Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage
Abstract
1. Introduction
2. Exercise-Induced Muscle Damage
2.1. Etiology and Cellular Dynamics of EIMD
2.2. Primary Muscle Damage—Mechanical Stress
2.3. Secondary Muscle Damage—Inflammatory Phase
2.4. Resolving Inflammation—Resolution Phase
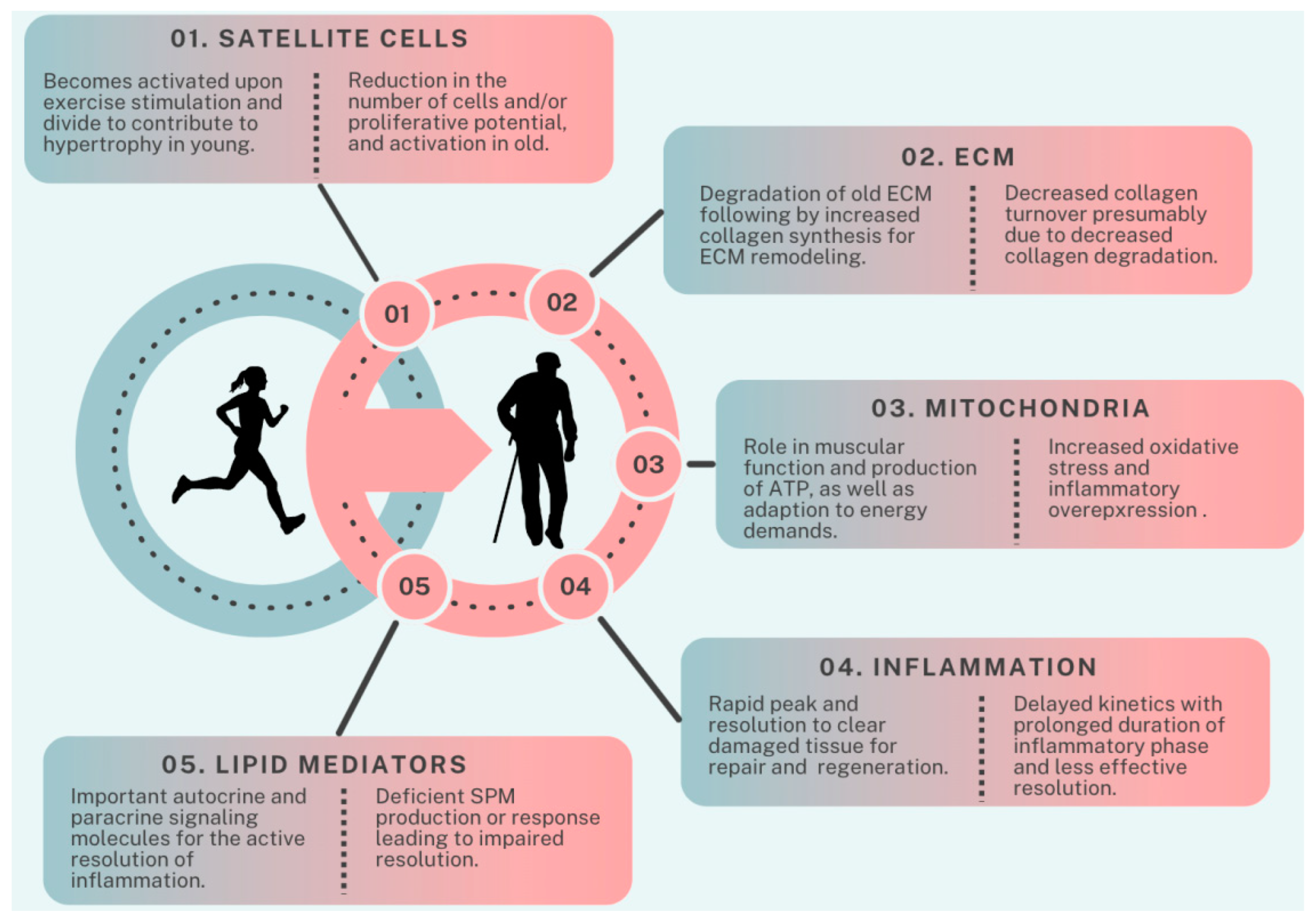
3. Factors Contributing to Age-Related Impairment in Exercise Recovery
3.1. Difference in Recovery from Resistance Exercise in Aged Muscles
3.2. Satellite Cell Contribution to Decreased Hypertrophic Potential
3.3. Transition of the ECM to a Fibrogenic Phenotype impacts Recovery
3.4. Mitochondrial Dysfunction and Implications on Oxidative Stress and Inflammation
3.5. Inflammatory Changes Post-Exercise
3.6. Lipid Mediator Involvement on Resolution of Inflammation
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Chronic Adaptations to Eccentric Training: A Systematic Review. Sports Med. 2017, 47, 917–941. [Google Scholar] [CrossRef]
- Paschalis, V.; Nikolaidis, M.G.; Theodorou, A.A.; Panayiotou, G.; Fatouros, I.G.; Koutedakis, Y.; Jamurtas, A.Z. A weekly bout of eccentric exercise is sufficient to induce health-promoting effects. Med. Sci. Sports Exerc. 2011, 43, 64–73. [Google Scholar] [CrossRef]
- Roig, M.; O’Brien, K.; Kirk, G.; Murray, R.; McKinnon, P.; Shadgan, B.; Reid, W.D. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: A systematic review with meta-analysis. Br. J. Sports Med. 2009, 43, 556–568. [Google Scholar] [CrossRef]
- LaStayo, P.; Marcus, R.; Dibble, L.; Frajacomo, F.; Lindstedt, S. Eccentric exercise in rehabilitation: Safety, feasibility, and application. J. Appl. Physiol. 2014, 116, 1426–1434. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, M.G. Effects of unaccustomed downhill running on muscle damage, oxidative stress, and leukocyte apoptosis. J. Exerc. Nutr. Biochem. 2015, 19, 55–63. [Google Scholar] [CrossRef]
- Marklund, P.; Mattsson, C.M.; Wahlin-Larsson, B.; Ponsot, E.; Lindvall, B.; Lindvall, L.; Ekblom, B.; Kadi, F. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J. Appl. Physiol. 2013, 114, 66–72. [Google Scholar] [CrossRef]
- Tee, J.C.; Bosch, A.N.; Lambert, M.I. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007, 37, 827–836. [Google Scholar] [CrossRef]
- Bridgeman, L.A.; Gill, N.D.; Dulson, D.K.; McGuigan, M.R. The Effect of Exercise-Induced Muscle Damage After a Bout of Accentuated Eccentric Load Drop Jumps and the Repeated Bout Effect. J. Strength Cond. Res. 2017, 31, 386–394. [Google Scholar] [CrossRef]
- Komi, P.V. Stretch-shortening cycle: A powerful model to study normal and fatigued muscle. J. Biomech. 2000, 33, 1197–1206. [Google Scholar] [CrossRef]
- Gonzalez-Bartholin, R.; Mackay, K.; Valladares, D.; Zbinden-Foncea, H.; Nosaka, K.; Penailillo, L. Changes in oxidative stress, inflammation and muscle damage markers following eccentric versus concentric cycling in older adults. Eur. J. Appl. Physiol. 2019, 119, 2301–2312. [Google Scholar] [CrossRef]
- Penailillo, L.; Blazevich, A.; Numazawa, H.; Nosaka, K. Metabolic and muscle damage profiles of concentric versus repeated eccentric cycling. Med. Sci. Sports Exerc. 2013, 45, 1773–1781. [Google Scholar] [CrossRef]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and its effects on inflammation in skeletal muscle at rest and following exercise-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495. [Google Scholar] [CrossRef]
- Skorski, S.; Mujika, I.; Bosquet, L.; Meeusen, R.; Coutts, A.J.; Meyer, T. The Temporal Relationship Between Exercise, Recovery Processes, and Changes in Performance. Int. J. Sports Physiol. Perform. 2019, 14, 1015–1021. [Google Scholar] [CrossRef]
- Damas, F.; Nosaka, K.; Libardi, C.A.; Chen, T.C.; Ugrinowitsch, C. Susceptibility to Exercise-Induced Muscle Damage: A Cluster Analysis with a Large Sample. Int. J. Sports Med. 2016, 37, 633–640. [Google Scholar] [CrossRef]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Exercise-Induced Muscle Damage and Recovery in Young and Middle-Aged Males with Different Resistance Training Experience. Sports 2019, 7, 132. [Google Scholar] [CrossRef]
- Chapman, D.; Newton, M.; Sacco, P.; Nosaka, K. Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int. J. Sports Med. 2006, 27, 591–598. [Google Scholar] [CrossRef]
- Byrne, C.; Eston, R.G.; Edwards, R.H. Characteristics of isometric and dynamic strength loss following eccentric exercise-induced muscle damage. Scand. J. Med. Sci. Sports 2001, 11, 134–140. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Med. 2017, 47, 663–675. [Google Scholar] [CrossRef]
- Chalchat, E.; Gaston, A.F.; Charlot, K.; Peñailillo, L.; Valdés, O.; Tardo-Dino, P.E.; Nosaka, K.; Martin, V.; Garcia-Vicencio, S.; Siracusa, J. Appropriateness of indirect markers of muscle damage following lower limbs eccentric-biased exercises: A systematic review with meta-analysis. PLoS ONE 2022, 17, e0271233. [Google Scholar] [CrossRef]
- Hayes, E.J.; Stevenson, E.; Sayer, A.A.; Granic, A.; Hurst, C. Recovery from Resistance Exercise in Older Adults: A Systematic Scoping Review. Sports Med. Open 2023, 9, 51. [Google Scholar] [CrossRef]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef]
- Enoka, R.M. Eccentric contractions require unique activation strategies by the nervous system. J. Appl. Physiol. 1996, 81, 2339–2346. [Google Scholar] [CrossRef]
- Friden, J.; Sjostrom, M.; Ekblom, B. Myofibrillar damage following intense eccentric exercise in man. Int. J. Sports Med. 1983, 4, 170–176. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef]
- Sorichter, S.; Puschendorf, B.; Mair, J. Skeletal muscle injury induced by eccentric muscle action: Muscle proteins as markers of muscle fiber injury. Exerc. Immunol. Rev. 1999, 5, 5–21. [Google Scholar]
- Gissel, H. The role of Ca2+ in muscle cell damage. Ann. N. Y. Acad. Sci. 2005, 1066, 166–180. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X. The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol. Res. 2016, 65, 547–560. [Google Scholar] [CrossRef]
- Allen, D.G. Eccentric muscle damage: Mechanisms of early reduction of force. Acta Physiol. Scand. 2001, 171, 311–319. [Google Scholar] [CrossRef]
- Stauber, W.T.; Clarkson, P.M.; Fritz, V.K.; Evans, W.J. Extracellular matrix disruption and pain after eccentric muscle action. J. Appl. Physiol. 1990, 69, 868–874. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The use of nonsteroidal anti-inflammatory drugs for exercise-induced muscle damage: Implications for skeletal muscle development. Sports Med. 2012, 42, 1017–1028. [Google Scholar] [CrossRef]
- Gissel, H.; Clausen, T. Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol. Scand. 2001, 171, 327–334. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; MacLaren, D.P. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: The role of reactive oxygen species. Eur. J. Appl. Physiol. 2004, 91, 615–621. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation during skeletal muscle regeneration and tissue remodeling: Application to exercise-induced muscle damage management. Immunol. Cell Biol. 2016, 94, 140–145. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Stožer, A.; Vodopivc, P.; Križančić Bombek, L. Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F., 3rd; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Peake, J.M.; Markworth, J.F.; Nosaka, K.; Raastad, T.; Wadley, G.D.; Coffey, V.G. Modulating exercise-induced hormesis: Does less equal more? J. Appl. Physiol. 2015, 119, 172–189. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar]
- Gallo, J.; Raska, M.; Kriegova, E.; Goodman, S.B. Inflammation and its resolution and the musculoskeletal system. J. Orthop. Translat. 2017, 10, 52–67. [Google Scholar] [CrossRef]
- Oh, S.F.; Pillai, P.S.; Recchiuti, A.; Yang, R.; Serhan, C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Investig. 2011, 121, 569–581. [Google Scholar] [CrossRef]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016, 22, 110–134. [Google Scholar]
- Hettinger, Z.R.; Hamagata, K.; Confides, A.L.; Lawrence, M.M.; Miller, B.F.; Butterfield, T.A.; Dupont-Versteegden, E.E. Age-Related Susceptibility to Muscle Damage Following Mechanotherapy in Rats Recovering From Disuse Atrophy. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2132–2140. [Google Scholar] [CrossRef]
- Rader, E.P.; Faulkner, J.A. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J. Appl. Physiol. 2006, 101, 887–892. [Google Scholar] [CrossRef]
- Rader, E.P.; Faulkner, J.A. Recovery from contraction-induced injury is impaired in weight-bearing muscles of old male mice. J. Appl. Physiol. 2006, 100, 656–661. [Google Scholar] [CrossRef]
- Brooks, S.V.; Faulkner, J.A. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988, 404, 71–82. [Google Scholar] [CrossRef]
- Faulkner, J.A.; Brooks, S.V.; Zerba, E. Skeletal muscle weakness and fatigue in old age: Underlying mechanisms. Annu. Rev. Gerontol. Geriatr. 1990, 10, 147–166. [Google Scholar] [CrossRef]
- McBride, T.A.; Gorin, F.A.; Carlsen, R.C. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech. Ageing Dev. 1995, 83, 185–200. [Google Scholar] [CrossRef]
- Close, G.L.; Kayani, A.; Vasilaki, A.; McArdle, A. Skeletal muscle damage with exercise and aging. Sports Med. 2005, 35, 413–427. [Google Scholar] [CrossRef]
- Lavender, A.P.; Nosaka, K. Responses of old men to repeated bouts of eccentric exercise of the elbow flexors in comparison with young men. Eur. J. Appl. Physiol. 2006, 97, 619–626. [Google Scholar] [CrossRef]
- Lavender, A.P.; Nosaka, K. Changes in markers of muscle damage of middle-aged and young men following eccentric exercise of the elbow flexors. J. Sci. Med. Sport 2008, 11, 124–131. [Google Scholar] [CrossRef]
- Nikolaidis, M.G. The Effects of Resistance Exercise on Muscle Damage, Position Sense, and Blood Redox Status in Young and Elderly Individuals. Geriatrics 2017, 2, 20. [Google Scholar] [CrossRef]
- Przybyla, B.; Gurley, C.; Harvey, J.F.; Bearden, E.; Kortebein, P.; Evans, W.J.; Sullivan, D.H.; Peterson, C.A.; Dennis, R.A. Aging alters macrophage properties in human skeletal muscle both at rest and in response to acute resistance exercise. Exp. Gerontol. 2006, 41, 320–327. [Google Scholar] [CrossRef]
- Buford, T.W.; MacNeil, R.G.; Clough, L.G.; Dirain, M.; Sandesara, B.; Pahor, M.; Manini, T.M.; Leeuwenburgh, C. Active muscle regeneration following eccentric contraction-induced injury is similar between healthy young and older adults. J. Appl. Physiol. 2014, 116, 1481–1490. [Google Scholar] [CrossRef]
- Sorensen, J.R.; Skousen, C.; Holland, A.; Williams, K.; Hyldahl, R.D. Acute extracellular matrix, inflammatory and MAPK response to lengthening contractions in elderly human skeletal muscle. Exp. Gerontol. 2018, 106, 28–38. [Google Scholar] [CrossRef]
- Lee, J.H.F.; Boland-Freitas, R.; Ng, K. Sarcolemmal excitability changes in normal human aging. Muscle Nerve 2018, 57, 981–988. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Cooper, I.; Kraev, I.; Lange, S.; Elliott, B.T. Preliminary Investigations Into the Effect of Exercise-Induced Muscle Damage on Systemic Extracellular Vesicle Release in Trained Younger and Older Men. Front. Physiol. 2021, 12, 723931. [Google Scholar] [CrossRef]
- Manfredi, T.G.; Fielding, R.A.; O’Reilly, K.P.; Meredith, C.N.; Lee, H.Y.; Evans, W.J. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med. Sci. Sports Exerc. 1991, 23, 1028–1034. [Google Scholar] [CrossRef]
- Houmard, J.A.; Weidner, M.L.; Gavigan, K.E.; Tyndall, G.L.; Hickey, M.S.; Alshami, A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J. Appl. Physiol. 1998, 85, 1337–1341. [Google Scholar] [CrossRef]
- McKay, B.R.; Ogborn, D.I.; Bellamy, L.M.; Tarnopolsky, M.A.; Parise, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012, 26, 2509–2521. [Google Scholar] [CrossRef]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef]
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. 1995, 268, E514–E520. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; Parise, G.; Bellamy, L.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS ONE 2014, 9, e89431. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.M.; Libardi, C.A.; Vechin, F.C.; Lixandrao, M.E.; Jannig, P.R.; Costa, L.A.; Bacurau, A.V.; Snijders, T.; Parise, G.; et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J. Physiol. 2016, 594, 5209–5222. [Google Scholar] [CrossRef]
- Dalle, S.; Hiroux, C.; Poffe, C.; Ramaekers, M.; Deldicque, L.; Koppo, K. Cardiotoxin-induced skeletal muscle injury elicits profound changes in anabolic and stress signaling, and muscle fiber type composition. J. Muscle Res. Cell Motil. 2020, 41, 375–387. [Google Scholar] [CrossRef]
- Joseph, G.A.; Wang, S.X.; Jacobs, C.E.; Zhou, W.; Kimble, G.C.; Tse, H.W.; Eash, J.K.; Shavlakadze, T.; Glass, D.J. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol. Cell. Biol. 2019, 39, e00141-19. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.T.; Mossakowski, A.A.; Sule, R.; Gomes, A.; Baar, K. Dominant-negative p53-overexpression in skeletal muscle induces cell death and fiber atrophy in rats. Cell Death. Dis. 2022, 13, 716. [Google Scholar] [CrossRef]
- Langer, H.T.; Senden, J.M.G.; Gijsen, A.P.; Kempa, S.; van Loon, L.J.C.; Spuler, S. Muscle Atrophy Due to Nerve Damage Is Accompanied by Elevated Myofibrillar Protein Synthesis Rates. Front. Physiol. 2018, 9, 1220. [Google Scholar] [CrossRef]
- Ploutz-Snyder, L.L.; Giamis, E.L.; Formikell, M.; Rosenbaum, A.E. Resistance training reduces susceptibility to eccentric exercise-induced muscle dysfunction in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B384–B390. [Google Scholar] [CrossRef]
- Kramer, I.F.; Verdijk, L.B.; Hamer, H.M.; Verlaan, S.; Luiking, Y.C.; Kouw, I.W.K.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Bierau, J.; et al. Both basal and post-prandial muscle protein synthesis rates, following the ingestion of a leucine-enriched whey protein supplement, are not impaired in sarcopenic older males. Clin. Nutr. 2017, 36, 1440–1449. [Google Scholar] [CrossRef]
- Ham, D.J.; Borsch, A.; Lin, S.; Thurkauf, M.; Weihrauch, M.; Reinhard, J.R.; Delezie, J.; Battilana, F.; Wang, X.; Kaiser, M.S.; et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat. Commun. 2020, 11, 4510. [Google Scholar] [CrossRef]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015, 29, 4485–4496. [Google Scholar] [CrossRef]
- Langer, H.T.; West, D.; Senden, J.; Spuler, S.; van Loon, L.J.C.; Baar, K. Myofibrillar protein synthesis rates are increased in chronically exercised skeletal muscle despite decreased anabolic signaling. Sci. Rep. 2022, 12, 7553. [Google Scholar] [CrossRef]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef]
- Phillips, B.E.; Williams, J.P.; Gustafsson, T.; Bouchard, C.; Rankinen, T.; Knudsen, S.; Smith, K.; Timmons, J.A.; Atherton, P.J. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet. 2013, 9, e1003389. [Google Scholar] [CrossRef]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef]
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 57–62. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Paez, H.G.; Pitzer, C.R.; Alway, S.E. Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells 2023, 12, 249. [Google Scholar] [CrossRef]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J.; Andersen, J.L.; Schjerling, P.; Olsen, S.; Kjaer, M. The behaviour of satellite cells in response to exercise: What have we learned from human studies? Pflugers Arch. 2005, 451, 319–327. [Google Scholar] [CrossRef]
- Crameri, R.M.; Langberg, H.; Magnusson, P.; Jensen, C.H.; Schroder, H.D.; Olesen, J.L.; Suetta, C.; Teisner, B.; Kjaer, M. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J. Physiol. 2004, 558, 333–340. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Snijders, T.; Joanisse, S.; Wavell, C.G.; Mitchell, C.J.; Johnston, L.M.; Baker, S.K.; Phillips, S.M.; Parise, G. Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R85–R92. [Google Scholar] [CrossRef]
- Carson, J.A.; Alway, S.E. Stretch overload-induced satellite cell activation in slow tonic muscle from adult and aged Japanese quail. Am. J. Physiol. 1996, 270, C578–C584. [Google Scholar] [CrossRef]
- Carson, J.A.; Yamaguchi, M.; Alway, S.E. Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J. Appl. Physiol. 1995, 78, 293–299. [Google Scholar] [CrossRef]
- Cutlip, R.G.; Baker, B.A.; Geronilla, K.B.; Mercer, R.R.; Kashon, M.L.; Miller, G.R.; Murlasits, Z.; Alway, S.E. Chronic exposure to stretch-shortening contractions results in skeletal muscle adaptation in young rats and maladaptation in old rats. Appl. Physiol. Nutr. Metab. 2006, 31, 573–587. [Google Scholar] [CrossRef]
- Shefer, G.; Van de Mark, D.P.; Richardson, J.B.; Yablonka-Reuveni, Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006, 294, 50–66. [Google Scholar] [CrossRef]
- Brooks, N.E.; Schuenke, M.D.; Hikida, R.S. No change in skeletal muscle satellite cells in young and aging rat soleus muscle. J. Physiol. Sci. 2009, 59, 465–471. [Google Scholar] [CrossRef]
- Snijders, T.; Verdijk, L.B.; Smeets, J.S.; McKay, B.R.; Senden, J.M.; Hartgens, F.; Parise, G.; Greenhaff, P.; van Loon, L.J. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age 2014, 36, 9699. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Moore, D.R.; McKay, B.R.; Tarnopolsky, M.A.; Parise, G. Blunted satellite cell response is associated with dysregulated IGF-1 expression after exercise with age. Eur. J. Appl. Physiol. 2018, 118, 2225–2231. [Google Scholar] [CrossRef]
- McKay, B.R.; Ogborn, D.I.; Baker, J.M.; Toth, K.G.; Tarnopolsky, M.A.; Parise, G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am. J. Physiol. Cell Physiol. 2013, 304, C717–C728. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Dye, D.E.; Kinnear, B.F.; van Kuppevelt, T.H.; Grounds, M.D.; Coombe, D.R. Interactions between Skeletal Muscle Myoblasts and their Extracellular Matrix Revealed by a Serum Free Culture System. PLoS ONE 2015, 10, e0127675. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Kirby, T.J.; Kosmac, K.; McCarthy, J.J.; Peterson, C.A. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem. Cell 2017, 20, 56–69. [Google Scholar] [CrossRef]
- Almada, A.E.; Wagers, A.J. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 267–279. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P.; Trotter, J.A. The morphology and mechanical properties of endomysium in series-fibred muscles: Variations with muscle length. J. Muscle. Res. Cell Motil. 1994, 15, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Kragstrup, T.W.; Kjaer, M.; Mackey, A.L. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand. J. Med. Sci. Sports 2011, 21, 749–757. [Google Scholar] [CrossRef]
- Hofmann, U.K.; Jordan, M.; Rondak, I.; Wolf, P.; Kluba, T.; Ipach, I. Osteoarthritis of the knee or hip significantly impairs driving ability (cross-sectional survey). BMC Musculoskelet. Disord. 2014, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Herchenhan, A.; Uhlenbrock, F.; Eliasson, P.; Weis, M.; Eyre, D.; Kadler, K.E.; Magnusson, S.P.; Kjaer, M. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J. Biol. Chem. 2015, 290, 16440–16450. [Google Scholar] [CrossRef]
- Wessner, B.; Liebensteiner, M.; Nachbauer, W.; Csapo, R. Age-specific response of skeletal muscle extracellular matrix to acute resistance exercise: A pilot study. Eur. J. Sport Sci. 2019, 19, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Chapman, M.A.; Bushong, E.A.; Deerinck, T.J.; Ellisman, M.H.; Lieber, R.L. High resolution three-dimensional reconstruction of fibrotic skeletal muscle extracellular matrix. J. Physiol. 2017, 595, 1159–1171. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patel, K.D. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp. Lung. Res. 2005, 31, 599–621. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.C.; Redden, J.T.; Schwartz, Z.; Cohen, D.J.; McClure, M.J. Advanced Glycation End-Products in Skeletal Muscle Aging. Bioengineering 2021, 8, 168. [Google Scholar] [CrossRef]
- Peng, Y.; Kim, J.M.; Park, H.S.; Yang, A.; Islam, C.; Lakatta, E.G.; Lin, L. AGE-RAGE signal generates a specific NF-kappaB RelA “barcode” that directs collagen I expression. Sci. Rep. 2016, 6, 18822. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Donato, R. HMGB1-RAGE regulates muscle satellite cell homeostasis through p38-MAPK- and myogenin-dependent repression of Pax7 transcription. J. Cell Sci. 2012, 125, 1440–1454. [Google Scholar] [CrossRef]
- Calve, S.; Simon, H.G. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 2012, 26, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Nelson, B.; Xin, L.; Welling, T.; Groscost, L.; Hubal, M.J.; Chipkin, S.; Clarkson, P.M.; Parcell, A.C. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J. 2015, 29, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Wilschut, K.J.; Haagsman, H.P.; Roelen, B.A. Extracellular matrix components direct porcine muscle stem cell behavior. Exp. Cell Res. 2010, 316, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Rasool Ali, T.; Habib, M. FIBRONECTIN EXPRESSION PATTERN IN SKELETAL MUSCLE REGENERATION. Int. J. Adv. Res. 2017, 5, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.A.; Angus, S.A.; Stokes, K.; Karpowicz, P.; Krause, M.P. Impaired ECM Remodeling and Macrophage Activity Define Necrosis and Regeneration Following Damage in Aged Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 4575. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Ritov, V.B.; Menshikova, E.V.; Kelley, D.E. High-performance liquid chromatography-based methods of enzymatic analysis: Electron transport chain activity in mitochondria from human skeletal muscle. Anal. Biochem. 2004, 333, 27–38. [Google Scholar] [CrossRef]
- Li, Z.; Peng, L.; Sun, L.; Si, J. A link between mitochondrial damage and the immune microenvironment of delayed onset muscle soreness. BMC Med. Genom. 2023, 16, 196. [Google Scholar] [CrossRef]
- Dos Santos, R.S.; Veras, F.P.; Ferreira, D.W.; Sant’Anna, M.B.; Lollo, P.C.B.; Cunha, T.M.; Galdino, G. Involvement of the Hsp70/TLR4/IL-6 and TNF-alpha pathways in delayed-onset muscle soreness. J. Neurochem. 2020, 155, 29–44. [Google Scholar] [CrossRef]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria transplant therapy improves regeneration and restoration of injured skeletal muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, J.S.; Yoon, Y.; Santiago, M.C.; Brown, M.D.; Park, J.Y. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R927–R938. [Google Scholar] [CrossRef]
- Badu-Mensah, A.; Valinski, P.; Parsaud, H.; Hickman, J.J.; Guo, X. Hyperglycemia Negatively Affects IPSC-Derived Myoblast Proliferation and Skeletal Muscle Regeneration and Function. Cells 2022, 11, 3674. [Google Scholar] [CrossRef]
- Fulco, M.; Cen, Y.; Zhao, P.; Hoffman, E.P.; McBurney, M.W.; Sauve, A.A.; Sartorelli, V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 2008, 14, 661–673. [Google Scholar] [CrossRef]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramirez-Pardo, I.; Segales, J.; Hernansanz-Agustin, P.; Curtabbi, A.; Deryagin, O.; Pollan, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e1210. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Fang, X.; Zhang, N.; Zhang, X.; Zhu, Z. Activation of autophagy inhibits the activation of NLRP3 inflammasome and alleviates sevoflurane-induced cognitive dysfunction in elderly rats. BMC Neurosci. 2023, 24, 9. [Google Scholar] [CrossRef]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Pedersen, B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. N. Am. 2003, 23, 15–39. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Pereira, M.A.; Curran, K.M. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med. Sci. Sports Exerc. 2000, 32, 1601–1609. [Google Scholar] [CrossRef]
- Driver, J.A.; Djousse, L.; Logroscino, G.; Gaziano, J.M.; Kurth, T. Incidence of cardiovascular disease and cancer in advanced age: Prospective cohort study. BMJ 2008, 337, a2467. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Eberhardt, M.S.; Flegal, K.M.; Engelgau, M.M.; Saydah, S.H.; Williams, D.E.; Geiss, L.S.; Gregg, E.W. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care 2006, 29, 1263–1268. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, inflammation and aging. Aging Dis. 2012, 3, 130–140. [Google Scholar] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Alturki, M.; Beyer, I.; Mets, T.; Bautmans, I. Impact of drugs with anti-inflammatory effects on skeletal muscle and inflammation: A systematic literature review. Exp. Gerontol. 2018, 114, 33–49. [Google Scholar] [CrossRef]
- Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J. Appl. Physiol. 2020, 129, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Della Gatta, P.A.; Garnham, A.P.; Peake, J.M.; Cameron-Smith, D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain. Behav. Immun. 2014, 39, 80–86. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.; Sadlier, D.M.; Godson, C. Pro-resolving lipid mediators: Agents of anti-ageing? Semin. Immunol. 2018, 40, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Crean, D.; Godson, C. Specialised lipid mediators and their targets. Semin. Immunol. 2015, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Dalli, J.; Colas, R.A.; Shinohara, M.; Serhan, C.N. Aging delays resolution of acute inflammation in mice: Reprogramming the host response with novel nano-proresolving medicines. J. Immunol. 2014, 193, 4235–4244. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.C.W.; Rudloff, S.; Langer, H.T.; Norman, K.; Herpich, C. Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage. Cells 2024, 13, 255. https://doi.org/10.3390/cells13030255
Li DCW, Rudloff S, Langer HT, Norman K, Herpich C. Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage. Cells. 2024; 13(3):255. https://doi.org/10.3390/cells13030255
Chicago/Turabian StyleLi, Donna Ching Wah, Stefan Rudloff, Henning Tim Langer, Kristina Norman, and Catrin Herpich. 2024. "Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage" Cells 13, no. 3: 255. https://doi.org/10.3390/cells13030255
APA StyleLi, D. C. W., Rudloff, S., Langer, H. T., Norman, K., & Herpich, C. (2024). Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage. Cells, 13(3), 255. https://doi.org/10.3390/cells13030255



