In Vivo Cellular Phosphatidylcholine Kinetics of CD15+ Leucocytes and CD3+ T-Lymphocytes in Adults with Acute Respiratory Distress Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Participant Selection
2.2. CD15+ Leucocytes and CD3+ Cell Isolation
2.3. Phospholipid Extraction
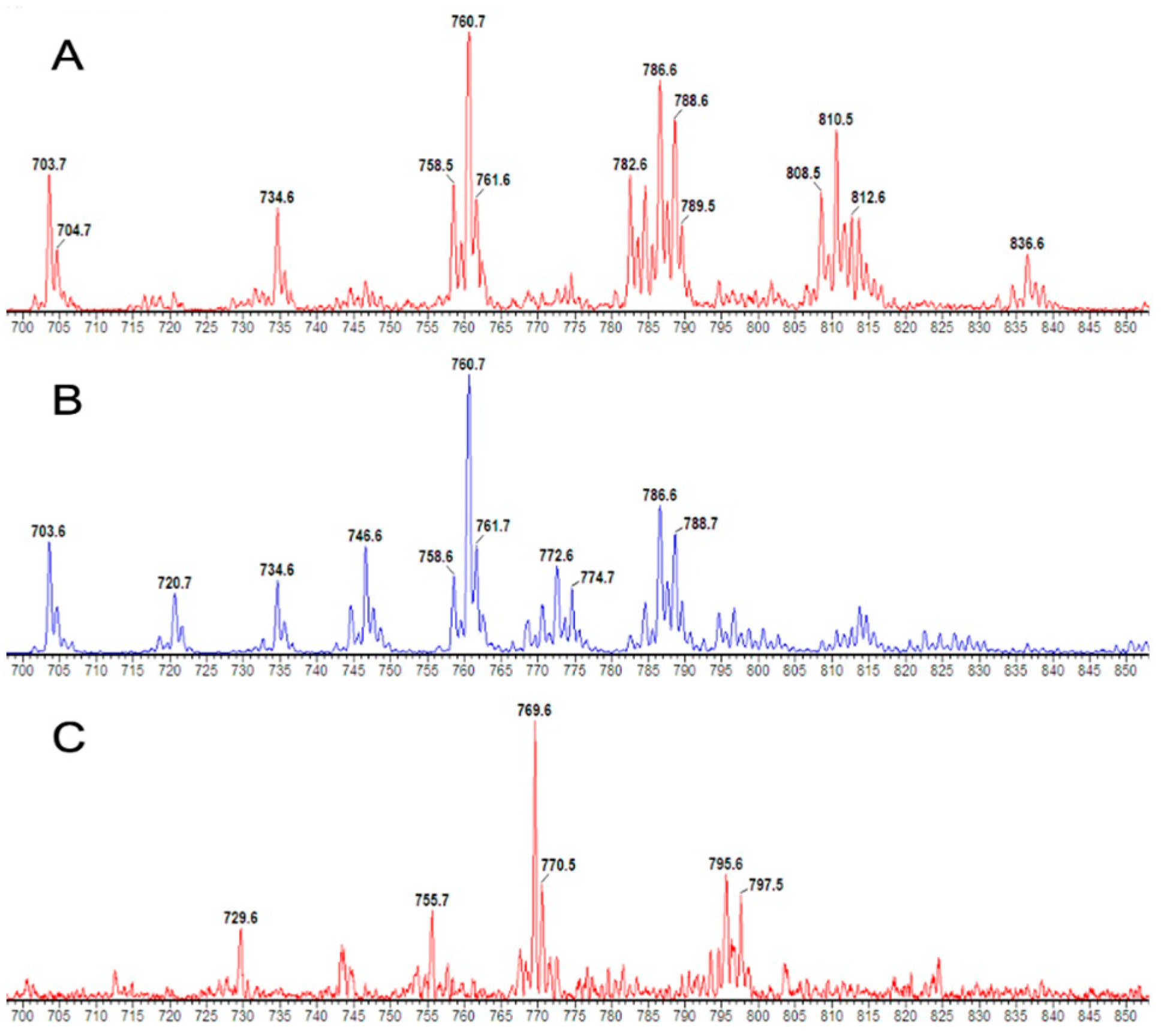
2.4. Nanoflow Electrospray Ionisation Mass Spectrometry (ESI-MS/MS)
2.5. Data Extraction and Statistical Analysis
3. Results
3.1. Participant Characteristics
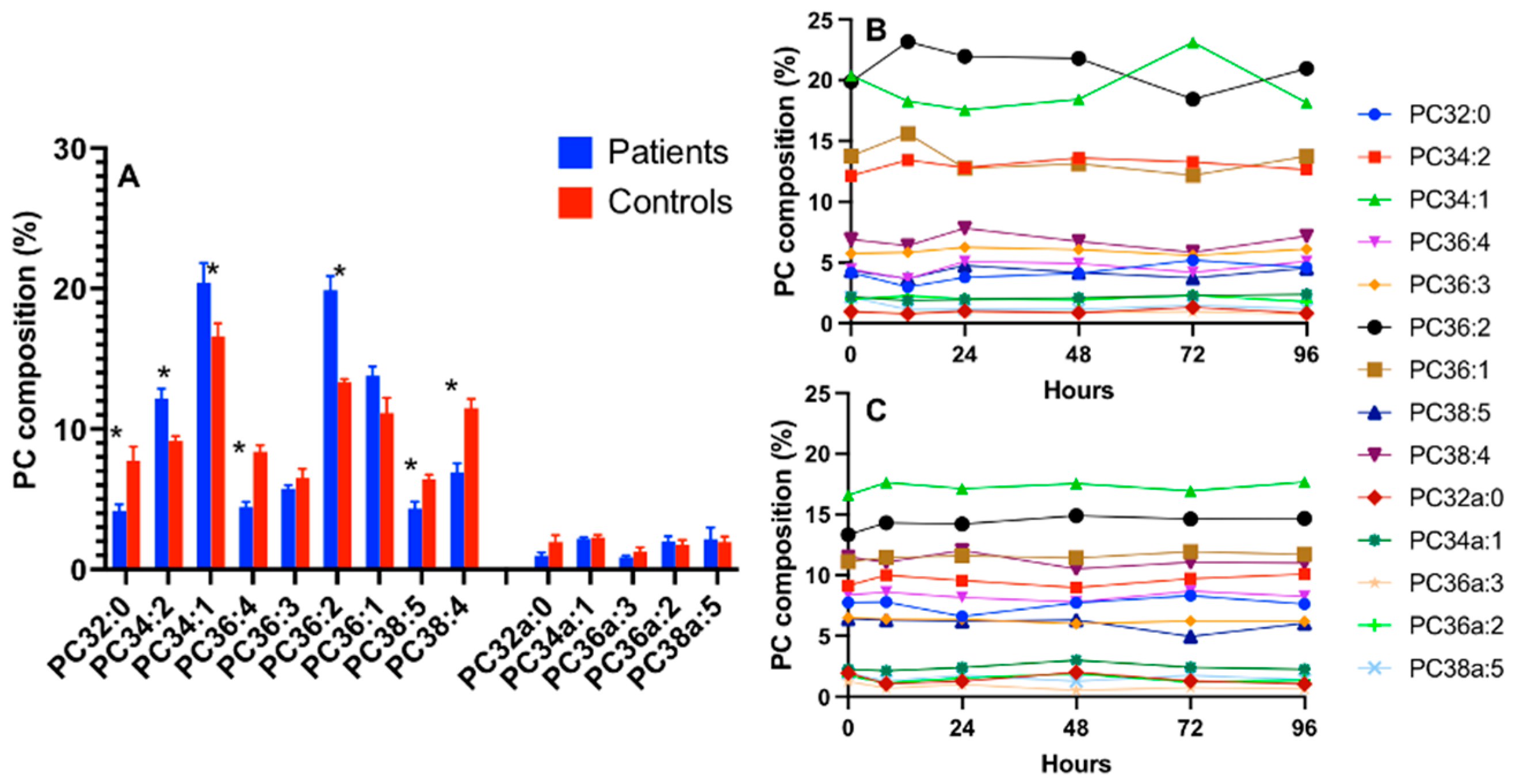
3.2. CD3+ and CD15+ Cellular PC Composition in Healthy Humans
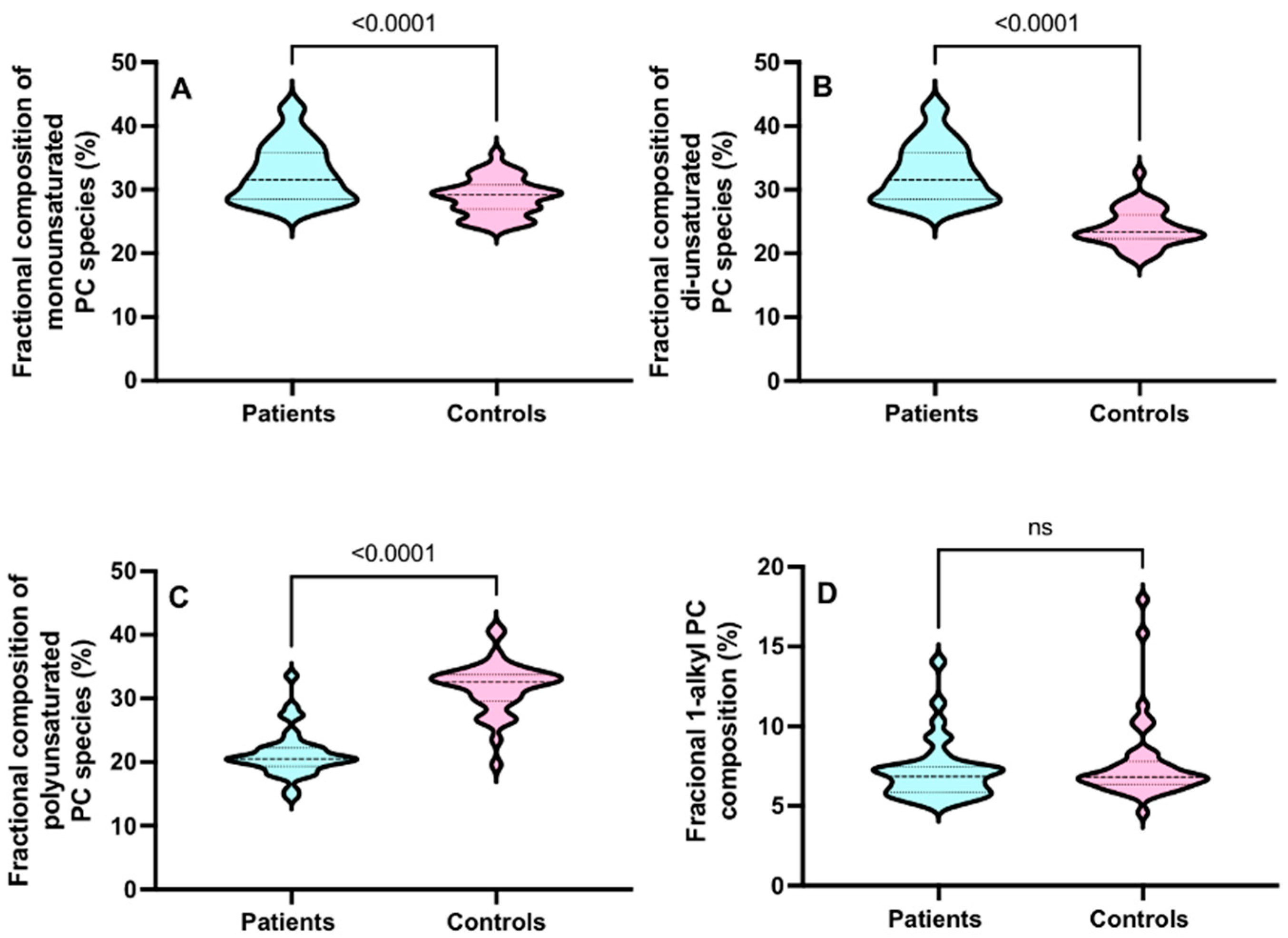
3.3. CD15+ Cellular PC Composition in ARDS
3.4. CD3+ Cellular PC Composition in ARDS
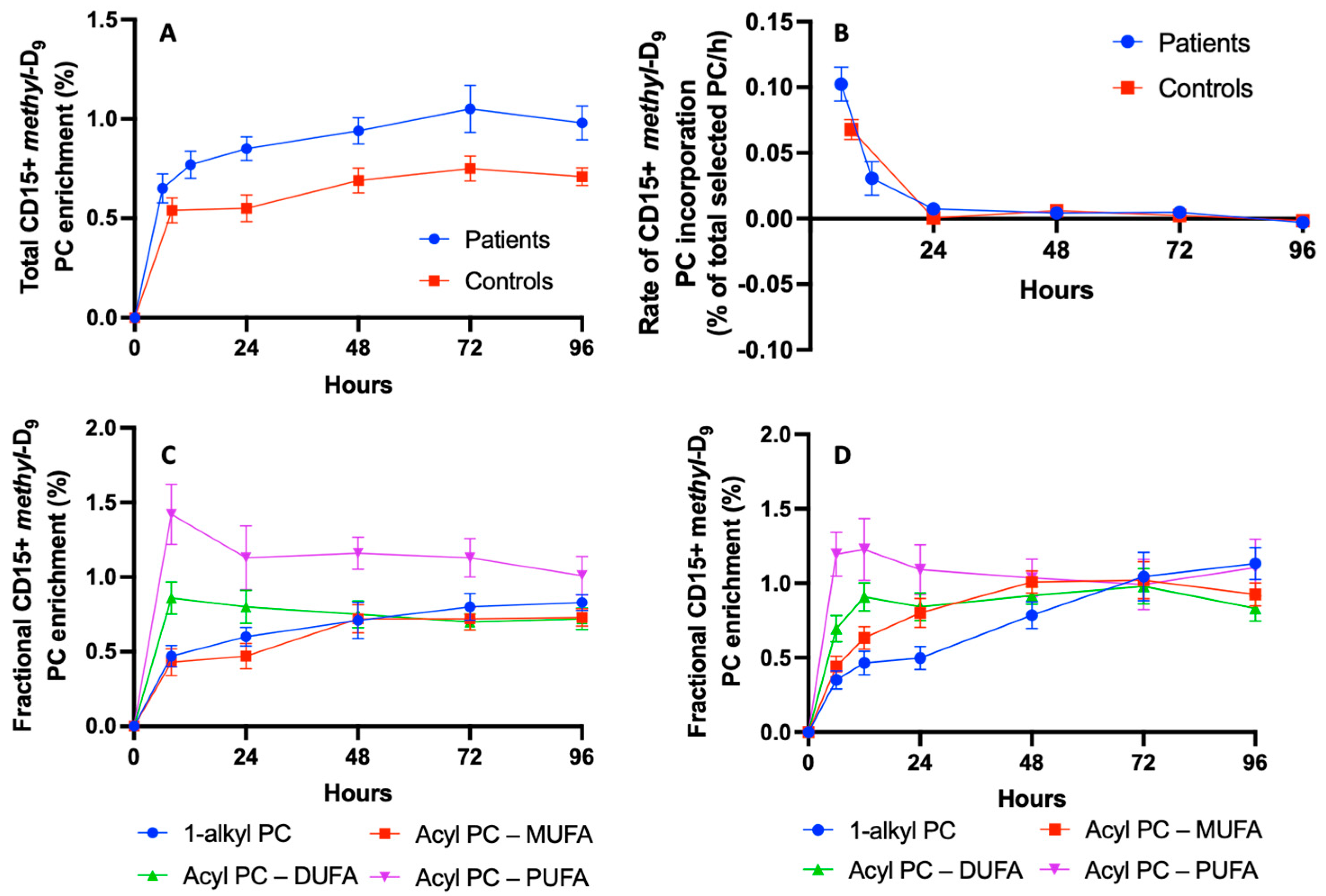
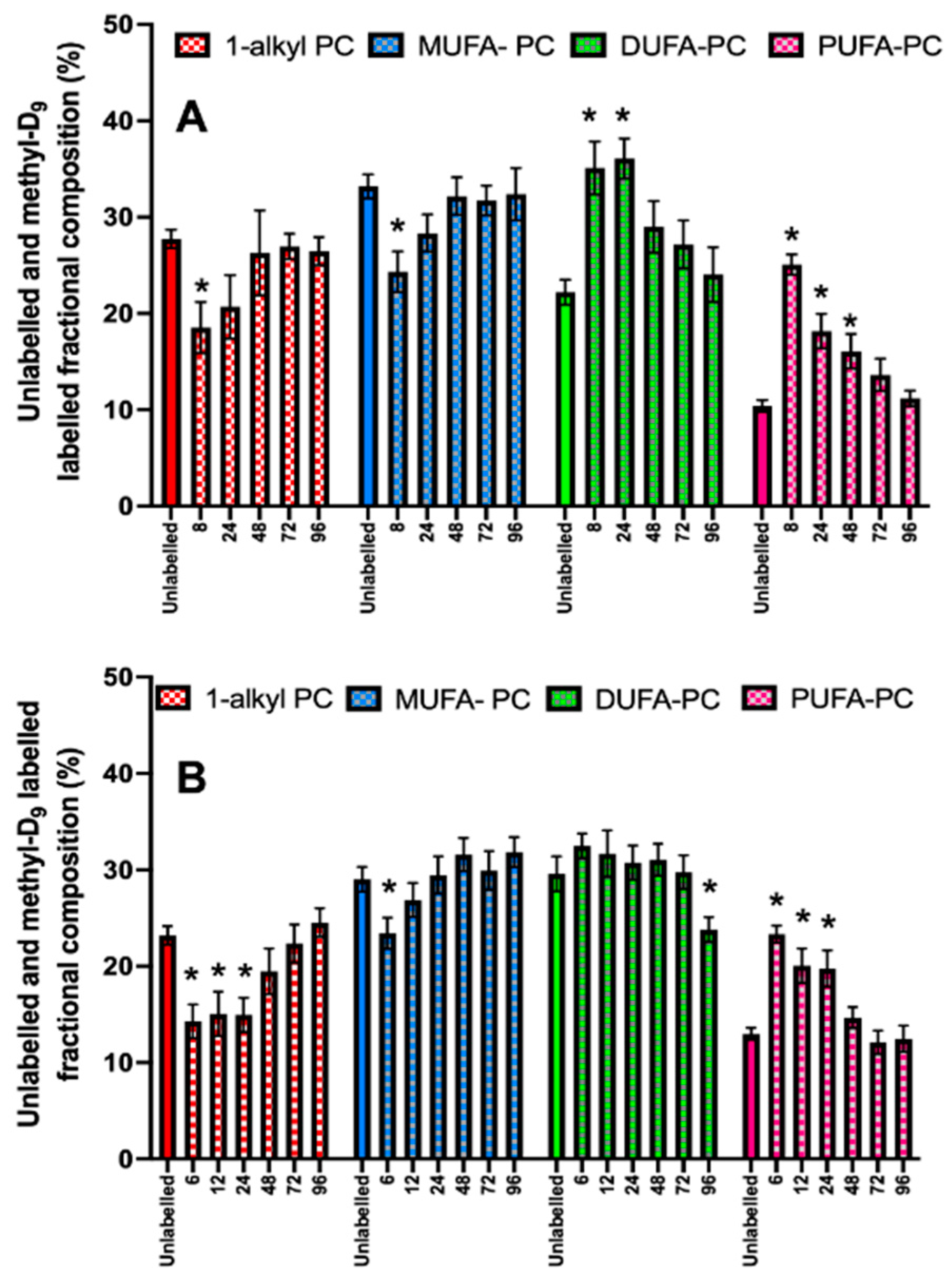
3.5. PC Kinetics in CD15+ Cells in Healthy Humans and Patients with ARDS
3.6. Acyl Remodelling of Newly Synthesised Phosphatidylcholine in CD15+ Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 2011, 50, 240–257. [Google Scholar] [CrossRef]
- Goss, V.; Hunt, A.N.; Postle, A.D. Regulation of lung surfactant phospholipid synthesis and metabolism. Biochim. Biophys. Acta 2013, 1831, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Marat, A.L.; Haucke, V. Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 2016, 35, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Postle, A.D.; Madden, J.; Clark, G.T.; Wright, S.M. Electrospray ionisation mass spectrometry analysis of differential turnover of phosphatidylcholine by human blood leukocytes. Phys. Chem. Chem. Phys. 2004, 6, 1018–1021. [Google Scholar] [CrossRef]
- Masclans, J.R.; Bermejo, B.; Pico, M.; de Latorre, F.J.; Rodriguez-Roisin, R.; Planas, M. The prognostic value of eicosanoids in the acute respiratory distress syndrome. Med. Clin. 1999, 112, 81–84. [Google Scholar]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Zemans, R.L.; Matthay, M.A. What drives neutrophils to the alveoli in ARDS? Thorax 2017, 72, 1–3. [Google Scholar] [CrossRef]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef]
- Capra, V.; Rovati, G.E.; Mangano, P.; Buccellati, C.; Murphy, R.C.; Sala, A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochim. Biophys. Acta 2015, 1851, 377–382. [Google Scholar] [CrossRef]
- Dushianthan, A.; Cusack, R.; Koster, G.; Grocott, M.P.W.; Postle, A.D. Insight into erythrocyte phospholipid molecular flux in healthy humans and in patients with acute respiratory distress syndrome. PLoS ONE 2019, 14, e0221595. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the CDP-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef]
- Galella, G.; Marangoni, F.; Rise, P.; Colombo, C.; Galli, G.; Galli, C. n − 6 and n − 3 fatty acid accumulation in thp-1 cell phospholipids. Biochim. Biophys. Acta 1993, 1169, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Ivanova, P.T.; Byrne, M.O.; Milne, S.B.; Marnett, L.J.; Brown, H.A. Lipid profiling reveals arachidonate deficiency in RAW264.7 cells: Structural and functional implications. Biochemistry 2006, 45, 14795–14808. [Google Scholar] [CrossRef]
- Rise, P.; Eligini, S.; Ghezzi, S.; Colli, S.; Galli, C. Fatty acid composition of plasma, blood cells and whole blood: Relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Dushianthan, A.; Goss, V.; Cusack, R.; Grocott, M.P.; Postle, A.D. Altered molecular specificity of surfactant phosphatidycholine synthesis in patients with acute respiratory distress syndrome. Respir. Res. 2014, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Postle, A.D.; Henderson, N.G.; Koster, G.; Clark, H.W.; Hunt, A.N. Analysis of lung surfactant phosphatidylcholine metabolism in transgenic mice using stable isotopes. Chem. Phys. Lipids 2011, 164, 549–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Cardinal-Fernandez, P.; Lorente, J.A.; Ballen-Barragan, A.; Matute-Bello, G. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship. Ann. Am. Thorac. Soc. 2017, 14, 844–850. [Google Scholar] [CrossRef]
- Vassallo, A.; Wood, A.J.; Subburayalu, J.; Summers, C.; Chilvers, E.R. The counter-intuitive role of the neutrophil in the acute respiratory distress syndrome. Br. Med. Bull. 2019, 131, 43–55. [Google Scholar] [CrossRef]
- Park, I.; Kim, M.; Choe, K.; Song, E.; Seo, H.; Hwang, Y.; Ahn, J.; Lee, S.H.; Lee, J.H.; Jo, Y.H.; et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur. Respir. J. 2019, 53, 1800786. [Google Scholar] [CrossRef]
- Parsons, P.E.; Fowler, A.A.; Hyers, T.M.; Henson, P.M. Chemotactic activity in bronchoalveolar lavage fluid from patients with adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1985, 132, 490–493. [Google Scholar] [PubMed]
- Chilton, F.H.; Connell, T.R. 1-ether-linked phosphoglycerides. Major endogenous sources of arachidonate in the human neutrophil. J. Biol. Chem. 1988, 263, 5260–5265. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Cluzel, M.; Triggiani, M. Recent advances in our understanding of the biochemical interactions between platelet-activating factor and arachidonic acid. Lipids 1991, 26, 1021–1027. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Barham, J.B.; Edens, M.B.; Fonteh, A.N.; Johnson, M.M.; Easter, L.; Chilton, F.H. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J. Nutr. 2000, 130, 1925–1931. [Google Scholar] [CrossRef]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Hagi, A.; Nakayama, M.; Shinzaki, W.; Haji, S.; Ohyanagi, H. Effects of the omega-6:omega-3 fatty acid ratio of fat emulsions on the fatty acid composition in cell membranes and the anti-inflammatory action. JPEN J. Parenter. Enter. Nutr. 2010, 34, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.A.; Wallace, F.A.; Miles, E.A.; Calder, P.C.; Newsholm, P. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 2000, 35, 763–768. [Google Scholar] [CrossRef] [PubMed]




| PC Species | ARDS Patients | Controls | % Change in ARDS Patients | ||
|---|---|---|---|---|---|
| Peak Methyl-D9 Enrichment (%) * | Time to Maximal Enrichment | Peak Methyl-D9 Enrichment (%) * | Time to Maximal Enrichment | ||
| PC32a:0 | 1.34 (0.29) | 72 h | 0.97 (0.08) | 96 h | +38.1% |
| PC32:0 | 1.38 (0.28) | 72 h | 1.07 (0.12) | 72 h | +29.0% |
| PC34a:1 | 1.13 (0.12) | 96 h | 0.81 (0.07) | 96 h | +39.5% |
| PC34:2 | 1.04 (0.14) | 12 h | 0.82 (0.12) | 24 h | +26.8% |
| PC34:1 | 1.01 (0.12) | 48 h | 0.69 (0.03) | 72 h | +46.4% |
| PC36a:3 | 1.17 (0.13) | 96 h | 0.82 (0.18) | 72 h | +42.7% |
| PC36a:2 | 1.00 (0.10) | 96 h | 0.67 (0.05) | 72 h | +49.3% |
| PC36:4 | 1.26 (0.25) | 6 h | 1.48 (0.18) | 8 h | −14.9% |
| PC36:3 | 1.10 (0.11) | 6 h | 1.28 (0.23) | 8 h | −14.1% |
| PC36:2 | 1.09 (0.12) | 72 h | 0.74 (0.08) | 72 h | +47.3% |
| PC36:1 | 1.06 (0.16 | 72 h | 0.81 (0.13) | 48 h | +30.9% |
| PC38a:5 | 1.26 (0.16) | 96 h | 1.17 (0.35) | 48 h | +7.7% |
| PC38:5 | 1.13 (0.19) | 6 h | 1.39 (0.34) | 8 h | −18.7% |
| PC38:4 | 1.09 (0.12) | 6 h | 1.22 (0.29) | 8 h | −10.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushianthan, A.; Cusack, R.; Goss, V.; Koster, G.; Grocott, M.P.W.; Postle, A.D. In Vivo Cellular Phosphatidylcholine Kinetics of CD15+ Leucocytes and CD3+ T-Lymphocytes in Adults with Acute Respiratory Distress Syndrome. Cells 2024, 13, 332. https://doi.org/10.3390/cells13040332
Dushianthan A, Cusack R, Goss V, Koster G, Grocott MPW, Postle AD. In Vivo Cellular Phosphatidylcholine Kinetics of CD15+ Leucocytes and CD3+ T-Lymphocytes in Adults with Acute Respiratory Distress Syndrome. Cells. 2024; 13(4):332. https://doi.org/10.3390/cells13040332
Chicago/Turabian StyleDushianthan, Ahilanandan, Rebecca Cusack, Victoria Goss, Grielof Koster, Michael P. W. Grocott, and Anthony D. Postle. 2024. "In Vivo Cellular Phosphatidylcholine Kinetics of CD15+ Leucocytes and CD3+ T-Lymphocytes in Adults with Acute Respiratory Distress Syndrome" Cells 13, no. 4: 332. https://doi.org/10.3390/cells13040332
APA StyleDushianthan, A., Cusack, R., Goss, V., Koster, G., Grocott, M. P. W., & Postle, A. D. (2024). In Vivo Cellular Phosphatidylcholine Kinetics of CD15+ Leucocytes and CD3+ T-Lymphocytes in Adults with Acute Respiratory Distress Syndrome. Cells, 13(4), 332. https://doi.org/10.3390/cells13040332






