Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Electroporation-Based Transient Transfection
2.3. Immunoprecipitation (IP) Experiments
2.4. Sample Preparation and Immunoblot Analyses
2.5. In-Gel Digestion, Mass Spectrometry, & Database Search
2.6. Bioinformatic Approaches
2.7. In Vitro Kinase Screens
2.8. Statistics & Data Analysis
3. Results
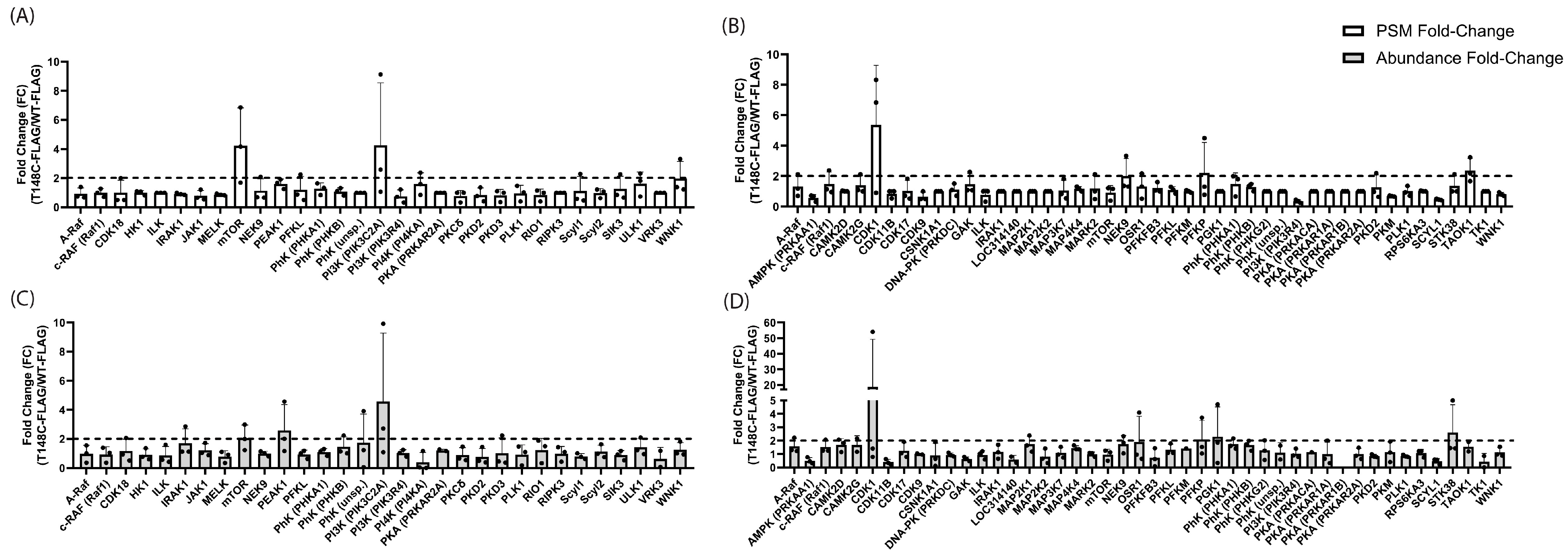
3.1. Phosphosite-Accurate Kinase-Substrate Crosslinking Assay (PhAXA)
3.2. Bioinformatics-Based Prediction of T148-Phosphorylating Kinases
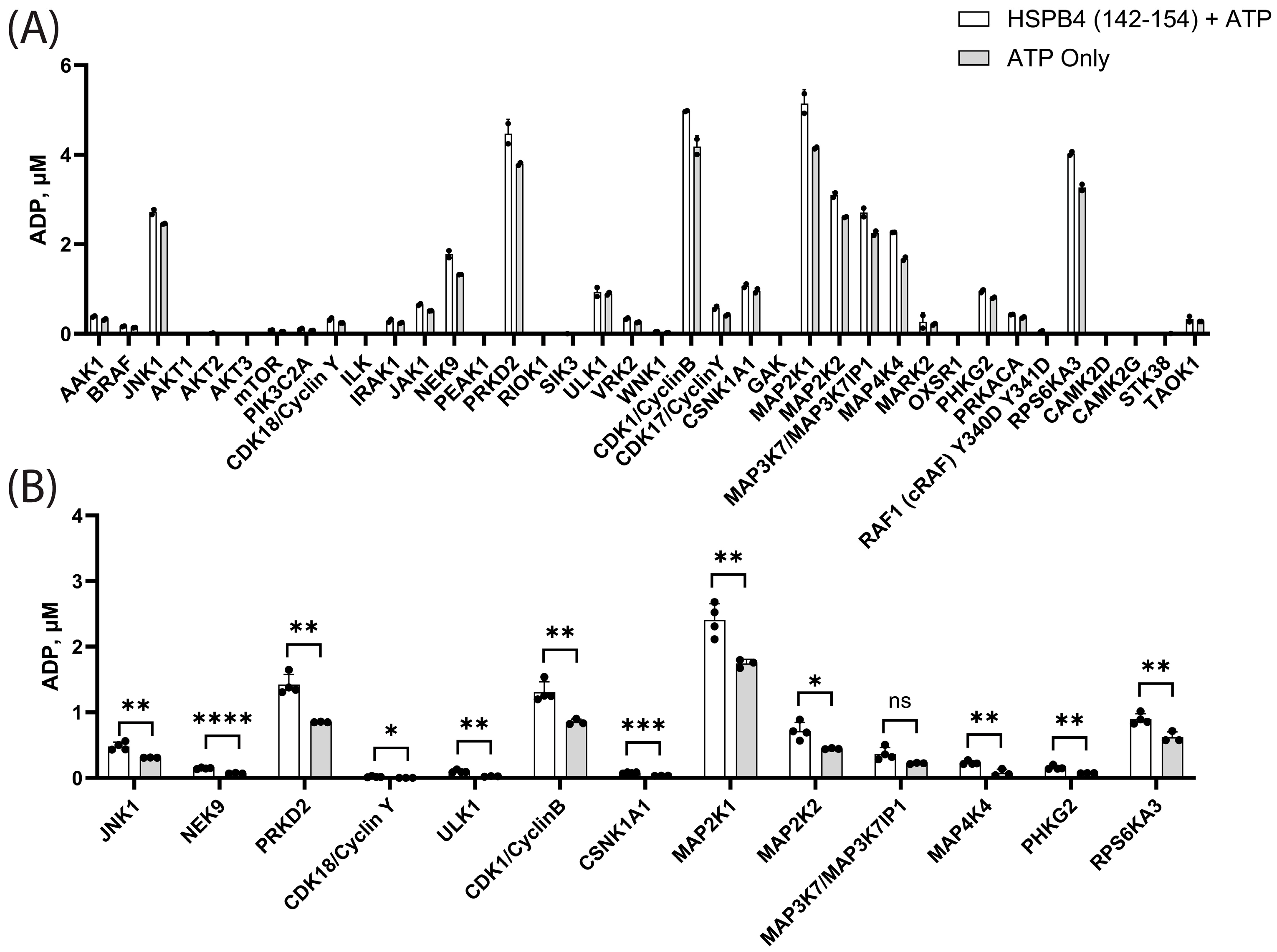
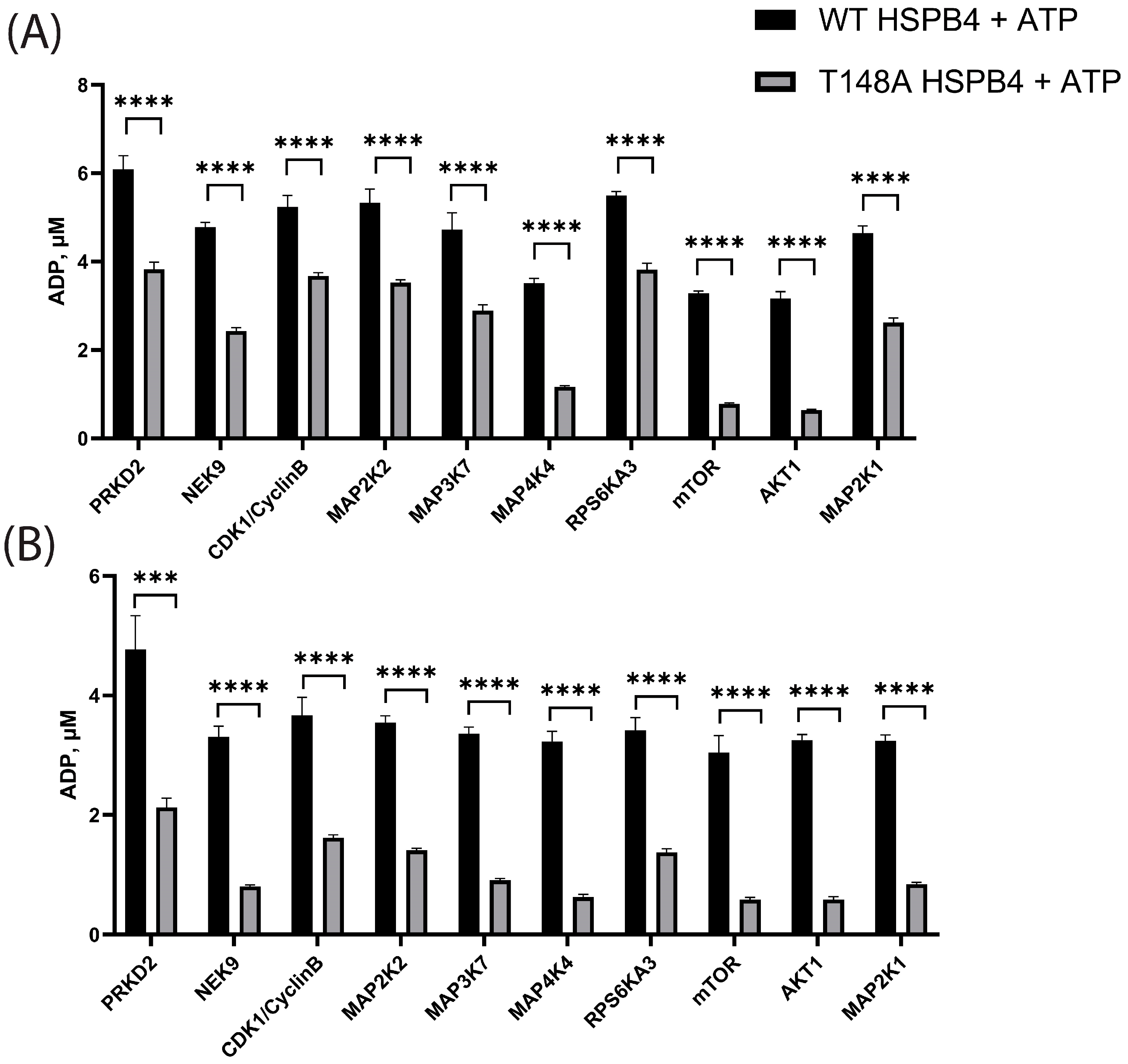
3.3. In Vitro Kinase Screens
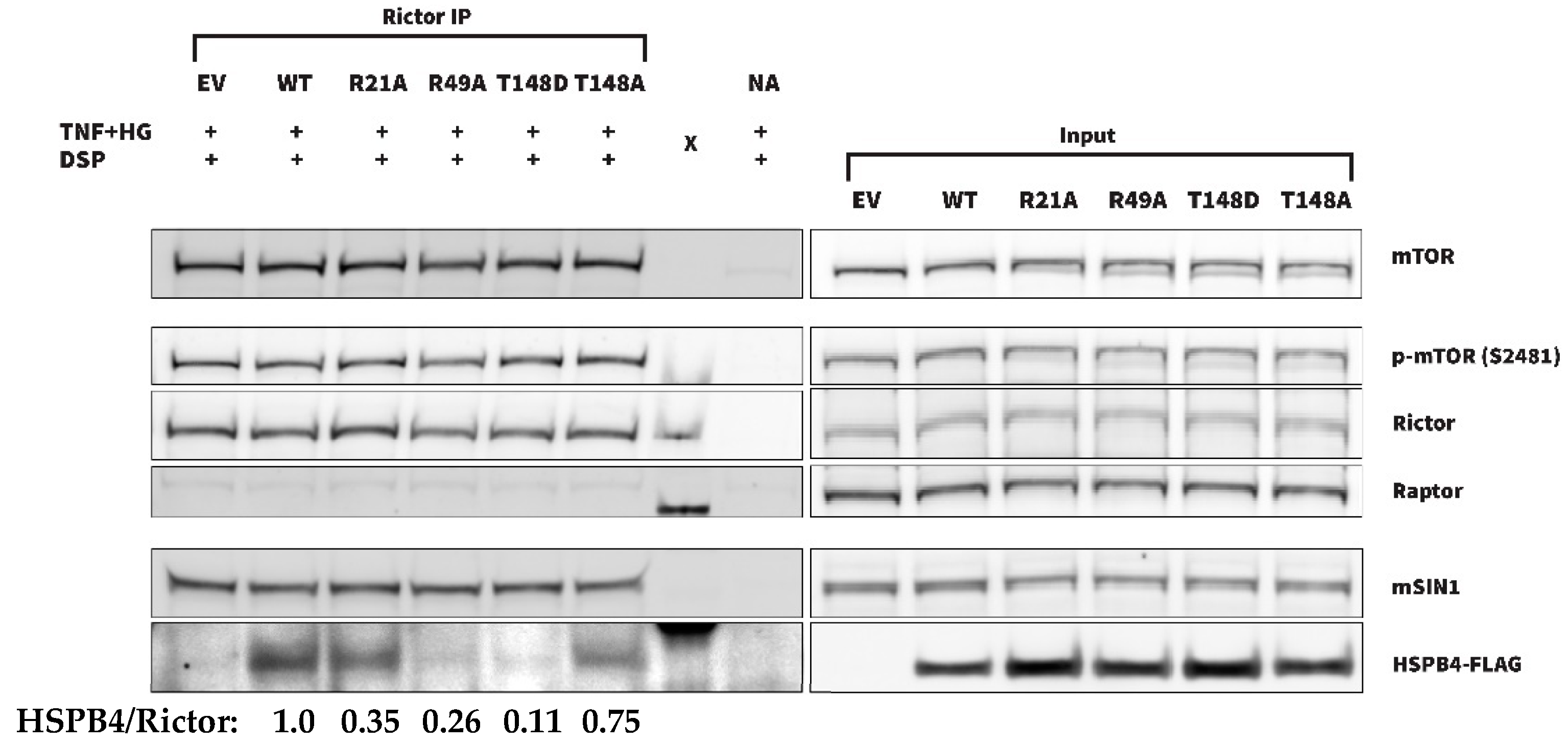
3.4. Characterization of mTOR-HSPB4 Interaction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kappé, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.M.; de Jong, W.W. The human genome encodes 10 α-crystallin–related small heat shock proteins: HspB1–10. Cell Stress Chaperones 2003, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Narberhaus, F. α-Crystallin-Type Heat Shock Proteins: Socializing Minichaperones in the Context of a Multichaperone Network. Microbiol. Mol. Biol. Rev. 2002, 66, 64–93. [Google Scholar] [CrossRef]
- Janowska, M.K.; Baughman, H.E.R.; Woods, C.N.; Klevit, R.E. Mechanisms of Small Heat Shock Proteins. Cold Spring Harb. Perspect. Biol. 2019, 11, a034025. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Weinkauf, S.; Buchner, J. Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 2019, 294, 2121–2132. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.A.; Reddy, G.B. Modulation of alpha-crystallin chaperone activity: A target to prevent or delay cataract? IUBMB Life 2009, 61, 485–495. [Google Scholar] [CrossRef]
- Sharma, K.K.; Santhoshkumar, P. Lens aging: Effects of crystallins. Biochim. Biophys. Acta 2009, 1790, 1095–1108. [Google Scholar] [CrossRef]
- Fort, P.E.; Lampi, K.J. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp. Eye Res. 2011, 92, 98–103. [Google Scholar] [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim. Biophys. Acta 2016, 1860, 258–268. [Google Scholar] [CrossRef]
- Alge, C.S.; Priglinger, S.G.; Neubauer, A.S.; Kampik, A.; Zillig, M.; Bloemendal, H.; Welge-Lussen, U. Retinal pigment epithelium is protected against apoptosis by alpha B-crystallin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3575–3582. [Google Scholar]
- Nishikawa, S.; Ishiguro, S.; Kato, K.; Tamai, M. A transient expression of alpha B-crystallin in the developing rat retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4159–4164. [Google Scholar]
- Robinson, M.L.; Overbeek, P.A. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2276–2284. [Google Scholar]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Farjo, R.; Yoshida, S.; Kern, T.S.; Swaroop, A.; Andley, U.P. A comprehensive analysis of the expression of crystallins in mouse retina. Mol. Vis. 2003, 9, 410–419. [Google Scholar]
- Sakaguchi, H.; Miyagi, M.; Darrow, R.M.; Crabb, J.S.; Hollyfield, J.G.; Organisciak, D.T.; Crabb, J.W. Intense light exposure changes the crystallin content in retina. Exp. Eye Res. 2003, 76, 131–133. [Google Scholar] [CrossRef]
- Shi, Y.; Su, C.; Wang, J.T.; Du, B.; Dong, L.J.; Liu, A.H.; Li, X.R. Temporal and spatial changes in VEGF, alpha A- and alpha B-crystallin expression in a mouse model of oxygen-induced retinopathy. Int. J. Clin. Exp. Med. 2015, 8, 3349–3359. [Google Scholar]
- Andley, U.P. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef]
- Deretic, D.; Aebersold, R.H.; Morrison, H.D.; Papermaster, D.S. Alpha A- and alpha B-crystallin in the retina. Association with the post-Golgi compartment of frog retinal photoreceptors. J. Biol. Chem. 1994, 269, 16853–16861. [Google Scholar] [CrossRef]
- Kase, S.; Meghpara, B.B.; Ishida, S.; Rao, N.A. Expression of alpha-crystallin in the retina of human sympathetic ophthalmia. Mol. Med. Rep. 2012, 5, 395–399. [Google Scholar] [CrossRef]
- Lewis, G.P.; Erickson, P.A.; Kaska, D.D.; Fisher, S.K. An immunocytochemical comparison of Muller cells and astrocytes in the cat retina. Exp. Eye Res. 1988, 47, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; Tomooka, B.H.; van Noort, J.M.; Wawrousek, E.F.; O’Connor, K.C.; Hafler, D.A.; Sobel, R.A.; Robinson, W.H.; Steinman, L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 2007, 448, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A.A.; Fox, L.; Smith, J.; Degenstein, L. Antiserum to lens antigens immunostains Muller glia cells in the neural retina. Proc. Natl. Acad. Sci. USA 1985, 82, 5570–5573. [Google Scholar] [CrossRef] [PubMed]
- Ruebsam, A.; Dulle, J.E.; Myers, A.M.; Sakrikar, D.; Green, K.M.; Khan, N.W.; Schey, K.; Fort, P.E. A specific phosphorylation regulates the protective role of alphaA-crystallin in diabetes. JCI Insight 2018, 3, e97919. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, Y.; Kwong, J.M.; Caprioli, J.; Piri, N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3869–3875. [Google Scholar] [CrossRef]
- Besirli, C.G.; Nath, M.; Yao, J.; Pawar, M.; Myers, A.M.; Zacks, D.; Fort, P.E. HSPB4/CRYAA Protect Photoreceptors during Retinal Detachment in Part through FAIM2 Regulation. Neurol. Int. 2024, 16, 905–917. [Google Scholar] [CrossRef]
- Ying, X.; Zhang, J.; Wang, Y.; Wu, N.; Wang, Y.; Yew, D.T. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J. Mol. Neurosci. 2008, 35, 253–258. [Google Scholar] [CrossRef]
- Anders, F.; Liu, A.; Mann, C.; Teister, J.; Lauzi, J.; Thanos, S.; Grus, F.H.; Pfeiffer, N.; Prokosch, V. The Small Heat Shock Protein alpha-Crystallin B Shows Neuroprotective Properties in a Glaucoma Animal Model. Int. J. Mol. Sci. 2017, 18, 2418. [Google Scholar] [CrossRef] [PubMed]
- Losiewicz, M.K.; Fort, P.E. Diabetes impairs the neuroprotective properties of retinal alpha-crystallins. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5034–5042. [Google Scholar] [CrossRef]
- Li, R.; Reiser, G. Phosphorylation of Ser45 and Ser59 of alphaB-crystallin and p38/extracellular regulated kinase activity determine alphaB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide- and staurosporine-induced cell death. J. Neurochem. 2011, 118, 354–364. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Z.; Reiser, G. Specific phosphorylation of alphaA-crystallin is required for the alphaA-crystallin-induced protection of astrocytes against staurosporine and C2-ceramide toxicity. Neurochem. Int. 2012, 60, 652–658. [Google Scholar] [CrossRef]
- Nath, M.; Fort, P.E. alphaA-Crystallin Mediated Neuroprotection in the Retinal Neurons Is Independent of Protein Kinase B. Front. Neurosci. 2022, 16, 912757. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Sluzala, Z.B.; Phadte, A.S.; Shan, Y.; Myers, A.M.; Fort, P.E. Evidence for Paracrine Protective Role of Exogenous αA-Crystallin in Retinal Ganglion Cells. Eneuro 2022, 9. [Google Scholar] [CrossRef]
- Ito, H.; Kamei, K.; Iwamoto, I.; Inaguma, Y.; Nohara, D.; Kato, K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J. Biol. Chem. 2001, 276, 5346–5352. [Google Scholar] [CrossRef]
- Peschek, J.; Braun, N.; Rohrberg, J.; Back, K.C.; Kriehuber, T.; Kastenmuller, A.; Weinkauf, S.; Buchner, J. Regulated structural transitions unleash the chaperone activity of alphaB-crystallin. Proc. Natl. Acad. Sci. USA 2013, 110, E3780–E3789. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Raman, B.; Ramakrishna, T.; Rao Ch, M. Effect of phosphorylation on alpha B-crystallin: Differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. J. Mol. Biol. 2008, 375, 1040–1051. [Google Scholar] [CrossRef]
- Aquilina, J.A.; Benesch, J.L.; Ding, L.L.; Yaron, O.; Horwitz, J.; Robinson, C.V. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem. 2004, 279, 28675–28680. [Google Scholar] [CrossRef] [PubMed]
- Muchowski, P.J.; Wu, G.J.; Liang, J.J.; Adman, E.T.; Clark, J.I. Site-directed mutations within the core “alpha-crystallin” domain of the small heat-shock protein, human alphaB-crystallin, decrease molecular chaperone functions. J. Mol. Biol. 1999, 289, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Ecroyd, H.; Meehan, S.; Horwitz, J.; Aquilina, J.A.; Benesch, J.L.; Robinson, C.V.; Macphee, C.E.; Carver, J.A. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem. J. 2007, 401, 129–141. [Google Scholar] [CrossRef]
- Kamradt, M.C.; Chen, F.; Sam, S.; Cryns, V.L. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J. Biol. Chem. 2002, 277, 38731–38736. [Google Scholar] [CrossRef]
- Jeong, W.J.; Rho, J.H.; Yoon, Y.G.; Yoo, S.H.; Jeong, N.Y.; Ryu, W.Y.; Ahn, H.B.; Park, W.C.; Rho, S.H.; Yoon, H.S.; et al. Cytoplasmic and nuclear anti-apoptotic roles of alphaB-crystallin in retinal pigment epithelial cells. PLoS ONE 2012, 7, e45754. [Google Scholar] [CrossRef]
- Aggeli, I.K.; Beis, I.; Gaitanaki, C. Oxidative stress and calpain inhibition induce alpha B-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell. Signal 2008, 20, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.E.; Hoover, H.E.; Thuerauf, D.J.; Glembotski, C.C. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ. Res. 2003, 92, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.; Marcus, K.; Sickmann, A.; Herrmann, M.; Klose, J.; Meyer, H.E. Identification of phosphorylation and acetylation sites in alphaA-crystallin of the eye lens ( mus musculus) after two-dimensional gel electrophoresis. Anal. Bioanal. Chem. 2003, 376, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Nakamura, M.; Wolpert, E.B.; Reiter, C.E.; Seigel, G.M.; Antonetti, D.A.; Gardner, T.W. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001, 276, 32814–32821. [Google Scholar] [CrossRef]
- Mehlen, P.; Mehlen, A.; Guillet, D.; Preville, X.; Arrigo, A.P. Tumor necrosis factor-alpha induces changes in the phosphorylation, cellular localization, and oligomerization of human hsp27, a stress protein that confers cellular resistance to this cytokine. J. Cell. Biochem. 1995, 58, 248–259. [Google Scholar] [CrossRef]
- Arrigo, A.P. Tumor necrosis factor induces the rapid phosphorylation of the mammalian heat shock protein hsp28. Mol. Cell. Biol. 1990, 10, 1276–1280. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Menon, A.; Garner, A.L. Chemoproteomic Profiling Uncovers CDK4-Mediated Phosphorylation of the Translational Suppressor 4E-BP1. Cell Chem. Biol. 2019, 26, 980–990.e8. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lee, T.Y.; Liang, H.K.; Huang, C.M.; Wang, T.Y.; Yang, Y.H.; Chu, C.H.; Huang, H.D.; Ko, M.T.; Hwang, J.K. KinasePhos 2.0: A web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 2007, 35, W588–W594. [Google Scholar] [CrossRef]
- Ma, R.; Li, S.; Li, W.; Yao, L.; Huang, H.D.; Lee, T.Y. KinasePhos 3.0: Redesign and expansion of the prediction on kinase-specific phosphorylation sites. Genom. Proteom. Bioinform. 2022, 21, 228–241. [Google Scholar] [CrossRef]
- Blom, N.; Sicheritz-Ponten, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef]
- Li, S.-H.; Zhang, J.; Zhao, Y.-W.; Dao, F.-Y.; Ding, H.; Chen, W.; Tang, H. iPhoPred: A Predictor for Identifying Phosphorylation Sites in Human Protein. IEEE Access 2019, 7, 177517–177528. [Google Scholar] [CrossRef]
- Dou, Y.; Yao, B.; Zhang, C. PhosphoSVM: Prediction of phosphorylation sites by integrating various protein sequence attributes with a support vector machine. Amino Acids 2014, 46, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- de Castro, E.; Sigrist, C.J.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Lin, S.; Deng, W.; Zhou, J.; Zhang, Y.; Shi, Y.; Peng, D.; Xue, Y. GPS 5.0: An Update on the Prediction of Kinase-specific Phosphorylation Sites in Proteins. Genom. Proteom. Bioinform. 2020, 18, 72–80. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 7, 310–322. [Google Scholar]
- Keshava Prasad, T.S.; Goel, R.; Kandasamy, K.; Keerthikumar, S.; Kumar, S.; Mathivanan, S.; Telikicherla, D.; Raju, R.; Shafreen, B.; Venugopal, A.; et al. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009, 37, D767–D772. [Google Scholar] [CrossRef]
- Amanchy, R.; Periaswamy, B.; Mathivanan, S.; Reddy, R.; Tattikota, S.G.; Pandey, A. A curated compendium of phosphorylation motifs. Nat. Biotechnol. 2007, 25, 285–286. [Google Scholar] [CrossRef]
- Safaei, J.; Manuch, J.; Gupta, A.; Stacho, L.; Pelech, S. Prediction of 492 human protein kinase substrate specificities. Proteome Sci. 2011, 9 (Suppl. S1), S6. [Google Scholar] [CrossRef]
- Dinkel, H.; Chica, C.; Via, A.; Gould, C.M.; Jensen, L.J.; Gibson, T.J.; Diella, F. Phospho.ELM: A database of phosphorylation sites--update 2011. Nucleic Acids Res. 2011, 39, D261–D267. [Google Scholar] [CrossRef]
- UniProt, C. The universal protein resource (UniProt). Nucleic Acids Res. 2008, 36, D190–D195. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Yaron, T.M.; Huntsman, E.M.; Kerelsky, A.; Song, J.; Regev, A.; Lin, T.Y.; Liberatore, K.; Cizin, D.M.; Cohen, B.M.; et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature 2023, 613, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Obenauer, J.C.; Cantley, L.C.; Yaffe, M.B. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003, 31, 3635–3641. [Google Scholar] [CrossRef]
- Riel-Mehan, M.M.; Shokat, K.M. A crosslinker based on a tethered electrophile for mapping kinase-substrate networks. Chem. Biol. 2014, 21, 585–590. [Google Scholar] [CrossRef][Green Version]
- Kemp, B.E.; Pearson, R.B. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 1990, 15, 342–346. [Google Scholar] [CrossRef]
- Voorter, C.E.; Mulders, J.W.; Bloemendal, H.; de Jong, W.W. Some aspects of the phosphorylation of alpha-crystallin A. Eur. J. Biochem. 1986, 160, 203–210. [Google Scholar] [CrossRef]
- Spector, A.; Chiesa, R.; Sredy, J.; Garner, W. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc. Natl. Acad. Sci. USA 1985, 82, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, Y.T.; Tsai, C.F.; Chen, Y.J.; Lee, J.S.; Chiou, S.H. Phosphoproteomics characterization of novel phosphorylated sites of lens proteins from normal and cataractous human eye lenses. Mol. Vis. 2011, 17, 186–198. [Google Scholar]
- Liu, J.P.; Schlosser, R.; Ma, W.Y.; Dong, Z.; Feng, H.; Liu, L.; Huang, X.Q.; Liu, Y.; Li, D.W. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp. Eye Res. 2004, 79, 393–403. [Google Scholar] [CrossRef]
- Thedieck, K.; Polak, P.; Kim, M.L.; Molle, K.D.; Cohen, A.; Jeno, P.; Arrieumerlou, C.; Hall, M.N. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE 2007, 2, e1217. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Ruegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Yang, Q.; Inoki, K.; Ikenoue, T.; Guan, K.L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes. Dev. 2006, 20, 2820–2832. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Huang, X.; Boudeau, J.; Pawlowski, R.; Wullschleger, S.; Deak, M.; Ibrahim, A.F.; Gourlay, R.; Magnuson, M.A.; Alessi, D.R. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 2007, 405, 513–522. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, J.; Takagi, E.; Wang, F.; Saha, B.; Liu, X.; Joubert, L.M.; Gleason, C.E.; Jin, M.; Li, C.; et al. Interactions between mTORC2 core subunits Rictor and mSin1 dictate selective and context-dependent phosphorylation of substrate kinases SGK1 and Akt. J. Biol. Chem. 2022, 298, 102288. [Google Scholar] [CrossRef]
- Losiewicz, M.K.; Elghazi, L.; Fingar, D.C.; Rajala, R.V.S.; Lentz, S.I.; Fort, P.E.; Abcouwer, S.F.; Gardner, T.W. mTORC1 and mTORC2 expression in inner retinal neurons and glial cells. Exp. Eye Res. 2020, 197, 108131. [Google Scholar] [CrossRef]
- Biswas, A.; Miller, A.; Oya-Ito, T.; Santhoshkumar, P.; Bhat, M.; Nagaraj, R.H. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry 2006, 45, 4569–4577. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Ito, H.; Kamei, K.; Inaguma, Y.; Iwamoto, I.; Saga, S. Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J. Biol. Chem. 1998, 273, 28346–28354. [Google Scholar] [CrossRef]
- Hoover, H.E.; Thuerauf, D.J.; Martindale, J.J.; Glembotski, C.C. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J. Biol. Chem. 2000, 275, 23825–23833. [Google Scholar] [CrossRef]
- Kasahara, K.; Ikuta, T.; Chida, K.; Asakura, R.; Kuroki, T. Rapid phosphorylation of 28-kDa heat-shock protein by treatment with okadaic acid and phorbol ester of BALB/MK-2 mouse keratinocytes. Eur. J. Biochem. 1993, 213, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Maizels, E.T.; Peters, C.A.; Kline, M.; Cutler, R.E., Jr.; Shanmugam, M.; Hunzicker-Dunn, M. Heat-shock protein-25/27 phosphorylation by the delta isoform of protein kinase C. Biochem. J. 1998, 332 Pt 3, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Butt, E.; Immler, D.; Meyer, H.E.; Kotlyarov, A.; Laass, K.; Gaestel, M. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: Phosphorylation-induced actin polymerization caused by HSP27 mutants. J. Biol. Chem. 2001, 276, 7108–7113. [Google Scholar] [CrossRef]
- Rane, M.J.; Pan, Y.; Singh, S.; Powell, D.W.; Wu, R.; Cummins, T.; Chen, Q.; McLeish, K.R.; Klein, J.B. Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 2003, 278, 27828–27835. [Google Scholar] [CrossRef]
- Scroggins, B.T.; Neckers, L. Post-translational modification of heat-shock protein 90: Impact on chaperone function. Expert. Opin. Drug Discov. 2007, 2, 1403–1414. [Google Scholar] [CrossRef]
- Fan, Q.; Huang, L.; Zhu, H.; Zhang, K.; Ye, H.; Luo, Y.; Sun, X.; Zhou, P.; Lu, Y. Identification of proteins that interact with alpha A-crystallin using a human proteome microarray. Mol. Vis. 2014, 20, 117–124. [Google Scholar]
- Martin, J.; Masri, J.; Bernath, A.; Nishimura, R.N.; Gera, J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. Biochem. Biophys. Res. Commun. 2008, 372, 578–583. [Google Scholar] [CrossRef]
- Cuellar, J.; Ludlam, W.G.; Tensmeyer, N.C.; Aoba, T.; Dhavale, M.; Santiago, C.; Bueno-Carrasco, M.T.; Mann, M.J.; Plimpton, R.L.; Makaju, A.; et al. Structural and functional analysis of the role of the chaperonin CCT in mTOR complex assembly. Nat. Commun. 2019, 10, 2865. [Google Scholar] [CrossRef]
- Kim, S.G.; Hoffman, G.R.; Poulogiannis, G.; Buel, G.R.; Jang, Y.J.; Lee, K.W.; Kim, B.Y.; Erikson, R.L.; Cantley, L.C.; Choo, A.Y.; et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 2013, 49, 172–185. [Google Scholar] [CrossRef]
- Takai, H.; Xie, Y.; de Lange, T.; Pavletich, N.P. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes. Dev. 2010, 24, 2019–2030. [Google Scholar] [CrossRef]
- Chou, P.C.; Rajput, S.; Zhao, X.; Patel, C.; Albaciete, D.; Oh, W.J.; Daguplo, H.Q.; Patel, N.; Su, B.; Werlen, G.; et al. mTORC2 Is Involved in the Induction of RSK Phosphorylation by Serum or Nutrient Starvation. Cells 2020, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, N.; Matsuyama, S.; Voss, O.; Doseff, A.I.; Song, K.; Danielpour, D.; Nagaraj, R.H. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010, 1, e31. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.J.; Coxon, P.Y.; Powell, D.W.; Webster, R.; Klein, J.B.; Pierce, W.; Ping, P.; McLeish, K.R. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J. Biol. Chem. 2001, 276, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Matsuzaki, H.; Tanaka, M.; Takemura, Y.; Kuroda, S.; Ono, Y.; Kikkawa, U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. Febs Lett. 1997, 410, 493–498. [Google Scholar] [CrossRef]
- Phadte, A.S.; Sluzala, Z.B.; Fort, P.E. Therapeutic Potential of alpha-Crystallins in Retinal Neurodegenerative Diseases. Antioxidants 2021, 10, 1001. [Google Scholar] [CrossRef]


| Phosphorylation Prediction Program | Kinase Predictions |
|---|---|
| HPRD PhosphoMotif Finder | PKA, PKC, GRK1, CK2 |
| Kinase Library | PBK, GRK7, LKB1, skMLCK, JNK1, GAK, JNK2, JNK3, MPSK1, p38D, BIKE, AAK1, BRAF, MPSK1, VRK2 |
| KinasePhos2.0 | PKB, GRK, PKC, CDK, CK2, PDK |
| KinasePhos3.0 | CMGC group, HT1_ARATH |
| PhosphoNet | MEK4, MEK7, MEK1, LKB1, SgK288, IRAK4, MEK2, IRAK1, ADCK3, MELK, IKKe, IKKb, SNRK, PKG2, PIM1, IKKa, p38A, MAPK |
| ScanProsite | CK2 |
| GPS 6.0 | TKL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sluzala, Z.B.; Shan, Y.; Elghazi, L.; Cárdenas, E.L.; Hamati, A.; Garner, A.L.; Fort, P.E. Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation. Cells 2024, 13, 2000. https://doi.org/10.3390/cells13232000
Sluzala ZB, Shan Y, Elghazi L, Cárdenas EL, Hamati A, Garner AL, Fort PE. Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation. Cells. 2024; 13(23):2000. https://doi.org/10.3390/cells13232000
Chicago/Turabian StyleSluzala, Zachary B., Yang Shan, Lynda Elghazi, Emilio L. Cárdenas, Angelina Hamati, Amanda L. Garner, and Patrice E. Fort. 2024. "Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation" Cells 13, no. 23: 2000. https://doi.org/10.3390/cells13232000
APA StyleSluzala, Z. B., Shan, Y., Elghazi, L., Cárdenas, E. L., Hamati, A., Garner, A. L., & Fort, P. E. (2024). Novel mTORC2/HSPB4 Interaction: Role and Regulation of HSPB4 T148 Phosphorylation. Cells, 13(23), 2000. https://doi.org/10.3390/cells13232000







