Mast Cells and Basophils in Major Viral Diseases: What Are the Correlations with SARS-CoV-2, Influenza A Viruses, HIV, and Dengue?
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Role of Mast Cells and Basophils in the Course of SARS-CoV-2 Infection
3.2. Role of Mast Cells and Basophils in the Course of Influenza a Virus Infection
3.3. Role of Mast Cells and Basophils in the Course of HIV Infection
3.4. Role of Mast Cells and Basophils in Dengue Virus Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murdaca, G.; Di Gioacchino, M.; Greco, M.; Borro, M.; Paladin, F.; Petrarca, C.; Gangemi, S. Basophils and Mast Cells in COVID-19 Pathogenesis. Cells 2021, 10, 2754. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential Association of Mast Cells with Coronavirus Disease 2019. Ann. Allergy Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast Cell Responses to Viruses and Pathogen Products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Wang, Y.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.; Chen, X.; Zhang, A.; et al. Recent Developments in the Immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Tetè, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast Cells Activated by SARS-CoV-2 Release Histamine Which Increases IL-1 Levels Causing Cytokine Storm and Inflammatory Reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated Levels of IL-6 and CRP Predict the Need for Mechanical Ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. COVID-19 and Multisystem Inflammatory Syndrome, or Is It Mast Cell Activation Syndrome? J. Biol. Regul. Homeost. Agents 2020, 34, 1633–1636. [Google Scholar]
- Li, X.; Zhang, C.; Bao, Z. Mast Cell Activation May Contribute to Adverse Health Transitions in COVID-19 Patients with Frailty. Emerg. Microbes Infect. 2023, 12, 2251589. [Google Scholar] [CrossRef]
- Tan, J.Y.J.; Anderson, D.E.; Rathore, A.P.S.; O’Neill, A.; Mantri, C.K.; Saron, W.A.A.; Lee, C.Q.E.; Cui, C.W.; Kang, A.E.Z.; Foo, R.; et al. Mast Cell Activation in Lungs during SARS-CoV-2 Infection Associated with Lung Pathology and Severe COVID-19. J. Clin. Investig. 2023, 133, e149834. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef]
- Cao, J.B.; Zhu, S.T.; Huang, X.S.; Wang, X.Y.; Wu, M.L.; Li, X.; Liu, F.L.; Chen, L.; Zheng, Y.T.; Wang, J.H. Mast Cell Degranulation-Triggered by SARS-CoV-2 Induces Tracheal-Bronchial Epithelial Inflammation and Injury. Virol. Sin. 2024, 39, 309–318. [Google Scholar] [CrossRef]
- MacCann, R.; Leon, A.A.G.; Gonzalez, G.; Carr, M.J.; Feeney, E.R.; Yousif, O.; Cotter, A.G.; de Barra, E.; Sadlier, C.; Doran, P.; et al. Dysregulated Early Transcriptional Signatures Linked to Mast Cell and Interferon Responses Are Implicated in COVID-19 Severity. Front. Immunol. 2023, 14, 1166574. [Google Scholar] [CrossRef]
- Wu, M.L.; Liu, F.L.; Sun, J.; Li, X.; He, X.Y.; Zheng, H.Y.; Zhou, Y.H.; Yan, Q.; Chen, L.; Yu, G.Y.; et al. SARS-CoV-2-Triggered Mast Cell Rapid Degranulation Induces Alveolar Epithelial Inflammation and Lung Injury. Signal Transduct. Target. Ther. 2021, 6, 428. [Google Scholar] [CrossRef]
- Liu, S.; Suzuki, Y.; Takemasa, E.; Watanabe, R.; Mogi, M. Mast Cells Promote Viral Entry of SARS-CoV-2 via Formation of Chymase/Spike Protein Complex. Eur. J. Pharmacol. 2022, 930, 175169. [Google Scholar] [CrossRef]
- Bonam, S.R.; Chauvin, C.; Levillayer, L.; Mathew, M.J.; Sakuntabhai, A.; Bayry, J. SARS-CoV-2 Induces Cytokine Responses in Human Basophils. Front. Immunol. 2022, 13, 838448. [Google Scholar] [CrossRef]
- Degenfeld-Schonburg, L.; Sadovnik, I.; Smiljkovic, D.; Peter, B.; Stefanzl, G.; Gstoettner, C.; Jaksch, P.; Hoetzenecker, K.; Aigner, C.; Radtke, C.; et al. Coronavirus Receptor Expression Profiles in Human Mast Cells, Basophils, and Eosinophils. Cells 2024, 13, 173. [Google Scholar] [CrossRef]
- Liu, J.; Gong, S.; Lv, J.; Wang, G.; Guo, Y.; Gao, D.; Zhang, D.; Ma, S.; Luo, H.; Yang, H.; et al. Clinical Features and Blood Indicators for Severity and Prognosis in COVID-19 Patients. Clin. Lab. 2023, 69, 742–748. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Xiang, N.; Wang, L.; Zheng, T.; Zhuo, X.; Shi, R.; Su, X.; Liu, Y.; Liao, G.; et al. Characterization and Trajectories of Hematological Parameters Prior to Severe COVID-19 Based on a Large-Scale Prospective Health Checkup Cohort in Western China: A Longitudinal Study of 13-Year Follow-Up. BMC Med. 2024, 22, 105. [Google Scholar] [CrossRef]
- Binsaleh, N.K.; Eltayeb, R.; Sherwani, S.; Almishaal, A.A.; Hindi, E.; Qanash, H.; Bazaid, A.; Alharbi, A.O.; Bazaid, M.; Altamimi, S.A. Comparison of Hematological Parameters Between Survivors and Non-Survivors COVID-19 Patients in Saudi Arabia. Int. J. Gen. Med. 2023, 16, 3955–3962. [Google Scholar] [CrossRef]
- Ben, S.; Gao, F.; Xu, Z.; Zhang, R.; Zhang, X.; Wang, N.; Zhang, M.; Hou, L. The Role of Hematological Parameters in Asymptomatic and Non-Severe Cases of Omicron Variant Infection. Virol. J. 2024, 21, 143. [Google Scholar] [CrossRef]
- Saeed, J.R.; Lal, A. Impact of Severity of Covid-19 on Haematological Parameters in Patients Reporting to Saidu Group of Teaching Hospitals, Swat. J. Ayub Med. Coll. Abbottabad 2023, 35, 80–83. [Google Scholar] [CrossRef]
- Danieli, M.G.; Brunetto, S.; Gammeri, L.; Palmeri, D.; Claudi, I.; Shoenfeld, Y.; Gangemi, S. Machine Learning Application in Autoimmune Diseases: State of Art and Future Prospectives. Autoimmun. Rev. 2024, 23, 103496. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, W.; Liu, T.; Li, J.; Xiao, K.; Ma, X.; Xie, L.; Jiang, J.; Zhou, H.; Liu, R.; et al. Early Prediction Model for Critical Illness of Hospitalized COVID-19 Patients Based on Machine Learning Techniques. Front. Public Health 2022, 10, 880999. [Google Scholar] [CrossRef]
- Chadaga, K.; Prabhu, S.; Sampathila, N.; Chadaga, R.; Umakanth, S.; Bhat, D.; GS, S.K. Explainable Artificial Intelligence Approaches for COVID-19 Prognosis Prediction Using Clinical Markers. Sci. Rep. 2024, 14, 1783. [Google Scholar] [CrossRef]
- de Souza, A.A.; de Almeida, D.C.; Barcelos, T.S.; Bortoletto, R.C.; Munoz, R.; Waldman, H.; Goes, M.A.; Silva, L.A. Simple Hemogram to Support the Decision-Making of COVID-19 Diagnosis Using Clusters Analysis with Self-Organizing Maps Neural Network. Soft Comput. 2023, 27, 3295–3306. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Xu, J.; Zhou, Q. A Complete Blood Count-Based Multivariate Model for Predicting the Recovery of Patients with Moderate COVID-19: A Retrospective Study. Sci. Rep. 2022, 12, 18262. [Google Scholar] [CrossRef]
- Baik, S.M.; Hong, K.S.; Park, D.J. Application and Utility of Boosting Machine Learning Model Based on Laboratory Test in the Differential Diagnosis of Non-COVID-19 Pneumonia and COVID-19. Clin. Biochem. 2023, 118, 110584. [Google Scholar] [CrossRef]
- Peteranderl, C.; Herold, S.; Schmoldt, C. Human Influenza Virus Infections. Semin. Respir. Crit. Care Med. 2016, 37, 487–500. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Ng, K.; Raheem, J.; St Laurent, C.D.; Marcet, C.T.; Vliagoftis, H.; Befus, A.D.; Moon, T.C. Responses of Human Mast Cells and Epithelial Cells Following Exposure to Influenza A Virus. Antivir. Res. 2019, 171, 104566. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, Y.; Han, D.; Zhang, G.; Cao, S.; Xie, J.; Xue, J.; Li, Y.; Meng, D.; Fan, X.; et al. Mast Cell-Induced Lung Injury in Mice Infected with H5N1 Influenza Virus. J. Virol. 2012, 86, 3347–3356. [Google Scholar] [CrossRef]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory Response of Mast Cells during Influenza A Virus Infection Is Mediated by Active Infection and RIG-I Signaling. J. Immunol. 2013, 190, 4676–4684. [Google Scholar] [CrossRef]
- Meng, D.; Huo, C.; Wang, M.; Xiao, J.; Liu, B.; Wei, T.; Dong, H.; Zhang, G.; Hu, Y.; Sun, L. Influenza A Viruses Replicate Productively in Mouse Mastocytoma Cells (P815) and Trigger Pro-Inflammatory Cytokine and Chemokine Production through TLR3 Signaling Pathway. Front. Microbiol. 2017, 7, 2130. [Google Scholar] [CrossRef]
- Liu, B.; Meng, D.; Wei, T.; Zhang, S.; Hu, Y.; Wang, M. Apoptosis and Pro-Inflammatory Cytokine Response of Mast Cells Induced by Influenza A Viruses. PLoS ONE 2014, 9, e100109. [Google Scholar] [CrossRef]
- Desheva, Y.; Mamontov, A.; Petkova, N.; Karev, V.; Nazarov, P. Mast Cell Degranulation and Histamine Release during A/H5N1 Influenza Infection in Influenza-Sensitized Mice. Life Sci. 2020, 258, 118230. [Google Scholar] [CrossRef]
- Han, D.; Wei, T.; Zhang, S.; Wang, M.; Tian, H.; Cheng, J.; Xiao, J.; Hu, Y.; Chen, M. The Therapeutic Effects of Sodium Cromoglycate against Influenza A Virus H5N1 in Mice. Influenza Other Respir. Viruses 2016, 10, 57–66. [Google Scholar] [CrossRef]
- Huo, C.; Tang, Y.; Li, X.; Han, D.; Gu, Q.; Su, R.; Liu, Y.; Reiter, R.J.; Liu, G.; Hu, Y.; et al. Melatonin Alleviates Lung Injury in H1N1-Infected Mice by Mast Cell Inactivation and Cytokine Storm Suppression. PLoS Pathog. 2023, 19, e1011406. [Google Scholar] [CrossRef]
- Huo, C.; Wu, H.; Xiao, J.; Meng, D.; Zou, S.; Wang, M.; Qi, P.; Tian, H.; Hu, Y. Genomic and Bioinformatic Characterization of Mouse Mast Cells (P815) upon Different Influenza A Virus (H1N1, H5N1, and H7N2) Infections. Front. Genet. 2019, 10, 461843. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Huo, C.; Zou, S.; Lian, Z.; Hu, Y. ITRAQ-Based Proteomic and Bioinformatic Characterization of Human Mast Cells upon Infection by the Influenza A Virus Strains H1N1 and H5N1. FEBS Lett. 2019, 593, 2612–2627. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, H.; Huo, C.; Zou, S.; Hu, Y.; Yang, H. Transcriptomic Profiling of Mouse Mast Cells upon Pathogenic Avian H5N1 and Pandemic H1N1 Influenza a Virus Infection. Viruses 2022, 14, 292. [Google Scholar] [CrossRef]
- Clementsen, P.; Jensen, C.B.; Hannoun, C.; Søborg, M.; Norn, S. Influenza A Virus Potentiates Basophil Histamine Release Caused by Endotoxin-Induced Complement Activation. Examination of Normal Individuals and Patients with Intrinsic Asthma. Allergy 1988, 43, 93–99. [Google Scholar] [CrossRef]
- Clementsen, P.; Douglas, A.R.; Skehel, J.J.; Hannoun, C.; Bach-Mortensen, N.; Norn, S. Influenza A Virus Enhances IgE-Mediated Histamine Release from Human Basophil Leukocytes. Examination of the Effect of Viral Neuraminidase and Haemagglutinin. Agents Actions 1989, 27, 58–61. [Google Scholar] [CrossRef]
- Clementsen, P.; Norn, S.; Kristensen, K.S.; Hannoun, C. Influenza A Virus Enhances Basophil Histamine Release and the Enhancement Is Abolished by Carbohydrates. Allergy 1990, 45, 471–476. [Google Scholar] [CrossRef]
- Huftel, M.A.; Swensen, C.A.; Borcherding, W.R.; Dick, E.C.; Hong, R.; Kita, H.; Gleich, G.J.; Busse, W.W. The Effect of T-Cell Depletion on Enhanced Basophil Histamine Release after In Vitro Incubation with Live Influenza A Virus. Am. J. Respir. Cell Mol. Biol. 2012, 7, 434–440. [Google Scholar] [CrossRef]
- Wang, F.; Bian, S.; Zhou, W.; Liu, S.; Shu, Y.; Chen, Y. Causal Relationship between Blood Traits and Severe Influenza A(H1N1)Pdm09 Infection in East Asian: A Mendelian Randomization Study. J. Med. Virol. 2024, 96, e29736. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef]
- Hemelaar, J. The Origin and Diversity of the HIV-1 Pandemic. Trends Mol. Med. 2012, 18, 182–192. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Rivellese, F.; De Paulis, A. Are Basophils and Mast Cells Masters in HIV Infection? Int. Arch. Allergy Immunol. 2017, 171, 158–165. [Google Scholar]
- Obeagu, E.I.; Obeagu, G.U.; Akinleye, C.A. Unveiling the Enigmatic Roles of Basophils in HIV Infection: A Narrative Review. Medicine 2024, 103, e40384. [Google Scholar] [CrossRef]
- Marone, G.; Florio, G.; Triggiani, M.; Petraroli, A.; De Paulis, A. Mechanisms of IgE Elevation in HIV-1 Infection. Crit. Rev. Immunol. 2000, 20, 477–496. [Google Scholar] [CrossRef]
- Marone, G.; Florio, G.; Petraroli, A.; Triggiani, M.; De Paulis, A. Role of Human FcepsilonRI+ Cells in HIV-1 Infection. Immunol. Rev. 2001, 179, 128–138. [Google Scholar] [CrossRef]
- Marone, G.; Florio, G.; Petraroli, A.; De Paulis, A. Dysregulation of the IgE/FcϵRI Network in HIV-1 Infection. J. Allergy Clin. Immunol. 2001, 107, 22–30. [Google Scholar] [CrossRef]
- Marone, G.; De Paulis, A.; Florio, G.; Petraroli, A.; Rossi, F.W.; Triggiani, M. Are Mast Cells MASTers in HIV-1 Infection? Int. Arch. Allergy Immunol. 2001, 125, 89–95. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wadley, R.; Reddel, S.W.; Qi, J.C.; Archis, C.; Collins, A.; Clark, E.; Cooley, M.; Kouts, S.; et al. Mast Cells/Basophils in the Peripheral Blood of Allergic Individuals Who Are HIV-1 Susceptible Due to Their Surface Expression of CD4 and the Chemokine Receptors CCR3, CCR5, and CXCR4. Blood 2001, 97, 3484–3490. [Google Scholar] [CrossRef]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Lobasso, A.; Napolitano, F.; Granata, F.; Selleri, C.; De Paulis, A. HIV-1 Nef Promotes Migration and Chemokine Synthesis of Human Basophils and Mast Cells through the Interaction with CXCR4. Clin. Mol. Allergy 2016, 14, 15. [Google Scholar] [CrossRef][Green Version]
- Sundstrom, J.B.; Hair, G.A.; Ansari, A.A.; Secor, W.E.; Gilfillan, A.M.; Metcalfe, D.D.; Kirshenbaum, A.S. IgE-FcepsilonRI Interactions Determine HIV Coreceptor Usage and Susceptibility to Infection during Ontogeny of Mast Cells. J. Immunol. 2009, 182, 6401–6409. [Google Scholar] [CrossRef]
- Song, S.T.; Wu, M.L.; Zhang, H.J.; Su, X.; Wang, J.H. Mast Cell Activation Triggered by Retrovirus Promotes Acute Viral Infection. Front. Microbiol. 2022, 13, 798660. [Google Scholar] [CrossRef]
- Marone, G.; Rossi, F.W.; Pecoraro, A.; Pucino, V.; Criscuolo, G.; de Paulis, A.; Spadaro, G.; Marone, G.; Varricchi, G. HIV Gp120 Induces the Release of Proinflammatory, Angiogenic, and Lymphangiogenic Factors from Human Lung Mast Cells. Vaccines 2020, 8, 208. [Google Scholar] [CrossRef]
- Obeagu, E.I. Diagnostic and Prognostic Significance of Mast Cell Markers in HIV/AIDS: Current Insights and Future Directions. Medicine 2024, 103, E38117. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Immune Responses to Dengue Virus in the Skin. Open Biol. 2018, 8, 180087. [Google Scholar] [CrossRef]
- Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J.V. Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef]
- Tuchinda, M.; Dhorranintra, B.; Tuchinda, P. Histamine Content in 24-Hour Urine in Patients with Dengue Haemorrhagic Fever. Southeast. Asian J. Trop. Med. Public Health 1977, 8, 80–83. [Google Scholar]
- Troupin, A.; Shirley, D.; Londono-Renteria, B.; Watson, A.M.; McHale, C.; Hall, A.; Hartstone-Rose, A.; Klimstra, W.B.; Gomez, G.; Colpitts, T.M. A Role for Human Skin Mast Cells in Dengue Virus Infection and Systemic Spread. J. Immunol. 2016, 197, 4382–4391. [Google Scholar] [CrossRef]
- Dalrymple, N.A.; Mackow, E.R. Endothelial Cells Elicit Immune-Enhancing Responses to Dengue Virus Infection. J. Virol. 2012, 86, 6408–6415. [Google Scholar] [CrossRef]
- John, A.L.S.; Rathore, A.P.S.; Yap, H.; Ng, M.L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune Surveillance by Mast Cells during Dengue Infection Promotes Natural Killer (NK) and NKT-Cell Recruitment and Viral Clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef]
- Chu, Y.T.; Wan, S.W.; Anderson, R.; Lin, Y.S. Mast Cell–Macrophage Dynamics in Modulation of Dengue Virus Infection in Skin. Immunology 2015, 146, 163–172. [Google Scholar] [CrossRef]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA Sensors Enable Human Mast Cell Anti-Viral Chemokine Production and IFN-Mediated Protection in Response to Antibody-Enhanced Dengue Virus Infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef]
- Morrison, J.; Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Nishida, A.; St.John, A.L. Transcriptional Profiling Confirms the Therapeutic Effects of Mast Cell Stabilization in a Dengue Disease Model. J. Virol. 2017, 91, e00617-17. [Google Scholar] [CrossRef]
- Mantri, C.K.; Soundarajan, G.; Saron, W.A.A.; Rathore, A.P.S.; Alonso, S.; John, A.L.S. Maternal Immunity and Vaccination Influence Disease Severity in Progeny in a Novel Mast Cell-Deficient Mouse Model of Severe Dengue. Viruses 2021, 13, 900. [Google Scholar] [CrossRef]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.O.; Azeredo, E.L.; Nogueira, R.M.R.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex Cytokine Profile from Dengue Patients: MIP-1beta and IFN-Gamma as Predictive Factors for Severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef]
- Pérez, A.B.; García, G.; Sierra, B.; Alvarez, M.; Vázquez, S.; Cabrera, M.V.; Rodríguez, R.; Rosario, D.; Martínez, E.; Denny, T.; et al. IL-10 Levels in Dengue Patients: Some Findings from the Exceptional Epidemiological Conditions in Cuba. J. Med. Virol. 2004, 73, 230–234. [Google Scholar] [CrossRef]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue Virus Selectively Induces Human Mast Cell Chemokine Production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef]
- Brown, M.G.; Hermann, L.L.; Issekutz, A.C.; Marshall, J.S.; Rowter, D.; Al-Afif, A.; Anderson, R. Dengue Virus Infection of Mast Cells Triggers Endothelial Cell Activation. J. Virol. 2011, 85, 1145–1150. [Google Scholar] [CrossRef]
- Chu, Y.T.; Wan, S.W.; Chang, Y.C.; Lee, C.K.; Wu-Hsieh, B.A.; Anderson, R.; Lin, Y.S. Antibodies against Nonstructural Protein 1 Protect Mice from Dengue Virus-Induced Mast Cell Activation. Lab. Investig. 2017, 97, 602–614. [Google Scholar] [CrossRef][Green Version]
- Tissera, H.; Rathore, A.P.S.; Leong, W.Y.; Pike, B.L.; Warkentien, T.E.; Farouk, F.S.; Syenina, A.; Ooi, E.E.; Gubler, D.J.; Wilder-Smith, A.; et al. Chymase Level Is a Predictive Biomarker of Dengue Hemorrhagic Fever in Pediatric and Adult Patients. J. Infect. Dis. 2017, 216, 1112–1121. [Google Scholar] [CrossRef]
- Sahu, A.K.; Aggarwal, P.; Ekka, M.; Nayer, J.; Bhoi, S.; Kumar, A.; Luthra, K. Assessing the Serum Chymase Level as an Early Predictor of Dengue Severity. J. Med. Virol. 2021, 93, 3330–3337. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S.; Raghavan, B.; Ng, M.L.; Abraham, S.N. Contributions of Mast Cells and Vasoactive Products, Leukotrienes and Chymase, to Dengue Virus-Induced Vascular Leakage. Elife 2013, 2013, e00481. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Syenina, A.; Ooi, J.; Jagaraj, C.J.; Goh, C.C.; Tissera, H.; Wilder-Smith, A.; Ng, L.G.; et al. Dengue Virus–Elicited Tryptase Induces Endothelial Permeability and Shock. J. Clin. Investig. 2019, 129, 4180–4193. [Google Scholar] [CrossRef]
- Vasquez Velasquez, C.; Roman, A.D.; Lan, N.T.P.; Huy, N.T.; Mercado, E.S.; Espino, F.E.; Perez, M.L.M.; Huong, V.T.Q.; Thuy, T.T.; Tham, V.D.; et al. Alpha Tryptase Allele of Tryptase 1 (TPSAB1) Gene Associated with Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) in Vietnam and Philippines. Hum. Immunol. 2015, 76, 318–323. [Google Scholar] [CrossRef]
- Masri, M.F.B.; Mantri, C.K.; Rathore, A.P.S.; St. John, A.L. Peripheral Serotonin Causes Dengue Virus–Induced Thrombocytopenia through 5HT2 Receptors. Blood 2019, 133, 2325–2337. [Google Scholar] [CrossRef]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of Vasoactive Cytokines by Antibody-Enhanced Dengue Virus Infection of a Human Mast Cell/Basophil Line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef]
- Brown, M.G.; King, C.A.; Sherren, C.; Marshall, J.S.; Anderson, R. A Dominant Role for FcγRII in Antibody-Enhanced Dengue Virus Infection of Human Mast Cells and Associated CCL5 Release. J. Leukoc. Biol. 2006, 80, 1242–1250. [Google Scholar] [CrossRef]
- Jeewandara, C.; Gomes, L.; Udari, S.; Paranavitane, S.A.; Shyamali, N.L.A.; Ogg, G.S.; Malavige, G.N. Secretory Phospholipase A2 in the Pathogenesis of Acute Dengue Infection. Immun. Inflamm. Dis. 2016, 5, 7–15. [Google Scholar] [CrossRef]
- Wedemeyer, J.; Tsai, M.; Galli, S.J. Roles of Mast Cells and Basophils in Innate and Acquired Immunity. Curr. Opin. Immunol. 2000, 12, 624–631. [Google Scholar] [CrossRef]
- Marone, G.; Florio, G.; Petraroli, A.; Triggiani, M.; De Paulis, A. Human Mast Cells and Basophils in HIV-1 Infection. Trends Immunol. 2001, 22, 229–232. [Google Scholar] [CrossRef]
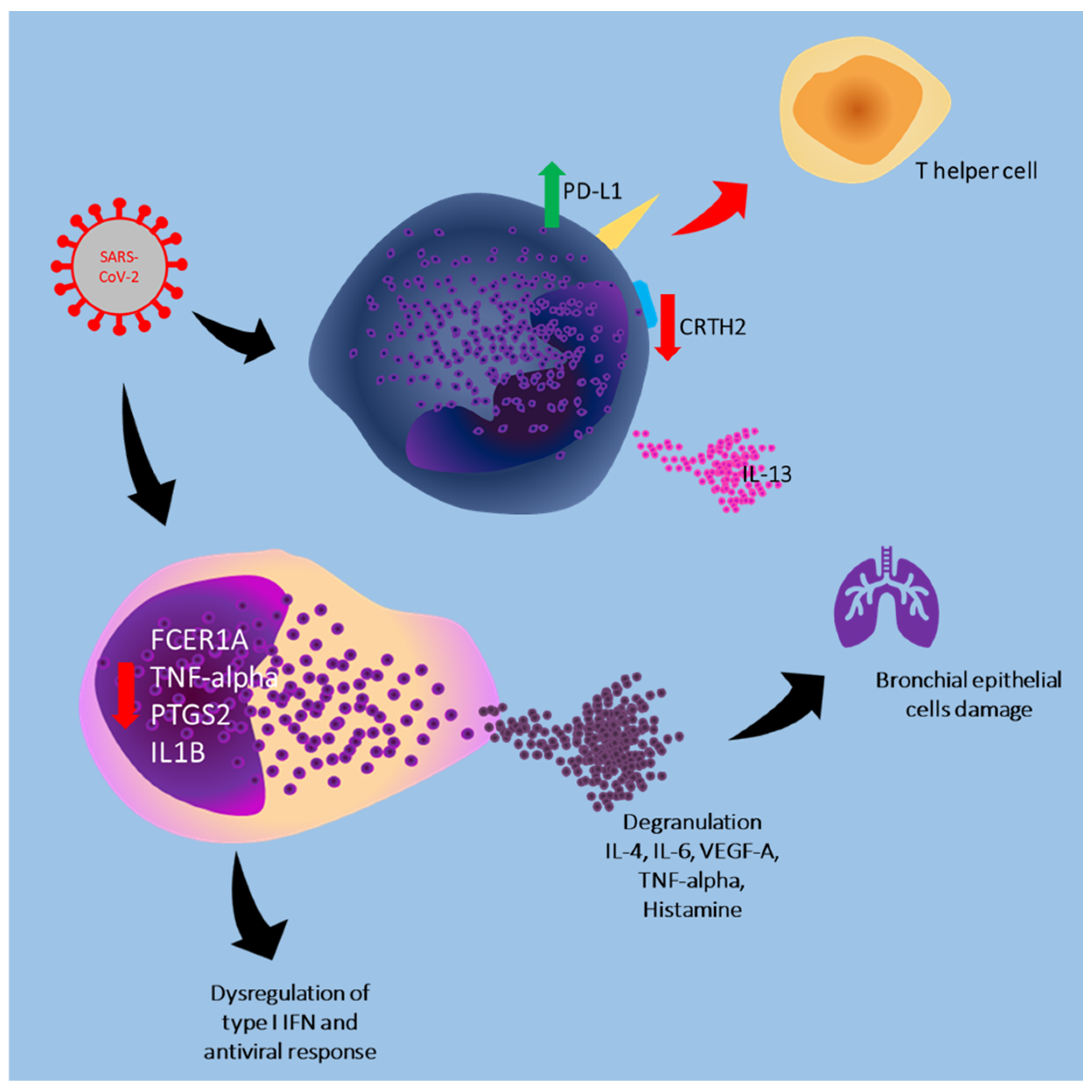
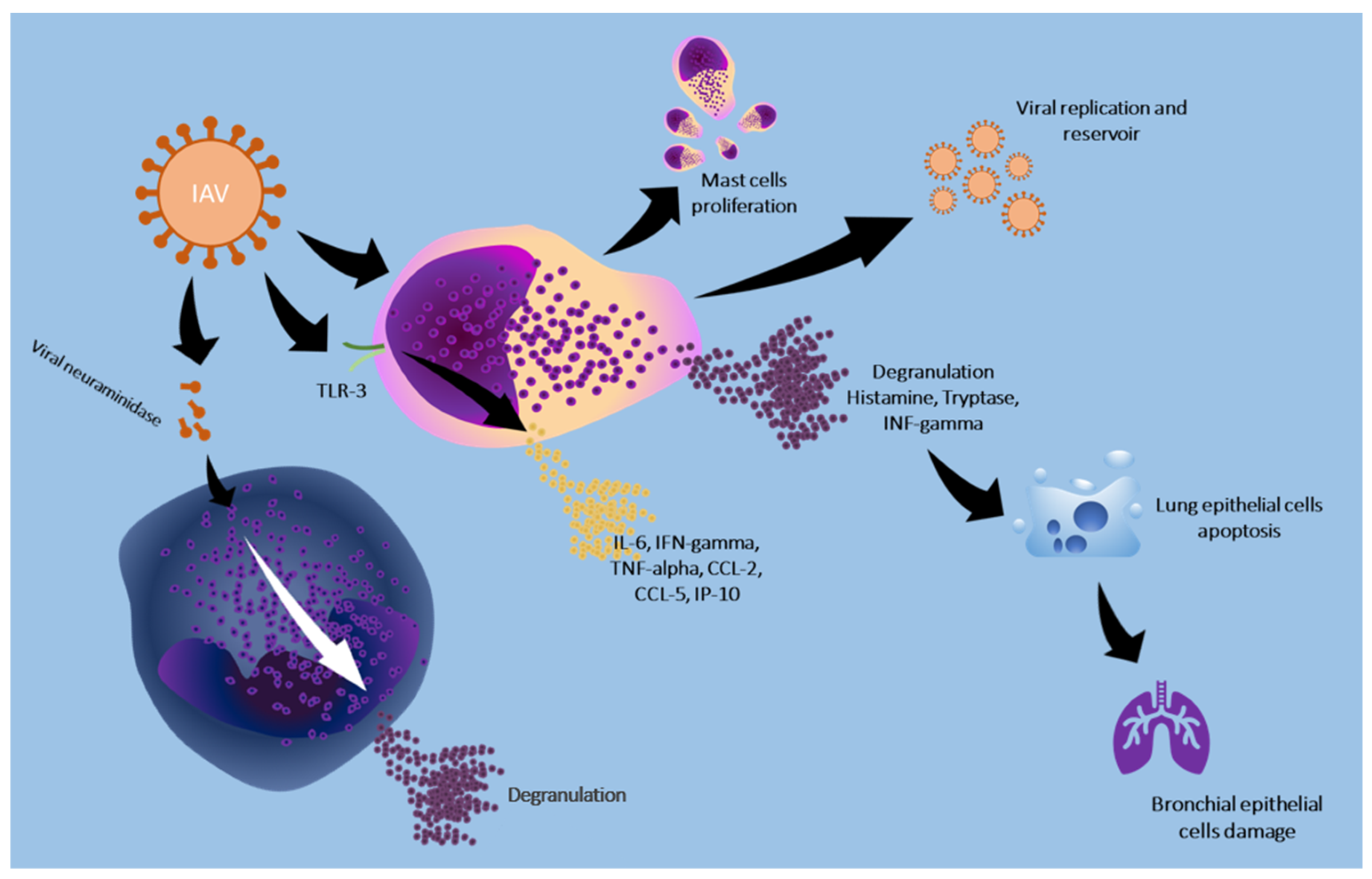


| Author, Year | Cell | Type of Study | Test Subject | Results |
|---|---|---|---|---|
| Li et al., 2023 [12] | Mast cell | Transcriptomics analysis | Human blood samples | NCAPG, MCM10 and CDC25C were identified as hub genes in peripheral blood, which could be used as diagnostic markers of poor prognosis |
| Tan et al., 2023 [13] | Mast cell | In vitro/transcriptomics analysis | Mice, non-human primate/human blood samples | Mouse chymase (MCPT1) levels were significantly elevated days 1, 3, 5, and 7 after SARS-CoV-2 infection. Infected primates experience lung pathology involving haemorrhagic manifestations and widespread MC activation. Patients with COVID-19 have significantly higher chymase serum level compared with controls (the highest levels were detected in severe group) |
| Gebremeskel et al., 2021 [14] | Mast cell | In vitro | Human blood samples | Patients with SARS-CoV-2 have significantly higher levels of CCL2, CCL3, CCL4, IP-10, IL-6, IL-8, VEGF, TNF-alpha, IFN-gamma, chymase, β-tryptase, and CPA3, suggesting systemic mast cell activation. Mast cell-derived proteases and eosinophil-associated mediators are increased in the lungs and/or serum of patients with COVID-19 |
| Cao et al., 2024 [15] | Mast cell | In vitro | Mice | SARS-CoV-2 infection induces mast cell accumulation and degranulation in the peri-trachea in mice. Mast cell activation induces the production of inflammatory factors in bronchial epithelial cells. Ebastine or loratadine reduces the induction of inflammatory factors and alleviate tracheal injury in mice |
| MacCann et al., 2023 [16] | Mast cell | In vitro | Human blood samples | Moderate/severe COVID-19 group, showed greater downregulation of FCER1A (gene encodes for FcεRI on mast cells) than the group with mild disease. Downregulation of the TNF-alpha, PTGS2 and IL1B genes was observed in the moderate/severe COVID-19 group, suggesting downregulation of mast cells activation. Circulating levels of IL-1β, IL-6, IL-17A and IL-10 were significantly elevated in the COVID-19 group compared to the SARS- group |
| Wu et al., 2021 [17] | Mast cell | In vitro | Mouse and non-human primate mast cells | Lung lesions, including inflammatory cells (lymphocytes and monocytes) infiltration, haemorrhage, alveolar septal thickening, and mucosa desquamation were observed around the areas of mast cell accumulation and degranulation in both mice and macaques, compared with controls. Spike-RBD binding to ACE2 is accompanied by an immediate mast cells degranulation, by the release of intracellular tryptase and chymase |
| Liu et al., 2022 [18] | Mast cell | In vivo (animal model) | Mice | SARS-CoV-2 spike glycoprotein triggers mast cell activation. MCP2 chymase has formed a complex with the spike protein, promoting protease-dependent viral entry. Mast cell stabilizers or chymase inhibitors reduce viral entry. The absence of mast cells affects early viral load in the upper respiratory tract, increasing the risk of viral invasion into the lower respiratory system |
| Reddy Bonam et al., 2022 [19] | Basophil | In vitro | Human blood samples | SARS-CoV-2 induces the release of IL-13 from basophils. The virus or infected epithelial cells do not alter the expression of CD69, CD13, or CD107a. SARS-CoV-2 does not induce PD-L1 on basophils |
| Degenfeld-Schonburg et al., 2024 [20] | Mast cell and Basophil | In vitro | Human blood samples | Primary mast cells, basophils, and eosinophils and their corresponding cell lines express CD13 and CD147 of CoV-R. Primary skin mast cells and basophils, as well as EOL-1 cells also express CD26, whereas the cell lines do not |
| Liu et al., 2023 [21] | Basophil | In vitro | Human blood samples | Increased levels of basophils, associated with other parameters including advanced age, high fever, increased white blood cells, CRP, LDH, high-sensitivity troponin, pro-BNP, and D-dimer are associated with severe disease and worse prognosis |
| Lin et al., 2024 [22] | Basophil | In vitro | Human blood samples | Higher levels of basophils (%) and monocytes (%) are associated with an increased risk of severe infection |
| Binsaleh et al., 2023 [23] | Basophil | Data analysis | Human blood samples | Disease-related mortality was significantly associated with a reduction in white blood cells and basophils |
| Ben et al., [24] 2024 | Basophil | Data analysis | Human blood samples | Individuals with non-severe disease showed reduced levels of basophils and basophils (%). Basophil counts combined with lymphocytes or platelet-to-lymphocyte ratio were more sensitive for detecting non-severe cases early |
| Rauf Saeed and Lal 2023 [25] | Basophil | Data analysis | Human blood samples | No correlations were found between basophil values and disease severity |
| Fu et al., 2022 [27] | Basophil | Data analysis | Human blood samples | The predictive model based on LASSO logistic regression identified 28 variables useful for the prognosis of critical illness. Among these factors, a low basophil count is among the parameters associated with the development of critical illness at the time of hospital admission |
| Chadaga et al., 2024 [28] | Basophil | Data analysis | Human blood samples | Basophils together with CRP, lymphocytes, albumin, D-dimer, and neutrophils have proven to be the best predictive markers of severity |
| De Souza et al., 2023 [29] | Basophil | Data analysis | Human blood samples | The unsupervised clustering analysis has been shown to be effective in facilitating the decision-making process in the patient with suspected infection through the use of simple parameters such as leukocytes, basophils, eosinophils, and red cell distribution width |
| Wang et al., 2022 [30] | Basophil | Data analysis | Human blood samples | Basophil levels associated with mean corpuscular volume, red blood cell distribution width, and platelet distribution width may be useful in predicting recovery in patients with moderate COVID-19. Small increases in these parameters within normal limits suggest improvement in these patients |
| Min Baik et al., 2023 [31] | Basophil | Data analysis | Human blood samples | Basophils, D-dimer, eosinophils, glucose, and aspartate aminotransferase are excellent markers for differentiating the two conditions |
| Authors, Year | Cell | Type of Study | Test Subject | Results |
|---|---|---|---|---|
| Ng et al., 2019 [34] | Mast cell | In vitro | Human mast cell (LAD2), human lung epithelial cell (Calu-3) | The three strains studied did not induce the release of histamine or β-hexosaminidase in LAD2. A/HK/8/68 induced the release of prostaglandin D2 in LAD2. CCL4 was released in TLR in a statistically significant way from LAD2 cells infected with A/PR/8/34. Increased expression of viral recognition receptors (RIG-I and MDA5) and viperin mRNA was found |
| Hu et al., 2012 [35] | Mast cell | In vitro/In vivo (animal model) | Mice/P815 cell line | Mast cells are significantly activated by the virus which promotes the release of histamine, tryptase and IFN-gamma |
| Graham et al., 2013 [36] | Mast cell | Ex vivo | C57BL/6 mice, B6.Cg-Kit(W-sh) mice and derived mast cells | A/WSN/33 causes the release of cytokines and chemokines from mast cells through a RIG-I/MAVS-dependent mechanism. Histamine release is independent of this mechanism |
| Meng et al., 2017 [37] | Mast cell | In vitro/In vivo (animal model) | P815 cell line, mice | H1N1, H5N1 and H7N2 viruses activated infected P815 cells via the Toll-like receptor 3 pathway, stimulating the production and release of proinflammatory cytokines and chemokines (IL-6, IFN-gamma, TNF-alpha, CCL-2, CCL-5 and IP-10) |
| Liu et al., 2014 [38] | Mast cell | In vitro | P815 cell line | Influenza viruses H1N1 (A/WSN/33), H5N1 (A/Chicken/Henan/1/04), and H7N2 (A/Chicken/Hebei/2/02) induce mast cell apoptosis through the intrinsic mitochondria/cytochrome c pathway |
| Desheva et al., 2020 [39] | Mast cell | In vivo/Ex vivo | Mice | Sixty-seven percent of vaccinated mice were protected from lethality compared to forty-three percent in the placebo group. Administration of antihistamines increased survival to 85–95%. More active mast cells were found in the lungs of immunized mice. |
| Han et al., 2016 [40] | Mast cell | In vivo (animal model) | Mice | The survival rate in treated mice was higher than in untreated mice and the expression of IL-6, TNF-alpha, TLR-3, and TIR-domain-containing adapter-inducing interferon-β was significantly reduced |
| Huo et al., 2023 [41] | Mast cell | In vivo (animal model) | AANAT−/− melatonin-deficient mice, wild type mice | Melatonin suppresses alveolar epithelial cell apoptosis both in vitro and in vitro, decreasing lung damage during infection. Melatonin suppresses the HIF-1 pathway and inhibits the release of proinflammatory cytokines from mast cells |
| Huo et al., 2019 [42] | Mast cell | In vitro | P815 cell line | The 5-HT and cyclic guanosine monophosphate (cGMP)/protein kinase G (PKG) signalling pathways are activated primarily in H1N1-infected P815 cells. The HIF-1 signalling pathway is preferentially activated in H7N2-infected P815 cells. Corresponding mRNA levels are also increased. |
| Wu et al., 2019 [43] | Mast cell | In vitro | Human mast cells | Forty-one differentially expressed proteins in human mast cells have been related to infection by H5N1 versus seasonal H1N1 virus. H1N1 significantly regulates the RNA degradation pathway via positive regulation of the CCR4-NOT transcription complex subunit 4. H5N1 suppresses apoptosis via negative regulation of the tumour protein p53 signalling pathway. The hypoxia-inducible factor-1 signalling pathway is more susceptible to H5N1 infection than to H1N1 virus |
| Tang et al., 2022 [44] | Mast cell | In vitro | P815 cell line | H1N1-infected mouse P815 mast cells exhibit more upregulated and downregulated genes than H5N1-infected cells. Differentially expressed genes in H1N1 infection were specifically enriched for FoxO and autophagy pathways. Differentially expressed genes in H5N1 infection were specifically enriched for NF-κB and necroptosis pathways. Nbeal2 is preferentially activated in H5N1-infected P815 cells |
| Clementsen et al., 1988 [45] | Basophil | In vivo (human) | Human basophils | Viral neuraminidase enhances histamine release from basophils |
| Clementsen et al., 1989 [46] | Basophil | In vivo (human) | Human basophils | In order for neuroaminidase to promote basophil activation, the virus must bind to some cell surface protein |
| Clementsen et al., 1990 [47] | Basophil | In vivo (human) | Human basophils | Influenza A virus causes an enhancement of mediator release. The enhancement is abolished by galactose, N-acetylglucosamine, alpha-methyl-D-glucoside, alpha-methyl-D-mannoside, N-acetylneuraminic acid and lactose. Sugars prevent the enhancement of mediator release by binding to the cell membrane of basophils |
| Huftel et al., 1992 [48] | Basophil | In vitro | Human mononuclear cells | Incubation with influenza A stimulates the release of LTC4 and histamine from basophils. Histamine release was enhanced in the virus-treated mononuclear cell group that had not undergone T-cell depletion |
| Wang et al., 2024 [49] | Basophil | In vitro | Human blood samples | Inverse variance weighted analysis revealed a correlation between low AST, LDL-C, and basophil levels with severe H1N1pdm09 disease. |
| Authors, Year | Cell | Type of Study | Test Subject | Results |
|---|---|---|---|---|
| Marone et al., 2001 [55] | Mast cell and Basophil | In vitro | Human mast cells and basophils | HIV-1 proteins gp120 and Tat trigger the release of proinflammatory cytokines and polarize the T(H)2 cell response through FcεRI (+) cells and the beta-chemokine receptor CCR3 on these cells |
| Li et al., 2001 [58] | Mast cell Basophil | Ex vivo/In vitro | Human mast cells and basophils | Metachromatic cells express on their surface FcεRI, CD4, and the chemokine receptors CCR3, CCR5, and CXCR4, but not CD3 and CD68. These cells are susceptible to HIV-1 |
| Rossi et al., 2016 [59] | Mast cell Basophil | In vitro | Human mast cells and basophils | Incubation of basophils and mast cells with Nef induced the release of CXCL8/IL-8 and CCL3/MIP-1α. Nef protein has a crucial role in basophils and mast cells recruitment at site of virus replication |
| Sundstrom et al., 2009 [60] | Mast cell | In vitro | Human progenitor mast cells | The interaction between IgE and their receptor, in the phases of mast cell ontogenesis, would positively regulate the functional expression of chemokines, influencing the composition of viral variants stored in the tissue reservoir of long-lived mast cells |
| Song et al., 2022 [61] | Mast cell | In vivo (animal model) | C57BL/6 wild type mice, C57BL/6-Kit W-sh/W-sh (Sash) mice | In in vivo models, retrovirus infection stimulates mast cell degranulation through activation of G-MDSCs |
| Marone et al., 2020 [62] | Mast cell | In vitro | Human lung mast cells | gp120 acts as a superantigen, interacting with FcεRI-bound IgE of VH3 family of mast cells and promoting the release of proinflammatory mediators, angiogenic and lymphangiogenic factors |
| Authors, Year | Cell | Type of Study | Test Subject | Results |
|---|---|---|---|---|
| Troupin et al., 2016 [67] | Mast cell | In vitro | Human skin mast cells | The skin and its resident cells, such as mast cells, are the first critical organ in the immune response to the dengue virus |
| Dalrymple et al., 2012 [68] | Mast cell | In vitro | Human endothelial cells | Endothelial cells infected with dengue virus produce chemokines that recall and activate mast cells |
| St. John et al., 2011 [69] | Mast cell | In vitro | Human, mice and monkey mast cells | Immune surveillance through the release of preformed mediators, the modification of gene expression by chemokines production and the recruitment of NK cells |
| Chu et al., 2015 [70] | Mast cell | In vivo (animal model) | Kit (W-sh/W-sh) mice | Mast cell-deficient mouse models show increased infection and macrophage infiltration at the skin injection site, as well as bleeding time compared to wild-type mice. |
| Brown et al., 2012 [71] | Mast cell | In vitro | Human cord blood-derived mast cells, KU812 and HMC-1 mast cell lines | Immunomodulation of antiviral response via secretion of IFN-I, TNF-alpha, and CCL4 and 5 |
| Morrison et al., 2017 [72] | Mast cell | In vivo (animal model) | Mice | Mast cell membrane stabilizers enhance the antiviral activity of the immune system through the release of INF-I |
| Mantri et al., 2021 [73] | Mast cell | In vivo (animal model) | Mast cell-deficient mouse model | IFN-I and II deficiency is related to comorbidity; IFN-gamma expression without IFN-I expression is related to increased mortality |
| Bozza et al., 2008 [74] | Mast cell | In vitro | Human blood samples | CCL4 and 5 chemokines are reduced in patients with dengue haemorrhagic fever |
| King et al., 2002 [76] | Mast cell and Basophil | In vitro | Human mast cells and basophils | Elevated levels of RANTES, MIP-1alpha, and MIP-1beta were observed following infection. Levels of IL-8 and ENA-78 were not increased |
| Brown et al., 2011 [77] | Mast cell | In vitro | Human cord blood-derived mast cells, HMC-1 | Infection results in the release of factors that activate human endothelial cells and increased expression of the adhesion molecules ICAM-1 and VCAM-1. Use of a specific TNF-blocking antibody blocked this effect, identifying TNF as an endothelial cell activating factor |
| Chu et al., 2017 [78] | Mast cell | In vivo (animal model) | Mice | Chymase contributes to dengue virus-induced vascular leakage |
| St. John et al., 2013 [81] | Mast cell | In vivo (animal model) | Mice | Leukotriens and proteases released by mast cells contribute to vascular damage |
| Rathore et al., 2019 [82] | Mast cell | In vitro | Human mast cells | Tryptase contributes to the degradation of fibrinogen and coagulation factors and probably to dengue virus-induced vascular permeability. |
| Masri et al., 2019 [84] | Mast cell | In vivo (animal model) | Mast cell-deficient mice, wild-type mice | Serotonin released by mast cells contributes to thrombocytopenia in dengue haemorrhagic fever |
| King et al., 2000 [85] | Mast cell and Basophil | In vitro | Human Mast cells and basophils | Vasoactive cytokine production by mast cells/basophils may contribute to the vascular pathology seen in severe dengue disease |
| Brown et al., 2006 [86] | Mast cell | In vitro | Human Mast cells | Mast cell activation is antibody-mediated via FcεRII |
| Jeewandara et al., 2016 [87] | Mast cell | In vivo (human) | Human blood samples | Phospholipase A2 activity significantly correlates with the degree of viraemia in patients with dengue haemorrhagic fever |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammeri, L.; Sanfilippo, S.; Alessandrello, C.; Gangemi, S.; Minciullo, P.L. Mast Cells and Basophils in Major Viral Diseases: What Are the Correlations with SARS-CoV-2, Influenza A Viruses, HIV, and Dengue? Cells 2024, 13, 2044. https://doi.org/10.3390/cells13242044
Gammeri L, Sanfilippo S, Alessandrello C, Gangemi S, Minciullo PL. Mast Cells and Basophils in Major Viral Diseases: What Are the Correlations with SARS-CoV-2, Influenza A Viruses, HIV, and Dengue? Cells. 2024; 13(24):2044. https://doi.org/10.3390/cells13242044
Chicago/Turabian StyleGammeri, Luca, Serena Sanfilippo, Clara Alessandrello, Sebastiano Gangemi, and Paola Lucia Minciullo. 2024. "Mast Cells and Basophils in Major Viral Diseases: What Are the Correlations with SARS-CoV-2, Influenza A Viruses, HIV, and Dengue?" Cells 13, no. 24: 2044. https://doi.org/10.3390/cells13242044
APA StyleGammeri, L., Sanfilippo, S., Alessandrello, C., Gangemi, S., & Minciullo, P. L. (2024). Mast Cells and Basophils in Major Viral Diseases: What Are the Correlations with SARS-CoV-2, Influenza A Viruses, HIV, and Dengue? Cells, 13(24), 2044. https://doi.org/10.3390/cells13242044







