Adaptive Optics-Transscleral Flood Illumination Imaging of Retinal Pigment Epithelium in Dry Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
3. Results
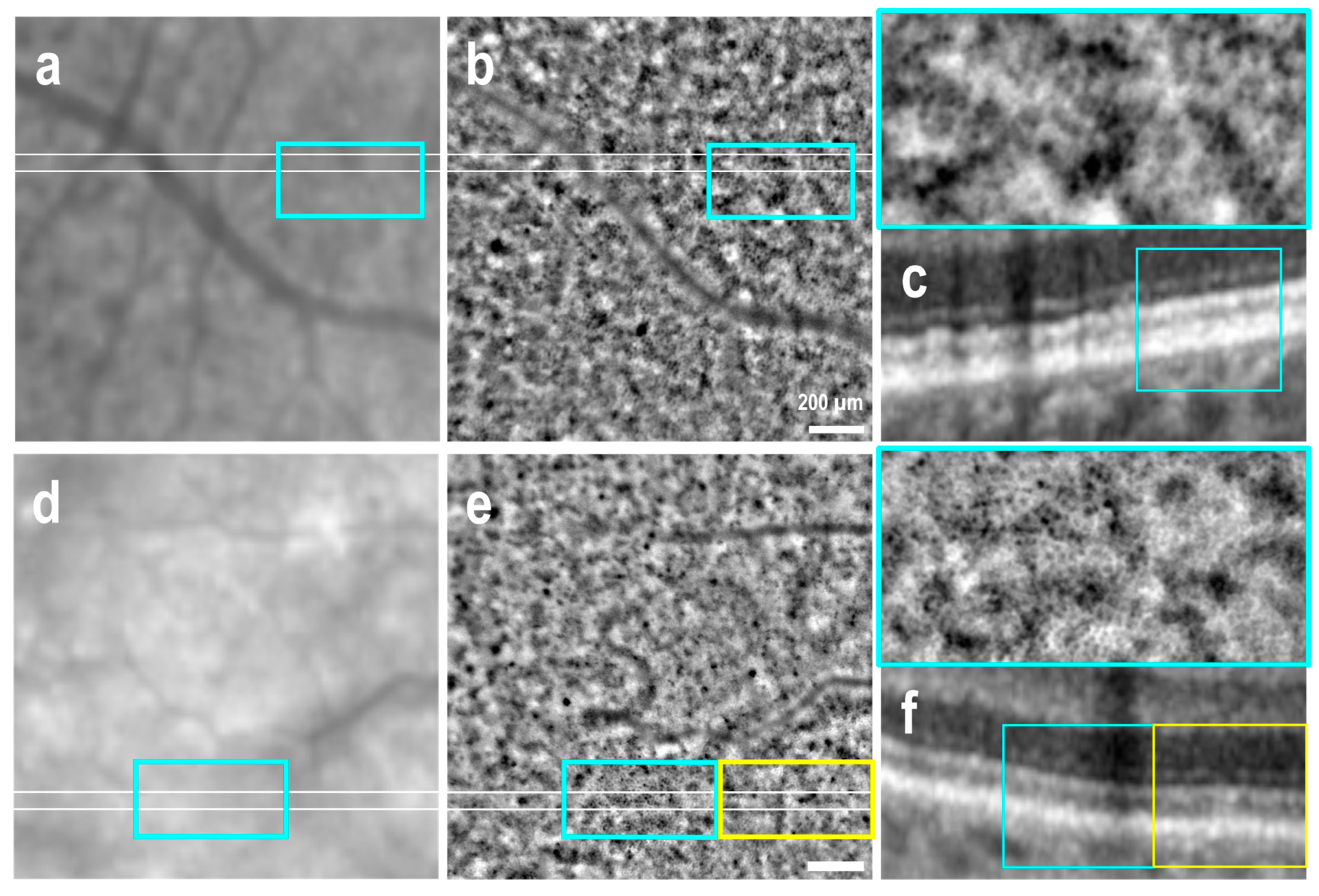
3.1. Clinically Healthy Areas
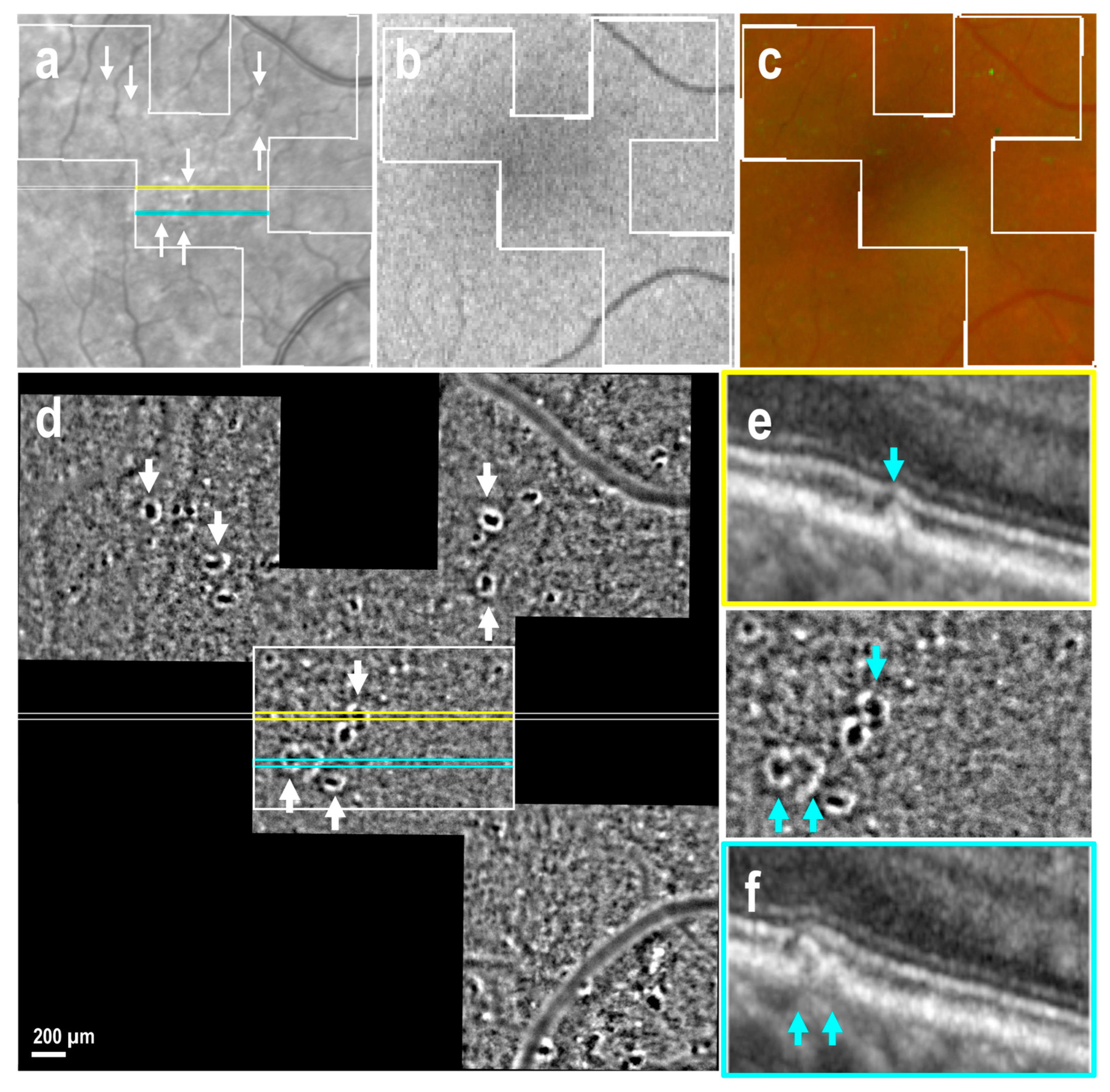
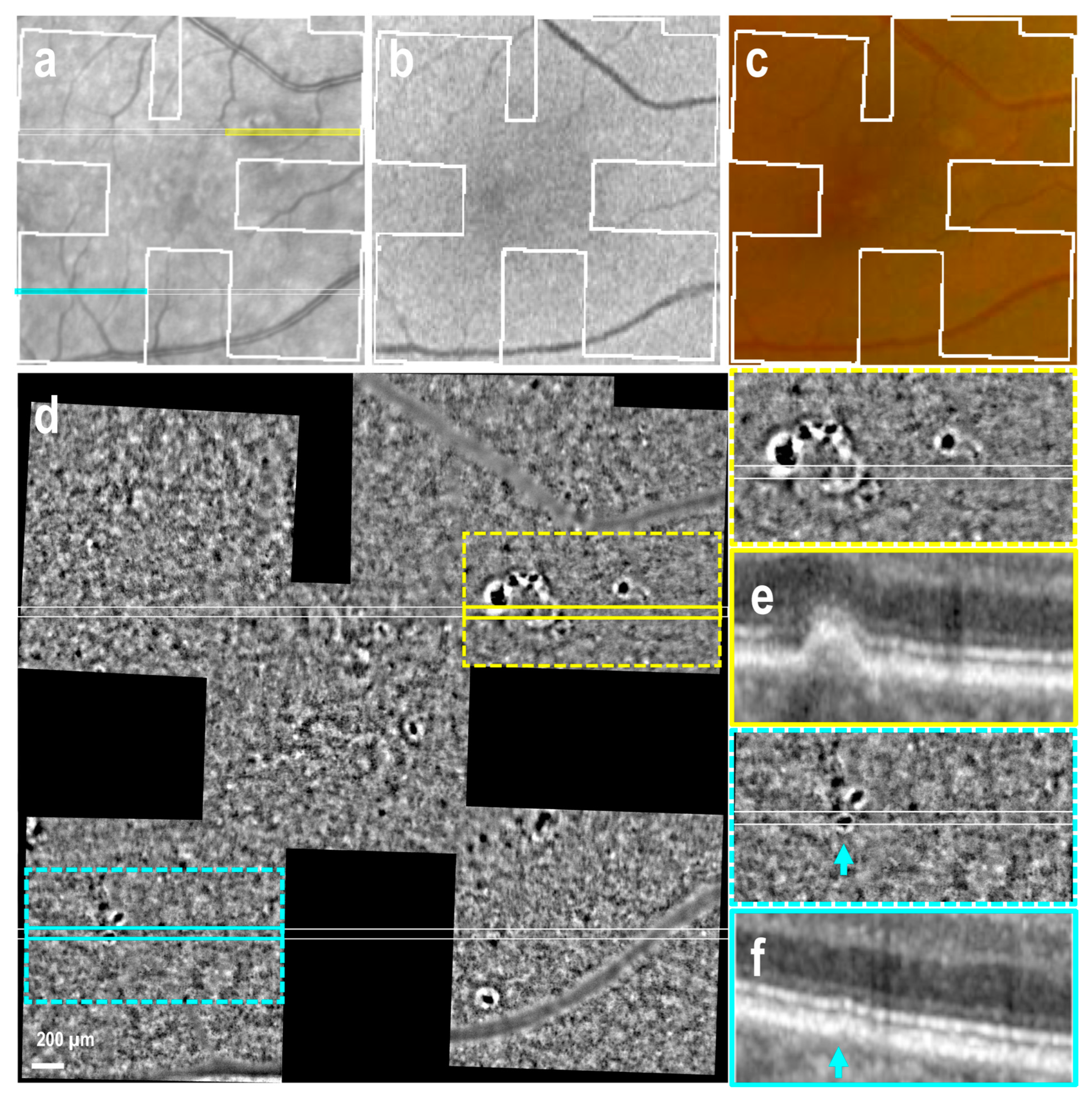
3.2. Drusen of Various Size
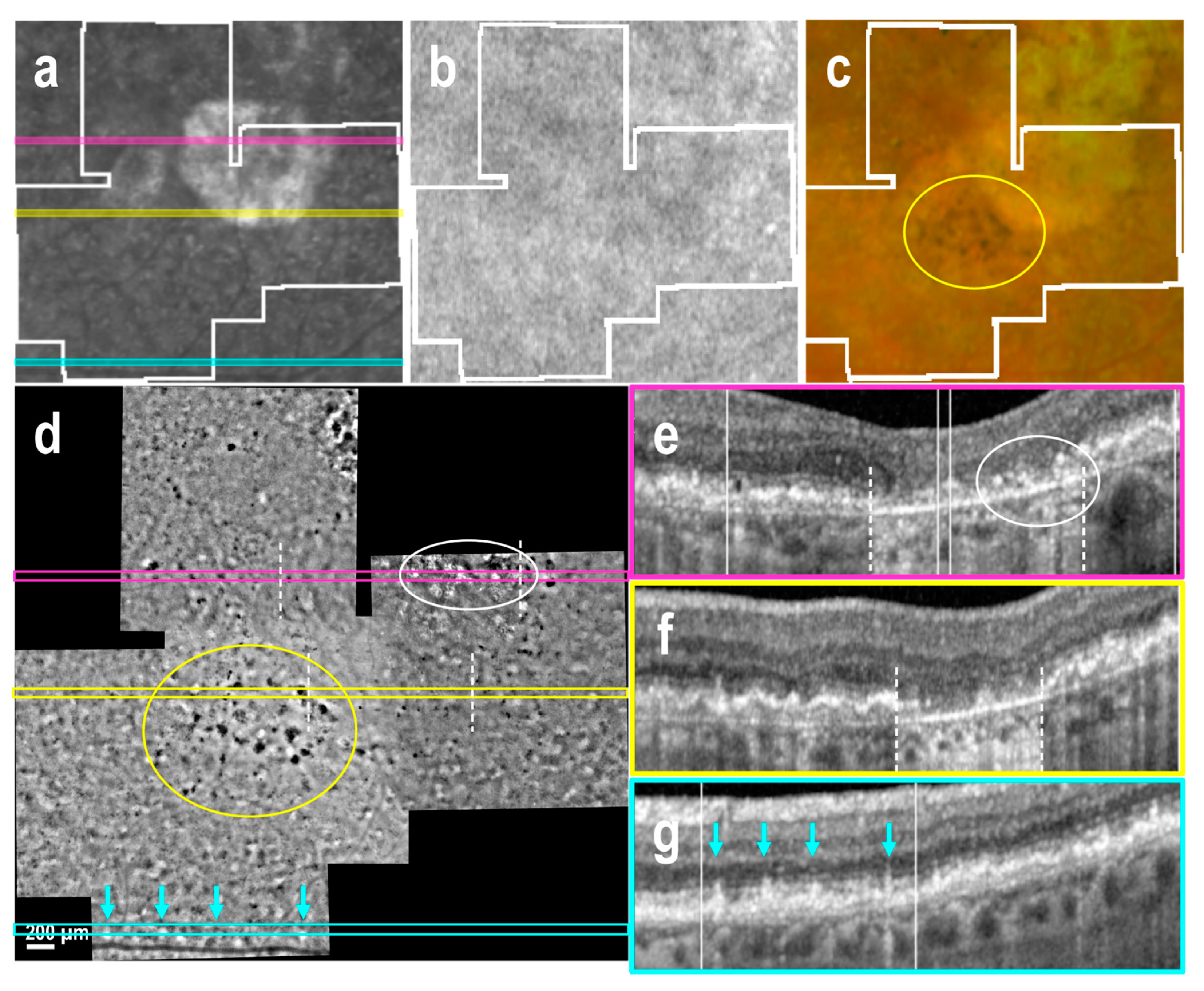
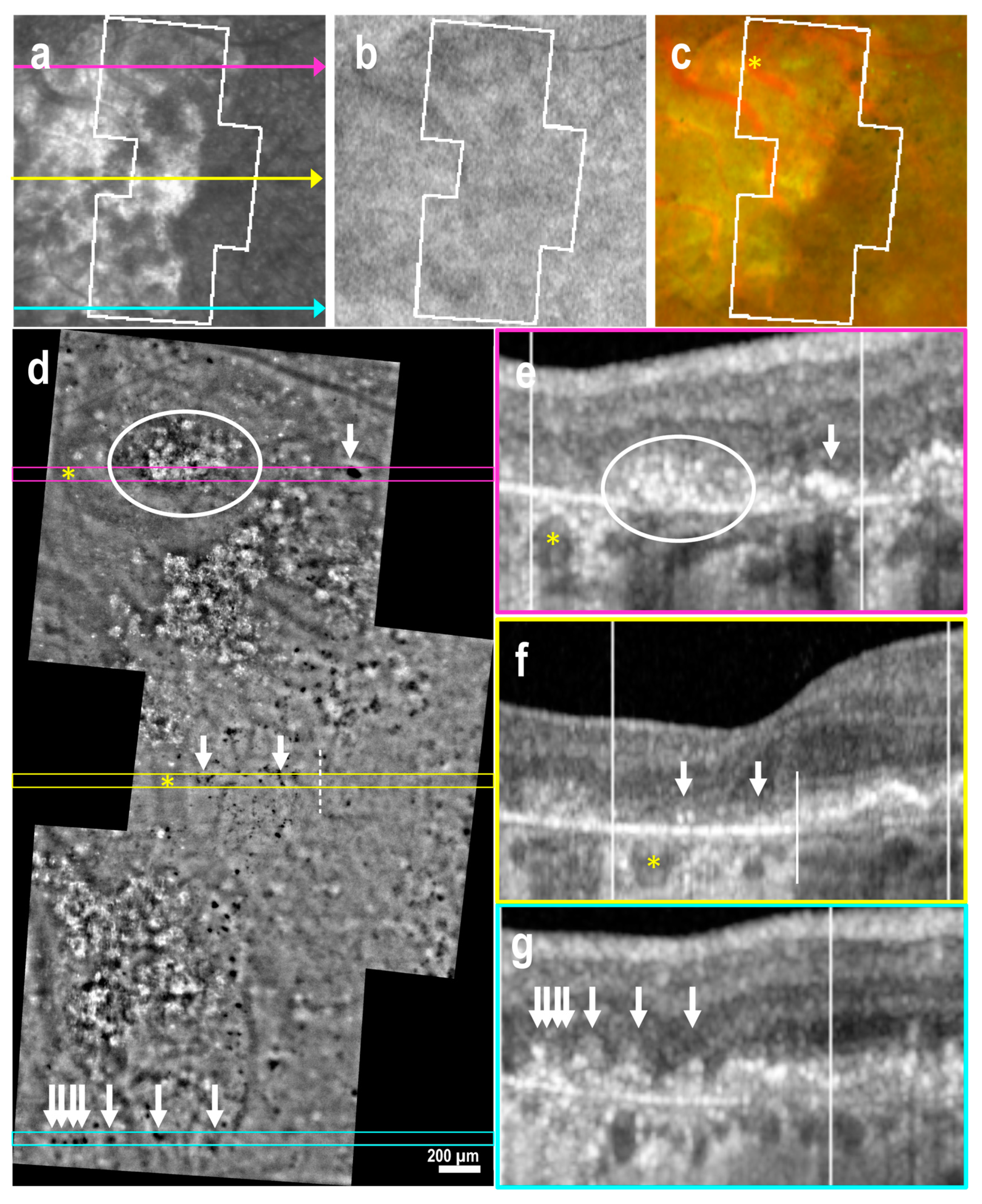
3.3. Subretinal Drusenoid Deposits
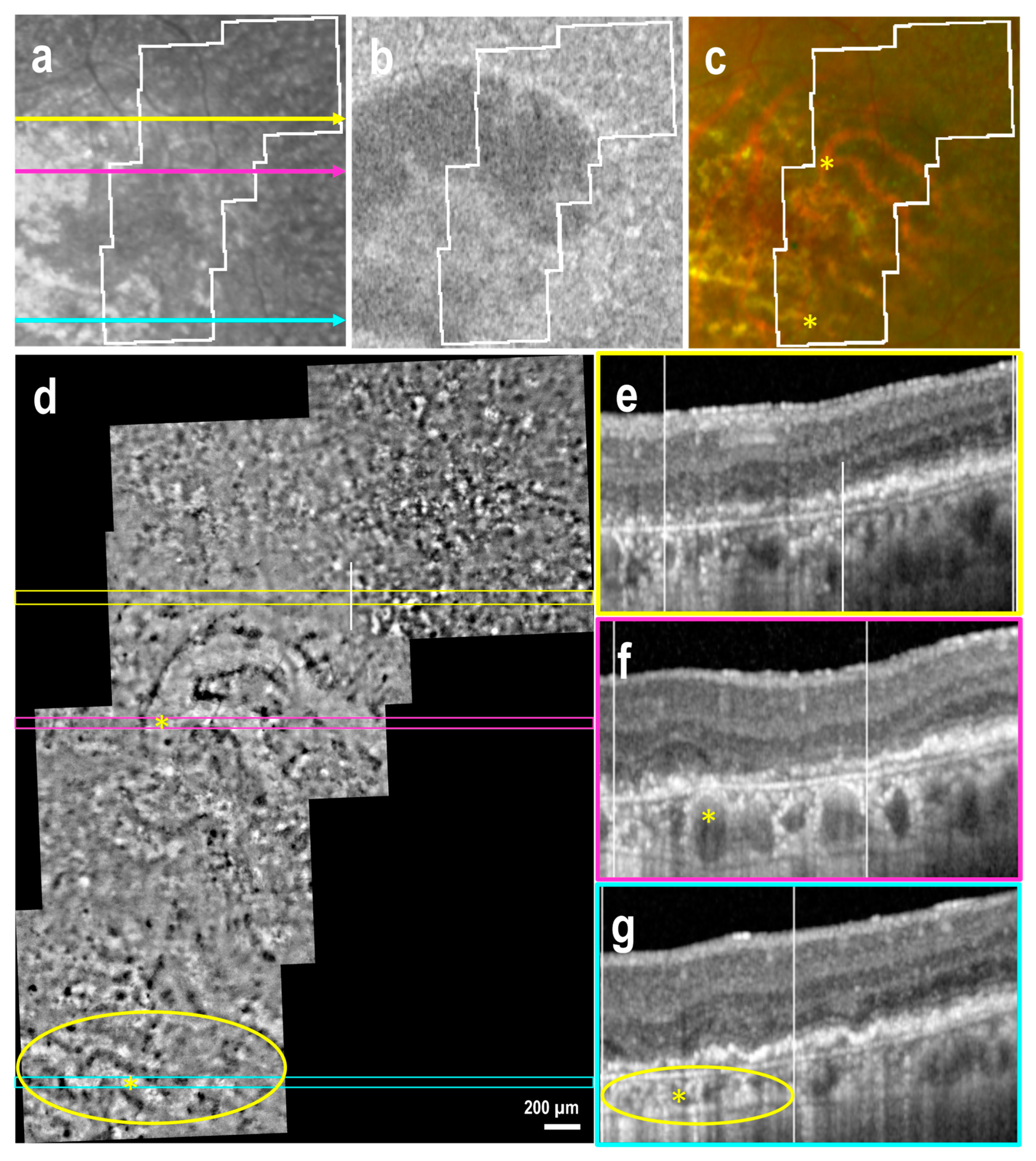
3.4. Atrophic Regions and Borders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | axial length |
| AMD | age-related macular degeneration |
| AO | adaptive optics |
| BCVA | best-corrected visual acuity |
| FAF | fundus autofluorescence |
| IOP | intraocular pressure |
| IR | infrared |
| OCT | optical coherence tomography |
| RE | refractory error |
| RORA | RPE and outer retinal atrophy |
| RPE | retinal pigment epithelium |
| SLO | scanning laser ophthalmoscopy |
References
- de Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef]
- Chaudhuri, M.; Hassan, Y.; Vemana, P.P.S.B.; Pattanashetty, M.S.B.; Abdin, Z.U.; Siddiqui, H.F.; Chaudhuri, M. Age-related macular degeneration: An exponentially emerging imminent threat of visual impairment and irreversible blindness. Cureus 2023, 15, e39624. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, A.C.; Holz, F.G.; Finger, R.P. Retinal Diseases in Europe: Prevalence, incidence and healthcare needs. In Proceedings of the European Society of Retina Specialists 17th EURETINA Congress, Barcelona, Spain, 7–10 September 2017; pp. 1–28. [Google Scholar]
- Keeling, E.; Lotery, A.J.; Tumbarello, D.A.; Ratnayaka, J.A. Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Cells 2018, 7, 16. [Google Scholar] [CrossRef]
- Ach, T.; Tolstik, E.; Messinger, J.D.; Zarubina, A.V.; Heintzmann, R.; Curcio, C.A. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3242–3252. [Google Scholar] [CrossRef]
- Tarau, I.S.; Berlin, A.; Curcio, C.A.; Ach, T. The cytoskeleton of the retinal pigment epithelium: From normal aging to age-related macular degeneration. Int. J. Mol. Sci. 2019, 20, 3578. [Google Scholar] [CrossRef]
- van Leeuwen, E.M.; Emri, E.; Merle, B.M.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Narimatsu, T.; Ozawa, Y.; Miyake, S.; Kubota, S.; Hirasawa, M.; Nagai, N.; Shimmura, S.; Tsubota, K. Disruption of cell-cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4555–4562. [Google Scholar] [CrossRef]
- Bruban, J.; Glotin, A.; Dinet, V.; Chalour, N.; Sennlaub, F.; Jonet, L.; An, N.; Faussat, A.M.; Mascarelli, F. Amyloid-β (1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell 2009, 8, 162–177. [Google Scholar] [CrossRef]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.-C.; et al. CFH exerts anti-oxidant effects on retinal pigment epithelial cells independently from protecting against membrane attack complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef]
- Zech, J.C.; Pouvreau, I.; Cotinet, A.; Goureau, O.; Le Varlet, B.; de Kozak, Y. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1600–1608. [Google Scholar]
- Brandstetter, C.; Holz, F.G.; Krohne, T.U. Complement component C5a primes retinal pigment epithelial cells for inflammasome activation by lipofuscin-mediated photooxidative damage. J. Biol. Chem. 2015, 290, 31189–31198. [Google Scholar] [CrossRef]
- Wynne, N.; Carroll, J.; Duncan, J.L. Promises and pitfalls of evaluating photoreceptor-based retinal disease with adaptive optics scanning light ophthalmoscopy (AOSLO). Prog. Retin. Eye Res. 2021, 83, 100920. [Google Scholar] [CrossRef]
- Laforest, T.; Künzi, M.; Kowalczuk, L.; Carpentras, D.; Behar-Cohen, F.; Moser, C. Transscleral optical phase imaging of the human retina. Nat. Photonics 2020, 14, 439–445. [Google Scholar] [CrossRef]
- Kowalczuk, L.; Dornier, R.; Kunzi, M.; Iskandar, A.; Misutkova, Z.; Gryczka, A.; Navarro, A.; Jeunet, F.; Mantel, I.; Behar-Cohen, F.; et al. In vivo retinal pigment epithelium imaging using transscleral optical imaging in healthy eyes. Ophthalmol. Sci. 2023, 3, 100234. [Google Scholar] [CrossRef]
- Govindahari, V.; Dornier, R.; Ferdowsi, S.; Moser, C.; Mantel, I.; Behar-Cohen, F.; Kowalczuk, L. High-resolution adaptive optics-trans-scleral flood illumination (AO-TFI) imaging of retinal pigment epithelium (RPE) in central serous chorioretinopathy (CSCR). Sci. Rep. 2024, 14, 13689. [Google Scholar] [CrossRef]
- Rossi, E.A.; Rangel-Fonseca, P.; Parkins, K.; Fischer, W.; Latchney, L.R.; Folwell, M.A.; Williams, D.R.; Dubra, A.; Chung, M.M. In vivo imaging of retinal pigment epithelium cells in age related macular degeneration. Biomed. Opt. Express 2013, 4, 2527–2539. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Rivero, E.B.; Clark, M.E.; Witherspoon, C.D.; Spaide, R.F.; Girkin, C.A.; Owsley, C.; Curcio, C.A. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. Am. J. Ophthalmol. 2014, 158, 584–596. [Google Scholar] [CrossRef]
- Vienola, K.V.; Zhang, M.; Snyder, V.C.; Sahel, J.A.; Dansingani, K.K.; Rossi, E.A. Microstructure of the retinal pigment epithelium near-infrared autofluorescence in healthy young eyes and in patients with AMD. Sci. Rep. 2020, 10, 9561. [Google Scholar] [CrossRef]
- Grieve, K.; Gofas-Salas, E.; Ferguson, R.D.; Sahel, J.A.; Paques, M.; Rossi, E.A. In vivo near-infrared autofluorescence imaging of retinal pigment epithelial cells with 757 nm excitation. Biomed. Opt. Express 2018, 9, 5946–5961. [Google Scholar] [CrossRef]
- Rossi, E.A.; Norberg, N.; Eandi, C.; Chaumette, C.; Kapoor, S.; Le, L.; Snyder, V.C.; Martel, J.N.; Gautier, J.; Gocho, K.; et al. A new method for visualizing drusen and their progression in flood-illumination adaptive optics ophthalmoscopy. Transl. Vis. Sci. Technol. 2021, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.M.; Holmes, C.; Raza, A.; Riedl, S.; Anders, P.; Kaye, R.; Prevost, T.; Fritsche, L.G.; Rueckert, D.; Bogunović, H.; et al. Features of intermediate and late dry age-related macular degeneration on adaptive optics ophthalmoscopy: Pinnacle Study Report 8. Eye 2025, 39, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., III; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Thévenaz, P.; Unser, M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc. Res. Tech. 2007, 70, 135–146. [Google Scholar] [CrossRef]
- Bogovic, J.A.; Hanslovsky, P.; Wong, A.; Saalfeld, S. Robust registration of calcium images by learned contrast synthesis. In Proceedings of the 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI), Prague, Czech Republic, 13–16 April 2016; pp. 1123–1126. [Google Scholar] [CrossRef]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312. [Google Scholar] [CrossRef]
- Manafi, N.; Mahmoudi, A.; Emamverdi, M.; Corradetti, G.; Corona, S.T.; Wykoff, C.C.; Sadda, S.R. Topographic analysis of local OCT biomarkers which predict progression to atrophy in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 2083–2091. [Google Scholar] [CrossRef]
- Paraoan, L.; Sharif, U.; Carlsson, E.; Supharattanasitthi, W.; Mahmud, N.M.; Kamalden, T.A.; Hiscott, P.; Jackson, M.; Grierson, I. Secretory proteostasis of the retinal pigmented epithelium: Impairment links to age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100859. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Won, J.Y. Molecular mechanisms of retinal pigment epithelium dysfunction in age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 12298. [Google Scholar] [CrossRef]
- Paques, M.; Meimon, S.; Rossant, F.; Rosenbaum, D.; Mrejen, S.; Sennlaub, F.; Grieve, K. Adaptive optics ophthalmoscopy: Application to age-related macular degeneration and vascular diseases. Prog. Retin. Eye Res. 2018, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.R.; Arathorn, D.W.; Roorda, A.; Parker, A. Retinal motion estimation in adaptive optics scanning laser ophthalmoscopy. Opt. Express 2006, 14, 487–497. [Google Scholar] [CrossRef]
- de Guimaraes, T.A.C.; Varela, M.D.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Chowers, I.; Lotery, A.J. Beyond the complement cascade: Insights into systemic immunosenescence and inflammaging in age-related macular degeneration and current barriers to treatment. Cells 2023, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Muste, J.C.; Russell, M.W.; Singh, R.P. Photobiomodulation therapy for age-related macular degeneration and diabetic retinopathy: A review. Clin. Ophthalmol. 2021, 15, 3709–3720. [Google Scholar] [CrossRef]
- Khateb, S.; Jha, S.; Bharti, K.; Banin, E. Cell-based therapies for age-related macular degeneration. Adv. Exp. Med. Biol. 2021, 1256, 265–293. [Google Scholar] [CrossRef]
| Patient | Gender | Age (Years) | Eye | Iris Color | Axial Length (mm) | Spherical Equivalent RE (Diopters) | BCVA (Logmar) | IOP (mm Hg) |
|---|---|---|---|---|---|---|---|---|
| P041 | F | 67 | OD | Blue | 22.82 | 0.00 | −0.097 | 14 |
| P042 | F | 72 | OD | Blue | 24.16 | −1.25 | 0.000 | 17 |
| P042 | F | 72 | OS | Blue | 23.49 | −0.50 | 0.000 | 15 |
| P058 | F | 77 | OD | Brown | 22.99 | 0.75 | 0.000 | 17 |
| P059 | F | 79 | OD | Brown | 24.07 | −2.38 | 0.097 | 16 |
| P066 | F | 80 | OD | Brown | 23.43 | −5.75 | 0.000 | 13 |
| P066 | F | 80 | OS | Brown | 23.54 | −3.25 | 0.000 | 12 |
| P072 | F | 56 | OS | Brown | 22.49 | 0.00 | −0.097 | 12 |
| P076 | M | 63 | OD | Blue | 26.25 | −3.13 | −0.097 | 12 |
| P079 | F | 72 | OS | Light brown | 22.63 | 1.25 | 0.000 | 16 |
| P085 | F | 62 | OD | Blue | 22.74 | 0.00 | −0.097 | 15 |
| Mean | 70 | 23.51 | −1.30 | −0.026 | 14.45 | |||
| SD | 8.3 | 1.07 | 2.11 | 0.063 | 1.97 | |||
| Min | 56 | 22.49 | −5.75 | −0.097 | 12 | |||
| Median | 72 | 23.43 | −0.5 | 0.000 | 15 | |||
| Max | 80 | 26.25 | 1.25 | 0.097 | 17 |
| Patient | Eye | Diagnosis | Lens Status | N (Images) | Quality Mean | Quality SD |
|---|---|---|---|---|---|---|
| P041 | OD | Early AMD | Non-significant Cataract | 5 | 0.09 | 0.04 |
| P058 | OD | Early AMD | Non-significant Cataract | 6 | 0.15 | 0.05 |
| P072 | OS | Early AMD | Transparent | 4 | 0.22 | 0.05 |
| P076 | OD | Early AMD | Non-significant Cataract | 6 | 0.39 | 0.03 |
| P079 | OS | Early AMD | Non-significant Cataract | 6 | 0.28 | 0.07 |
| P085 | OD | Intermediate AMD | Non-significant Cataract | 6 | 0.19 | 0.07 |
| P042 | OD | Atrophic AMD | Pseudophakia | 4 | 0.13 | 0.03 |
| P042 | OS | Atrophic AMD | Pseudophakia | 7 | 0.13 | 0.02 |
| P059 | OD | Atrophic AMD | Non-significant Cataract | 6 | 0.17 | 0.03 |
| P066 | OD | Atrophic AMD | Pseudophakia | 4 | 0.13 | 0.02 |
| P066 | OS | Atrophic AMD | Pseudophakia | 6 | 0.13 | 0.03 |
| Total: | 60 | 0.18 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczuk, L.; Dornier, R.; Navarro, A.; Jeunet, F.; Moser, C.; Behar-Cohen, F.; Mantel, I. Adaptive Optics-Transscleral Flood Illumination Imaging of Retinal Pigment Epithelium in Dry Age-Related Macular Degeneration. Cells 2025, 14, 633. https://doi.org/10.3390/cells14090633
Kowalczuk L, Dornier R, Navarro A, Jeunet F, Moser C, Behar-Cohen F, Mantel I. Adaptive Optics-Transscleral Flood Illumination Imaging of Retinal Pigment Epithelium in Dry Age-Related Macular Degeneration. Cells. 2025; 14(9):633. https://doi.org/10.3390/cells14090633
Chicago/Turabian StyleKowalczuk, Laura, Rémy Dornier, Aurélie Navarro, Fanny Jeunet, Christophe Moser, Francine Behar-Cohen, and Irmela Mantel. 2025. "Adaptive Optics-Transscleral Flood Illumination Imaging of Retinal Pigment Epithelium in Dry Age-Related Macular Degeneration" Cells 14, no. 9: 633. https://doi.org/10.3390/cells14090633
APA StyleKowalczuk, L., Dornier, R., Navarro, A., Jeunet, F., Moser, C., Behar-Cohen, F., & Mantel, I. (2025). Adaptive Optics-Transscleral Flood Illumination Imaging of Retinal Pigment Epithelium in Dry Age-Related Macular Degeneration. Cells, 14(9), 633. https://doi.org/10.3390/cells14090633








