Adenosine in Intestinal Epithelial Barrier Function
Abstract
1. Introduction
2. The Adenosine Alarm System
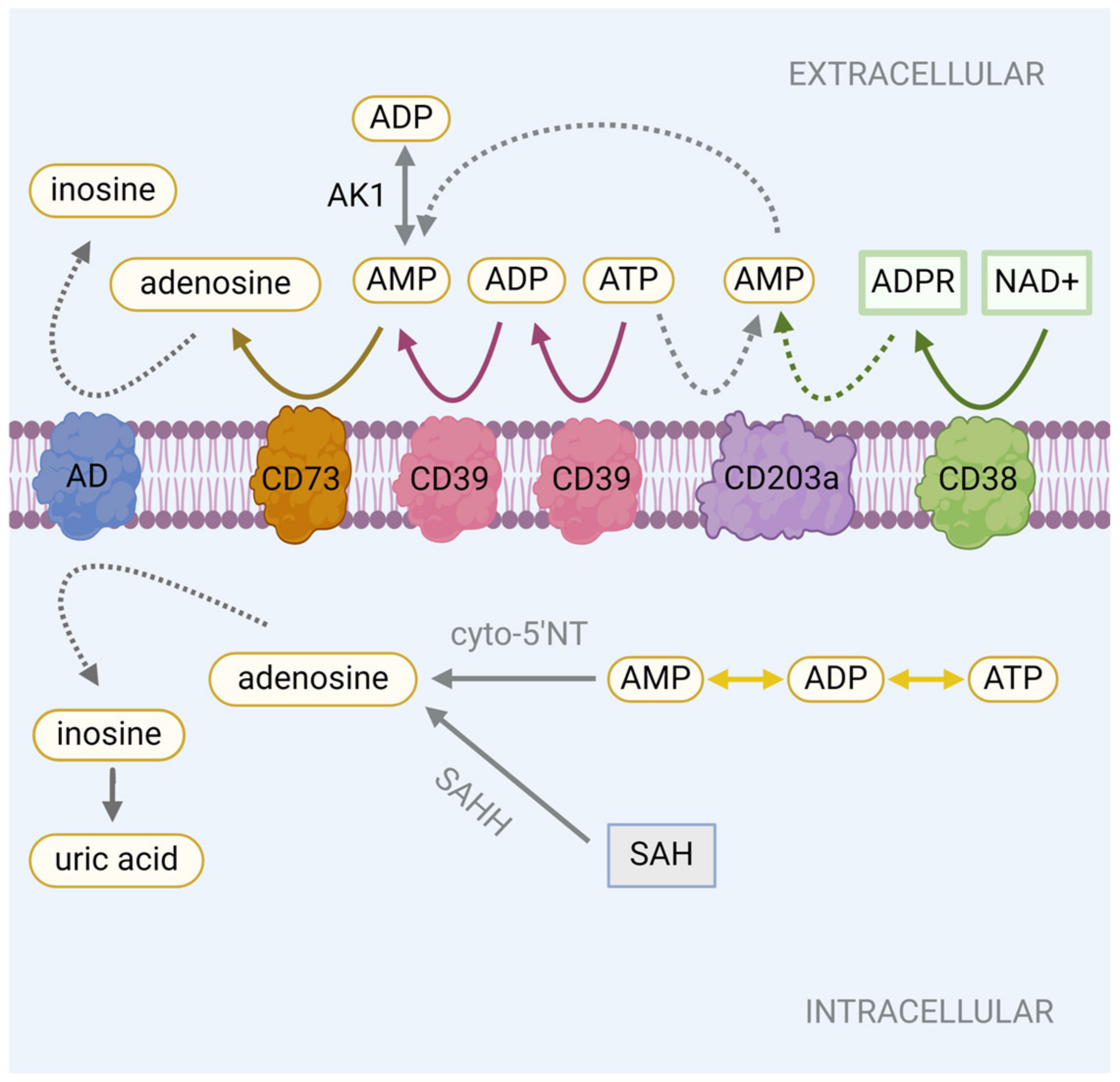
2.1. Adenosine Production
2.2. Modulation of Adenosine Levels
2.3. Adenosine Receptors
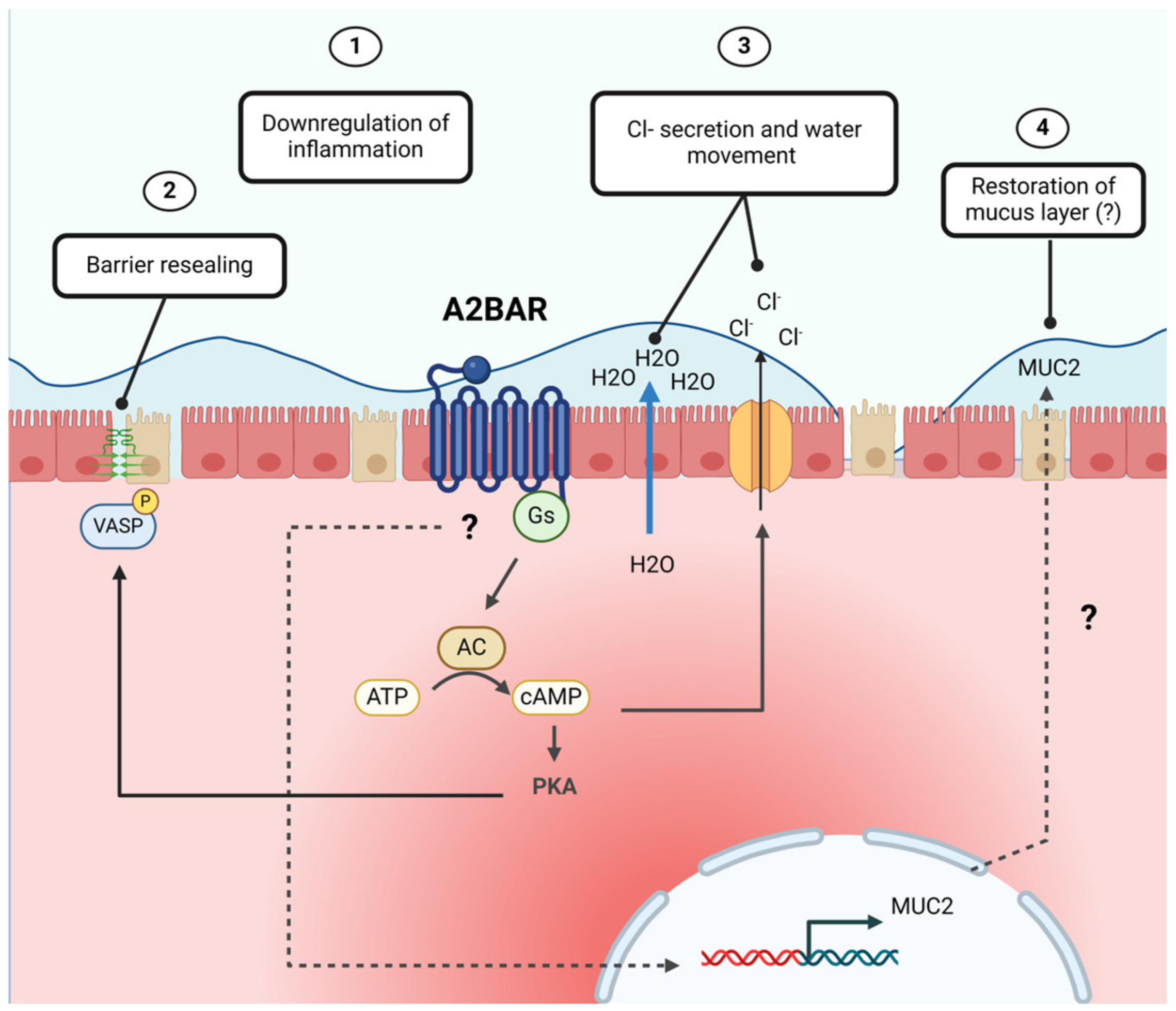
2.4. Adenosine Receptor Signaling
3. Adenosine in the Intestinal Mucus Layer
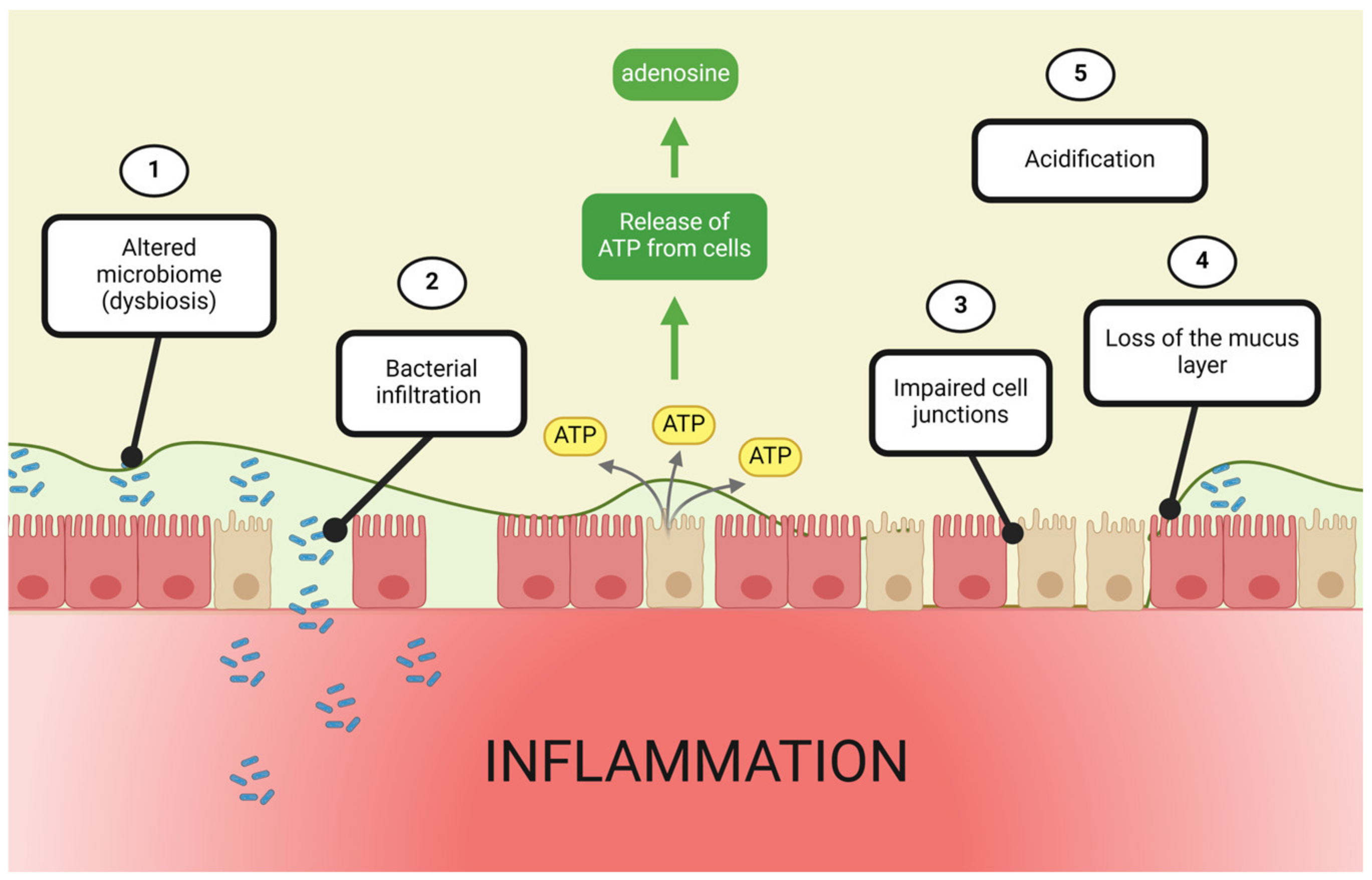
4. Contributions of the Gut Microbiome to Adenosine-Mediated Barrier Protection
| Bacteria | Regulates Adenosine Signaling | Affected by Adenosine Signaling | Effect | Reference |
|---|---|---|---|---|
| Commensal Bacteria: | ||||
| Lactobacillus reuteri | ✓ |
| [122] | |
| Bifidobacterium pseudolongum | ✓ |
| [126,127,128,129] | |
| Akkermansia muciniphila | ✓ |
| [126,127,128,129] | |
| Pathogenic Bacteria | ||||
| Salmonella enterica (serovar Typhimurium) | ✓ |
| [124] | |
5. Adenosine Reinforces Epithelial Tight Junctions
5.1. Tight Junction Architecture
5.2. CD39 and CD73 Contributions to Barrier Function
5.3. Adenosine Receptor Signaling in Tight Junction Dynamics
6. Adenosine Promotes Intestinal Chloride Secretion
7. Adenosine Restores Intestinal Acid–Base Balance
8. Contribution of ENTs to Intestinal Barrier Function
9. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered Defense: How Mucus and Tight Junctions Seal the Intestinal Barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal Mucus Components and Secretion Mechanisms: What We Do and Do Not Know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The Inner of the Two Muc2 Mucin-Dependent Mucus Layers in Colon Is Devoid of Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.; Dowdell, A.S.; Lee, J.S.; Colgan, S.P. Microbial Metabolite Regulation of Epithelial Cell-Cell Interactions and Barrier Function. Cells 2022, 11, 944. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Tsolis, R.M.; Bäumler, A.J. The Microbiome and Gut Homeostasis. Science 2022, 377, eabp9960. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Lechuga, S.; Ivanov, A.I. Disruption of the Epithelial Barrier during Intestinal Inflammation: Quest for New Molecules and Mechanisms. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 1183–1194. [Google Scholar] [CrossRef]
- Thoo, L.; Noti, M.; Krebs, P. Keep Calm: The Intestinal Barrier at the Interface of Peace and War. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Pathway Paradigms Revealed from the Genetics of Inflammatory Bowel Disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Zielińska, M.; Sokal, A.; Filip, R. Genetic and Epigenetic Etiology of Inflammatory Bowel Disease: An Update. Genes 2022, 13, 2388. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.A.; Magnusson, M.K.; Rydström, A.; Jonstrand, C.; Hengst, J.; Johansson, M.E.V.; Velcich, A.; Öhman, L.; Strid, H.; Sjövall, H.; et al. Spontaneous Colitis in Muc2-Deficient Mice Reflects Clinical and Cellular Features of Active Ulcerative Colitis. PLoS ONE 2014, 9, e100217. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.L.; Ka-Shu Wong, G.; Dieleman, L.A.; et al. Mucosal Barrier Depletion and Loss of Bacterial Diversity Are Primary Abnormalities in Paediatric Ulcerative Colitis. J. Crohns Colitis 2016, 10, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.D.; Bentzel, C.J.; Riecken, E.O.; Schulzke, J.D. Altered Tight Junction Structure Contributes to the Impaired Epithelial Barrier Function in Ulcerative Colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in Expression and Distribution of Claudin 2, 5 and 8 Lead to Discontinuous Tight Junctions and Barrier Dysfunction in Active Crohn’s Disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bowser, J.L.; Phan, L.H.; Eltzschig, H.K. The Hypoxia–Adenosine Link during Intestinal Inflammation. J. Immunol. 2018, 200, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine, an Endogenous Distress Signal, Modulates Tissue Damage and Repair. Cell Death Differ. 2007, 14, 1315–1323. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Haskó, G. Adenosine and Inflammation: It’s Time to (Re)Solve the Problem. Trends Pharmacol. Sci. 2022, 43, 43–55. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Aherne, C.M.; Saeedi, B.; Collins, C.B.; Masterson, J.C.; Mcnamee, E.N.; Perrenoud, L.; Rapp, C.R.; Curtis, V.F.; Bayless, A.; Fletcher, A.; et al. Epithelial-Specific A2B Adenosine Receptor Signaling Protects the Colonic Epithelial Barrier during Acute Colitis. Mucosal Immunol. 2015, 8, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Frick, J.-S.; MacManus, C.F.; Scully, M.; Glover, L.E.; Eltzschig, H.K.; Colgan, S.P. Contribution of Adenosine A2B Receptors to Inflammatory Parameters of Experimental Colitis. J. Immunol. 2009, 182, 4957–4964. [Google Scholar] [CrossRef]
- Odashima, M.; Bamias, G.; Rivera-Nieves, J.; Linden, J.; Nast, C.C.; Moskaluk, C.A.; Marini, M.; Sugawara, K.; Kozaiwa, K.; Otaka, M.; et al. Activation of A2A Adenosine Receptor Attenuates Intestinal Inflammation in Animal Models of Inflammatory Bowel Disease. Gastroenterology 2005, 129, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Aherne, C.M.; Collins, C.B.; Rapp, C.R.; Olli, K.E.; Perrenoud, L.; Jedlicka, P.; Bowser, J.L.; Mills, T.W.; Karmouty-Quintana, H.; Blackburn, M.R.; et al. Coordination of ENT2-Dependent Adenosine Transport and Signaling Dampens Mucosal Inflammation. JCI Insight 2018, 3, e121521. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Yegutkin, G.G. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 2019, 36, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.; Braun, N.; Sévigny, J.; Robson, S.C.; Servos, J.; Zimmermann, H. The C-Terminal Cysteine-Rich Region Dictates Specific Catalytic Properties in Chimeras of the Ectonucleotidases NTPDase1 and NTPDase2. Eur. J. Biochem. 2001, 268, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Elsaadi, S.; Misund, K.; Abdollahi, P.; Vandsemb, E.N.; Moen, S.H.; Kusnierczyk, A.; Slupphaug, G.; Standal, T.; Waage, A.; et al. Conversion of ATP to Adenosine by CD39 and CD73 in Multiple Myeloma Can Be Successfully Targeted Together with Adenosine Receptor A2A Blockade. J. Immunother. Cancer 2020, 8, e000610. [Google Scholar] [CrossRef]
- Francois, V.; Shehade, H.; Acolty, V.; Preyat, N.; Delrée, P.; Moser, M.; Oldenhove, G. Intestinal Immunopathology Is Associated with Decreased CD73-Generated Adenosine during Lethal Infection. Mucosal Immunol. 2015, 8, 773–784. [Google Scholar] [CrossRef]
- Yegutkin, G.G.; Henttinen, T.; Samburski, S.S.; Spychala, J.; Jalkanen, S. The Evidence for Two Opposite, ATP-Generating and ATP-Consuming, Extracellular Pathways on Endothelial and Lymphoid Cells. Biochem. J. 2002, 367 Pt 1, 121–128. [Google Scholar] [CrossRef]
- Morandi, F.; Marimpietri, D.; Horenstein, A.L.; Bolzoni, M.; Toscani, D.; Costa, F.; Castella, B.; Faini, A.C.; Massaia, M.; Pistoia, V.; et al. Microvesicles Released from Multiple Myeloma Cells Are Equipped with Ectoenzymes Belonging to Canonical and Non-Canonical Adenosinergic Pathways and Produce Adenosine from ATP and NAD+. Oncoimmunology 2018, 7, e1458809. [Google Scholar] [CrossRef]
- Dzeja, P.; Terzic, A. Adenylate Kinase and AMP Signaling Networks: Metabolic Monitoring, Signal Communication and Body Energy Sensing. Int. J. Mol. Sci. 2009, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Zeleznikar, R.J.; Goldberg, N.D. Adenylate Kinase: Kinetic Behavior in Intact Cells Indicates It Is Integral to Multiple Cellular Processes. Mol. Cell. Biochem. 1998, 184, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Vitkevicius, K.T.; Redfield, M.M.; Burnett, J.C.; Terzic, A. Adenylate Kinase–Catalyzed Phosphotransfer in the Myocardium. Circ. Res. 1999, 84, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203a/CD73 Ectoenzymatic Pathway Independent of CD39 Drives a Novel Adenosinergic Loop in Human T Lymphocytes. Oncoimmunology 2013, 2, e26246. [Google Scholar] [CrossRef] [PubMed]
- Garvey, E.P.; Prus, K.L. A Specific Inhibitor of Heart Cytosolic 5’-Nucleotidase I Attenuates Hydrolysis of Adenosine 5’-Monophosphate in Primary Rat Myocytes. Arch. Biochem. Biophys. 1999, 364, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.L.; Abeles, R.H. The Mechanism of Action of S-Adenosylhomocysteinase. J. Biol. Chem. 1979, 254, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Deussen, A.; Lloyd, H.G.E.; Schrader, J. Contribution of S-Adenosylhomocysteine to Cardiac Adenosine Formation. J. Mol. Cell. Cardiol. 1989, 21, 773–782. [Google Scholar] [CrossRef]
- Ritzel, M.W.L.; Ng, A.M.L.; Yao, S.Y.M.; Graham, K.; Loewen, S.K.; Smith, K.M.; Ritzel, R.G.; Mowles, D.A.; Carpenter, P.; Chen, X.Z.; et al. Molecular Identification and Characterization of Novel Human and Mouse Concentrative Na+-Nucleoside Cotransporter Proteins (HCNT3 and MCNT3) Broadly Selective for Purine and Pyrimidine Nucleosides (System Cib). J. Biol. Chem. 2001, 276, 2914–2927. [Google Scholar] [CrossRef]
- Damaraju, S.; Zhang, J.; Visser, F.; Tackaberry, T.; Dufour, J.; Smith, K.M.; Slugoski, M.; Ritzel, M.W.L.; Baldwin, S.A.; Young, J.D.; et al. Identification and Functional Characterization of Variants in Human Concentrative Nucleoside Transporter 3, HCNT3 (SLC28A3), Arising from Single Nucleotide Polymorphisms in Coding Regions of the HCNT3 Gene. Pharmacogenet. Genom. 2005, 15, 173–182. [Google Scholar] [CrossRef]
- Errasti-Murugarren, E.; Cano-Soldado, P.; Pastor-Anglada, M.; Casado, F.J. Functional Characterization of a Nucleoside-Derived Drug Transporter Variant (HCNT3C602R) Showing Altered Sodium-Binding Capacity. Mol. Pharmacol. 2008, 73, 379–386. [Google Scholar] [CrossRef]
- Larráyoz, I.M.; Casado, F.J.; Pastor-Anglada, M.; Lostao, M.P. Electrophysiological Characterization of the Human Na+/Nucleoside Cotransporter 1 (HCNT1) and Role of Adenosine on HCNT1 Function. J. Biol. Chem. 2004, 279, 8999–9007. [Google Scholar] [CrossRef] [PubMed]
- Gorraitz, E.; Pastor-Anglada, M.; Lostao, M.P. Effects of Na+ and H+ on Steady-State and Presteady-State Currents of the Human Concentrative Nucleoside Transporter 3 (HCNT3). Pflügers Arch. 2010, 460, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Slugoski, M.D.; Cass, C.E.; Baldwin, S.A.; Karpinski, E.; Young, J.D. Cation Coupling Properties of Human Concentrative Nucleoside Transporters HCNT1, HCNT2 and HCNT3. Mol. Membr. Biol. 2007, 24, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Slugoski, M.D.; Loewen, S.K.; Ng, A.M.L.; Yao, S.Y.M.; Chen, X.Z.; Karpinski, E.; Cass, C.E.; Baldwin, S.A.; Young, J.D. The Broadly Selective Human Na+/Nucleoside Cotransporter(HCNT3) Exhibits Novel Cation-Coupled Nucleoside TransportCharacteristics. J. Biol. Chem. 2005, 280, 25436–25449. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharmacol. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Bakken, A.H.; Hudkins, K.L.; Lai, Y.; Casado, F.J.; Pastor-Anglada, M.; Tse, C.M.; Hayashi, J.; Unadkat, J.D. In Situ Hybridization and Immunolocalization of Concentrative and Equilibrative Nucleoside Transporters in the Human Intestine, Liver, Kidneys, and Placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 1809–1822. [Google Scholar] [CrossRef]
- Mangravite, L.M.; Lipschutz, J.H.; Mostov, K.E.; Giacomini, K.M. Localization of GFP-Tagged Concentrative Nucleoside Transporters in a Renal Polarized Epithelial Cell Line. Am. J. Physiol. Ren. Physiol. 2001, 280, F879–F885. [Google Scholar] [CrossRef]
- Fernández-Calotti, P.; Casulleras, O.; Antolin, M.; Guarner, F.; Pastor-Anglada, M. Galectin-4 Interacts with the Drug Transporter Human Concentrative Nucleoside Transporter 3 to Regulate Its Function. FASEB J. 2016, 30, 544–554. [Google Scholar] [CrossRef]
- Young, J.D.; Yao, S.Y.M.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The Human Concentrative and Equilibrative Nucleoside Transporter Families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef]
- Ward, J.L.; Sherali, A.; Mo, Z.P.; Tse, C.M. Kinetic and Pharmacological Properties of Cloned Human Equilibrative Nucleoside Transporters, ENT1 and ENT2, Stably Expressed in Nucleoside Transporter-Deficient PK15 Cells: ENT2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. J. Biol. Chem. 2000, 275, 8375–8381. [Google Scholar] [CrossRef]
- Muñoz, G.; San Martín, R.; Farías, M.; Cea, L.; Vecchiola, A.; Casanello, P.; Sobrevia, L. Insulin Restores Glucose Inhibition of Adenosine Transport by Increasing the Expression and Activity of the Equilibrative Nucleoside Transporter 2 in Human Umbilical Vein Endothelium. J. Cell. Physiol. 2006, 209, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Casanello, P.; Sobrevia, L. Human Equilibrative Nucleoside Transporters 1 and 2 May Be Differentially Modulated by A2B Adenosine Receptors in Placenta Microvascular Endothelial Cells from Pre-Eclampsia. Placenta 2008, 29, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Boleti, H.; Coe, I.R.; Baldwin, S.A.; Young, J.D.; Cass, C.E. Molecular Identification of the Equilibrative NBMPR-Sensitive (Es) Nucleoside Transporter and Demonstration of an Equilibrative NBMPR- Insensitive (Ei) Transport Activity in Human Erythroleukemia (K562) Cells. Neuropharmacology 1997, 36, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Dobrzynski, H.; Foppolo, S.; Beal, P.R.; Ismat, F.; Scullion, E.R.; Sun, L.; Tellez, J.; Ritzel, M.W.L.; Claycomb, W.C.; et al. Distribution and Functional Characterization of Equilibrative Nucleoside Transporter-4, a Novel Cardiac Adenosine Transporter Activated at Acidic PH. Circ. Res. 2006, 99, 510–519. [Google Scholar] [CrossRef]
- Baldwin, S.A.; Yao, S.Y.M.; Hyde, R.J.; Ng, A.M.L.; Foppolo, S.; Barnes, K.; Ritzel, M.W.L.; Cass, C.E.; Young, J.D. Functional Characterization of Novel Human and Mouse Equilibrative Nucleoside Transporters (HENT3 and MENT3) Located in Intracellular Membranes. J. Biol. Chem. 2005, 280, 15880–15887. [Google Scholar] [CrossRef]
- Govindarajan, R.; Leung, G.P.H.; Zhou, M.; Tse, C.M.; Wang, J.; Unadkat, J.D. Facilitated Mitochondrial Import of Antiviral and Anticancer Nucleoside Drugs by Human Equilibrative Nucleoside Transporter-3. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G910–G922. [Google Scholar] [CrossRef]
- Mangravite, L.M.; Xiao, G.; Giacomini, K.M. Localization of Human Equilibrative Nucleoside Transporters, HENT1 and HENT2, in Renal Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2003, 284, 902–910. [Google Scholar] [CrossRef]
- Morote-Garcia, J.C.; Rosenberger, P.; Nivillac, N.M.I.; Coe, I.R.; Eltzschig, H.K. Hypoxia-Inducible Factor-Dependent Repression of Equilibrative Nucleoside Transporter 2 Attenuates Mucosal Inflammation During Intestinal Hypoxia. Gastroenterology 2009, 136, 607–618. [Google Scholar] [CrossRef]
- Senyavina, N.V.; Gerasimenko, T.N.; Fomicheva, K.A.; Tonevitskaya, S.A.; Kaprin, A.D. Localization and Expression of Nucleoside Transporters ENT1 and ENT2 in Polar Cells of Intestinal Epithelium. Bull. Exp. Biol. Med. 2016, 160, 771–774. [Google Scholar] [CrossRef]
- Xia, L.; Engel, K.; Zhou, M.; Wang, J. Membrane Localization and PH-Dependent Transport of a Newly Cloned Organic Cation Transporter (PMAT) in Kidney Cells. Am. J. Physiol. Ren. Physiol. 2007, 292, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Celis, N.; Araos, J.; Sanhueza, C.; Toledo, F.; Beltrán, A.R.; Pardo, F.; Leiva, A.; Ramírez, M.A.; Sobrevia, L. Intracellular Acidification Increases Adenosine Transport in Human Umbilical Vein Endothelial Cells. Placenta 2017, 51, 10–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, M.; Duan, H.; Engel, K.; Xia, L.; Wang, J. Adenosine Transport by Plasma Membrane Monoamine Transporter: Reinvestigation and Comparison with Organic Cations. Drug Metab. Dispos. 2010, 38, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Yoshida, D.; Miki, K.; Usui, T.; Ikeda, T. An Amperometric-Enzymatic Method for Assays of Inorganic Phosphate and Adenosine Deaminase in Serum Based on the Measurement of Uric Acid with a Dialysis Membrane-Covered Carbon Electrode. Anal. Chim. Acta 1995, 303, 301–307. [Google Scholar] [CrossRef]
- Lloyd, H.G.E.; Fredholm, B.B. Involvement of Adenosine Deaminase and Adenosine Kinase in Regulating Extracellular Adenosine Concentration in Rat Hippocampal Slices. Neurochem. Int. 1995, 26, 387–395. [Google Scholar] [CrossRef] [PubMed]
- van Calker, D.; Müller, M.; Hamprecht, B. Adenosine Regulates via Two Different Types of Receptors, the Accumulation of Cyclic AMP in Cultured Brain Cells. J. Neurochem. 1979, 33, 999–1005. [Google Scholar] [CrossRef]
- Bruns, R.F.; Lu, G.H.; Pugsley, T.A. Adenosine Receptor Subtypes: Binding Studies. In Topics and Perspectives in Adenosine Research, Proceedings of the 3rd International Symposium on Adenosine, Munich, Germany, 15–19 June 1986; Springer: Berlin/Heidelberg, Germany, 1987; pp. 59–73. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Irenius, E.; Kull, B.; Schulte, G. Comparison of the Potency of Adenosine as an Agonist at Human Adenosine Receptors Expressed in Chinese Hamster Ovary Cells. Biochem. Pharmacol. 2001, 61, 443–448. [Google Scholar] [CrossRef]
- Aherne, C.M.; Kewley, E.M.; Eltzschig, H.K. The Resurgence of A2B Adenosine Receptor Signaling. Biochim. Biophys. Acta 2011, 1808, 1329–1339. [Google Scholar] [CrossRef]
- Sitaraman, S.V.; Si-Tahar, M.; Merlin, D.; Strohmeier, G.R.; Madara, J.L. Polarity of A2b Adenosine Receptor Expression Determines Characteristics of Receptor Desensitization. Am. J. Physiol. Cell Physiol. 2000, 278, C1230–C1236. [Google Scholar] [CrossRef]
- Strohmeier, G.R.; Reppert, S.M.; Lencer, W.I.; Madara, J.L. The A2B Adenosine Receptor Mediates CAMP Responses to Adenosine Receptor Agonists in Human Intestinal Epithelia. J. Biol. Chem. 1995, 270, 2387–2394. [Google Scholar] [CrossRef]
- Aherne, C.; Collins, C.; McNamee, E.; Clambey, E.; Aly, T.; de Zoeten, E.; Eltzschig, H. A2B Adenosine Receptor Signaling Influences Epithelial Cell-Leukocyte Crosstalk to Induce Tissue Protection in Acute and Chronic Experimental Colitis: P-195. Inflamm. Bowel Dis. 2011, 17 (Suppl. 2), S70. [Google Scholar] [CrossRef]
- Peakman, M.-C.; Hill, S.J. Adenosine A2B-Receptor-Mediated Cyclic AMP Accumulation in Primary Rat Astrocytes. Br. J. Pharmacol. 1994, 111, 191–198. [Google Scholar] [CrossRef]
- Darashchonak, N.; Koepsell, B.; Bogdanova, N.; Von Versen-Höynck, F. Adenosine A2B Receptors Induce Proliferation, Invasion and Activation of CAMP Response Element Binding Protein (CREB) in Trophoblast Cells. BMC Pregnancy Childbirth 2014, 14, 2. [Google Scholar] [CrossRef]
- Munshi, R.; Pang, I.H.; Sternweis, P.C.; Linden, J. A1 Adenosine Receptors of Bovine Brain Couple to Guanine Nucleotide-Binding Proteins Gi1, Gi2, and Go. J. Biol. Chem. 1991, 266, 22285–22289. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Gettys, T.W.; Stiles, G.L. Differential Interaction with and Regulation of Multiple G-Proteins by the Rat A3 Adenosine Receptor. J. Biol. Chem. 1995, 270, 16895–16902. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Okajima, F.; Tomura, H.; Shimegi, S.; Kondo, Y. A Single Species of A1 Adenosine Receptor Expressed in Chinese Hamster Ovary Cells Not Only Inhibits CAMP Accumulation but Also Stimulates Phospholipase C and Arachidonate Release. Mol. Pharmacol. 1994, 45, 1036–1042. [Google Scholar]
- Freund, S.; Ungerer, M.; Lohse, M.J. A1 Adenosine Receptors Expressed in CHO-Cells Couple to Adenylyl Cyclase and to Phospholipase C. Naunyn Schmiedeberg’s Arch. Pharmacol. 1994, 350, 49–56. [Google Scholar] [CrossRef]
- Dickenson, J.M.; Hill, S.J. Involvement of G-Protein Βγ Subunits in Coupling the Adenosine A1 Receptor to Phospholipase C in Transfected CHO Cells. Eur. J. Pharmacol. 1998, 355, 85–93. [Google Scholar] [CrossRef]
- Schmitz, E.A.; Takahashi, H.; Karakas, E. Structural Basis for Activation and Gating of IP3 Receptors. Nat. Commun. 2022, 13, 1408. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Inoue, A.; Jacobson, K.A. On the G Protein-Coupling Selectivity of the Native A2B Adenosine Receptor. Biochem. Pharmacol. 2018, 151, 201–213. [Google Scholar] [CrossRef]
- Gao, Z.G.; Jacobson, K.A. A2B Adenosine Receptor and Cancer. Int. J. Mol. Sci. 2019, 20, 5139. [Google Scholar] [CrossRef]
- Schulte, G.; Fredholm, B.B. Signalling from Adenosine Receptors to Mitogen-Activated Protein Kinases. Cell. Signal. 2003, 15, 813–827. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, T.; Weber, M.J.; Linden, J. A2B Adenosine and P2Y2 Receptors Stimulate Mitogen-Activated Protein Kinase in Human Embryonic Kidney-293 Cells. Cross-Talk between Cyclic AMP and Protein Kinase c Pathways. J. Biol. Chem. 1999, 274, 5972–5980. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, Y.; Eisenstein, A.S.; Hu, W.; Quintana, A.; Lam, W.K.; Wang, Y.; Wu, Z.; Ravid, K.; Huang, P. A Novel Mechanism of Control of NFκB Activation and Inflammation Involving A2B Adenosine Receptors. J. Cell Sci. 2012, 125, 4507–4517. [Google Scholar] [CrossRef]
- Vecchio, E.A.; Tan, C.Y.R.; Gregory, K.J.; Christopoulos, A.; White, P.J.; May, L.T. Ligand-Independent Adenosine A2B Receptor Constitutive Activity as a Promoter of Prostate Cancer Cell Proliferation. J. Pharmacol. Exp. Ther. 2016, 357, 36–44. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The Adherent Gastrointestinal Mucus Gel Layer: Thickness and Physical State in Vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Ermund, A.; Schütte, A.; Johansson, M.E.V.; Gustafsson, J.K.; Hansson, G.C. Studies of Mucus in Mouse Stomach, Small Intestine, and Colon. I. Gastrointestinal Mucus Layers Have Different Prop-erties Depending on Location as Well as over the Peyer’s Patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G341–G347. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171. [Google Scholar] [CrossRef] [PubMed]
- Velcich, A.; Yang, W.C.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Zarepour, M.; Bhullar, K.; Montero, M.; Ma, C.; Huang, T.; Velcich, A.; Xia, L.; Vallance, B.A. The Mucin Muc2 Limits Pathogen Burdens and Epithelial Barrier Dysfunction during Salmonella Enterica Serovar Typhimurium Colitis. Infect. Immun. 2013, 81, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Kim, G.; Shafer, S.; Chen, Z.; Kubo, S.; Ji, Y.; Luo, J.; Yang, W.; Perner, S.P.; Kanellopoulou, C.; et al. Mucus Sialylation Determines Intestinal Host-Commensal Homeostasis. Cell 2022, 185, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Wu, W.; Mishra, P.K.; Chen, F.; Millman, A.; Csóka, B.; Koscsó, B.; Eltzschig, H.K.; Haskó, G.; Gause, W.C. A2B Adenosine Receptor Induces Protective Antihelminth Type 2 Immune Responses. Cell Host Microbe 2014, 15, 339–350. [Google Scholar] [CrossRef]
- Pedroza, M.; Schneider, D.J.; Karmouty-Quintana, H.; Coote, J.; Shaw, S.; Corrigan, R.; Molina, J.G.; Alcorn, J.L.; Galas, D.; Gelinas, R.; et al. Interleukin-6 Contributes to Inflammation and Remodeling in a Model of Adenosine Mediated Lung Injury. PLoS ONE 2011, 6, e22667. [Google Scholar] [CrossRef]
- McNamara, N.; Gallup, M.; Khong, A.; Sucher, A.; Maltseva, I.; Fahy, J.V.; Basbaum, C. Adenosine Up-Regulation of the Mucin Gene, MUC2, in Asthma. FASEB J. 2004, 18, 1770–1772. [Google Scholar] [CrossRef]
- Young, H.W.J.; Molina, J.G.; Dimina, D.; Zhong, H.; Jacobson, M.; Chan, L.-N.L.; Chan, T.-S.; Lee, J.J.; Blackburn, M.R. A3 Adenosine Receptor Signaling Contributes to Airway Inflammation and Mucus Production in Adenosine Deaminase-Deficient Mice. J. Immunol. 2004, 173, 1380–1389. [Google Scholar] [CrossRef]
- Mohsenin, A.; Mi, T.; Xia, Y.; Kellems, R.E.; Chen, J.F.; Blackburn, M.R. Genetic Removal of the A2A Adenosine Receptor Enhances Pulmonary Inflammation, Mucin Production, and Angiogenesis in Adenosine Deaminase-Deficient Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Young, H.W.; Molina, J.G.; Volmer, J.B.; Schnermann, J.; Blackburn, M.R. A Protective Role for the A1 Adenosine Receptor in Adenosine-Dependent Pulmonary Injury. J. Clin. Investig. 2005, 115, 35–43. [Google Scholar] [CrossRef]
- Luis, A.S.; Hansson, G.C. Intestinal Mucus and Their Glycans: A Habitat for Thriving Microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin Glycan Foraging in the Human Gut Microbiome. Front. Genet. 2015, 6, 81. [Google Scholar] [CrossRef]
- Glover, J.S.; Ticer, T.D.; Engevik, M.A. Characterizing the Mucin-Degrading Capacity of the Human Gut Microbiota. Sci. Rep. 2022, 12, 8456. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Caetano, M.; Antunes, L.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut Microbiota: Role in Pathogen Colonization, Immune Responses and Inflammatory Disease. Immunol. Rev. 2017, 279, 70. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLoS ONE 2013, 8, 80604. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function Through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-Derived Butyrate Dynamically Regulates Intestinal Homeostasis through Regulation of Actin-Associated Protein Synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780. [Google Scholar] [CrossRef]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-Throughput Clone Library Analysis of the Mucosa-Associated Microbiota Reveals Dysbiosis and Differences between Inflamed and Non-Inflamed Regions of the Intestine in Inflammatory Bowel Disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut Microbiome Structure and Metabolic Activity in Inflammatory Bowel Disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef]
- Perruzza, L.; Gargari, G.; Proietti, M.; Fosso, B.; D’Erchia, A.M.; Faliti, C.E.; Rezzonico-Jost, T.; Scribano, D.; Mauri, L.; Colombo, D.; et al. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 2017, 18, 2566–2575. [Google Scholar] [CrossRef]
- Proietti, M.; Perruzza, L.; Scribano, D.; Pellegrini, G.; D’Antuono, R.; Strati, F.; Raffaelli, M.; Gonzalez, S.F.; Thelen, M.; Hardt, W.D.; et al. ATP Released by Intestinal Bacteria Limits the Generation of Protective IgA against Enteropathogens. Nat. Commun. 2019, 10, 250. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, B.W.; Li, R.; Yang, P.; Leng, P.; Huang, Y. Exploration of the Link between Gut Microbiota and Purinergic Signalling. Purinergic Signal. 2022, 19, 315–327. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, X.; He, B.; Hoang, T.K.; Taylor, C.M.; Blanchard, E.; Freeborn, J.; Park, S.; Luo, M.; Couturier, J.; et al. Lactobacillus Reuteri DSM 17938 Feeding of Healthy Newborn Mice Regulates Immune Responses While Modulating Gut Microbiota and Boosting Beneficial Metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G824–G838. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-Tunable Engineered Yeast Probiotics for the Treatment of Inflammatory Bowel Disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef]
- Kao, D.J.; Saeedi, B.J.; Kitzenberg, D.; Burney, K.M.; Dobrinskikh, E.; Battista, K.D.; Vázquez-Torres, A.; Colgan, S.P.; Kominsky, D.J. Intestinal Epithelial Ecto-5’-Nucleotidase (CD73) Regulates Intestinal Colonization and Infection by Nontyphoidal Salmonella. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Kitzenberg, D.A.; Lee, J.S.; Mills, K.B.; Kim, J.S.; Liu, L.; Vázquez-Torres, A.; Colgan, S.P.; Kao, D.J. Adenosine Awakens Metabolism to Enhance Growth-Independent Killing of Tolerant and Persister Bacteria across Multiple Classes of Antibiotics. mBio 2022, 13, e00480-22. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-Derived Inosine Modulates Response to Checkpoint Inhibitor Immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut Microbiota-Derived Inosine from Dietary Barley Leaf Supplementation Attenuates Colitis through PPARγ Signaling Activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Mabley, J.G.; Pacher, P.; Liaudet, L.; Soriano, F.G.; Hasko, G.; Marton, A.; Szabo, C.; Salzman, A.L. Inosine Reduces Inflammation and Improves Survival in a Murine Model of Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G138–G144. [Google Scholar] [CrossRef]
- Rahimian, R.; Fakhfouri, G.; Daneshmand, A.; Mohammadi, H.; Bahremand, A.; Rasouli, M.R.; Mousavizadeh, K.; Dehpour, A.R. Adenosine A2A Receptors and Uric Acid Mediate Protective Effects of Inosine against TNBS-Induced Colitis in Rats. Eur. J. Pharmacol. 2010, 649, 376–381. [Google Scholar] [CrossRef]
- da Rocha Lapa, F.; de Oliveira, A.P.L.; Accetturi, B.G.; de Oliveira Martins, I.; Domingos, H.V.; de Almeida Cabrini, D.; de Lima, W.T.; Santos, A.R.S. Anti-Inflammatory Effects of Inosine in Allergic Lung Inflammation in Mice: Evidence for the Participation of Adenosine A2A and A3 Receptors. Purinergic Signal. 2013, 9, 325–336. [Google Scholar] [CrossRef]
- Welihinda, A.A.; Kaur, M.; Greene, K.; Zhai, Y.; Amento, E.P. The Adenosine Metabolite Inosine Is a Functional Agonist of the Adenosine A2A Receptor with a Unique Signaling Bias. Cell. Signal. 2016, 28, 552–560. [Google Scholar] [CrossRef]
- Gomez, G.; Sitkovsky, M.V. Differential Requirement for A2a and A3 Adenosine Receptors for the Protective Effect of Inosine in Vivo. Blood 2003, 102, 4472–4478. [Google Scholar] [CrossRef]
- Haskó, G.; Kuhel, D.G.; Németh, Z.H.; Mabley, J.G.; Stachlewitz, R.F.; Virág, L.; Lohinai, Z.; Southan, G.J.; Salzman, A.L.; Szabó, C. Inosine Inhibits Inflammatory Cytokine Production by a Posttranscriptional Mechanism and Protects Against Endotoxin-Induced Shock. J. Immunol. 2000, 164, 1013–1019. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Wang, T.; Ferris, M.; Taylor, C.M.; Tian, X.; Luo, M.; Tran, D.Q.; Zhou, J.; Tatevian, N.; et al. Resetting Microbiota by Lactobacillus Reuteri Inhibits T Reg Deficiency–Induced Autoimmunity via Adenosine A2A Receptors. J. Exp. Med. 2017, 214, 107–123. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Garssen, J.; Fink-Gremmels, J.; Braber, S. Epithelial Integrity, Junctional Complexes, and Biomarkers Associated with Intestinal Functions. Tissue Barriers 2021, 10, 1996830. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional Strands in Tight Junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Ma, Y.; Shen, L.; Xu, Y.; Liu, L.; Bu, X.; Guo, Z.; Qin, H.; Li, Z.; Wang, Z.; et al. NDRG2 Regulates Adherens Junction Integrity to Restrict Colitis and Tumourigenesis. eBioMedicine 2020, 61, 103068. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Nijhuis, A.; Kumagai, T.; Lindsay, J.; Silver, A. Defects in the Adherens Junction Complex (E-Cadherin/β-Catenin) in Inflammatory Bowel Disease. Cell Tissue Res. 2015, 360, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Naydenov, N.G. Dynamics and Regulation of Epithelial Adherens Junctions: Recent Discoveries and Controversies. Int. Rev. Cell Mol. Biol. 2013, 303, 27–99. [Google Scholar] [CrossRef]
- Spindler, V.; Meir, M.; Vigh, B.; Flemming, S.; Hütz, K.; Germer, C.T.; Waschke, J.; Schlegel, N. Loss of Desmoglein 2 Contributes to the Pathogenesis of Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2349–2359. [Google Scholar] [CrossRef]
- Raya-Sandinoa, A.; Luissint, A.C.; Kusters, D.H.M.; Narayanan, V.; Flemming, S.; Garcia-Hernandez, V.; Godsel, L.M.; Green, K.J.; Hagen, S.J.; Conway, D.E.; et al. Regulation of Intestinal Epithelial Intercellular Adhesion and Barrier Function by Desmosomal Cadherin Desmocollin-2. Mol. Biol. Cell 2021, 32, 753–768. [Google Scholar] [CrossRef]
- Schlegel, N.; Meir, M.; Heupel, W.M.; Holthöfer, B.; Leube, R.E.; Waschke, J. Desmoglein 2-Mediated Adhesion Is Required for Intestinal Epithelial Barrier Integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, 774–783. [Google Scholar] [CrossRef]
- Gross, A.; Pack, L.A.P.; Schacht, G.M.; Kant, S.; Ungewiss, H.; Meir, M.; Schlegel, N.; Preisinger, C.; Boor, P.; Guldiken, N.; et al. Desmoglein 2, but Not Desmocollin 2, Protects Intestinal Epithelia from Injury. Mucosal Immunol. 2018, 11, 1630–1639. [Google Scholar] [CrossRef]
- Nagler, S.; Ghoreishi, Y.; Kollmann, C.; Kelm, M.; Gerull, B.; Waschke, J.; Burkard, N.; Schlegel, N. Plakophilin 2 Regulates Intestinal Barrier Function by Modulating Protein Kinase C Activity in Vitro. Tissue Barriers 2023, 11, 2138061. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Marchiando, A.M.; Shen, L.; Vallen Graham, W.; Weber, C.R.; Schwarz, B.T.; Austin, J.R.; Raleigh, D.R.; Guan, Y.; Watson, A.J.M.; Montrose, M.H.; et al. Caveolin-1–Dependent Occludin Endocytosis Is Required for TNF-Induced Tight Junction Regulation in Vivo. J. Cell Biol. 2010, 189, 111. [Google Scholar] [CrossRef] [PubMed]
- Utech, M.; Ivanov, A.I.; Samarin, S.N.; Bruewer, M.; Turner, J.R.; Mrsny, R.J.; Parkos, C.A.; Nusrat, A. Mechanism of IFN-γ-Induced Endocytosis of Tight Junction Proteins: Myosin II-Dependent Vacuolarization of the Apical Plasma Membrane. Mol. Biol. Cell 2005, 16, 5040. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Utech, M.; Ivanov, A.I.; Hopkins, A.M.; Parkos, C.A.; Nusrat, A. Interferon-Gamma Induces Internalization of Epithelial Tight Junction Proteins via a Macropinocytosis-like Process. FASEB J. 2005, 19, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Chumki, S.A.; Stephenson, R.E.; Misterovich, E.R.; Wu, J.L.; Dudley, C.E.; Erofeev, I.S.; Goryachev, A.B.; Miller, A.L. Mechanosensitive Calcium Flashes Promote Sustained RhoA Activation during Tight Junction Remodeling. J. Cell Biol. 2022, 221, e202105107. [Google Scholar] [CrossRef] [PubMed]
- Nusrat, A.; Turner, J.R.; Madara, J.L. Molecular Physiology and Pathophysiology of Tight Junctions. IV. Regulation of Tight Junctions by Extracellular Stimuli: Nutrients, Cytokines, and Immune Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G851–G857. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Krug, S.M.; Bojarski, C.; Fromm, A.; Lee, I.M.; Dames, P.; Richter, J.F.; Turner, J.R.; Fromm, M.; Schulzke, J.D. Tricellulin Is Regulated via Interleukin-13-Receptor A2, Affects Macromolecule Uptake, and Is Decreased in Ulcerative Colitis. Mucosal Immunol. 2018, 11, 345–356. [Google Scholar] [CrossRef]
- Sun, X.; Cárdenas, A.; Wu, Y.; Enjyoji, K.; Robson, S.C. Vascular Stasis, Intestinal Hemorrhage, and Heightened Vascular Permeability Complicate Acute Portal Hypertension in Cd39-Null Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 306–311. [Google Scholar] [CrossRef][Green Version]
- Yegutkin, G.G.; Auvinen, K.; Rantakari, P.; Hollmén, M.; Karikoski, M.; Grénman, R.; Elima, K.; Jalkanen, S.; Salmi, M. Ecto-5′-Nucleotidase/CD73 Enhances Endothelial Barrier Function and Sprouting in Blood but Not Lymphatic Vasculature. Eur. J. Immunol. 2014, 45, 562–573. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Ibla, J.C.; Furuta, G.T.; Leonard, M.O.; Jacobson, K.A.; Enjyoji, K.; Robson, S.C.; Colgan, S.R. Coordinated Adenine Nucleotide Phosphohydrolysis and Nucleoside Signaling in Posthypoxic Endothelium Role of Ectonucleotidases and Adenosine A2B Receptors. J. Exp. Med. 2003, 198, 783–796. [Google Scholar] [CrossRef]
- Thompson, L.F.; Eltzschig, H.K.; Ibla, J.C.; Van De Wiele, C.J.; Resta, R.; Morote-Garcia, J.C.; Colgan, S.P. Crucial Role for Ecto-5′-Nucleotidase (CD73) in Vascular Leakage during Hypoxia. J. Exp. Med. 2004, 200, 1395. [Google Scholar] [CrossRef]
- Friedman, D.J.; Künzli, B.M.; A-Rahim, Y.I.; Sevigny, J.; Berberat, P.O.; Enjyoji, K.; Csizmadia, E.; Friess, H.; Robson, S.C. CD39 Deletion Exacerbates Experimental Murine Colitis and Human Polymorphisms Increase Susceptibility to Inflammatory Bowel Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16788–16793. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, M.S.; Waickman, A.T.; Mahamed, D.A.; Mueller, C.; Mills, J.H.; Czopik, A. CD73 Is Critical for the Resolution of Murine Colonic Inflammation. J. Biomed. Biotechnol. 2012, 2012, 260983. [Google Scholar] [CrossRef]
- Patrick Schenck, L.; Hirota, S.A.; Hirota, C.L.; Boasquevisque, P.; Tulk, S.E.; Li, Y.; Wadhwani, A.; Doktorchik, C.T.A.; Macnaughton, W.K.; Beck, P.L.; et al. Attenuation of Clostridium Difficile Toxin-Induced Damage to Epithelial Barrier by Ecto-5′-Nucleotidase (CD73) and Adenosine Receptor Signaling. Neurogastroenterol. Motil. 2013, 25, e441–e453. [Google Scholar] [CrossRef]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-Nucleotidase (CD73) Regulation by Hypoxia-Inducible Factor-1 Mediates Permeability Changes in Intestinal Epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef]
- Comerford, K.M.; Lawrence, D.W.; Synnestvedt, K.; Levi, B.P.; Colgan, S.P. Role of Vasodilator-Stimulated Phosphoprotein in Protein Kinase A-Induced Changes in Endothelial Junctional Permeability. FASEB J. 2002, 16, 583–585. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Comerford, K.M.; Colgan, S.P. Role of VASP in Reestablishment of Epithelial Tight Junction Assembly after Ca2+ Switch. Am. J. Physiol. Cell Physiol. 2002, 282, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Jacobi, B.; Schittenhelm, J.; Henn, M.; Eltzschig, H.K. Cutting Edge: A2B Adenosine Receptor Signaling Provides Potent Protection during Intestinal Ischemia/Reperfusion Injury. J. Immunol. 2009, 182, 3965–3968. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.L.; Portwich, M.; Schmidt, A.; Utepbergenov, D.I.; Huber, O.; Blasig, I.E.; Krause, G. The Tight Junction Protein Occludin and the Adherens Junction Protein α-Catenin Share a Common Interaction Mechanism with ZO-1. J. Biol. Chem. 2005, 280, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Yang, S.; Li, H.; Yao, H.; Zhang, Y.; Zhang, J.; Xu, G.; Jin, H.; Wang, F. The Interplay Between E-Cadherin, Connexin 43, and Zona Occludens 1 in Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5104–5111. [Google Scholar] [CrossRef] [PubMed]
- Maiers, J.L.; Peng, X.; Fanning, A.S.; DeMali, K.A. ZO-1 Recruitment to α-Catenin -a Novel Mechanism for Coupling the Assembly of Tight Junctions to Adherens Junctions. J. Cell Sci. 2013, 126, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nagafuchi, A.; Moroi, S.; Tsukita, S. Involvement of ZO-1 in Cadherin-Based Cell Adhesion through Its Direct Binding to α Catenin and Actin Filaments. J. Cell Biol. 1997, 138, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Umeda, K.; Tsukita, S.; Furuse, M.; Tsukita, S. Requirement of ZO-1 for the Formation of Belt-like Adherens Junctions during Epithelial Cell Polarization. J. Cell Biol. 2007, 176, 779–786. [Google Scholar] [CrossRef]
- Schlegel, N.; Boerner, K.; Waschke, J. Targeting Desmosomal Adhesion and Signalling for Intestinal Barrier Stabilization in Inflammatory Bowel Diseases—Lessons from Experimental Models and Patients. Acta Physiol. 2021, 231, e13492. [Google Scholar] [CrossRef]
- Kolachala, V.L.; Vijay-Kumar, M.; Dalmasso, G.; Yang, D.; Linden, J.; Wang, L.; Gewirtz, A.; Ravid, K.; Merlin, D.; Sitaraman, S.V. A2B Adenosine Receptor Gene Deletion Attenuates Murine Colitis. Gastroenterology 2008, 135, 861–870. [Google Scholar] [CrossRef]
- Kolachala, V.L.; Ruble, B.K.; Vijay-Kumar, M.; Wang, L.; Mwangi, S.; Figler, H.E.; Figler, R.A.; Srinivasan, S.; Gewirtz, A.T.; Linden, J.; et al. Blockade of Adenosine A2B Receptors Ameliorates Murine Colitis. Br. J. Pharmacol. 2008, 155, 127–137. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Laroui, H.; Kolachala, V.L.; Wang, L.; Garg, P.; Denning, T.L.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. A2BAR Expression in Non-Immune Cells Plays an Important Role in the Development of Murine Colitis. Dig. Liver Dis. 2012, 44, 819. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Qiu, Y.; Wang, W.; Xiao, W.; Liang, H.; Zhang, C.; Yang, H.; Teitelbaum, D.H.; Sun, L.H.; Yang, H. Adenosine A2B Receptor Modulates Intestinal Barrier Function under Hypoxic and Ischemia/Reperfusion Conditions. Int. J. Clin. Exp. Pathol. 2014, 7, 2006–2018. [Google Scholar]
- Negussie, A.B.; Dell, A.C.; Davis, B.A.; Geibel, J.P. Colonic Fluid and Electrolyte Transport 2022: An Update. Cells 2022, 11, 1712. [Google Scholar] [CrossRef]
- Barrett, K.E.; Keely, S.J. Chloride Secretion by the Intestinal Epithelium: Molecular Basis and Regulatory Aspects. Annu. Rev. Physiol. 2003, 62, 535–572. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Mall, M. Electrolyte Transport in the Mammalian Colon: Mechanisms and Implications for Disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Donowitz, M.; Verkman, A.S. Secretory Diarrhoea: Mechanisms and Emerging Therapies. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 446. [Google Scholar] [CrossRef]
- Dobbins, J.W.; Laurenson, J.P.; Forrest, J.N. Adenosine and Adenosine Analogues Stimulate Adenosine Cyclic 3’, 5’-Monophosphate-Dependent Chloride Secretion in the Mammalian Ileum. J. Clin. Investig. 1984, 74, 929–935. [Google Scholar] [CrossRef]
- Barrett, K.E.; Huott, P.A.; Shah, S.S.; Dharmsathaphorn, K.; Wasserman, S.I. Differing Effects of Apical and Basolateral Adenosine on Colonic Epithelial Cell Line T84. Am. J. Physiol.-Cell Physiol. 1989, 256, C197–C203. [Google Scholar] [CrossRef]
- Madara, J.L.; Patapoff, T.W.; Gillece-Castro, B.; Colgan, S.P.; Parkos, C.A.; Delp, C.; Mrsny, R.J. 5’-Adenosine Monophosphate Is the Neutrophil-Derived Paracrine Factor That Elicits Chloride Secretion from T84 Intestinal Epithelial Cell Monolayers. J. Clin. Investig. 1993, 91, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K.E.; Cohn, J.A.; Huott, P.A.; Wasserman, S.I.; Dharmsathaphorn, K. Immune-Related Intestinal Chloride Secretion. II. Effect of Adenosine on T84 Cell Line. Am. J. Physiol.-Cell Physiol. 1990, 258, C902–C912. [Google Scholar] [CrossRef]
- Ghanem, E.; Lövdahl, C.; Daré, E.; Ledent, C.; Fredholm, B.B.; Boeynaems, J.M.; Van Driessche, W.; Beauwens, R. Luminal Adenosine Stimulates Chloride Secretion through A1 Receptor in Mouse Jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, 972–977. [Google Scholar] [CrossRef]
- Cartwright, I.M.; Dowdell, A.S.; Lanis, J.M.; Brink, K.R.; Mu, A.; Kostelecky, R.E.; Schaefer, R.E.M.; Welch, N.; Onyiah, J.C.; Hall, C.H.T.; et al. Mucosal Acidosis Elicits a Unique Molecular Signature in Epithelia and Intestinal Tissue Mediated by GPR31-Induced CREB Phosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023871118. [Google Scholar] [CrossRef]
- Roediger, W.E.W.; Lawson, M.J.; Kwok, V. Colonic Bicarbonate Output as a Test of Disease Activity in Ulcerative Colitis. J. Clin. Pathol. 1984, 37, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J.; Christensen, L.A.; Jacobsen, B.A.; Rasmussen, S.N. Very Low Intraluminal Colonic PH in Patients with Active Ulcerative Colitis. Dig. Dis. Sci. 1993, 38, 1989–1993. [Google Scholar] [CrossRef]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal Luminal PH in Inflammatory Bowel Disease: Possible Determinants and Implications for Therapy with Aminosalicylates and Other Drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef]
- Cartwright, I.M.; Curtis, V.F.; Lanis, J.M.; Alexeev, E.E.; Welch, N.; Goldberg, M.S.; Schaefer, R.E.M.; Gao, R.Y.; Chun, C.; Fennimore, B.; et al. Adaptation to Inflammatory Acidity through Neutrophil-Derived Adenosine Regulation of SLC26A3. Mucosal Immunol. 2020, 13, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal Lactate and Ulcerative Colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Hove, H.; Mortensen, P.B. Influence of Intestinal Inflammation (IBD) and Small and Large Bowel Length on Fecal Short-Chain Fatty Acids and Lactate. Dig. Dis. Sci. 1995, 40, 1372–1380. [Google Scholar] [CrossRef]
- Hayashi, H.; Nagai, H.; Ohba-ichiro, K.; Soleimani, M.; Suzuki, Y. Segmental Differences in Slc26a3-Dependent Cl− Absorption and HCO3− Secretion in the Mouse Large Intestine in Vitro in Ussing Chambers. J. Physiol. Sci. 2021, 71, 5. [Google Scholar] [CrossRef]
- Walker, N.M.; Simpson, J.E.; Brazill, J.M.; Gill, R.K.; Dudeja, P.K.; Schweinfest, C.W.; Clarke, L.L. Role of Down-Regulated in Adenoma Anion Exchanger in HCO3– Secretion Across Murine Duodenum. Gastroenterology 2009, 136, 893–901.e2. [Google Scholar] [CrossRef]
- Walker, N.M.; Simpson, J.E.; Yen, P.F.; Gill, R.K.; Rigsby, E.V.; Brazill, J.M.; Dudeja, P.K.; Schweinfest, C.W.; Clarke, L.L. Down-Regulated in Adenoma Cl/HCO3 Exchanger Couples With Na/H Exchanger 3 for NaCl Absorption in Murine Small Intestine. Gastroenterology 2008, 135, 1645–1653.e3. [Google Scholar] [CrossRef]
- Xiao, F.; Juric, M.; Li, J.; Riederer, B.; Yeruva, S.; Kumar Singh, A.; Zheng, L.; Glage, S.; Kollias, G.; Dudeja, P.; et al. Loss of Downregulated in Adenoma (DRA) Impairs Mucosal HCO3- Secretion in Murine Ileocolonic Inflammation. Inflamm. Bowel Dis. 2012, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Rossmann, H.; Lamprecht, G.; Kretz, A.; Neff, C.; LinWu, E.; Gregor, M.; Groneberg, D.A.; Kere, J.; Seidler, U. Down-Regulated in Adenoma Mediates Apical Cl−/HCO3− Exchange in Rabbit, Rat, and Human Duodenum. Gastroenterology 2002, 122, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. Slc26a3 (Dra)-Deficient Mice Display Chloride-Losing Diarrhea, Enhanced Colonic Proliferation, and Distinct Up-Regulation of Ion Transporters in the Colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed]
- Höglund, P.; Haila, S.; Socha, J.; Tomaszewski, L.; Saarialho-Kere, U.; Karjalainen-Lindsberg, M.L.; Airola, K.; Holmberg, C.; de la Chapelle, A.; Kere, J. Mutations of the Down–Regulated in Adenoma (DRA) Gene Cause Congenital Chloride Diarrhoea. Nat. Genet. 1996, 14, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, W.; Furth, E.E.; Wen, X.; Katz, J.P.; Sellon, R.K.; Silberg, D.G.; Antalis, T.M.; Schweinfest, C.W.; Wu, G.D. Intestinal Inflammation Reduces Expression of DRA, a Transporter Responsible for Congenital Chloride Diarrhea. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G1445–G1453. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, D.; Li, M.; Tian, D.; Yu, H.; Yu, Q. Tumor Necrosis Factor-α Acts Reciprocally with Solute Carrier Family 26, Member 3, (Downregulated-in-Adenoma) and Reduces Its Expression, Leading to Intestinal Inflammation. Int. J. Mol. Med. 2018, 41, 1224. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Yu, Q.; Li, J.; Johansson, M.E.V.; Singh, A.K.; Xia, W.; Riederer, B.; Engelhardt, R.; Montrose, M.; Soleimani, M.; et al. Slc26a3 Deficiency Is Associated with Loss of Colonic HCO3− Secretion, Absence of a Firm Mucus Layer and Barrier Impairment in Mice. Acta Physiol. 2014, 211, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, D.; Li, M.; Wang, H.; He, Q.; Wang, Y.; Yu, H.; Tian, D.; Yu, Q. SLC26A3 (DRA) Prevents TNF-Alpha-Induced Barrier Dysfunction and Dextran Sulfate Sodium-Induced Acute Colitis. Lab. Investig. 2018, 98, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.M.; Yin, J.; Singh, V.; Sarker, R.; Lin, R.; Verkman, A.S.; Turner, J.R.; Donowitz, M. CAMP Stimulates SLC26A3 Activity in Human Colon by a CFTR-Dependent Mechanism That Does Not Require CFTR Activity. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wojtal, K.A.; Eloranta, J.J.; Hruz, P.; Gutmann, H.; Drewe, J.; Staumann, A.; Beglinger, C.; Fried, M.; Kullak-Ublick, G.A.; Vavricka, S.R. Changes in MRNA Expression Levels of Solute Carrier Transporters in Inflammatory Bowel Disease Patients. Drug Metab. Dispos. 2009, 37, 1871–1877. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, M.; Aherne, C.M. Adenosine in Intestinal Epithelial Barrier Function. Cells 2024, 13, 381. https://doi.org/10.3390/cells13050381
Stepanova M, Aherne CM. Adenosine in Intestinal Epithelial Barrier Function. Cells. 2024; 13(5):381. https://doi.org/10.3390/cells13050381
Chicago/Turabian StyleStepanova, Mariya, and Carol M. Aherne. 2024. "Adenosine in Intestinal Epithelial Barrier Function" Cells 13, no. 5: 381. https://doi.org/10.3390/cells13050381
APA StyleStepanova, M., & Aherne, C. M. (2024). Adenosine in Intestinal Epithelial Barrier Function. Cells, 13(5), 381. https://doi.org/10.3390/cells13050381





