Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. OA Patients and Adipose Tissues Obtained
2.2. Isolation and Characterization of Adipose-Derived Mesenchymal Stem Cells (ASCs)
2.3. Immunofluorescence (IF) Analysis
2.4. Quantitative Real-Time PCR
2.5. Conditioned Medium Collection
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Isolation and Stimulation of PBMC
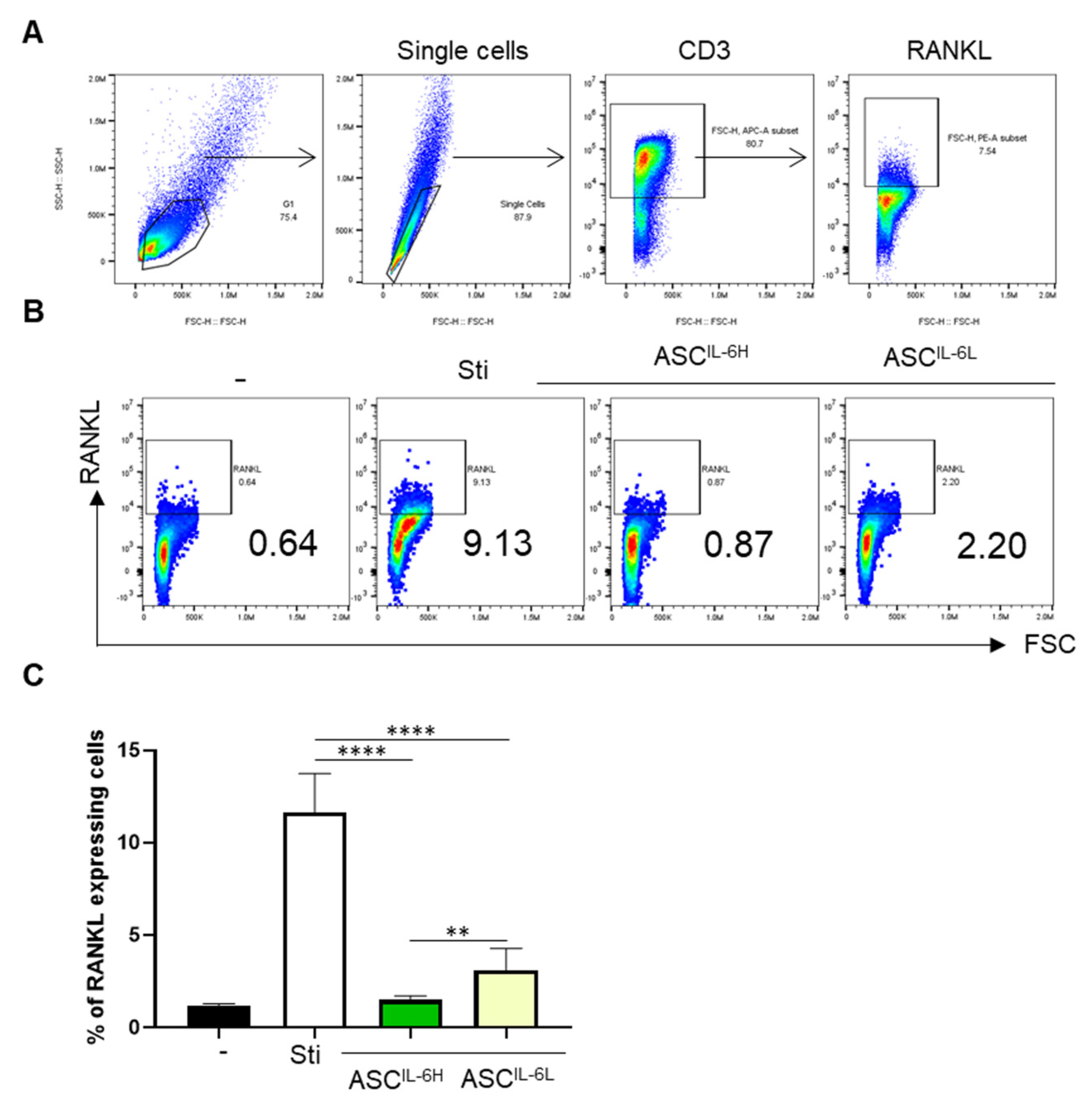
2.8. Flow Cytometry
2.9. Cytometric Bead Array
2.10. Statistical Analysis
3. Results
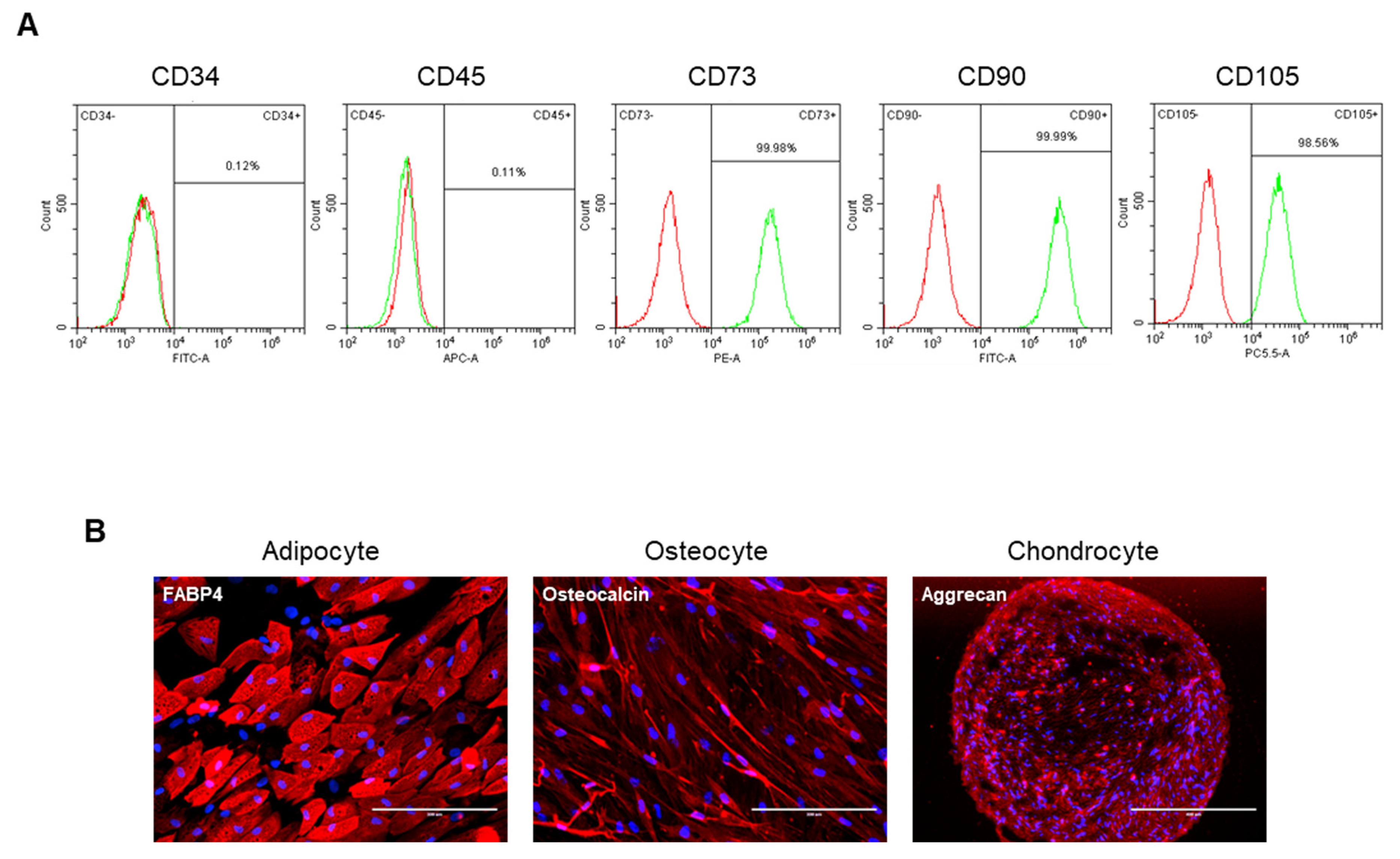
3.1. Characterization of ASCs Isolated from Osteoarthritis Patients Reveals Mesenchymal Stem Cell-like Properties
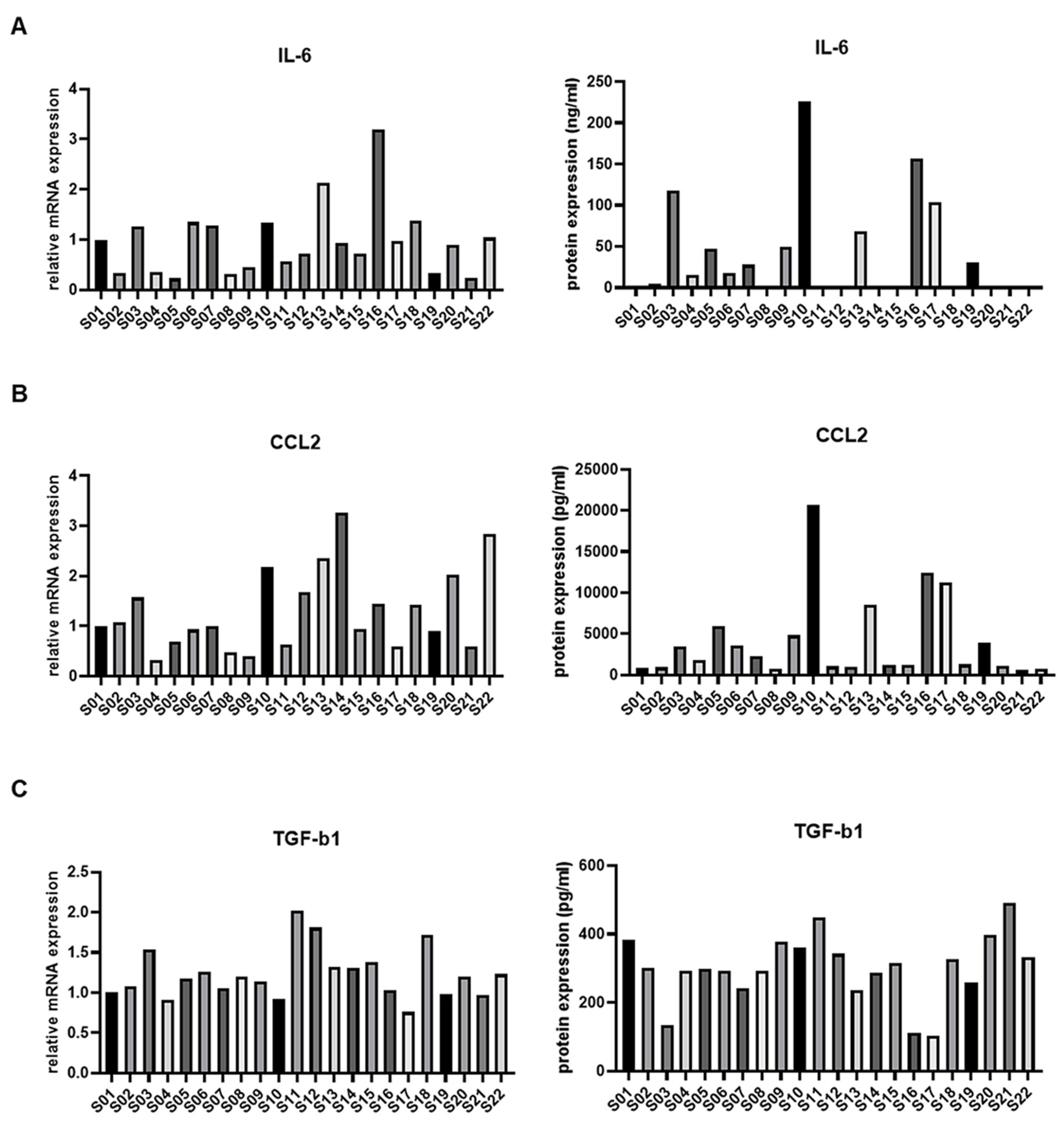
3.2. IL-6 Expression in ASCs from Osteoarthritis Patients and Immune Modulation
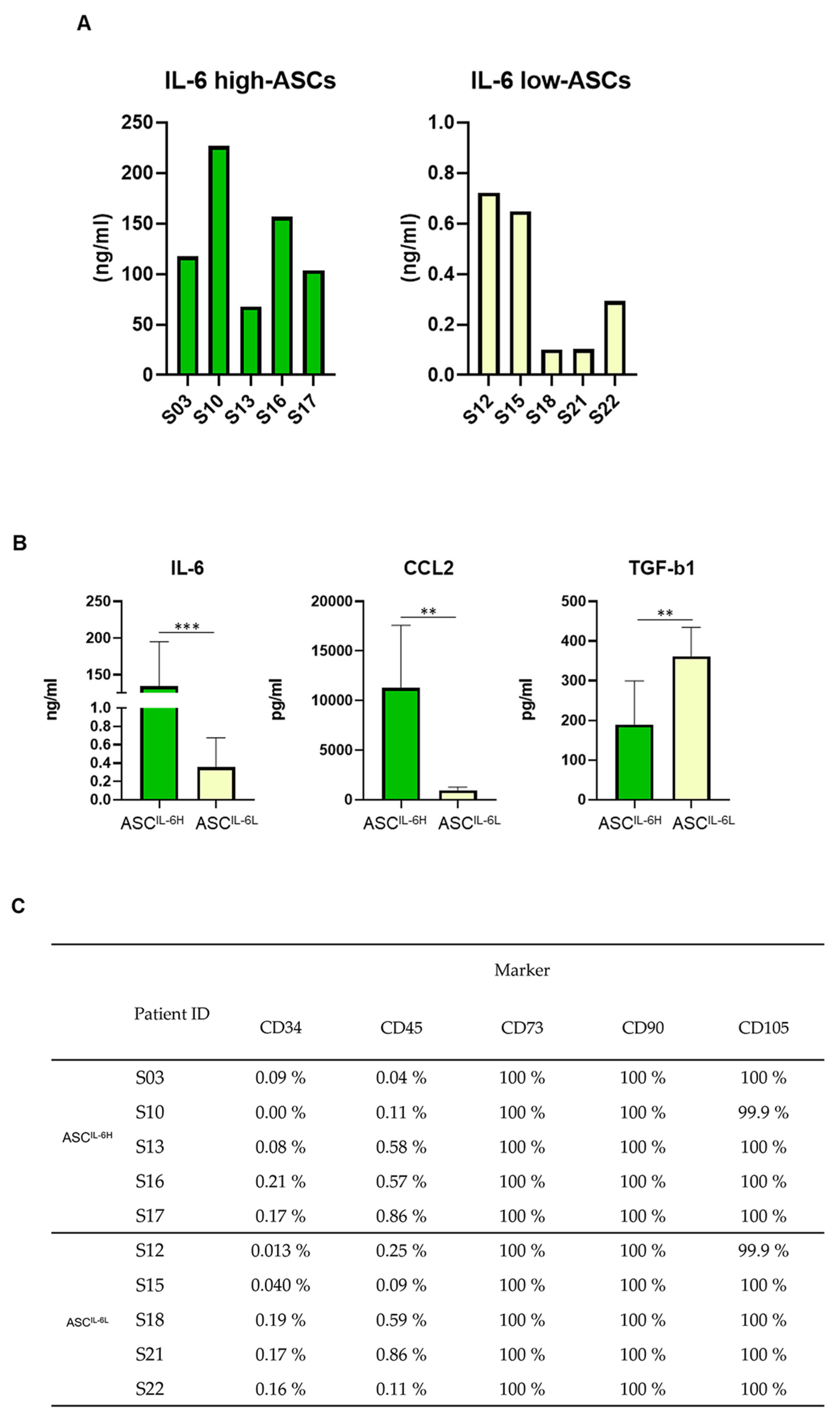
3.3. Grouping of ASC Based on IL-6 Expression and Its Impact on MSC Characteristics
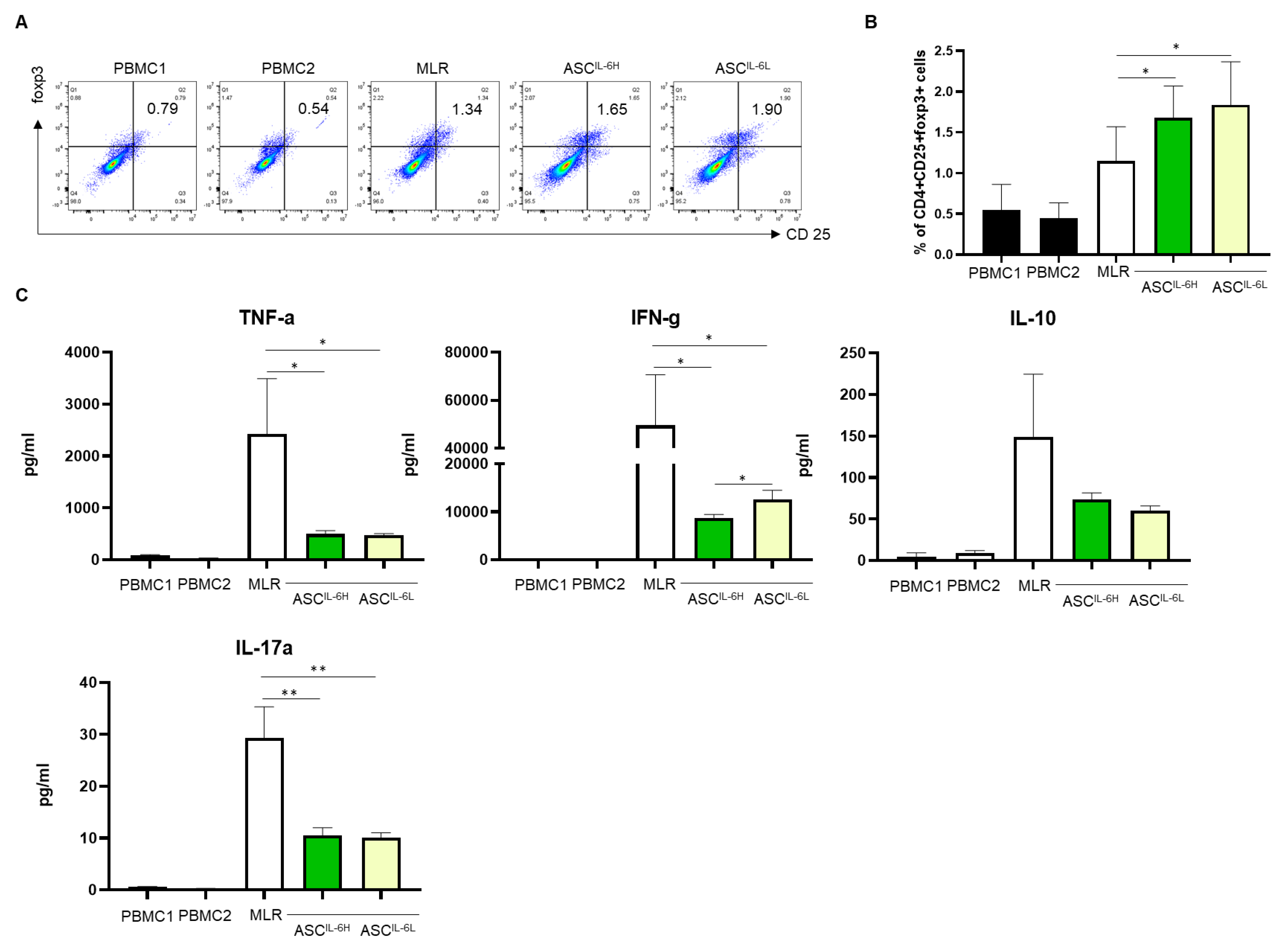
3.4. Impact of IL-6 Expression on Treg Induction by ASCs
3.5. Impact of IL-6 Expression on the Suppression of PHA-Induced PBMC Proliferation by ASCs
3.6. Impact of IL-6 Expression on the Suppression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the art: The immunomodulatory role of MSCs for osteoarthritis. Int. J. Mol. Sci. 2023, 23, 1618. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, M.H.J. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The Immunological Facets of Chondrocytes in Osteoarthritis: A Narrative Review. J. Rheumatol. 2023, 51, jrheum.2023-0816. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Shin, J.-S.; Lee, H.; Kim, S.H.; Noh, K.-C.; Kim, S.J.; Kim, H.N.; Choi, J.Y.; Song, S.Y. Identification of plasma and urinary inflammatory markers in severe knee osteoarthritis: Relations with synovial fluid markers. Knee Surg. Relat. Res. 2024, 36, 19. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Prockop, D.J. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin. Pharmacol. Ther. 2007, 82, 241–243. [Google Scholar] [CrossRef]
- Kang, J.S.; Suh, Y.J.; Moon, K.H.; Park, J.S.; Roh, T.H.; Park, M.H.; Ryu, D.J. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: A matched pair control study with simple core decompression. Stem Cell Res. Ther. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Ryu, D.J.; Jeon, Y.S.; Park, J.S.; Bae, G.C.; Kim, J.-S.; Kim, M.K. Comparison of bone marrow aspirate concentrate and allogenic human umbilical cord blood derived mesenchymal stem cell implantation on chondral defect of knee: Assessment of Clinical and Magnetic Resonance Imaging Outcomes at 2-Year Follow-Up. Cell Transplant. 2020, 29, 963689720943581. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Jung, M.; Chung, K.; Jung, S.-H.; Choi, C.-H.; Kim, S.-H. Effects of concurrent cartilage procedures on cartilage regeneration in high tibial osteotomy: A systematic review. Knee Surg. Relat. Res. 2024, 36, 13. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non redundant roles in human mesenchymal stem cell induction of CD4+CD25highFoxp3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Eitner, A.; König, C.; Kohler, F.C.; Hofmann, G.O.; Wildemann, B.; Aurich, M.; Schaible, H.-G. Importance of IL-6 trans-signaling and high autocrine IL-6 production in human osteoarthritic chondrocyte metabolism. Osteoarthr. Cartil. 2024, 32, 561–573. [Google Scholar] [CrossRef]
- Kondo, M.; Yamaoka, K.; Sakata, K.; Sonomoto, K.; Lin, L.; Nakano, K.; Tanaka, Y. Contribution of the Interleukin-6/STAT-3 Signaling Pathway to Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Arthritis Rheumatol. 2015, 67, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, J.; Miller, J.; Leventhal, J.; Huang, X.; Flaa, C.; Wang, E.; Tambur, A.; Burt, R.K.; Gallon, L.; Mathew, J.M. The human “Treg MLR”: Immune monitoring for FOXP3+ T regulatory cell generation. Transplantation 2009, 88, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.-Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, X.; Liu, Y.; Jie, P.; Mazhar, M.; Liu, Y.; Dechsupa, N.; Wang, L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2023, 19, 1214–1231. [Google Scholar] [CrossRef]
- Bowles, A.C.; Wise, R.M.; Bunnell, B.A. Anti-inflammatory Effects of Adipose-Derived Stem Cells (ASCs). In Mesenchymal Stem Cells: Immunomodulation; Springer: Cham, Switzerland, 2016; pp. 43–60. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Skalska, U.; Kontny, E. Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity 2015, 49, 124–131. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef]
- Na, H.; Im, K.-I.; Kim, N.; Lee, J.; Gil, S.; Min, G.J.; Cho, S.G. The IL-6 signaling pathway contributes critically to the immunomodulatory mechanism of human Decidua-derived mesenchymal stromal cells. iScience 2024, 27, 109783. [Google Scholar] [CrossRef]
- Ding, Q.; Fang, H.; Jin, P.; Lv, J.; Ding, S.; Zhu, W.; Chen, C. Pretreating mesenchymal stem cells with IL-6 regulates the inflammatory response of DSS-induced ulcerative colitis in rats. Transpl. Immunol. 2022, 76, 101765. [Google Scholar] [CrossRef]
- Suzdaltseva, Y.; Goryunov, K.; Silina, E.; Manturova, N.; Stupin, V.; Kiselev, S.L. Equilibrium among Inflammatory Factors Determines Human MSC-Mediated Immunosuppressive Effect. Cells 2022, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, L.; Feng, H.; Yue, Y.; Zhang, Y.; Wang, Q.; Zhao, H. Therapeutics of osteoarthritis and pharmacological mechanisms: A focus on RANK/RANKL signaling. Biomed. Pharmacother. 2023, 167, 115646. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Ha, H.; Kim, H.-N.; Lee, J.-H.; Kim, H.S.; Lee, S.; Kim, H.-M.; Kim, J.Y.; Kim, H.-H.; Song, Y.W.; et al. Reciprocal cross-talk between RANKL and interferon-γ–inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis Rheum. 2008, 58, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Dai, A.; Pan, L.; Zhang, L.; Wang, Z.; Ke, T.; Sun, W.; Wu, Y.; Ding, P.-H.; Chen, L. Inflamm-Aging-Related Cytokines of IL-17 and IFN-γ Accelerate Osteoclastogenesis and Periodontal Destruction. J. Immunol. Res. 2021, 2021, 9919024. [Google Scholar] [CrossRef]
- Chang, Q.; Li, C.; Lu, Y.; Geng, R.; Wei, J.; Hu, J. Adipose-derived mesenchymal stromal cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis. Scand. J. Immunol. 2020, 92, e12877. [Google Scholar] [CrossRef]
- Abe, T.; Sumi, K.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Nakajima, K.; Ando, K.; Tanimoto, K. The effect of mesenchymal stem cells on osteoclast precursor cell differentiation. J. Oral Sci. 2019, 61, 30–35. [Google Scholar] [CrossRef]

| Donor No. | Age (Years) | Sex (F/M) | BMI | OA Symptom Duration (Months) | VAS Pain Score | K-L Grade |
|---|---|---|---|---|---|---|
| 1 | 58 | F | 26.9 | 6 | 6 | II |
| 2 | 48 | F | 21.5 | 6 | 6 | II |
| 3 | 56 | F | 29.4 | 6 | 6 | IV |
| 4 | 60 | F | 22.7 | 5 | 4 | II |
| 5 | 59 | M | 25.1 | 7 | 5 | IV |
| 6 | 70 | F | 27.7 | 13 | 5 | II |
| 7 | 52 | F | 29.1 | 40 | 6 | IV |
| 8 | 62 | F | 33.0 | 18 | 7 | III |
| 9 | 63 | M | 28.1 | 10 | 5 | II |
| 10 | 67 | F | 26.4 | 16 | 5 | III |
| 11 | 55 | M | 26.9 | 180 | 5 | II |
| 12 | 67 | F | 23.0 | 2 | 7 | III |
| 13 | 65 | F | 24.9 | 6 | 5 | III |
| 14 | 57 | M | 27.0 | 27 | 6 | II |
| 15 | 60 | M | 27.8 | 6 | 5 | III |
| 16 | 64 | F | 26.9 | 2 | 5 | IV |
| 17 | 57 | M | 31.4 | 3 | 6 | III |
| 18 | 63 | M | 30.1 | 17 | 8 | IV |
| 19 | 58 | M | 26.6 | 26 | 6 | III |
| 20 | 59 | M | 30.6 | 3 | 6 | III |
| 21 | 63 | F | 27.2 | 27 | 4 | II |
| 22 | 62 | F | 26.4 | 2 | 7 | II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Kim, D.-Y.; Noh, H.j.; Lee, S.Y.; Yoo, J.A.; Won, S.J.; Jeon, Y.S.; Baek, J.H.; Ryu, D.J. Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis. Cells 2024, 13, 2046. https://doi.org/10.3390/cells13242046
Lee H-J, Kim D-Y, Noh Hj, Lee SY, Yoo JA, Won SJ, Jeon YS, Baek JH, Ryu DJ. Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis. Cells. 2024; 13(24):2046. https://doi.org/10.3390/cells13242046
Chicago/Turabian StyleLee, Hyun-Joo, Dae-Yong Kim, Hyeon jeong Noh, Song Yi Lee, Ji Ae Yoo, Samuel Jaeyoon Won, Yoon Sang Jeon, Ji Hoon Baek, and Dong Jin Ryu. 2024. "Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis" Cells 13, no. 24: 2046. https://doi.org/10.3390/cells13242046
APA StyleLee, H.-J., Kim, D.-Y., Noh, H. j., Lee, S. Y., Yoo, J. A., Won, S. J., Jeon, Y. S., Baek, J. H., & Ryu, D. J. (2024). Elevated IL-6 Expression in Autologous Adipose-Derived Stem Cells Regulates RANKL Mediated Inflammation in Osteoarthritis. Cells, 13(24), 2046. https://doi.org/10.3390/cells13242046








