Levels of Circulating Ketone Bodies in Patients Undergoing Cardiac Surgery on Cardiopulmonary Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sampling and Analysis
2.3. Data collection
2.4. Study Outcomes
2.5. Statistical Analysis
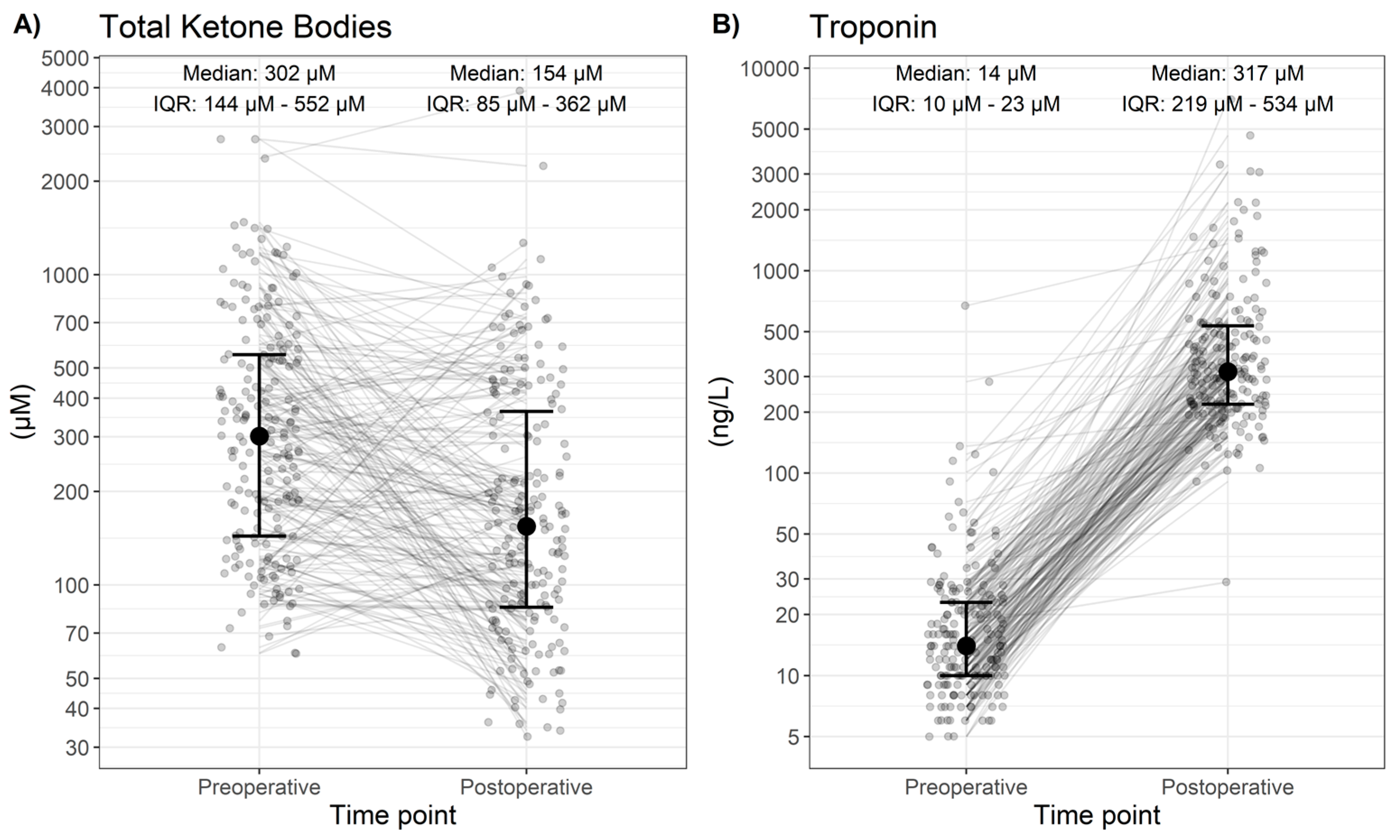
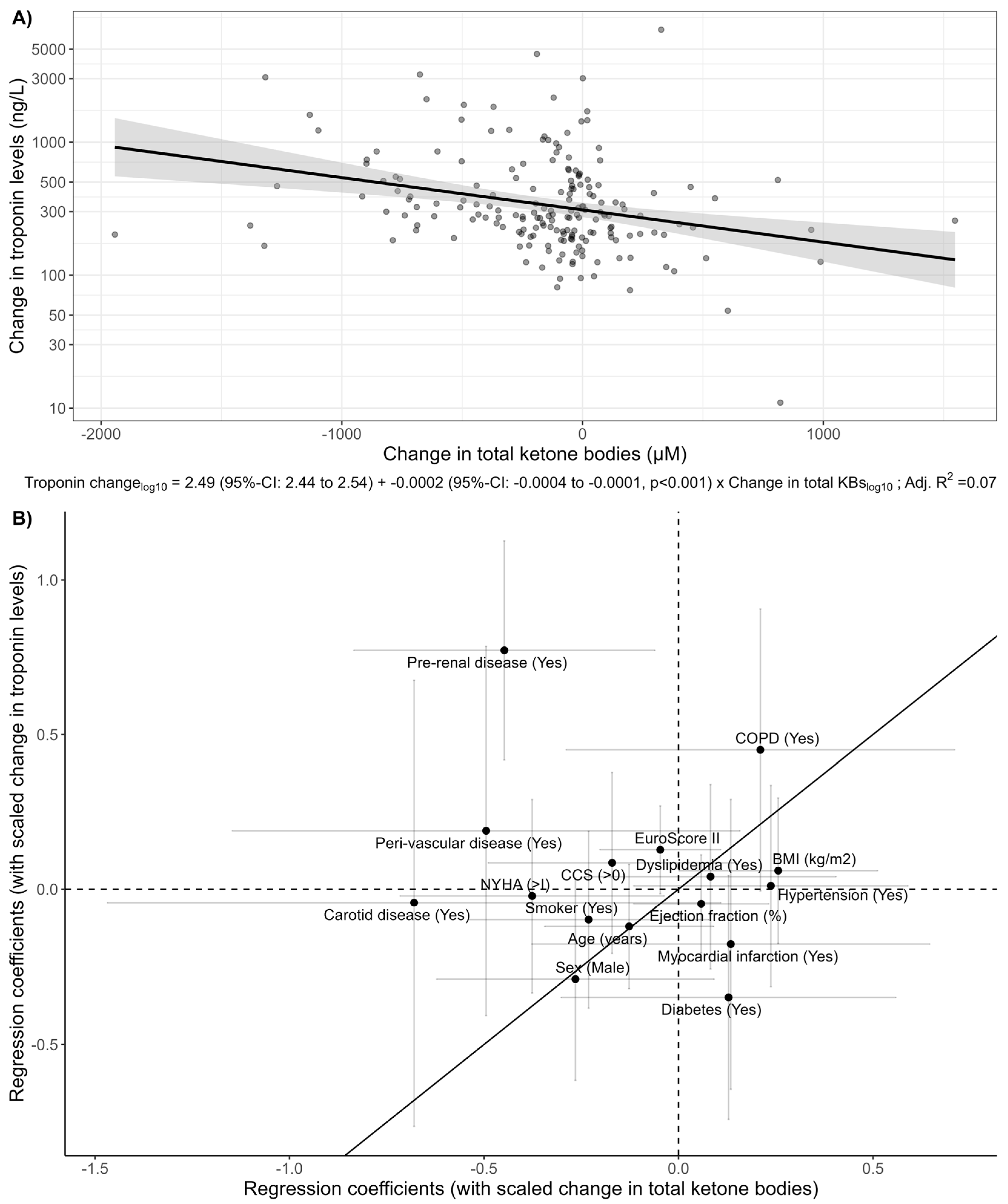
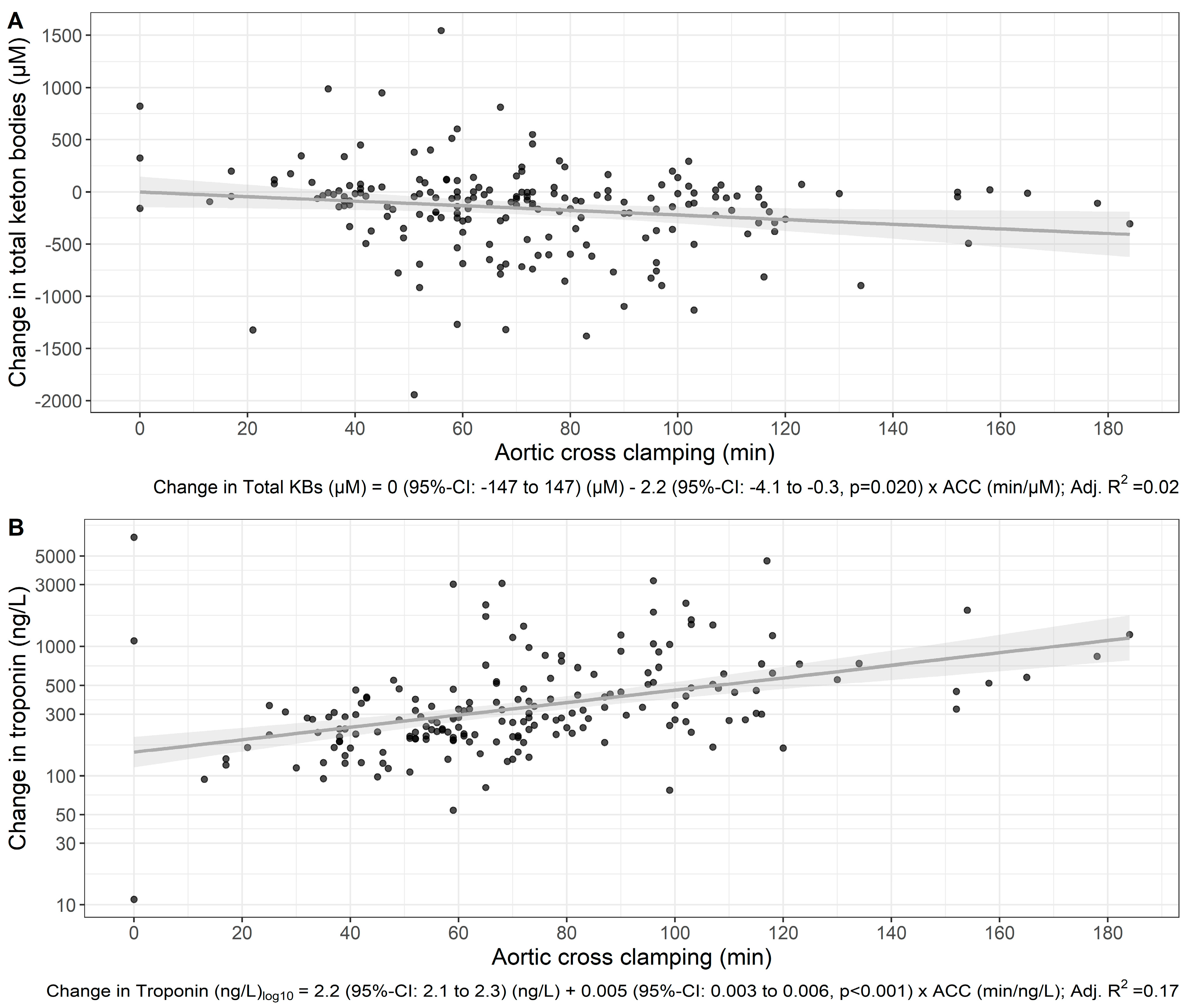
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulze, P.C.; Wu, J.M.F. Ketone bodies for the starving heart. Nat. Metab. 2020, 2, 1183–1185. [Google Scholar] [CrossRef]
- Ho, K.L.; Zhang, L.; Wagg, C.; Al Batran, R.; Gopal, K.; Levasseur, J.; Leone, T.; Dyck, J.R.B.; Ussher, J.R.; Muoio, D.M.; et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 2019, 115, 1606–1616. [Google Scholar]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef]
- Lommi, J.; Kupari, M.; Koskinen, P.; Näveri, H.; Leinonen, H.; Pulkki, K.; Härkönen, M. Blood ketone bodies in congestive heart failure. J. Am. Coll. Cardiol. 1996, 28, 665–672. [Google Scholar] [CrossRef]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Ho, K.L.; Pherwani, S.; Ketema, E.B. Ketone metabolism in the failing heart. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158813. [Google Scholar] [CrossRef]
- de Koning, M.L.Y.; Westenbrink, B.D.; Assa, S.; Garcia, E.; Connelly, M.A.; van Veldhuisen, D.J.; Dullaart, R.P.F.; Lipsic, E.; van der Harst, P. Association of Circulating Ketone Bodies With Functional Outcomes After ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 78, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, Z.; Liu, J.; Ma, L.; Sun, X.; Gao, R.; Pan, L.; Zhang, J.; Dilan, A.; An, J.; et al. β-Hydroxybutyrate Exacerbates Hypoxic Injury by Inhibiting HIF-1α-Dependent Glycolysis in Cardiomyocytes-Adding Fuel to the Fire? Cardiovasc. Drugs Ther. 2022, 36, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, P.P.; Mihalj, M.; Huber, M.; Schefold, J.C.; Hartmann, A.; Walter, M.; Steinhagen-Thiessen, E.; Schmidli, J.; Stüber, F.; Räber, L.; et al. Impact of Lipoprotein(a) Levels on Perioperative Outcomes in Cardiac Surgery. Cells 2021, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Heinisch, P.P.; Huber, M.; Schefold, J.C.; Hartmann, A.; Walter, M.; Steinhagen-Thiessen, E.; Schmidli, J.; Stüber, F.; Räber, L.; et al. Effect of Perioperative Lipid Status on Clinical Outcomes after Cardiac Surgery. Cells 2021, 10, 2717. [Google Scholar] [CrossRef]
- Heinisch, P.P.; Mihalj, M.; Haliguer, E.; Gahl, B.; Winkler, B.; Venetz, P.; Jenni, H.; Schober, P.; Erdoes, G.; Luedi, M.M.; et al. Initial experience with minimally invasive extracorporeal circulation in coronary artery bypass graft reoperations. Swiss Med. Wkly. 2022, 152, w30101. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- McGarry, J.D.; Foster, D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980, 49, 395–420. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Rosenzweig, A.; Nguyen, C.T. Ketone Bodies: Universal Cardiac Response to Stress? J. Am. Coll. Cardiol. 2021, 78, 1433–1436. [Google Scholar] [CrossRef]
- Øvrum, E.; Abdelnoor, M.; Forfang, K. Effect of Aortic Cross-Clamp Time on Myocardial Infarction after Coronary Bypass Surgery. Asian Cardiovasc. Thorac. Ann. 1997, 5, 77–82. [Google Scholar] [CrossRef]
- Canty, J.M., Jr. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1–H15. [Google Scholar] [CrossRef]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.K.; Georgopoulos, M.M.; Katsilambros, N.L. Ketone bodies and the heart. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, e209–e214. [Google Scholar] [CrossRef]
- Schade, D.S.; Eaton, R.P. The regulation of plasma ketone body concentration by counterregulatory hormones in man. Diabetes 1977, 26, 989–996. [Google Scholar] [CrossRef]
- Schade, D.S.; Eaton, R.P. The regulation of plasma ketone body concentration by counter-regulatory hormones in man. III. Effects of norepinephrine in normal man. Diabetes 1979, 28, 5–10. [Google Scholar] [CrossRef]
- Nomoto, S.; Shimahara, Y.; Kumada, K.; Ogino, H.; Okamoto, Y.; Ban, T. Arterial ketone body ratio during and after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1992, 103, 1164–1167. [Google Scholar] [CrossRef]
- Liu, G.; Wang, M.Q.; Kagawa, T.; Wada, S.; Morita, S.; Ohno, Y.; Shinbara, K.; Nomimura, T.; Hayashi, S.; Orihashi, K. Effects of extracorporeal circulation on blood ketone body ratio reflecting hepatic energy metabolism during cardiac operation. Surg. Gynecol. Obstet. 1993, 177, 507–512. [Google Scholar]
- Nakamura, K.; Onitsuka, T.; Yano, M.; Yano, Y. Study in three different types of cardiopulmonary bypass on arterial ketone body ratio: Its prognostic implication and participation of body temperature. Interact. Cardiovasc. Thorac. Surg. 2003, 2, 25–29. [Google Scholar] [CrossRef][Green Version]
- Hashimoto, K.; Sasaki, T.; Hachiya, T.; Onoguchi, K.; Takakura, H.; Oshiumi, M.; Takeuchi, S. Superior hepatic mitochondrial oxidation-reduction state in normothermic cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2001, 121, 1179–1186. [Google Scholar] [CrossRef][Green Version]

| Variable | All Patients | Low Preoperative Total Ketone Bodies (<Median of 302 µM) | High Preoperative Total Ketone Bodies (≥Median of 302 µM) | p | n |
|---|---|---|---|---|---|
| n = 192 | n = 96 | n = 96 | |||
| Age (years) | 67.0 [60.0;73.0] | 65.5 [59.0;72.0] | 68.0 [60.8;75.0] | 0.059 | 192 |
| Sex (female) | 47 (24.5%) | 21 (21.9%) | 26 (27.1%) | 0.502 | 192 |
| Body Mass Index (BMI; kg/m2) | 26.1 [23.7;30.4] | 28.0 [25.0;31.7] | 24.7 [22.7;28.0] | <0.001 | 192 |
| Diabetes on insulin (Yes) | 35 (18.2%) | 17 (17.7%) | 18 (18.8%) | >0.999 | 192 |
| Hypertension (Yes) | 130 (68.4%) | 68 (71.6%) | 62 (65.3%) | 0.435 | 190 |
| Dyslipidaemia (Yes) | 111 (58.1%) | 59 (62.1%) | 52 (54.2%) | 0.334 | 191 |
| Smoker: | 0.880 | 188 | |||
| Non-smoker | 97 (51.6%) | 48 (50.5%) | 49 (52.7%) | ||
| Previous/current smoker | 91 (48.4%) | 47 (49.5%) | 44 (47.3%) | ||
| Obesity (Yes) | 52 (27.1%) | 33 (34.4%) | 19 (19.8%) | 0.035 | 192 |
| Preoperative renal disease (Yes) | 43 (22.4%) | 13 (13.5%) | 30 (31.2%) | 0.006 | 192 |
| Peripheral vascular disease (Yes) | 11 (6.2%) | 6 (6.7%) | 5 (5.7%) | >0.999 | 178 |
| Carotid disease (Yes) | 6 (3.7%) | 3 (3.9%) | 3 (3.6%) | >0.999 | 162 |
| Myocardial infarction (Yes) | 20 (10.5%) | 9 (9.5%) | 11 (11.5%) | 0.832 | 191 |
| COPD (Yes) | 23 (12.1%) | 15 (15.8%) | 8 (8.42%) | 0.182 | 190 |
| NYHA (>1) | 131 (68.6%) | 59 (62.1%) | 72 (75.0%) | 0.078 | 191 |
| CCS (>0) | 71 (37.6%) | 33 (35.5%) | 38 (39.6%) | 0.666 | 189 |
| Ejection Fraction (%) | 60.0 [55.0;65.0] | 60.0 [56.5;65.0] | 60.0 [55.0;65.0] | 0.638 | 191 |
| EuroSCORE 2 | 1.73 [0.90;2.93] | 1.52 [0.83;2.68] | 2.19 [1.08;3.46] | 0.035 | 184 |
| Betablocker (Yes) | 86 (44.8%) | 43 (44.8%) | 43 (44.8%) | >0.999 | 192 |
| ECC or MiECC: | 0.299 | 192 | |||
| ECC | 149 (77.6%) | 71 (74.0%) | 78 (81.2%) | ||
| MiECC | 43 (22.4%) | 25 (26.0%) | 18 (18.8%) | ||
| Deep hypothermic cardiac arrest (Yes) | 19 (10.0%) | 8 (8.4%) | 11 (11.5%) | 0.646 | 191 |
| Aortic valve (Yes) | 86 (44.8%) | 36 (37.5%) | 50 (52.1%) | 0.059 | 192 |
| Mitral valve (Yes) | 45 (23.4%) | 22 (22.9%) | 23 (24.0%) | >0.999 | 192 |
| Tricuspid valve (Yes) | 17 (8.85%) | 7 (7.29%) | 10 (10.4%) | 0.611 | 192 |
| Coronary artery bypass (Yes) | 77 (40.1%) | 45 (46.9%) | 32 (33.3%) | 0.077 | 192 |
| Ascending Aortic (Yes) | 38 (19.8%) | 19 (19.8%) | 19 (19.8%) | >0.999 | 192 |
| Aortic Arch (Yes) | 11 (5.7%) | 5 (5.2%) | 6 (6.3%) | >0.999 | 192 |
| Bypass time (min) | 104 [80.0;132] | 100 [82.8;133] | 108 [80.0;132] | 0.793 | 192 |
| Aortic cross-clamping (min) | 68.5 [52.0;91.8] | 68.0 [49.8;95.5] | 69.0 [52.0;90.2] | 0.653 | 192 |
| Lowest body temperature (deg C) | 33.2 [32.1;33.8] | 33.4 [32.1;33.9] | 33.0 [32.1;33.7] | 0.260 | 192 |
| Operation duration (min) | 234 [195;276] | 238 [200;274] | 227 [181;276] | 0.147 | 192 |
| All Patients | No Cardiotomy | With Cardiotomy | p | |
|---|---|---|---|---|
| n = 192 | n = 59 | n = 133 | ||
| Preoperative (descriptive) [Median; IQR] | ||||
| Troponin (ng/L) | 14.0 [10.0; 23.0] | 14.5 [10.0; 23.0] | 14.0 [10.0; 24.0] | 0.962 |
| Total Ketone Bodies (µM) | 302 [144; 552] | 267 [137; 526] | 337 [148; 577] | 0.378 |
| Postoperative (descriptive) [Median; IQR] | ||||
| Troponin (ng/L) | 317 [219; 534] | 236 [170; 349] | 358 [242; 640] | <0.001 |
| Total Ketone Bodies (µM) | 154 [85; 362] | 173 [102; 390] | 154 [79; 336] | 0.398 |
| Change (postoperative–preoperative, descriptive) [Median; IQR] | ||||
| Troponin (ng/L) | 286 [204; 511] | 216 [135; 310] | 329 [228; 624] | <0.001 |
| Total Ketone Bodies (µM) | −87 [−311; 20] | −78 [−285; 28] | −98 [−348; 20] | 0.734 |
| Change (postoperative–preoperative, inference) [Change in median; 95% confidence interval] | ||||
| Troponin (ng/L) | 340 (302 to 391, p < 0.001) | 233 (200 to 274, p < 0.001) | 411 (350 to 496, p < 0.001) | |
| Total Ketone Bodies (µM) | −123 (−175 to −79, p < 0.001) | −119 (−223 to −37, p = 0.003) | −126 (−191 to −77, p < 0.001) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levis, A.; Huber, M.; Mathis, D.; Filipovic, M.G.; Stieger, A.; Räber, L.; Stueber, F.; Luedi, M.M. Levels of Circulating Ketone Bodies in Patients Undergoing Cardiac Surgery on Cardiopulmonary Bypass. Cells 2024, 13, 294. https://doi.org/10.3390/cells13040294
Levis A, Huber M, Mathis D, Filipovic MG, Stieger A, Räber L, Stueber F, Luedi MM. Levels of Circulating Ketone Bodies in Patients Undergoing Cardiac Surgery on Cardiopulmonary Bypass. Cells. 2024; 13(4):294. https://doi.org/10.3390/cells13040294
Chicago/Turabian StyleLevis, Anja, Markus Huber, Déborah Mathis, Mark G. Filipovic, Andrea Stieger, Lorenz Räber, Frank Stueber, and Markus M. Luedi. 2024. "Levels of Circulating Ketone Bodies in Patients Undergoing Cardiac Surgery on Cardiopulmonary Bypass" Cells 13, no. 4: 294. https://doi.org/10.3390/cells13040294
APA StyleLevis, A., Huber, M., Mathis, D., Filipovic, M. G., Stieger, A., Räber, L., Stueber, F., & Luedi, M. M. (2024). Levels of Circulating Ketone Bodies in Patients Undergoing Cardiac Surgery on Cardiopulmonary Bypass. Cells, 13(4), 294. https://doi.org/10.3390/cells13040294











