [13] | N = 338, 68 weeks | Obese or overweight cohort without T2D Drug: Sema 2.4 mg or Lira 3.0 mg or placebo (matching for both conditions) SC injection once weekly (Sema), SC injection once daily (Lira) | Objectives: Assess the efficacy of once-weekly Sema vs. once-daily Lira on weight loss. Change in body weight (%) by week 68. Mean wt. change from baseline: −15.8% with Sema, −6.4% with Lira, and −1.9% with placebo. G-I adverse events: 84.1% with Sema, 82.7% with Lira. |
| Wilding J, STEP 1-extension [NCT03548935], 2022 [25] | N = 327, 1 year after withdrawal from STEP-1 | Extension analysis Previous drug: Sema 2.4 mg or placebo | Objectives: body weight changes and cardio-metabolic factors following Sema withdrawal. Primary outcome: One year after withdrawal of weekly Sema 2.4 mg + lifestyle intervention, participants regained two-thirds of their prior weight loss. |
| Heerspink H, SURPASS-4 Post Hoc Analysis, 2022 [17] | N = 2002, Median 85 weeks (104 weeks max) | Metformin-treated, sulfonylurea-treated, SGLT2i-treated T2D cohort Drug: Tirzep 5 mg, 10 mg, 15 mg, or glargine 100 U/mL (titrated) SC injection once weekly (Tirzep), SC injection once daily (glargine) | Objectives: Compare the effects of Tirzep and insulin glargine on the kidney. Primary outcome: Tirzep slowed the eGFR decline (1.4 vs. 3.6 mL/min) and UACR increased with insulin while with Tirzep it decreased by −6.8% compared with insulin glargine. |
| Dahl D, SURPASS-5 [NCT04039503], 2022, phase 3 [26] | N = 475, 40 weeks | T2D with titrated insulin glargine on glycemic control cohort Drug: Tirzep 5 mg, 10 mg, 15 mg, or placebo SC injection once weekly | Objectives: Assess efficacy and safety of Tirzep in T2D patients receiving inadequate glycemic control. Primary outcome: Mean changes in HbA1c were −2.40%, −2.34%, and −0.86% with 10 mg, 15-mg Tirzep, and placebo, respectively. |
| Lincoff AM, SELECT [NCT03574597], 2023, phase 3 [27] | N = 17,604, Mean 137 weeks (Mean follow up 160 weeks) | Obese or overweight cohort with CVD and without T2D Drug: Sema 2.4 mg or placebo SC injection once weekly | Objectives: Assess reduction in risk of having cardiovascular events. Primary outcome = MACE (CVD mortality + nonfatal MI + nonfatal stroke). MACE 6.5% in Sema, 8.0% in placebo (Risk Diff. = 1.5%). SAE leading to permanent discontinuation was doubled in Sema. (16.6% in Sema, 8.2% in placebo). |
| Jastreboff AM, [NCT04881760], 2023, phase 2 [22] | N = 338, 48 weeks | Obese or overweight with weight-related comorbidities cohort without T2D Drug: Reta 1 mg, 4 mg, 8 mg, 12 mg, or placebo SC injection once weekly Retatrutide = multireceptor agonist of (GLP-1 + GIP + glucagon) | Objectives: Assess efficacy of Reta on body weight loss. Primary outcome: Change in body weight (%) by week 24. Results: Wt. change at 24 weeks −7.2% (1-mg), −12.9% (4-mg), −17.3% (8-mg), and 17.5% in the 12-mg retatrutide groups, −1.6% placebo. HR peaked at 24 weeks and declined thereafter. NB: Comparator should have been Tirzep, not placebo, to show adding glucagon would be safe. |
| Aronne L, SURMOUNT-4 [NCT04660643], 2024, phase 3 [28] | N = 670, 88 weeks (36 weeks onward placebo could be administered) | Cohort: Obese or overweight without T2D Drug: Tirzep 10 mg, 15 mg Mean wt. loss 20.9%. At week 36, randomized to continue Sema or placebo. | Objectives: Assess Tirzep effect on maintenance of body weight reduction. Primary outcome: Mean change in weight from week 36 until week 88 (%). Results: Those switched to placebo group regained 14% wt. (67%), those continuing tirzepatide lost an additional 5.5%. |
| Loomba R, [NCT04166773], 2024, phase 2 [29] | N = 190, 52 weeks | Cohort: Confirmed-MASH with liver fibrosis Drug: Tirzep 5 mg, 10 mg, 15 mg, or placebo SC injection once weekly | Objectives: Assess safety and efficacy of Tirzep as a MASH treatment. Primary outcome: Resolution of MASH without worsening of fibrosis by week 52. Results: Risk diff. 34%, 46%, and 53% at Tirzep 5-mg, 10-mg, and 15-mg, respectively. |
| Sanyal AJ, [NCT04771273], 2024, phase 2 [30] | N = 293, 48 weeks (24 weeks rapid-dose phase, 24 weeks maintenance phase) | Confirmed-MASH with fibrosis cohort Drug: Survo 2.4 mg, 4.8 mg, 6.0 mg, or placebo SC injection once weekly | Objectives: Assess safety, tolerability, and efficacy of Survo (dual agonist of glucagon and GLP-1 ra) as a MASH treatment. Primary outcome: Reduction in MASH with no worsening of fibrosis by week 48. Results: Risk diff. of liver fat decrease were 49%, 53%, and 43% in the 2.4 mg, 4.8 mg, and 6.0 mg Survo groups, respectively. More nausea in Survo (66% vs. 23%), diarrhea (49% vs. 23%), and vomiting (41% vs. 4%); SAEs were similar. |
| Sanyal AJ, [NCT04881760], 2024, phase 2a [31] | N = 98, 48 weeks | Obese or overweight with weight-related comorbidities cohort without T2D Drug: Reta 1 mg, 4 mg, 8 mg, 12 mg, or placebo SC injection once weekly | Objectives: Assess safety, tolerability, and efficacy of Reta for body weight loss, assess liver fat at 24 weeks. Results: At 24 weeks, normal LF was achieved by 27%, 52%, 79%, and 86% with 1 mg, 4 mg, 8 mg, and 12 mg of Reta and 0% (placebo). LF reductions were related to changes in wt., abdominal fat, and metabolic measures of insulin sensitivity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janket, S.-J.; Chatanaka, M.K.; Sohaei, D.; Tamimi, F.; Meurman, J.H.; Diamandis, E.P. Does Incretin Agonism Have Sustainable Efficacy? Cells 2024, 13, 1842. https://doi.org/10.3390/cells13221842
Janket S-J, Chatanaka MK, Sohaei D, Tamimi F, Meurman JH, Diamandis EP. Does Incretin Agonism Have Sustainable Efficacy? Cells. 2024; 13(22):1842. https://doi.org/10.3390/cells13221842
Chicago/Turabian StyleJanket, Sok-Ja, Miyo K. Chatanaka, Dorsa Sohaei, Faleh Tamimi, Jukka H. Meurman, and Eleftherios P. Diamandis. 2024. "Does Incretin Agonism Have Sustainable Efficacy?" Cells 13, no. 22: 1842. https://doi.org/10.3390/cells13221842
APA StyleJanket, S.-J., Chatanaka, M. K., Sohaei, D., Tamimi, F., Meurman, J. H., & Diamandis, E. P. (2024). Does Incretin Agonism Have Sustainable Efficacy? Cells, 13(22), 1842. https://doi.org/10.3390/cells13221842








