Abstract
Mitochondria, the energy suppliers of the cells, play a central role in a variety of cellular processes essential for survival or leading to cell death. Consequently, mitochondrial dysfunction is implicated in numerous general and CNS disorders. The clinical manifestations of mitochondrial dysfunction include metabolic disorders, dysfunction of the immune system, tumorigenesis, and neuronal and behavioral abnormalities. In this review, we focus on the mitochondrial role in the CNS, which has unique characteristics and is therefore highly dependent on the mitochondria. First, we review the role of mitochondria in neuronal development, synaptogenesis, plasticity, and behavior as well as their adaptation to the intricate connections between the different cell types in the brain. Then, we review the sparse knowledge of the mechanisms of exogenous mitochondrial uptake and describe attempts to determine their half-life and transplantation long-term effects on neuronal sprouting, cellular proteome, and behavior. We further discuss the potential of mitochondrial transplantation to serve as a tool to study the causal link between mitochondria and neuronal activity and behavior. Next, we describe mitochondrial transplantation’s therapeutic potential in various CNS disorders. Finally, we discuss the basic and reverse—translation challenges of this approach that currently hinder the clinical use of mitochondrial transplantation.
1. Introduction
The traditional view of the mitochondria, the double-membrane organelle, was based on their central role in cellular respiration, a process that converts nutrients into adenosine triphosphate (ATP), the primary energy currency of the cell. At present, mitochondria are conceptualized as a cellular hub that maintains cellular homeostasis, regulates apoptosis, calcium signaling, and cellular oxidative stress states [1,2,3]. In addition, the mitochondrial tricarboxylic acid (TCA) cycle provides metabolites that serve as building blocks for a variety of macromolecules including proteins, lipids, carbohydrates, and nucleotides. Mitochondria also control the innate immune response and are essential mediators of epigenetic processes [4] (Figure 1). Serving as a hub, the mitochondria respond to the cellular state and its energy demands, which are transmitted by cytosolic signaling molecules such as Ca2+ and protein kinases and by neurotransmitters [5,6,7,8,9,10]. Maintaining overall cellular and mitochondrial homeostasis is strongly dependent on the coordination between mitochondrial and nuclear DNA [11]. The nuclear genes coordinate the synthesis of proteins essential for mitochondrial structure and function and both nuclear and mitochondrial DNA-encoded factors are involved in the regulation of mitochondrial DNA replication and repair [12]. This review focuses on mitochondrial role in the central nervous system (CNS) in health and disease and the challenges of the transplantation of exogenous mitochondria in the brain. The brain is a unique organ by virtue of comprising different cell types (neurons, oligodendrocytes, and glial cells such as astrocytes, microglia, and ependymal cells) and functional areas with intricate interconnections. Moreover, neurons are post-mitotic cells and therefore their protein and mitochondrial turnover rates are lower than those of other tissues. The neuronal cells are flexible and adaptive along life’s path, being highly sensitive to changes in the environment and to insults. Finally, changes in brain neuronal activity and circuitry can have behavioral manifestations. Therefore, the mitochondrial role in the CNS calls for specific consideration. In addition, we review the therapeutic potential of mitochondrial transplantation in the brain and use this manipulation to enlighten the mitochondrial role in neuronal development, activity, synaptic plasticity, and ultimately behavior. The open questions and challenges relevant to mitochondrial transplantation strategies are discussed as well.
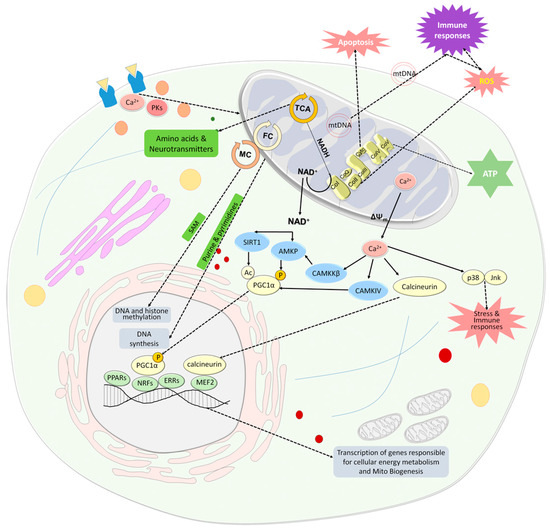
Figure 1.
Mitochondria are an important cellular hub. Mitochondria produce energy in the form of ATP through the OxPhos system, which is also a major source of ROS and is involved in apoptosis. The TCA cycle not only provides reduced substrates in the form of NADH and NADPH for the respiratory chain, but also provides amino acids, that are converted to neurotransmitters and serve as building blocks for proteins. The folate cycle, which is located in the mitochondria and the cytosol, provides purines and pyrimidines for DNA synthesis and, together with the methionine cycle, provides methyl groups for DNA and histone methylation. In addition, mitochondria communicate with the nucleus in homeostasis and stress. Changes in the NAD+/NADH ratio and extracellular Ca2+ concentrations are two major inducers of signaling pathways that activate PGC-1α. An increase in NAD+ levels is associated with reduced ATP levels and increased extracellular Ca2+ concentration is linked to dissipation of ΔΨm and enhanced leak of mitochondrial Ca2+. NAD+ activates SIRT1 which in turn activates PGC-1α by its deacetylation. NAD+ also activates AMKP which in turn activates PGC-1α by its phosphorylation. AMPK can also be activated by CaMKKβ, which is activated by Ca2+. PGC-1α is an upstream inducer of mitochondrial genes. It positively affects the activity of some hormone nuclear receptors, PPARs and ERRs, which regulate multiple pathways involved in cellular energy metabolism within and outside mitochondria, and nuclear transcription factors, NRFs, which leads to the transcription of nuclear coded respiratory chain components and Tfam. PGC1α can be also activated by CaMKIV which in turn is activated by Ca2+. Ca2+ also activates the phosphatase calcineurin, which activates the nuclear MEF2, which in turn regulates many muscle-specific genes and lipogenic and glycogenic enzyme genes. Ca2+ released from the mitochondria induces a stress state via the activation of stress-related MAPKs, p38 and Jnk, which leads to the activation of the immune system and oxidative stress state as do cell-free mtDNAs. Mitochondria are also involved in hemoglobin and steroid production and respond to the cellular state and its energy demands, transmitted by cytosolic signaling molecules such as Ca2+ and protein kinases and by neurotransmitters. Abbreviations: Ac—acetyl group; AMPK—AMP-activated protein kinase; CaMKIV—Ca2+/calmodulin-dependent protein kinase type IV; CaMKKβ—Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ); Co—complex; ERRs—estrogen-related receptors; FC—folate cycle; Jnk—c-Jun NH2-terminal kinase; MC—methionine cycle; MEF2—myocyte-specific enhancer factor 2; mtDNA- mitochondrial DNA; NAD—nicotinamide adenine dinucleotide; NFRs—nuclear respiratory factors; OxPhos-oxidative phosphorylation; P—phosphate group; p38—p38 mitogen-activated protein kinase; PGC-1α—PPARγ co-activator 1α; PK—protein kinases; PPARs—peroxisome proliferator-activated receptors; ROS—reactive oxygen species; SAM—S-adenosylmethionine; TCA—tricarboxylic acid; Tfam—mitochondria transcription factor A; ΔΨm—mitochondrial membrane potential.
2. Mitochondrial Homeostasis
Mitochondrial biogenesis, together with fission and fusion, is essential for the maintenance of mitochondrial homeostasis, and they play crucial roles in neuronal development and synaptic function. In fission, mitochondria are divided into smaller organelles, leading to the generation of new mitochondria, aiding in the distribution of energy throughout the neurons, and isolating and removing damaged or dysfunctional mitochondria through autophagy (mitophagy). Fusion, on the other hand, involves the merging of mitochondria, which facilitates the exchange of their components and assists in the efficient distribution of energy [13]. The balance between mitochondrial fission and fusion is regulated by evolutionarily conserved proteins. Mitochondria have two closely apposed boundary membranes. Fusion of adjacent mitochondria involves the fusion of the outer mitochondrial membrane (OMM) and is regulated by the mitofusin 1–2 (MFN1 and MFN2) proteins, and that of the inner mitochondrial membrane (IMM) is regulated by the Optic atrophy 1 (Opa1) protein [14,15]. MFN2 is also involved in ER–mitochondria communication for calcium homeostasis [16], while MFN1 partners with Opa1 [17,18]. The dynamin-related protein 1 (Drp1) protein, cycling between the cytosol and the mitochondrial outer membrane, and the fission 1 protein (Fis1) are involved in mitochondrial fission [15]. Drp1’s fission-promoting activity is controlled by various post-translational modifications in its variable domain, such as phosphorylation, SUMOylation, ubiquitination, and S-nitrosylation [19]. The crucial role of mitochondrial fusion for neuronal function was demonstrated, for example in Purkinje cells (PCs), in which MFN2 deficiency led to impaired respiratory complexes’ activity and inner membrane defects, causing cerebellar dysfunction. MFN1 and MFN2 overexpression restored PC viability [20]. Reduction of MFN2 in mitochondria also affects their movement and distribution in axons, leading to abnormal mitochondrial clustering in cell bodies and proximal axons [21]. Opa1 undergoes alternative splicing and proteolytic processing which results in multiple variants that can play different roles in fusion, cristae integrity, and maintaining mitochondrial DNA (mtDNA). Inhibition of Opa1-mediated mitochondrial fusion reduced synaptic marker proteins, decreased synapse numbers, and affected dendritic growth [22,23]. Drp1 is enriched at neuronal terminals and is essential for synapse formation and synaptic sprouting as discussed previously [24]. It was also shown that the inhibition of Drp1 is neuroprotective under toxic conditions such as glutamate toxicity or oxygen–glucose deprivation and ischemic brain damage in neuronal cultures and the brain, respectively [25]. Studies suggest an additional mitochondrial outer membrane protein the ganglioside-induced differentiation-associated protein 1(GDAP1), preferentially expressed in neurons, that, together with Drp1, MFN1–2, and Opa1, is essential for mitochondrial fusion/fission dynamics [26].
Mitochondrial biogenesis is an additional process that regulates mitochondrial homeostasis and is stimulated by cell energy demands and by mitochondrial dysfunction. One key downstream effector of nicotinamide adenine dinucleotide (NAD+) is the NAD+-dependent histone deacetylase Sirtuin 1 (SIRT1), which regulates the transcription factor peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α), a master regulator of mitochondrial biogenesis. Another PGC-1α activator affected by increased NAD+ levels is AMP-activated protein kinase (AMPK), which, in concert with SIRT1, improves metabolic fitness. PGC-1α is an upstream inducer of mitochondrial genes by positively affecting the activity of some hormone nuclear receptors [Peroxisome proliferator-activated receptor gamma (PPARγ) and estrogen-related receptor alpha (ERRα)], which regulate multiple pathways involved in cellular energy metabolism within and outside mitochondria, and of nuclear transcription factors 1 and 2 (NRF-1 and 2). Activation of NRF-1 and 2 leads to the transcription of nuclear-encoded respiratory chain components and mitochondrial transcription factor A (Tfam), thus promoting the synthesis of mitochondrial proteins, mtDNA replication, and transcription (Figure 1). PGC-1α also mediates the crosstalk between fission/fusion processes and mitochondrial biogenesis [20,27]. Although highly important for neuronal homeostasis, development, and survival there is limited knowledge on the mechanism evoking mitochondrial biogenesis in neuron endings away from the cell body. One suggestion is that additional regulatory mechanisms have to be involved for axon-localized mitochondrial biogenesis to occur, such as mechanisms that utilize axonal-localized mRNAs translated into mitochondrial proteins and form contacts between mitochondria and endosome/lysosome platforms [28,29]. Although unlikely, there is evidence that mitochondrial biogenesis can occur in the cell body with the new mitochondria transported by an anterograde mechanism while the damaged mitochondria are moved by retrograde transport and then subjected to mitophagy. Mitochondrial biogenesis is usually assessed by mtDNA content and levels of PGC-1α and its affected transcripts. Variations in mitochondrial biogenesis were shown to be associated with differences in schizophrenia penetrance in a 22q11.2 deletion syndrome [30]. In AD for example, there are reports on enhanced and reduced mitochondrial biogenesis, suggesting that neurons in different stages of degeneration or different brain areas show different patterns of biogenesis [31,32,33,34]. In sum, mitochondrial fission and fusion together with mitochondrial biogenesis affect cellular signaling pathways, gene expression, and cell fate in the complex landscape of neuronal development, synaptogenesis, and astrocyte-neuronal concerted regulation of synaptic plasticity [15,26,35,36].
3. Mitochondria and CNS Development
The mature brain, although constituting just 2–3% of the total body weight, consumes nearly 20% of the body’s basal metabolic rate; while early in development, the human brain consumes more than 40% of the body’s basal energy metabolism [37,38,39]. Glucose is the main energy substrate in the brain [40]. Its metabolism, through glycolysis, the non-oxidative breakdown of glucose to pyruvate and lactate in the cytoplasm, and through the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OxPhos) system in the mitochondria, produces the high-energy molecule ATP. Hence, it is not surprising that mitochondria play an integral role in neuronal development, migration, and synaptic function during CNS development. Neurulation is the series of processes transforming the neural plate into the neural tube, requires organelle biogenesis, including ribosomes and mitochondria, which demands the synthesis of nucleic acid, epigenetic modifications, and changes in precursor cell transcriptomes [41]. During progressive differentiation, both nucleic acid synthesis and epigenetic modifications rely on de novo purine synthesis and raw material generation for methyltransferases. These processes are partially dependent on mitochondrial folate-mediated one-carbon metabolism (FMOCM) (Figure 1). An additional important enzyme involved in the de novo synthesis of pyrimidine nucleotides and is important for neuronal development is dihydroorotate dehydrogenase (DHODH), located in the inner mitochondrial membrane [42,43]. As the neural tube closes, the expression of folate pathway genes is upregulated [44]. It was reported that low levels of dietary folate and mutations in genes encoding FMOCM enzymes are associated with neural tube closure defects [45,46]. Such mutations include those in Slc25a32, which encodes for the mitochondrial folate transporter/carrier (MFTC) [47]. Mitochondrial FMOCM, therefore, plays an essential role in ensuring that mitochondria supply the substantial demands for nucleic acids during this early period of neural development. The role of mitochondria in nuclear DNA methylation is not only linked to the folate/methionine cycle activities but also to mtDNA haplotypes. This has been demonstrated by the different degrees of nuclear DNA methylation in tissues and cells containing identical nuclei. According to recent findings, epigenetic histone methylation is also regulated by mtDNA heteroplasmy, [48,49].
At the cellular level, dividing neuronal stem cells (NSCs) in the neuronal tube differentiate into neuronal progenitor cells (NPCs), which either proliferate or further differentiate into neurons or glial cells that constitute the functioning neural network. This process is termed developmental neurogenesis. The determination of NPCs to undergo either proliferation or further differentiation is controlled by both mitochondrial biogenesis and bioenergetics. During the switch from glycolytic metabolism to mitochondrial OxPhos, NSCs become NPCs and subsequently differentiate into various neuronal cell types [50,51,52]. Mitochondrial structure and network dynamics are also crucial for neurogenesis. As NSCs differentiate, the morphology and dynamics of mitochondria undergo significant alterations [53,54]. In the initial state of mouse NSCs, mitochondria appear simple, with a round shape and few cristae. However, during differentiation, they transform into a more complex tubular network consisting of elongated mitochondria with abundant cristae and a dense matrix [55,56,57].
It was further demonstrated that the manipulation of the fission/fusion dynamics of the mitochondrial network, by interfering with the fission and fusion proteins Drp1 and MFN1/2, respectively, impairs neurogenesis and differentiation [58,59]. We have shown that deficits in mitochondrial function and the fusion protein Opa1 were associated with impaired differentiation of dopaminergic and glutamatergic neurons from schizophrenia-derived iPSCs [60]. Neuronal polarity is fundamental for the construction of neuronal networks and circuitry. It was reported that depletion of mitochondria prevented axon formation, while interference with its Ca2+ buffering impaired the formation of neuronal polarity [61].
Recently, it was demonstrated that mitochondrial metabolism sets the tempo of neuronal development. To our opinion, this elegant study is a fundamental proof for a mitochondrial role in neurodevelopment. In human and mouse cortical neurons which were xenotransplanted into the mouse brain developed along their species-specific timeline, suggesting that species-specific developmental timing is controlled by cell-intrinsic mechanisms. Furthermore, mitochondria contributed to species-specific developmental rates, as increasing mitochondrial metabolism in cultured human neurons enhanced neuronal maturation, while decreasing mitochondrial metabolism of mouse neurons reduced their maturation rate [62].
Understanding the underlying mechanisms of mitochondrial cross-talk with the cell’s intrinsic mechanism is essentially unknown, yet could be crucial in assessing the involvement of mitochondria in neurodevelopmental and neurodegenerative diseases.
4. Mitochondrial Migration in Neurons
Neurons rely on mitochondria for their growth, survival, and functionality; consequently, it is imperative for neurons to exhibit specialized mechanisms facilitating the distribution of mitochondria to distal synapses where energy demand is notably high. The redistribution of mitochondria is subjected to modifications induced by activity-dependent remodeling of both axons and synapses. Mitochondrial transport in neurons is a dynamic and complex process, it involves both anterograde and retrograde movement along microtubules [63]. The anterograde movement depends on a kinesin motor protein, specifically kinesin 1. Kinesins (KIFs) bind to mitochondrial surfaces and use ATP to move them to microtubule ends, which are usually axon terminals. Microtubules are polarized structures that control the direction of transport. The plus ends of microtubules point towards axon terminals, while minus ends are typically located near cell bodies. The mitochondrial retrograde transport from axon terminals to cell bodies is facilitated by a dynein motor protein. Dynein moves along microtubules in the opposite direction of KIFs, utilizing ATP for energy. Thus, the retrograde transport facilitates mitochondrial recycling to maintain their health [31]. Mitochondria attach to the motors through their respective motor adaptor proteins and mitochondrial receptors. In response to increased action potential firing rates or glutamate receptor activation, the complex of KIF5 (member of kinesin-1 family)–Milton (a motor adaptor protein)–MIRO (mitochondria rho family GTPase) mediates the movements of mitochondria along axons and dendrites. Elevated Ca2+ concentrations suppresses mitochondrial mobility through a MIRO Ca2+-sensing pathway [64,65]. Axonal mitochondria are anchored by syntaphilin (SNPH), a neuron-specific axonal protein that associates with the mitochondrial outer membrane. Studies showed that SNPH deletion significantly increased the percentage of mobile axonal mitochondria [31]. Interestingly, a study in Caenorhabditis elegans showed that during regeneration, mitochondria translocate into injured axons to increase the average mitochondrial density upon injury, whereas axons that fail to increase the mitochondrial density exhibit poor regeneration [66]. In mice, enhancing axonal mitochondrial transport by deletion of SNPH, improved the regeneration process [67,68]. In developing neurons, SNPH expression is reduced, while during maturation its expression is enhanced, suggesting a high mitochondrial transport demand during development [69]. Armcx1, a mammalian-specific protein that encodes a mitochondria-localized protein, also regulates mitochondrial movement and thereby neuronal health. Hence, its overexpression enhanced mitochondrial transport in axons and the regeneration and neuronal repair of axon outgrowth in retinal ganglionic cells and in cultured cortical neurons, respectively [70]. In dendrites, experimental reduction of mitochondrial number resulted in a loss of synapses and dendritic spines, whereas increasing mitochondrial content or activity increased spine number and synapse plasticity [71]. In all, the ability of mitochondria to move forwards and backwards along axons and dendrites in response to neuronal local-specific demands is essential for neuronal function, development, and repair, as well as for mitochondrial quality control.
5. Mitochondria and the Synapse
Mitochondria are involved in synaptic functions through energy and ATP production, as constant release of neurotransmitters and maintenance of ion gradients requires energy. In addition, Ca2+, which is firmly regulated by mitochondria within the presynaptic terminal, is a key mediator in neurotransmitter release. The TCA cycle is involved in the metabolism of the main excitatory and inhibitory neurotransmitters, glutamate and GABA, respectively (Figure 1). Notably, mitochondria and the TCA cycle act differently in astrocytes and neurons [72,73]. For example, GABA conversion to glutamine occurs through the astrocytic TCA cycle, since the enzymes glutamine synthetase and pyruvate carboxylase are expressed only in astrocytes, and not in neurons [74]. In neurons, the TCA cycle is involved in converting glucose to glutamate in presynaptic neurons [71,75]. Disorders linked to GABAergic transmissions, such as epilepsy, show compromised synaptic GABA concentrations. Notably, antiepileptic drugs like tiagabine hinder the astrocytic uptake of GABA, leading to an increase in synaptic GABA concentrations [76], suggesting that the astrocyte-specific mitochondrial TCA cycle becomes hyperactive in GABA-impaired disorders. This hyperactivity in mitochondrial energy supply could potentially disrupt the balance of energy distribution between neuronal and astrocytic TCA cycles.
Synaptic activity can generate reactive oxygen species (ROS), which are potentially harmful molecules for the cell. Mitochondria help to buffer and detoxify ROS to protect the delicate structures within the synapse from oxidative damage, while dysfunctional mitochondria produce ROS. We have shown that mitochondrial dysfunction is associated with impaired neuronal sprouting, synaptic connectivity, and neurotransmission in SZ-iPSCs-derived dopaminergic and glutamatergic neurons [60]. In addition, an experimental reduction of mitochondrial number in dendrites resulted in a loss of synapses and dendritic spines, whereas increasing mitochondrial content or activity increased spine number and synapse plasticity [77]. Overall, impairment in mitochondrial function and morphology can influence synaptic transmission and intricate neuron–astrocyte connections, which can lead to either synapse malfunction or neurodegeneration.
6. Mitochondria in Behavior
The impact of mitochondrial dysfunction on behavioral impairments has gathered significant attention in the field of neuroscience. As discussed in the previous sections, mitochondrial dysfunction can lead to disruptions in synaptic transmission, impaired neuronal plasticity, and increased susceptibility to neuroinflammation. These factors collectively contribute to the manifestation of various behavioral impairments, including cognitive decline, motor deficits, and mood disorders [12,59].
Cognitive impairment is a common behavioral abnormality in several neurological disorders, such as Alzheimer’s disease (AD), fronto-temporal dementia, and schizophrenia. Ample evidence implicates a role for mitochondrial dysfunction in cognitive impairments, primarily in AD and its progression. A role for synaptic mitochondria and tau proteins in stress-induced cognitive impairment was shown [78]. Thus, long-term stress caused tau-dependent atrophy in dendrites and spines in the prefrontal cortex, associated with alterations in mitochondrial OxPhos, transport, and localization, as well as cognitive impairments. A large-scale proteomic analysis of the human brain identified mitochondrial proteins that were associated with cognitive stability in aging [79]. Numerous studies have linked mitochondrial ROS production, which is mainly induced by the inhibition of the OxPhos system, to age-related and AD cognitive decline, specifically as treatment with mitochondria-targeted antioxidants tends to improve cognition in experimental models and humans (for a reviews see for example [80,81]). In an elegant study using pharmacological means, a causal link was shown between anxiety and social behavior under stress and impaired mitochondrial respiration, ATP deficits, and enhanced ROS production in the nucleus accumbens [82]. In the honeybee, brain mitochondrial function has been found to change with aggressive social interactions [83,84,85]. In humans, age-related increases in aggressive tendency was associated with increased baseline brain mitochondrial respiration [86,87]. Additionally, diet restriction and ketone body feeding, which enhance mammalian brain mitochondrial function, can also increase aggression, suggesting that mitochondrial function plays a role in modulating aggression-related neuronal signaling [84]. Interestingly, mitochondrial dysfunction has been observed in various psychiatric primary behavioral disorders including schizophrenia, bipolar disorder, major depressive disorder, and autism [88,89,90]. Almost all studies show an association between mitochondria and behavior, while the causative link is missing.
7. Mitochondria and CNS Diseases
Forming such an important cellular hub and being essential players in cellular survival and death, it is comprehensible that mitochondria are strongly involved in pathological processes leading to numerous general and CNS diseases. Mitochondrial malfunction can be attributed to multiple mitochondrial factors including their enzymes’ function and their mtDNA. Mitochondria in cells are not a homogenous population. A recent study showed, for the first time, two distinct populations of mitochondria in rat liver, cytoplasmic mitochondria and lipid droplet-associated mitochondria. The lipid droplet mitochondria were more efficient in fatty acid oxidation and exhibited specific molecular markers such as carnitine palmitoyl transferase (CPT1), phosphorylated acetyl- CoA carboxylase (ACC), and MFN2, while cytoplasmic mitochondria had higher respiration capacity. Interestingly, mitochondria associated with lipid droplets in a non-alcoholic fatty liver disease (NAFLD) rat model showed compromised fatty acid oxidation, highlighting the role of droplet-associated mitochondria in NAFLD and the significance of functional separation of mitochondria to treat NAFLD [91] and possibly other disorders.
The essential role of mitochondria in neurons has been described above. Any distortion in their activity can lead to changes in synaptic plasticity and neuronal network connectivity, eventually resulting in behavioral changes and neurodegeneration. Astrocytes have a crucial role in supporting neurons, and changes in their mitochondrial function are linked to the maintenance of brain health. Astrocytes typically have low activity of mitochondrial OxPhos as compared to neurons. Nevertheless, it was demonstrated that their OxPhos is essential for degrading fatty acids and maintaining lipid balance in the brain. Astrocytic OxPhos malfunctions can cause accumulation of lipid droplets and elevated acetyl-CoA levels, inducing astrocyte reactivity. The astrocytes’ lipid-centric mechanism was shown to stimulate neuronal fatty acid oxidation and oxidative stress, to activate microglia, and hinder the synthesis of essential lipids for myelin replenishment. As a consequence synaptic loss, demyelination, and neuroinflammation were observed, leading to cognitive decline and neurodegeneration relevant to AD and maybe also to multiple sclerosis (MS) [92]. Ample evidence from genetic, transcriptomic, and proteomic studies supports an association between mitochondrial enzyme deficiencies, specifically those of the OxPhos, and the pathophysiology of CNS disorders, including mental illness, such as schizophrenia, major depression, and bipolar disorders as well as neurodegenerative disorders such as Parkinson’s disease (PD) and AD. For example, in schizophrenia, major depression, and bipolar disorder, a deficiency in the first complex of the OxPhos system, NADH/ubiquinone oxidoreductase (complex I) is repeatedly reported, while in PD and AD, as well as in aging, a deficit is observed in both complex I and the fourth complex, cytochrome-c-oxidase [93,94,95,96,97,98,99,100,101,102].
Mitochondrial TCA cycle intermediates have an essential role in the synthesis of various biomolecules and signaling molecules, which are involved in chromatin modifications, DNA methylation, immunity, and hypoxic response (for a review see [103]). Hence, TCA cycle enzymes have been associated with various CNS dysfunctions. For example, in schizophrenia, enzymes of the first half of the TCA cycle (aconitase, alpha-ketoglutarate dehydrogenase complex, and succinyl coenzyme A synthetase) showed lower activity, while those in the second half (succinate dehydrogenase and malate dehydrogenase) showed higher activities, probably compensating for the lower activity levels of the first half of the cycle [104]. In AD, deficits in brain TCA cycle enzymes, specifically pyruvate dehydrogenase subunit beta (PDHB), succinate-CoA ligase [ADP-Forming] Subunit Beta (SUCLA2), and malate dehydrogenase 1 (MDH1) were observed [105]. Finally, patients with inherited deficiencies in TCA enzymes such as fumarate hydratase (FH) and α-ketoglutarate dehydrogenase (α-KGDH) present, among other symptoms, progressive and severe encephalopathy, psychiatric and pyramidal symptoms, and developmental delay [106,107].
mtDNA also contributes to CNS diseases, mainly due to the strong inflammation associated with increased circulating cell-free (cf)-mtDNA levels (Figure 1). Similar to cf-nuclear DNA [108], the role of cf-mtDNA is increasingly recognized in immune-mediated systemic and central inflammatory diseases [109,110]. The measurement of cf-mtDNA concentration emerges as a potential biomarker for acute inflammatory stress. Damaged mtDNA released into the cytosol activates inflammatory pathways, including the inflammasome and NF-κB, contributing to inflammation at various levels [111,112]. Notably, elevated levels of mtDNA are observed in plasma in CNS disorders with strong inflammatory responses, such as relapsing–remitting multiple sclerosis [113,114]. An increase in cf-mtDNA may reflect early inflammatory activity, leading to mitochondrial damage, neural loss, and brain atrophy and its change has been suggested as a predictor for treatment outcome [115,116]. Interestingly, a reduction in melatonin, an antioxidant produced by neurons and shown to be involved in mitochondrial homeostasis, can lead to disruption of mitochondria, the release of mtDNA, and activation of a cytosolic inflammatory response in neurons, by triggering the cGAS/STING/IRF3 pathway [117]. In PD, it has been suggested that mitochondrial dysfunction can lead to the release of mitochondrial damage-associated molecular patterns (mtDAMPs), triggering neuroinflammation through microglial immune receptors. Conversely, inflammatory molecules released by the glial cells can further damage mitochondrial function, triggering a vicious circle that can lead to neurodegeneration [118,119]. In mental disorders such as major depression and schizophrenia, higher levels of cf-mtDNA were associated with symptoms of depression and schizophrenia. Concomitantly, treatment with certain mood stabilizers (lamotrigine, valproic acid, or lithium) was associated with lower cf-mtDNA [120,121,122,123]. In addition to alterations in cf-mtDNA, changes in the mtDNA genome were also shown to be associated with CNS disorders. For example, SNPs in two control regions in the mtDNA (T16519C and T195C) as well as in mtDNA-encoded cytochrome B (CYTB), which protein is a component of the ubiquinol–cytochrome c reductase complex (complex III), were associated with schizophrenia and bipolar disorders. A12308G was associated with psychosis in both disorders [124]. Several point mtDNA mutation deletions and methylation, as well as changes in mtDNA copy numbers, have been associated with neurodegenerative disorders including PD and AD, amyotrophic lateral sclerosis, and Huntington’s disease (for a review see [125]). In monozygotic twins discordant for schizophrenia, a discordance in mtDNA copy number estimates was observed suggesting post-zygotic mtDNA changes that may contribute to the discordance in the disease between monozygotic twins [126]. mtDNA copy number in peripheral blood cells has been suggested as a potential biomarker as it was reduced in PD, increased in attention-deficit hyperactivity disorder (ADHD), and associated with psychosis severity and antipsychotic treatment in schizophrenia [127,128,129]. Taken together, all these findings corroborate that mitochondrial multifaceted dysfunction contributes to the pathogenesis of CNS disorders.
8. Mitochondrial Intercellular Transfer and Uptake Mechanism of Transplanted Mitochondria
Mitochondria transfer between cells to reach a homeostatic bioenergetic state in nearby cells or to support the energy demands of injured cells. Studies have shown that intercellular mitochondrial transfer occurs through various contact modes between cells, including gap junctions, cell fusion, and tunneling nanotubes (TNTs) forming between heterogeneous cells. The specific transfer method can vary depending on the cell type involved. TNTs are filled with actin bundles that enable cargo transfer, while microtubules are essential to allow the transfer of organelles as big as mitochondria [130,131,132,133]. Mitochondria, traditionally known as intercellular organelles, were shown to be secreted into the extracellular space, probably via extracellular vesicles (EVs), under both physiological and pathological conditions. These EVs can be taken up by cells and play diverse roles, regulating metabolism, provoking immune response, and inducing cell differentiation in the target cells [134,135]. For example, in mice it was shown that labeled mitochondria transferred from infused bone marrow stem cells to injured lung cells, apparently through the formation of nanotubes and EVs [136,137]. In the CNS, it has been shown that mitochondria can be transferred from neurons to adjacent astrocytes to be degraded or recycled [138]. Astrocytes, on the other hand, were shown to release functional mitochondria that enter nearby neurons by a calcium-dependent mechanism involving CD38 and cyclic-ADP-ribose signaling. These astrocyte-derived mitochondria rescued the nearby neurons in mice subjected to focal cerebral ischemia [139]. Mitochondrial transfer between cells has been studied as a potential therapeutic tool, mostly using mesenchymal stromal cells (MSCs) as donors of healthy mitochondria, in various disease models including respiratory, cardiac, and neurological diseases [68,136,140,141,142,143,144].
An additional route to affect the intercellular mitochondrial state is the transplantation of isolated mitochondria. As early as 1974, Knowles showed that transplantation of isolated mitochondria to Paramecium aurelia can transfer erythromycin-resistance to sensitive cells [145]. In 1982, Clark and Shay showed in mammalian cells that chloramphenicol resistance could be transferred to sensitive cells by mitochondrial transplantation [146]. Thereafter, ample evidence substantiated the ability of exogenous mitochondria to enter cells and improve their bioenergetic state. Several mechanisms have been suggested for the penetration of isolated mitochondria into cells including caveolae-dependent clathrin-dependent endocytosis, actin-meditated endocytosis, and micropinocytosis [147,148,149,150,151]. However, in contrast to intracellular transfer and export/uptake of mitochondria containing EVs, the mechanism by which isolated extracellular mitochondria enter the cell is still obscure. Regardless, transplanted exogenous mitochondria can fuse with the recipient cell’s endogenous mitochondrial network, which in cells results in enhanced cellular respiration and ATP content [152,153]. Attempts have been carried out to increase the penetration efficiency of the exogenous mitochondria. For example, it was shown that mitochondria packaged in cell membrane lipid rafts were taken up by cultured cells twice as much as unpackaged mitochondria. In another study, labeling mitochondria with Pep-1, an amphipathic peptide carrier and a member of the cell-penetrating peptide family, significantly increased the efficiency of mitochondrial penetration in pheochromocytoma (PC12) cells and mouse brains treated with 6-hydroxydopamine (6-OHDA), a PD-inducing agent, and in a mitochondrial disease model of myoclonic epilepsy with ragged red fibers. In both models, mitochondrial function was improved [152,154]. The question remains whether artificially increasing mitochondrial penetration is beneficial in all cell types and physiological states, as cellular control on uptake is bypassed.
9. Mitochondrial Transplantation in CNS Disorder Models
During the past decade, studies examining the therapeutic potential of transplantation of isolated extracellular mitochondria in a variety of general and CNS disease models have thrived (for reviews see [134,155,156,157] and Table 1). The beneficial effects of exogenous mitochondrial transplantation have been demonstrated in diverse pathological models including cardiac, lung, liver, and CNS pathologies [158,159,160,161,162,163,164,165]. In humans, a few clinical trials showed beneficial effects of autologous mitochondrial transplantation, but the follow-up period was relatively short. In infants who required ECMO support for ischemia–reperfusion-associated myocardial dysfunction, autologous mitochondrial transplantation led to an improvement in ventricular function and release from ECMO support. However, there is no information on how stable the improvement was [166]. Another human study was performed on six children with single large-scale mitochondrial DNA (mtDNA) de novo deletion syndromes (SLSMDs), a rare and severe multisystemic disease. Mitochondrial augmentation therapy, in which the maternal mitochondria, mostly similar to those of the recipient, were transplanted into the patients’ enriched hematopoietic cells following leukapheresis, was employed. Following the transplantation procedure, partial and limited improvement in mitochondrial number and clinical symptoms was observed during 6–12 months of follow-up [167]. In women with recurrent pregnancy failures, transplantation of autologous mitochondria to mature human oocytes with sperm at the time of intracytoplasmic sperm injection resulted in a significant improvement in the ratio of good-quality embryos and healthy normal babies [168]. All of these studies used autologous or allogeneic maternal-derived mitochondria for transplantation rather than allogeneic mitochondria to reduce immune response and avoid heteroplasmy.
Several studies have transplanted exogenous mitochondria in various CNS experimental models. In 6-OHDA Parkinson’s disease rat model, transplantation of exogenous allogeneic and xenogeneic mitochondria coupled with Pep-1 into the medial forebrain bundle (MFB) reduced loss of dopaminergic neurons in the substantia nigra three months later, suggesting that the effect of the transplanted mitochondria spread beyond the injection site, probably due to the characteristics of the MFB, being a neural pathway containing fibers. The restoration of the dopaminergic neurons was associated with enhanced mitochondrial functions, reduced neuroinflammation, and enhanced locomotive activity [155]. In this study, no significant difference was observed between Pep-1-coupled xenogeneic and allogeneic mitochondria-induced effects. The same group showed similar beneficial bioenergetical and behavioral effects at three to four weeks following repeated chronic (once a week for three months) intranasal injection of Pep-1-labeled mitochondria [165]. Another study used the MPTP null mouse model of PD and intravenously injected them with exogenous mitochondria. The GFP-labeled transplanted mitochondria were distributed in various organs including the liver, kidney, muscle, and brain. MPTP mice systemically injected with mitochondria showed improved locomotion, reduced ROS generation, and restored ATP levels and complex I activity. Systemically injected mitochondria in healthy mice did not affect ATP levels nor spontaneous locomotion but significantly increased latency to immobility in the forced swimming test [169]. Both studies showed improvements in cell survival and mitochondrial functions in cell cultures treated with the relevant toxins. Injection of exogenous mitochondria into the tail vein was also performed in a mouse model of AD, produced by intracerebroventricular injection with amyloid-beta 1–42 peptide. One to two weeks after the intravenous injections of fresh human-isolated mitochondria, AD mice exhibited significantly improved cognitive performance. Furthermore, there was a notable reduction in neuronal loss and gliosis in the hippocampus and increased activities of citrate synthase and cytochrome c oxidase in treated AD mice compared to non-treated AD mice [162]. Acute effects of intravenous repeated transplantation of mitochondria isolated from young mice into aged mice included improved mitochondrial bioenergetics and reduced redox state, associated with ameliorated learning and motor functions in the aged mice [170]. The protective effect of exogenous mitochondrial transplantation was also studied in experimental spinal cord injury (SCI). One study reported that up to 7 days post-transplantation, mitochondria were observed in multiple resident cell types but not in neurons. The other study detected the transplanted mitochondria up to 28 days post-transplantation in the vicinity of the lesion. One day after transplantation, a partial restoration of mitochondrial respiration as well as amelioration of mitochondrial fragmentation and cellular apoptosis were observed. Partial functional protection, assessed by tissue paring and recovery of sensory and motor function, was observed four weeks after transplantation; however, it faded after 6 weeks of follow-up [171,172]. In traumatic brain injury, which caused mitochondrial impairments, anxiety and cognitive deficits, transplantation of allogeneic liver-isolated mitochondria restored astrocytic brain-derived neurotrophic factor (BDNF) levels and the behavioral deficits [173].
The past decade has witnessed an abundance of studies focusing on mitochondrial abnormalities in several mental disorders including major depression and schizophrenia. A wide array of methodologies ranging from imaging through genetic, biochemical, and molecular to histological and structural techniques were used to reveal multifaceted mitochondrial dysfunction in mental disorders, specifically in schizophrenia [88,174,175]. Hence, mitochondrial transplantation effects were also assessed in experimental models of mental disorders. In a lipopolysaccharide-induced mouse model of inflammation-induced depression, intravenous injection of exogenous mitochondria acutely reduced depressive-like behaviors assessed by forced swim, tail suspension, and sucrose preference tests. This was associated with an acute reduction in astrocyte and microglia activation and cytokines levels, higher levels of BDNF transcripts and neurogenesis, and restored mitochondrial dysfunction measured by ATP and ROS production in mouse hippocampi [176]. We have studied the effect of exogenous allogeneic mitochondrial transplantation in schizophrenia patient-derived iPSCs (SZ-iPSCs) and in the maternal immune activation Poly I:C rat model of schizophrenia. Transplantation of mitochondria into SZ-iPSCs restored mitochondrial deficits including mitochondria respiration, ΔΨm, mitochondrial network dynamics, and transcript levels of specific subunits of complex I and of OPA1. Concomitantly, an enhanced efficiency of SZ-iPSCs differentiating into functional dopaminergic and glutamatergic neurons was observed. In the rat model of SZ, intra-medial prefrontal cortex (mPFC) single injection of mitochondria, in adolescent rats (34 days old), restored mitochondrial impairments and neuronal outgrowth and activity. assessed by monoamines’ transmission. in adulthood (100–120 days old). Proteomics analysis of the mPFC showed an association between the beneficial neuronal and mitochondrial effects and improved metabolic and neuronal development and plasticity pathways. Finally, mitochondrial transplantation in Poly I:C rats restored schizophrenia-related behavioral deficits such as attentional deficit and spontaneous locomotor activity in a novel environment. The behavioral changes showed a significant correlation with changes in monoamine and neuronal structural alterations. Unlike the intra-MFB injection in the PD model, our data suggest a localized effect of the transplanted mitochondria. Unexpectedly, a similar injection protocol to healthy rats induced detrimental effects in all parameters mentioned above, including behavioral, bioenergetical, and neuronal-related features. These data emphasize the importance of the bioenergetical and physiological states of the recipient in the outcome of mitochondrial transplantation. Furthermore, the opposite effects induced by mitochondrial transplantation in the schizophrenia model and healthy rats advocate for a causal link between the mitochondria and behavior, neuronal activity and plasticity [163,164].


Table 1.
Research reports on mitochondrial transplantation in CNS disease models.
Table 1.
Research reports on mitochondrial transplantation in CNS disease models.
| Disease | Species/Applied Model | Source of Mitochondria | Route of Transplantation | Ref. |
|---|---|---|---|---|
| Parkinson’s disease | Rat | Allogeneic/xenogeneic Pep-1-labeled mitochondria | Injection into the medial forebrain bundle | [154] |
| Parkinson’s disease | Mouse | Human hepatoma cells | Intravenous injection | [169] |
| Parkinson’s disease | Rat | Liver allogeneic mitochondria conjugated with Pep-1 | Intranasal infusion | [165] |
| Alzheimer’s disease | Mouse | HeLa cell-derived mitochondria | Tail intravenous injection | [162,177] |
| Cognitively impaired aged mice | Mouse | Liver allogeneic mitochondria isolated from young mice | Injected into the hippocampus of aged mice | [178] |
| Diabetes-associated cognitively impaired mice | Mouse | Platelet-derived mitochondria | Intracerebroventricular injection | [179] |
| Chronic mild stressed—aged rats | Rat | Brain-derived mitochondria from young rats | Injected intracerebroventricularly in aged rats | [180] |
| Aging | Mouse | Liver-derived allogeneic mitochondria from young rats | Intravenous injection | [170] |
| CNS injury | Mouse | Cerebral cortex-derived allogeneic mitochondria whose DJ1 protein was modified with O-GlcNAcylation | Intraventricular injection | [181] |
| Traumatic brain injury | Mouse | Liver/muscle-derived autologous mitochondria | Injected into the cerebral cortex | [173] |
| Brain stroke | Mouse | Bone marrow mesenchymal stem cell-derived allogenic mitochondria | Intranasal administration | [182] |
| Schizophrenia | Rat | Rat brain-derived allogeneic mitochondria | Bilateral injection of into medial prefrontal cortex | [163,164] |
| Lipopolysaccharide (LPS)-induced model of depression | Mouse | Hippocampus-derived allogeneic mitochondria | Intravenous injection | [176] |
| Spinal cord injury | Rat | Soleus muscle-derived allogeneic mitochondria | Injected into spinal cord | [171] |
| Spinal cord ischemia | Rat | Soleus muscle-derived allogeneic mitochondria | Intravenous transplantation via the jugular vein | [183] |
| Spinal cord injury | Rat | Soleus muscle-derived allogenic mitochondria | Injection into spinal cord via intraparenchymal route | [172] |
| Neuromuscular limb injury | Mouse | NR | Systemic injection | [184] |
| Lower limb ischemia–reperfusion injury | Mouse | Human umbilical cord mesenchymal stem cell-derived mitochondria | Gastrocnemius muscle injection | [185] |
| Optic nerve injury | Rat | Liver-derived allogeneic mitochondria | Intravitreal injections | [186] |
| Mitochondria transplantation in humans | ||||
| Recurrent pregnancy failure cases | Human | Ovarian cortical tissue-derived autologous mitochondria | Mitochondria transferred to human oocytes, intracytoplasmic sperm injection | [168] |
| Ischemia–reperfusion-associated myocardial dysfunction | Human (infants) | Rectus abdominis muscle-derived autologous mitochondria | Injected into the myocardium affected by ischemia–reperfusion | [166] |
| Single large-scale mitochondrial DNA (mtDNA) de novo deletion syndromes (SLSMDs) | Human (infants) | Maternal PBMC-derived mitochondria transplanted into the patients’ enriched CD34+ cells after leukapheresis | Transfused into patients | [167] |
NR—not reported.
10. Challenges of Mitochondrial Transplantation
The evidence described above supports the potential of mitochondrial transplantation to become a strategy for reducing behavioral and cellular injuries associated with mitochondrial dysfunction in general and CNS disorders. However, there are still concerns that need to be addressed both at the level of basic mechanisms and at the level of reverse-translational or bedside-to-benchtop research, which are summarized in Figure 2.
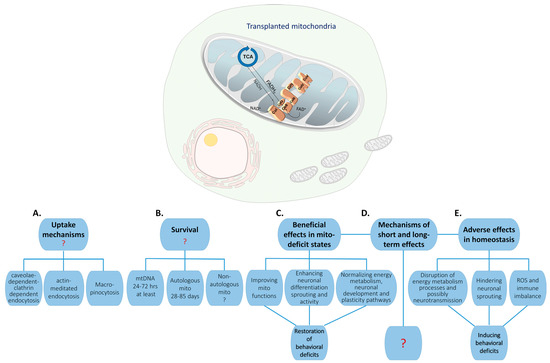
Figure 2.
Challenges in understanding the fate and the mechanism of action of transplanted mitochondria in the CNS: Transplanted mitochondria (mito), whether autologous or allogeneic, are taken up by cells. Several uptake mechanisms have been suggested (A), but none has been systematically investigated. The survival rate of the transplanted mitochondria (B) is still obscure as it depends on many factors including cell type, the methodology used to measure survival, mitochondrial proteins or mtDNA, and the source of the mitochondria, i.e., whether they are autologous or non-autologous. Regardless, it is accepted that the survival of the transplanted mitochondria has a shorter half-life than their induced long-term morphological, molecular, and behavioral effects have (C,D). Notably, it was shown that the changes induced by the transplanted mitochondria into the CNS or iPSCs depend on the endogenous mitochondrial bioenergetics state and probably on the physiological state of the recipient ((C) vs. (D)). The mechanisms controlling the long-term changes, which ultimately lead to behavioral alterations, are still an enigma (E).
The first relates to the ability of mitochondria to penetrate into organs’ cells and to cross the blood–brain barrier. As discussed earlier, several uptake mechanisms have been proposed, although none has clearly described the molecular mechanism by which mitochondria are taken up or how they escape the endosomes or macrophagosomes. Another related question is how mitochondria retain their integrity. The answer to this question is still an enigma. The integrity of the exogenous mitochondria is of special concern in blood, where Ca2+ concentrations are around 1.8 mM as opposed to 100 and 200 nM in the cytosol. Such high Ca2+ concentrations are known to induce the opening of permeability transition pores and thereby blunting mitochondrial membrane potential eventually leading to mitochondrial swelling and destruction [187]. In line with the integrity of the transplanted mitochondria is the question of their half-life survival in cells.
There have been attempts to follow the exogenous mitochondria both in cell cultures and in vivo. Different means for follow-up have been reported, including labeled mitochondrial proteins, their labeling with a cationic mitotracker that indicates the maintenance of mitochondrial membrane potential, and donor mtDNA, which differs from that of the recipient. As discussed previously, data on the survival rates of exogenous mitochondria range from a few days up to 12 weeks. This diversity is probably linked to the means used to follow the mitochondria, as mtDNA survival differs from that of mitochondrial proteins, which also show a high diversity in their half-life rates and are tissue-dependent. It is accepted that half-lives of intact mitochondria, their DNA, and proteins are in the range of days, ~8–28, 20–30, and ~7–30 days, respectively, depending on the tissue and the specific labeled protein used for follow-up. An additional issue in following mitochondrial integrity is the existence of EVs containing free mtDNA and mitochondrial proteins [135,188,189,190], suggesting that following mitochondrial integrity is challenging. Given thisthe above mentioned time scale, it is conceivable that transplanted exogenous mitochondria will stay intact for no more than several days. mtDNA may last longer than integral mitochondria, yet it is conceivable that the foreign mtDNA will be eliminated by the host cell upon mismatch, as the nDNA–mtDNA coordination is essential to cell survival [191].
Still, mitochondrial transplantation exerted long-term bioenergetical and behavioral effects. An attractive hypothesis is that the transplanted mitochondria induce local bioenergetics or immune responses, being strongly immunogenic [192]. A plausible complementary hypothesis is that the exogenous mitochondria or their content are transferred to adjacent cells from the recipient cells either via TNTs or by secreted EVs, both changing the physiological homeostasis in the recipient cells, which eventually lead to long-term effects. In line with the latter are the findings that intravenously injected mitochondria, observed in the liver but not in the brain, improved cognitive functions and our findings of a crosstalk between the physiological state of the recipient and exogenous mitochondria determining the long-term beneficial/detrimental outcomes [162,164].
The therapeutic potential of mitochondrial transplantation has also been questioned [193,194,195]. One major problem is the duration of the beneficial effect and whether treatment can be repeated in humans. Another concern is the source of the transplanted mitochondria, as in many mitochondrial-related disorders the malfunction of mitochondria is observed throughout the body, and therefore allogeneic mitochondria will be necessary for transplantation. Such mitochondria can increase the susceptibility to provoking inflammation, oxidative stress, and the risks of heteroplasmy [181,196]. In addition, it brings up the ethical problems of introducing a foreign DNA. A foreign mitochondrial genome may interfere with the nuclear genome, causing mitochondrial genetic drift and thereby mitochondrial dysfunction [197]. Opinions are widely divergent about the extent of this risk, with some arguing that human studies will almost certainly show adverse effects [198], while others argue there will be little risk [199]. Certainly, the lack of clarity on this matter poses a risk and is a subject of further research, which is currently underway [200]. The above-described concerns are augmented in systemic administration, which necessitates a considerably higher dose of mitochondria and a much longer delivery time to reach a target cell, and as many different organs receive the mitochondria it is less specific [201].
Finally, mitochondrial transplantation can induce both beneficial and detrimental bioenergetical, neuron structural, and behavioral alterations depending on the recipients’ mitochondrial homeostasis as well as on their physiological state, of which the specific relevant factors are currently an enigma. Therefore, any future use of mitochondrial transplantation for therapeutic aims will have to consider the possible toxic effects induced by the two-edge sword mitochondria.
11. Conclusions
Mitochondria are integral to cellular processes, particularly within the CNS, which emphasizes their vital importance in maintaining overall brain function. The multifaceted functions of CNS mitochondria, discussed in this review, range from their involvement in neuronal development, synaptogenesis, and plasticity to their adaptation to the complex interactions between brain cells. Mitochondrial transplantation is an innovative approach for the treatment of CNS disorders. However, the application of translational research to clinical implementation needs to be carefully considered in the light of the concerns discussed above. Nevertheless, mitochondrial transplantation provides an essential tool to study the causal link between mitochondrial function and neuronal development, sprouting, and plasticity, as well as behavior in health and disease. Given the essential role of mitochondria in numerous pathologies, interference with mitochondrial activity is of utmost importance and thus requires future intensive research.
Author Contributions
Both authors K.T. and D.B.-S. contributed to the conceptions of the review drafted and substantively revised it; Both approved the submitted version and agrees to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the review are appropriately investigated, resolved, and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a grant from the Israel Science Foundation (ISF) (2483/22) granted to D.B.-S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ricci, J.-E.; Muñoz-Pinedo, C.; Fitzgerald, P.; Bailly-Maitre, B.; Perkins, G.A.; Yadava, N.; Scheffler, I.E.; Ellisman, M.H.; Green, D.R. Disruption of Mitochondrial Function during Apoptosis Is Mediated by Caspase Cleavage of the P75 Subunit of Complex I of the Electron Transport Chain. Cell 2004, 117, 773–786. [Google Scholar] [CrossRef]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Diano, S.; Horvath, T.L. Mitochondrial Uncoupling Protein 2 (UCP2) in Glucose and Lipid Metabolism. Trends Mol. Med. 2012, 18, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, O.; Quirós, P.M.; Auwerx, J. Mitochondria and Epigenetics–Crosstalk in Homeostasis and Stress. Trends Cell Biol. 2017, 27, 453–463. [Google Scholar] [CrossRef]
- Hüttemann, M.; Lee, I.; Samavati, L.; Yu, H.; Doan, J.W. Regulation of Mitochondrial Oxidative Phosphorylation through Cell Signaling. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2007, 1773, 1701–1720. [Google Scholar] [CrossRef]
- Bergman, O.; Ben-Shachar, D. Mitochondrial Oxidative Phosphorylation System (OXPHOS) Deficits in Schizophrenia: Possible Interactions with Cellular Processes. Can. J. Psychiatry 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Papa, S.; Rasmo, D.D.; Technikova-Dobrova, Z.; Panelli, D.; Signorile, A.; Scacco, S.; Petruzzella, V.; Papa, F.; Palmisano, G.; Gnoni, A.; et al. Respiratory Chain Complex I, a Main Regulatory Target of the cAMP/PKA Pathway Is Defective in Different Human Diseases. FEBS Lett. 2012, 586, 568–577. [Google Scholar] [CrossRef]
- White, R.J.; Reynolds, I.J. Mitochondrial Depolarization in Glutamate-Stimulated Neurons: An Early Signal Specific to Excitotoxin Exposure. J. Neurosci. 1996, 16, 5688–5697. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. Serotonin in Pre-Implantation Mouse Embryos Is Localized to the Mitochondria and Can Modulate Mitochondrial Potential. Reproduction 2008, 135, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Brenner-Lavie, H.; Klein, E.; Ben-Shachar, D. Mitochondrial Complex I as a Novel Target for Intraneuronal DA: Modulation of Respiration in Intact Cells. Biochem. Pharmacol. 2009, 78, 85–95. [Google Scholar] [CrossRef]
- Blank, H.M.; Li, C.; Mueller, J.E.; Bogomolnaya, L.M.; Bryk, M.; Polymenis, M. An Increase in Mitochondrial DNA Promotes Nuclear DNA Replication in Yeast. PLoS Genet. 2008, 4, e1000047. [Google Scholar] [CrossRef]
- Roubertoux, P.L.; Sluyter, F.; Carlier, M.; Marcet, B.; Maarouf-Veray, F.; Chérif, C.; Marican, C.; Arrechi, P.; Godin, F.; Jamon, M.; et al. Mitochondrial DNA Modifies Cognition in Interaction with the Nuclear Genome and Age in Mice. Nat. Genet. 2003, 35, 65–69. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.A.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and Selective Fusion Govern Mitochondrial Segregation and Elimination by Autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Malka, F.; Guillery, O.; Cifuentes-Diaz, C.; Guillou, E.; Belenguer, P.; Lombès, A.; Rojo, M. Separate Fusion of Outer and Inner Mitochondrial Membranes. EMBO Rep. 2005, 6, 853–859. [Google Scholar] [CrossRef]
- Van Der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Farmer, T.; Naslavsky, N.; Caplan, S. Tying Trafficking to Fusion and Fission at the Mighty Mitochondria. Traffic 2018, 19, 569–577. [Google Scholar] [CrossRef]
- Cipolat, S.; De Brito, O.M.; Dal Zilio, B.; Scorrano, L. OPA1 Requires Mitofusin 1 to Promote Mitochondrial Fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 15927–15932. [Google Scholar] [CrossRef] [PubMed]
- Naon, D.; Zaninello, M.; Giacomello, M.; Varanita, T.; Grespi, F.; Lakshminaranayan, S.; Serafini, A.; Semenzato, M.; Herkenne, S.; Hernández-Alvarez, M.I.; et al. Critical Reappraisal Confirms That Mitofusin 2 Is an Endoplasmic Reticulum–Mitochondria Tether. Proc. Natl. Acad. Sci. USA 2016, 113, 11249–11254. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Choi, S.Y.; Cho, H.M.; Kim, H.J.; Sun, W. Physiological and Pathological Significance of Dynamin-Related Protein 1 (Drp1)-Dependent Mitochondrial Fission in the Nervous System. Exp. Neurobiol. 2013, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; McCaffery, J.M.; Chan, D.C. Mitochondrial Fusion Protects against Neurodegeneration in the Cerebellum. Cell 2007, 130, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.H.; Schmidt, R.E.; Pestronk, A.; Milbrandt, J. Altered Axonal Mitochondrial Transport in the Pathogenesis of Charcot-Marie-Tooth Disease from Mitofusin 2 Mutations. J. Neurosci. 2007, 27, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Del Dotto, V.; Mishra, P.; Vidoni, S.; Fogazza, M.; Maresca, A.; Caporali, L.; McCaffery, J.M.; Cappelletti, M.; Baruffini, E.; Lenaers, G.; et al. OPA1 Isoforms in the Hierarchical Organization of Mitochondrial Functions. Cell Rep. 2017, 19, 2557–2571. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Millet, A.M.E.; Guillermin, O.; Daloyau, M.; Davezac, N.; Miquel, M.-C.; Belenguer, P. OPA1 Loss of Function Affects in Vitro Neuronal Maturation. Brain 2013, 136, 1518–1533. [Google Scholar] [CrossRef]
- Ishihara, N.; Nomura, M.; Jofuku, A.; Kato, H.; Suzuki, S.O.; Masuda, K.; Otera, H.; Nakanishi, Y.; Nonaka, I.; Goto, Y.; et al. Mitochondrial Fission Factor Drp1 Is Essential for Embryonic Development and Synapse Formation in Mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Grohm, J.; Kim, S.-W.; Mamrak, U.; Tobaben, S.; Cassidy-Stone, A.; Nunnari, J.; Plesnila, N.; Culmsee, C. Inhibition of Drp1 Provides Neuroprotection in Vitro and in Vivo. Cell Death Differ. 2012, 19, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, A.M.; Delerue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, M.; Arnauné-Pelloquin, L.; Davezac, N.; Mils, V.; Miquel, M.C.; et al. Mitochondrial Fusion/Fission Dynamics in Neurodegeneration and Neuronal Plasticity. Neurobiol. Dis. 2016, 90, 3–19. [Google Scholar] [CrossRef]
- Peng, K.; Yang, L.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Cui, Z.; Ao, L.; Liu, J.; et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1α Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol. Neurobiol. 2017, 54, 3783–3797. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.T.; Harbauer, A.B. Metabolic Regulation of Mitochondrial Protein Biogenesis from a Neuronal Perspective. Biomolecules 2022, 12, 1595. [Google Scholar] [CrossRef]
- Cioni, J.-M.; Lin, J.Q.; Holtermann, A.V.; Koppers, M.; Jakobs, M.A.H.; Azizi, A.; Turner-Bridger, B.; Shigeoka, T.; Franze, K.; Harris, W.A.; et al. Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons. Cell 2019, 176, 56–72.e15. [Google Scholar] [CrossRef]
- Li, J.; Tran, O.T.; Crowley, T.B.; Moore, T.M.; Zackai, E.H.; Emanuel, B.S.; McDonald-McGinn, D.M.; Gur, R.E.; Wallace, D.C.; Anderson, S.A. Association of Mitochondrial Biogenesis with Variable Penetrance of Schizophrenia. JAMA Psychiatry 2021, 78, 911. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Cai, Q. Mitochondrial Transport in Neurons: Impact on Synaptic Homeostasis and Neurodegeneration. Nat. Rev. Neurosci. 2012, 13, 77–93. [Google Scholar] [CrossRef]
- Diana, A.; Simić, G.; Sinforiani, E.; Orrù, N.; Pichiri, G.; Bono, G. Mitochondria Morphology and DNA Content upon Sublethal Exposure to Beta-Amyloid(1-42) Peptide. Coll. Antropol. 2008, 32 (Suppl. S1), 51–58. [Google Scholar]
- De La Monte, S.M.; Luong, T.; Neely, T.R.; Robinson, D.; Wands, J.R. Mitochondrial DNA Damage as a Mechanism of Cell Loss in Alzheimer’s Disease. Lab. Investig. 2000, 80, 1323–1335. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Morais, V.A. Mitochondrial Biogenesis in Neurons: How and Where. Int. J. Mol. Sci. 2021, 22, 13059. [Google Scholar] [CrossRef]
- Zehnder, T.; Petrelli, F.; Romanos, J.; De Oliveira Figueiredo, E.C.; Lewis, T.L.; Déglon, N.; Polleux, F.; Santello, M.; Bezzi, P. Mitochondrial Biogenesis in Developing Astrocytes Regulates Astrocyte Maturation and Synapse Formation. Cell Rep. 2021, 35, 108952. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Tang, Y.; Mou, C.; Hu, X.; Shao, F.; Yan, W.; Wu, Q. Mitochondrial Fusion and Fission in Neuronal Death Induced by Cerebral Ischemia-Reperfusion and Its Clinical Application: A Mini-Review. Med. Sci. Monit. 2020, 26, e928651. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef]
- Goyal, M.S.; Raichle, M.E. Glucose Requirements of the Developing Human Brain. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S46–S49. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Chugani, H.T.; Grossman, L.I.; Lipovich, L.; Muzik, O.; Hof, P.R.; Wildman, D.E.; Sherwood, C.C.; Leonard, W.R.; Lange, N. Metabolic Costs and Evolutionary Implications of Human Brain Development. Proc. Natl. Acad. Sci. USA 2014, 111, 13010–13015. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Chau, K.F.; Shannon, M.L.; Fame, R.M.; Fonseca, E.; Mullan, H.; Johnson, M.B.; Sendamarai, A.K.; Springel, M.W.; Laurent, B.; Lehtinen, M.K. Downregulation of Ribosome Biogenesis during Early Forebrain Development. eLife 2018, 7, e36998. [Google Scholar] [CrossRef]
- Heidari, R.; Ommati, M.M.; Niknahad, H. Mitochondria as Biosynthetic Centers and Targeted Therapeutics. In Mitochondrial Metabolism; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–47. ISBN 978-0-12-822416-8. [Google Scholar]
- White, R.M.; Cech, J.; Ratanasirintrawoot, S.; Lin, C.Y.; Rahl, P.B.; Burke, C.J.; Langdon, E.; Tomlinson, M.L.; Mosher, J.; Kaufman, C.; et al. DHODH Modulates Transcriptional Elongation in the Neural Crest and Melanoma. Nature 2011, 471, 518–522. [Google Scholar] [CrossRef]
- Keuls, R.A.; Kojima, K.; Lozzi, B.; Steele, J.W.; Chen, Q.; Gross, S.S.; Finnell, R.H.; Parchem, R.J. MiR-302 Regulates Glycolysis to Control Cell-Cycle during Neural Tube Closure. Int. J. Mol. Sci. 2020, 21, 7534. [Google Scholar] [CrossRef]
- Boot, M.J.; Steegers-Theunissen, R.P.M.; Poelmann, R.E.; Van Iperen, L.; Lindemans, J.; Gittenberger-De Groot, A.C. Folic Acid and Homocysteine Affect Neural Crest and Neuroepithelial Cell Outgrowth and Differentiation in Vitro. Dev. Dyn. 2003, 227, 301–308. [Google Scholar] [CrossRef]
- Steele, J.W.; Kim, S.-E.; Finnell, R.H. One-Carbon Metabolism and Folate Transporter Genes: Do They Factor Prominently in the Genetic Etiology of Neural Tube Defects? Biochimie 2020, 173, 27–32. [Google Scholar] [CrossRef]
- Kim, J.; Lei, Y.; Guo, J.; Kim, S.-E.; Wlodarczyk, B.J.; Cabrera, R.M.; Lin, Y.L.; Nilsson, T.K.; Zhang, T.; Ren, A.; et al. Formate Rescues Neural Tube Defects Caused by Mutations in Slc25a32. Proc. Natl. Acad. Sci. USA 2018, 115, 4690–4695. [Google Scholar] [CrossRef]
- Kopinski, P.K.; Janssen, K.A.; Schaefer, P.M.; Trefely, S.; Perry, C.E.; Potluri, P.; Tintos-Hernandez, J.A.; Singh, L.N.; Karch, K.R.; Campbell, S.L.; et al. Regulation of Nuclear Epigenome by Mitochondrial DNA Heteroplasmy. Proc. Natl. Acad. Sci. USA 2019, 116, 16028–16035. [Google Scholar] [CrossRef]
- Fetterman, J.L.; Ballinger, S.W. Mitochondrial Genetics Regulate Nuclear Gene Expression through Metabolites. Proc. Natl. Acad. Sci. USA 2019, 116, 15763–15765. [Google Scholar] [CrossRef]
- Chen, C.-T.; Hsu, S.-H.; Wei, Y.-H. Upregulation of Mitochondrial Function and Antioxidant Defense in the Differentiation of Stem Cells. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2010, 1800, 257–263. [Google Scholar] [CrossRef]
- Facucho-Oliveira, J.M.; Alderson, J.; Spikings, E.C.; Egginton, S.; St. John, J.C. Mitochondrial DNA Replication during Differentiation of Murine Embryonic Stem Cells. J. Cell Sci. 2007, 120, 4025–4034. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Keeney, P.M.; Bennett, J.P. Differentiation of Human Neural Stem Cells into Motor Neurons Stimulates Mitochondrial Biogenesis and Decreases Glycolytic Flux. Stem Cells Dev. 2015, 24, 1984–1994. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Wang, L.; Cai, Y.; Zhong, X.; He, X.; Hu, L.; Tian, S.; Wu, M.; Hui, L.; et al. Fatty Acid Synthesis Is Critical for Stem Cell Pluripotency via Promoting Mitochondrial Fission. EMBO J. 2017, 36, 1330–1347. [Google Scholar] [CrossRef]
- Wilkerson, D.C.; Sankar, U. Mitochondria: A Sulfhydryl Oxidase and Fission GTPase Connect Mitochondrial Dynamics with Pluripotency in Embryonic Stem Cells. Int. J. Biochem. Cell Biol. 2011, 43, 1252–1256. [Google Scholar] [CrossRef]
- Folmes, C.D.L.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic Oxidative Bioenergetics Transitions into Pluripotency-Dependent Glycolysis to Facilitate Nuclear Reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Mils, V.; Bosch, S.; Roy, J.; Bel-Vialar, S.; Belenguer, P.; Pituello, F.; Miquel, M.-C. Mitochondrial Reshaping Accompanies Neural Differentiation in the Developing Spinal Cord. PLoS ONE 2015, 10, e0128130. [Google Scholar] [CrossRef]
- Prieto, J.; León, M.; Ponsoda, X.; Sendra, R.; Bort, R.; Ferrer-Lorente, R.; Raya, A.; López-García, C.; Torres, J. Early ERK1/2 Activation Promotes DRP1-Dependent Mitochondrial Fission Necessary for Cell Reprogramming. Nat. Commun. 2016, 7, 11124. [Google Scholar] [CrossRef]
- Fang, D.; Yan, S.; Yu, Q.; Chen, D.; Yan, S.S. Mfn2 Is Required for Mitochondrial Development and Synapse Formation in Human Induced Pluripotent Stem Cells/hiPSC Derived Cortical Neurons. Sci. Rep. 2016, 6, 31462. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; MacLaurin, J.G.; Lagace, D.C.; Park, D.S.; Slack, R.S. Mitochondrial Dysfunction Underlies Cognitive Defects as a Result of Neural Stem Cell Depletion and Impaired Neurogenesis. Hum. Mol. Genet. 2017, 26, 3327–3341. [Google Scholar] [CrossRef]
- Robicsek, O.; Karry, R.; Petit, I.; Salman-Kesner, N.; Müller, F.-J.; Klein, E.; Aberdam, D.; Ben-Shachar, D. Abnormal Neuronal Differentiation and Mitochondrial Dysfunction in Hair Follicle-Derived Induced Pluripotent Stem Cells of Schizophrenia Patients. Mol. Psychiatry 2013, 18, 1067–1076. [Google Scholar] [CrossRef]
- Mattson, M.P.; Partin, J. Evidence for Mitochondrial Control of Neuronal Polarity. J. Neurosci. Res. 1999, 56, 8–20. [Google Scholar] [CrossRef]
- Iwata, R.; Casimir, P.; Erkol, E.; Boubakar, L.; Planque, M.; Gallego López, I.M.; Ditkowska, M.; Gaspariunaite, V.; Beckers, S.; Remans, D.; et al. Mitochondria Metabolism Sets the Species-Specific Tempo of Neuronal Development. Science 2023, 379, eabn4705. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, M.-Y.; Sun, T.; Knight, A.L.; Sheng, Z.-H. Characterization of Mitochondrial Transport in Neurons. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 547, pp. 75–96. ISBN 978-0-12-801415-8. [Google Scholar]
- MacAskill, A.F.; Rinholm, J.E.; Twelvetrees, A.E.; Arancibia-Carcamo, I.L.; Muir, J.; Fransson, A.; Aspenstrom, P.; Attwell, D.; Kittler, J.T. Miro1 Is a Calcium Sensor for Glutamate Receptor-Dependent Localization of Mitochondria at Synapses. Neuron 2009, 61, 541–555. [Google Scholar] [CrossRef]
- Saotome, M.; Safiulina, D.; Szabadkai, G.; Das, S.; Fransson, Å.; Aspenstrom, P.; Rizzuto, R.; Hajnóczky, G. Bidirectional Ca2+-Dependent Control of Mitochondrial Dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. USA 2008, 105, 20728–20733. [Google Scholar] [CrossRef]
- Han, S.M.; Baig, H.S.; Hammarlund, M. Mitochondria Localize to Injured Axons to Support Regeneration. Neuron 2016, 92, 1308–1323. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, P.; Lin, M.-Y.; Sun, T.; Chen, Y.; Sheng, Z.-H. Facilitation of Axon Regeneration by Enhancing Mitochondrial Transport and Rescuing Energy Deficits. J. Cell Biol. 2016, 214, 103–119. [Google Scholar] [CrossRef]
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.-H.; Xu, X.-M. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 2020, 31, 623–641.e8. [Google Scholar] [CrossRef]
- Kang, J.-S.; Tian, J.-H.; Pan, P.-Y.; Zald, P.; Li, C.; Deng, C.; Sheng, Z.-H. Docking of Axonal Mitochondria by Syntaphilin Controls Their Mobility and Affects Short-Term Facilitation. Cell 2008, 132, 137–148. [Google Scholar] [CrossRef]
- Cartoni, R.; Norsworthy, M.W.; Bei, F.; Wang, C.; Li, S.; Zhang, Y.; Gabel, C.V.; Schwarz, T.L.; He, Z. The Mammalian-Specific Protein Armcx1 Regulates Mitochondrial Transport during Axon Regeneration. Neuron 2016, 92, 1294–1307. [Google Scholar] [CrossRef]
- Li, H.; Guglielmetti, C.; Sei, Y.J.; Zilberter, M.; Le Page, L.M.; Shields, L.; Yang, J.; Nguyen, K.; Tiret, B.; Gao, X.; et al. Neurons Require Glucose Uptake and Glycolysis in Vivo. Cell Rep. 2023, 42, 112335. [Google Scholar] [CrossRef]
- Turner, D.A.; Adamson, D.C. Neuronal-Astrocyte Metabolic Interactions: Understanding the Transition Into Abnormal Astrocytoma Metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial Metabolic Networks Sustain Hippocampal Synaptic Transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Schousboe, A.; Westergaard, N.; Waagepetersen, H.S.; Larsson, O.M.; Bakken, I.J.; Sonnewald, U. Trafficking between Glia and Neurons of TCA Cycle Intermediates and Related Metabolites. Glia 1997, 21, 99–105. [Google Scholar] [CrossRef]
- Sonnay, S.; Gruetter, R.; Duarte, J.M.N. How Energy Metabolism Supports Cerebral Function: Insights from 13C Magnetic Resonance Studies In Vivo. Front. Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef]
- Leach, J.P.; Sills, G.J.; Majid, A.; Butler, E.; Carswell, A.; Thompson, G.G.; Brodie, M.J. Effects of Tiagabine and Vigabatrin on GABA Uptake into Primary Cultures of Rat Cortical Astrocytes. Seizure 1996, 5, 229–234. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.-I.; Hayashi, Y.; Sheng, M. The Importance of Dendritic Mitochondria in the Morphogenesis and Plasticity of Spines and Synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef]
- Lopes, S.; Teplytska, L.; Vaz-Silva, J.; Dioli, C.; Trindade, R.; Morais, M.; Webhofer, C.; Maccarrone, G.; Almeida, O.F.X.; Turck, C.W.; et al. Tau Deletion Prevents Stress-Induced Dendritic Atrophy in Prefrontal Cortex: Role of Synaptic Mitochondria. Cereb. Cortex 2016, 27, bhw057. [Google Scholar] [CrossRef]
- Wingo, A.P.; Dammer, E.B.; Breen, M.S.; Logsdon, B.A.; Duong, D.M.; Troncosco, J.C.; Thambisetty, M.; Beach, T.G.; Serrano, G.E.; Reiman, E.M.; et al. Large-Scale Proteomic Analysis of Human Brain Identifies Proteins Associated with Cognitive Trajectory in Advanced Age. Nat. Commun. 2019, 10, 1619. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Kitner-Triolo, M.H.; Beydoun, H.A.; Kaufman, J.S.; Mason, M.A.; Evans, M.K.; Zonderman, A.B. Dietary Antioxidant Intake and Its Association with Cognitive Function in an Ethnically Diverse Sample of US Adults. Psychosom. Med. 2015, 77, 68–82. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef]
- Hollis, F.; Van Der Kooij, M.A.; Zanoletti, O.; Lozano, L.; Cantó, C.; Sandi, C. Mitochondrial Function in the Brain Links Anxiety with Social Subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef]
- Alaux, C.; Sinha, S.; Hasadsri, L.; Hunt, G.J.; Guzmán-Novoa, E.; DeGrandi-Hoffman, G.; Uribe-Rubio, J.L.; Southey, B.R.; Rodriguez-Zas, S.; Robinson, G.E. Honey Bee Aggression Supports a Link between Gene Regulation and Behavioral Evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 15400–15405. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Vekaria, H.J.; Palmer, J.H.; Sullivan, P.G. Brain Mitochondrial Bioenergetics Change with Rapid and Prolonged Shifts in Aggression in the Honey Bee, Apis Mellifera. J. Exp. Biol. 2018, 221, jeb176917. [Google Scholar] [CrossRef]
- Cervoni, M.S.; Cardoso-Júnior, C.A.M.; Craveiro, G.; Souza, A.D.O.; Alberici, L.C.; Hartfelder, K. Mitochondrial Capacity, Oxidative Damage and Hypoxia Gene Expression Are Associated with Age-Related Division of Labor in Honey Bee, Apis Mellifera L., Workers. J. Exp. Biol. 2017, 220, 4035–4046. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Gozal, D. Elevated Plasma Oxidative Stress Markers in Individuals with Intermittent Explosive Disorder and Correlation with Aggression in Humans. Biol. Psychiatry 2016, 79, 127–135. [Google Scholar] [CrossRef]
- Fanning, J.R.; Lee, R.; Gozal, D.; Coussons-Read, M.; Coccaro, E.F. Childhood Trauma and Parental Style: Relationship with Markers of Inflammation, Oxidative Stress, and Aggression in Healthy and Personality Disordered Subjects. Biol. Psychol. 2015, 112, 56–65. [Google Scholar] [CrossRef]
- Ben-Shachar, D. Mitochondrial Multifaceted Dysfunction in Schizophrenia; Complex I as a Possible Pathological Target. Schizophr. Res. 2017, 187, 3–10. [Google Scholar] [CrossRef]
- Kim, Y.; Vadodaria, K.C.; Lenkei, Z.; Kato, T.; Gage, F.H.; Marchetto, M.C.; Santos, R. Mitochondria, Metabolism, and Redox Mechanisms in Psychiatric Disorders. Antioxid. Redox Signal. 2019, 31, 275–317. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired Mitochondrial Function in Psychiatric Disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef]
- Talari, N.K.; Mattam, U.; Meher, N.K.; Paripati, A.K.; Mahadev, K.; Krishnamoorthy, T.; Sepuri, N.B.V. Lipid-Droplet Associated Mitochondria Promote Fatty-Acid Oxidation through a Distinct Bioenergetic Pattern in Male Wistar Rats. Nat. Commun. 2023, 14, 766. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Vitali, F.; Shang, Y.; Raikes, A.C.; Wang, T.; Jin, Y.; Brinton, R.D.; Gu, H.; Yin, F. Loss of Fatty Acid Degradation by Astrocytic Mitochondria Triggers Neuroinflammation and Neurodegeneration. Nat. Metab. 2023, 5, 445–465. [Google Scholar] [CrossRef]
- Holper, L.; Ben-Shachar, D.; Mann, J. Multivariate Meta-Analyses of Mitochondrial Complex I and IV in Major Depressive Disorder, Bipolar Disorder, Schizophrenia, Alzheimer Disease, and Parkinson Disease. Neuropsychopharmacology 2019, 44, 837–849. [Google Scholar] [CrossRef]
- Rollins, B.L.; Morgan, L.; Hjelm, B.E.; Sequeira, A.; Schatzberg, A.F.; Barchas, J.D.; Lee, F.S.; Myers, R.M.; Watson, S.J.; Akil, H.; et al. Mitochondrial Complex I Deficiency in Schizophrenia and Bipolar Disorder and Medication Influence. Complex Psychiatry 2017, 3, 157–169. [Google Scholar] [CrossRef]
- Ni, P.; Ma, Y.; Chung, S. Mitochondrial Dysfunction in Psychiatric Disorders. Schizophr. Res. 2022; in press. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial Medicine for Aging and Neurodegenerative Diseases. Neuromol. Med. 2008, 10, 291–315. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Dror, N.; Klein, E.; Karry, R.; Sheinkman, A.; Kirsh, Z.; Mazor, M.; Tzukerman, M.; Ben-Shachar, D. State-Dependent Alterations in Mitochondrial Complex I Activity in Platelets: A Potential Peripheral Marker for Schizophrenia. Mol. Psychiatry 2002, 7, 995–1001. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Karry, R. Sp1 Expression Is Disrupted in Schizophrenia; A Possible Mechanism for the Abnormal Expression of Mitochondrial Complex I Genes, NDUFV1 and NDUFV2. PLoS ONE 2007, 2, e817. [Google Scholar] [CrossRef]
- Lunnon, K.; Keohane, A.; Pidsley, R.; Newhouse, S.; Riddoch-Contreras, J.; Thubron, E.B.; Devall, M.; Soininen, H.; Kłoszewska, I.; Mecocci, P.; et al. Mitochondrial Genes Are Altered in Blood Early in Alzheimer’s Disease. Neurobiol. Aging 2017, 53, 36–47. [Google Scholar] [CrossRef]
- Mastroeni, D.; Khdour, O.M.; Delvaux, E.; Nolz, J.; Olsen, G.; Berchtold, N.; Cotman, C.; Hecht, S.M.; Coleman, P.D. Nuclear but Not Mitochondrial-encoded Oxidative Phosphorylation Genes Are Altered in Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. Alzheimer’s Dement. 2017, 13, 510–519. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Ni, J.; Zhang, Y.; Chen, M.; Cai, J.; Li, X.; Zhang, W.; Zhang, C. Genetic Variant in NDUFS1 Gene Is Associated with Schizophrenia and Negative Symptoms in Han Chinese. J. Hum. Genet. 2015, 60, 11–16. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Bubber, P.; Hartounian, V.; Gibson, G.E.; Blass, J.P. Abnormalities in the Tricarboxylic Acid (TCA) Cycle in the Brains of Schizophrenia Patients. Eur. Neuropsychopharmacol. 2011, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Wang, F.; Yu, H. Systemic Alterations of Tricarboxylic Acid Cycle Enzymes in Alzheimer’s Disease. Front. Neurosci. 2023, 17, 1206688. [Google Scholar] [CrossRef]
- Bourgeron, T.; Chretien, D.; Poggi-Bach, J.; Doonan, S.; Rabier, D.; Letouzé, P.; Munnich, A.; Rötig, A.; Landrieu, P.; Rustin, P. Mutation of the Fumarase Gene in Two Siblings with Progressive Encephalopathy and Fumarase Deficiency. J. Clin. Investig. 1994, 93, 2514–2518. [Google Scholar] [CrossRef]
- Guffon, N.; Lopez-Mediavilla, C.; Dumoulin, R.; Mousson, B.; Godinot, C.; Carrier, H.; Collombet, J.M.; Divry, P.; Mathieu, M.; Guibaud, P. 2-Ketoglutarate Dehydrogenase Deficiency, a Rare Cause of Primary Hyperlactataemia: Report of a New Case. J. Inherit. Metab. Dis. 1993, 16, 821–830. [Google Scholar] [CrossRef]
- Stortz, J.A.; Hawkins, R.B.; Holden, D.C.; Raymond, S.L.; Wang, Z.; Brakenridge, S.C.; Cuschieri, J.; Moore, F.A.; Maier, R.V.; Moldawer, L.L.; et al. Cell-Free Nuclear, but Not Mitochondrial, DNA Concentrations Correlate with the Early Host Inflammatory Response after Severe Trauma. Sci. Rep. 2019, 9, 13648. [Google Scholar] [CrossRef]
- Hajizadeh, S.; DeGroot, J.; TeKoppele, J.M.; Tarkowski, A.; Collins, L.V. Extracellular Mitochondrial DNA and Oxidatively Damaged DNA in Synovial Fluid of Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2003, 5, R234. [Google Scholar] [CrossRef]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.-M.; Tarkowski, A. Endogenously Oxidized Mitochondrial DNA Induces in Vivo and in Vitro Inflammatory Responses. J. Leukoc. Biol. 2004, 75, 995–1000. [Google Scholar] [CrossRef]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The Relationship between Circulating Mitochondrial DNA and Inflammatory Cytokines in Patients with Major Depression. J. Affect. Disord. 2018, 233, 15–20. [Google Scholar] [CrossRef]
- Ibrahim, M.; Strah, H.; Huang, H.; Krupnick, A.; Kreisel, D.; Hachem, R.; Trulock, E.; Alouch, A.; Gelman, A. Circulating Mitochondrial DNA Is Elevated in Patients with Immediate Primary Graft Dysfunction. J. Heart Lung Transplant. 2013, 32, S33. [Google Scholar] [CrossRef]
- Varhaug, K.N.; Barro, C.; Bjørnevik, K.; Myhr, K.-M.; Torkildsen, Ø.; Wergeland, S.; Bindoff, L.A.; Kuhle, J.; Vedeler, C. Neurofilament Light Chain Predicts Disease Activity in Relapsing-Remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e422. [Google Scholar] [CrossRef] [PubMed]
- Leurs, C.E.; Podlesniy, P.; Trullas, R.; Balk, L.; Steenwijk, M.D.; Malekzadeh, A.; Piehl, F.; Uitdehaag, B.M.; Killestein, J.; Van Horssen, J.; et al. Cerebrospinal Fluid mtDNA Concentration Is Elevated in Multiple Sclerosis Disease and Responds to Treatment. Mult. Scler. 2018, 24, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. A Biomarker and a Target for Progressive MS. Nat. Rev. Drug Discov. 2009, 8, 771. [Google Scholar] [CrossRef]
- Gambardella, S.; Limanaqi, F.; Ferese, R.; Biagioni, F.; Campopiano, R.; Centonze, D.; Fornai, F. Ccf-mtDNA as a Potential Link Between the Brain and Immune System in Neuro-Immunological Disorders. Front. Immunol. 2019, 10, 1064. [Google Scholar] [CrossRef]
- Jauhari, A.; Baranov, S.V.; Suofu, Y.; Kim, J.; Singh, T.; Yablonska, S.; Li, F.; Wang, X.; Oberly, P.; Minnigh, M.B.; et al. Melatonin Inhibits Cytosolic Mitochondrial DNA–Induced Neuroinflammatory Signaling in Accelerated Aging and Neurodegeneration. J. Clin. Investig. 2021, 131, e150328. [Google Scholar] [CrossRef]
- Tresse, E.; Marturia-Navarro, J.; Sew, W.Q.G.; Cisquella-Serra, M.; Jaberi, E.; Riera-Ponsati, L.; Fauerby, N.; Hu, E.; Kretz, O.; Aznar, S.; et al. Mitochondrial DNA Damage Triggers Spread of Parkinson’s Disease-like Pathology. Mol. Psychiatry, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Galizzi, G.; Di Carlo, M. Mitochondrial DNA and Inflammation in Alzheimer’s Disease. Curr. Issues Mol. Biol. 2023, 45, 8586–8606. [Google Scholar] [CrossRef]
- Daniels, T.E.; Zitkovsky, E.K.; Laumann, L.E.; Kunicki, Z.J.; Price, D.J.; Peterson, A.L.; Dennery, P.A.; Kao, H.-T.; Parade, S.H.; Price, L.H.; et al. Circulating Cell-Free Mitochondrial DNA and Depressive Symptoms Among Low-Active Adults Who Smoke. Psychosom. Med. 2024, 86, 37–43. [Google Scholar] [CrossRef]
- Fernström, J.; Ohlsson, L.; Asp, M.; Lavant, E.; Holck, A.; Grudet, C.; Westrin, Å.; Lindqvist, D. Plasma Circulating Cell-Free Mitochondrial DNA in Depressive Disorders. PLoS ONE 2021, 16, e0259591. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Huang, M.; Xu, Y.; Yao, Q.; Wu, X.; Zhou, D. Reduced Cell-Free Mitochondrial DNA Levels Were Induced by Antipsychotics Treatment in First-Episode Patients with Schizophrenia. Front. Psychiatry 2021, 12, 652314. [Google Scholar] [CrossRef] [PubMed]
- Melamud, M.M.; Buneva, V.N.; Ermakov, E.A. Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 3402. [Google Scholar] [CrossRef] [PubMed]
- Schulmann, A.; Ryu, E.; Goncalves, V.; Rollins, B.; Christiansen, M.; Frye, M.A.; Biernacka, J.; Vawter, M.P. Novel Complex Interactions between Mitochondrial and Nuclear DNA in Schizophrenia and Bipolar Disorder. Complex Psychiatry 2019, 5, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Huang, M.; Wang, B.; Yan, X.; Wu, Z.; Zhang, X. mtDNA Maintenance and Alterations in the Pathogenesis of NeurodegenerativeDiseases. Curr. Neuropharmacol. 2023, 21, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Win, P.W.; Singh, S.M.; Castellani, C.A. Mitochondrial DNA Copy Number and Heteroplasmy in Monozygotic Twins Discordant for Schizophrenia. Twin Res. Hum. Genet. 2023, 26, 280–289. [Google Scholar] [CrossRef]
- Pyle, A.; Anugrha, H.; Kurzawa-Akanbi, M.; Yarnall, A.; Burn, D.; Hudson, G. Reduced Mitochondrial DNA Copy Number Is a Biomarker of Parkinson’s Disease. Neurobiol. Aging 2016, 38, e7–e216. [Google Scholar] [CrossRef] [PubMed]
- Öğütlü, H.; Selçuk Esin, İ.; Bağış Erdem, H.; Tatar, A.; Burak Dursun, O. Mitochondrial DNA Copy Number Is Associated with Attention Deficit Hyperactivity Disorder. Psychiatr. Danub. 2020, 32, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Efstathopoulos, P.; Millischer, V.; Olsson, E.; Wei, Y.B.; Brüstle, O.; Schalling, M.; Villaescusa, J.C.; Ösby, U.; Lavebratt, C. Mitochondrial DNA Copy Number Is Associated with Psychosis Severity and Anti-Psychotic Treatment. Sci. Rep. 2018, 8, 12743. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Vignais, M.-L.; Caicedo, A.; Brondello, J.-M.; Jorgensen, C. Cell Connections by Tunneling Nanotubes: Effects of Mitochondrial Trafficking on Target Cell Metabolism, Homeostasis, and Response to Therapy. Stem Cells Int. 2017, 2017, 6917941. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef]
- Önfelt, B.; Nedvetzki, S.; Benninger, R.K.P.; Purbhoo, M.A.; Sowinski, S.; Hume, A.N.; Seabra, M.C.; Neil, M.A.A.; French, P.M.W.; Davis, D.M. Structurally Distinct Membrane Nanotubes between Human Macrophages Support Long-Distance Vesicular Traffic or Surfing of Bacteria. J. Immunol. 2006, 177, 8476–8483. [Google Scholar] [CrossRef]
- Suh, J.; Lee, Y.-S. Mitochondria as Secretory Organelles and Therapeutic Cargos. Exp. Mol. Med. 2024, 56, 66–85. [Google Scholar] [CrossRef]
- Amari, L.; Germain, M. Mitochondrial Extracellular Vesicles—Origins and Roles. Front. Mol. Neurosci. 2021, 14, 767219. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial Transfer from Bone-Marrow–Derived Stromal Cells to Pulmonary Alveoli Protects against Acute Lung Injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Islam, M.N.; Bhattacharya, S.; Bhattacharya, J. Intercellular Mitochondrial Transfer: Bioenergetic Crosstalk between Cells. Curr. Opin. Genet. Dev. 2016, 38, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.O.; Kim, K.-Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular Degradation of Axonal Mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of Mitochondria from Astrocytes to Neurons after Stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Yeung, S.C.; Liang, Y.; Liang, X.; Ding, Y.; Ip, M.S.M.; Tse, H.-F.; Mak, J.C.W.; Lian, Q. Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage. Am. J. Respir. Cell. Mol. Biol. 2014, 51, 455–465. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes Is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Mukkala, A.N.; Jerkic, M.; Khan, Z.; Szaszi, K.; Kapus, A.; Rotstein, O. Therapeutic Effects of Mesenchymal Stromal Cells Require Mitochondrial Transfer and Quality Control. Int. J. Mol. Sci. 2023, 24, 15788. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Jiang, D.; Liang, X.; Liao, S.; Zhang, Z.; Yue, W.; Li, X.; Chiu, S.-M.; Chai, Y.-H.; et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Rep. 2016, 7, 749–763. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-Derived Exosomes Promote Recovery from Traumatic Brain Injury via Microglia/Macrophages in Rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef]
- Knowles, J.K.C. An Improved Microinjection Technique in Paramecium Aurelia. Exp. Cell Res. 1974, 88, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Shay, J.W. Mitochondrial Transformation of Mammalian Cells. Nature 1982, 295, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Kitani, T.; Kami, D.; Matoba, S.; Gojo, S. Internalization of Isolated Functional Mitochondria: Involvement of Macropinocytosis. J. Cell. Mol. Med. 2014, 18, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Pacak, C.A.; Preble, J.M.; Kondo, H.; Seibel, P.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B.; McCully, J.D. Actin-Dependent Mitochondrial Internalization in Cardiomyocytes: Evidence for Rescue of Mitochondrial Function. Biol. Open 2015, 4, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, X.; Wang, B.; Wang, Z.; Liu, Y.; Di, C.; Si, J.; Li, H.; Wu, Q.; Xu, D.; et al. Endocytosis-Mediated Mitochondrial Transplantation: Transferring Normal Human Astrocytic Mitochondria into Glioma Cells Rescues Aerobic Respiration and Enhances Radiosensitivity. Theranostics 2019, 9, 3595–3607. [Google Scholar] [CrossRef] [PubMed]
- Kesner, E.E.; Saada-Reich, A.; Lorberboum-Galski, H. Characteristics of Mitochondrial Transformation into Human Cells. Sci. Rep. 2016, 6, 26057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Miao, C. Mitochondrial Transplantation as a Promising Therapy for Mitochondrial Diseases. Acta Pharm. Sin. B 2023, 13, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- McPhie, D.; Sargent, L.; Babb, S.; Ben-Shachar, D.; Cataldo, A.; Cohen, B. Alternative Methods for Mitochondrial Transplantation: Efficiency of Unpackaged and Lipid-Packaged Preparations; Molecular Biology. bioRxiv 2017, 102913. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D. Transit and Integration of Extracellular Mitochondria in Human Heart Cells. Sci. Rep. 2017, 7, 17450. [Google Scholar] [CrossRef]
- Chang, J.-C.; Wu, S.-L.; Liu, K.-H.; Chen, Y.-H.; Chuang, C.-S.; Cheng, F.-C.; Su, H.-L.; Wei, Y.-H.; Kuo, S.-J.; Liu, C.-S. Allogeneic/Xenogeneic Transplantation of Peptide-Labeled Mitochondria in Parkinson’s Disease: Restoration of Mitochondria Functions and Attenuation of 6-Hydroxydopamine–Induced Neurotoxicity. Transl. Res. 2016, 170, 40–56.e3. [Google Scholar] [CrossRef]
- Roushandeh, A.M.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial Transplantation as a Potential and Novel Master Key for Treatment of Various Incurable Diseases. Cytotechnology 2019, 71, 647–663. [Google Scholar] [CrossRef]
- McCully, J.D.; Del Nido, P.J.; Emani, S.M. Mitochondrial Transplantation for Organ Rescue. Mitochondrion 2022, 64, 27–33. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Chen, L.; Li, W.; Chen, L.; Wang, Y.-P. Mitochondria Transfer and Transplantation in Human Health and Diseases. Mitochondrion 2022, 65, 80–87. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of Isolated Mitochondria during Early Reperfusion for Cardioprotection. Am. J. Physiol.—Heart Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef]
- Shi, X.; Bai, H.; Zhao, M.; Li, X.; Sun, X.; Jiang, H.; Fu, A. Treatment of Acetaminophen-Induced Liver Injury with Exogenous Mitochondria in Mice. Transl. Res. 2018, 196, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-Derived Autologous Mitochondrial Transplantation: A Novel Strategy for Treating Cerebral Ischemic Injury. Behav. Brain Res. 2019, 356, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-J.; Kuo, C.-C.; Lee, H.-C.; Shen, C.-I.; Cheng, F.-C.; Wu, S.-F.; Chang, J.-C.; Pan, H.-C.; Lin, S.-Z.; Liu, C.-S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection against Ischemic Stress in Ischemic Rat Brains. Cell Transpl. 2016, 25, 913–927. [Google Scholar] [CrossRef]
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2019, 72, 587–604. [Google Scholar] [CrossRef]
- Robicsek, O.; Ene, H.M.; Karry, R.; Ytzhaki, O.; Asor, E.; McPhie, D.; Cohen, B.M.; Ben-Yehuda, R.; Weiner, I.; Ben-Shachar, D. Isolated Mitochondria Transfer Improves Neuronal Differentiation of Schizophrenia-Derived Induced Pluripotent Stem Cells and Rescues Deficits in a Rat Model of the Disorder. Schizophr. Bull. 2018, 44, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Ene, H.M.; Karry, R.; Farfara, D.; Ben-Shachar, D. Mitochondria Play an Essential Role in the Trajectory of Adolescent Neurodevelopment and Behavior in Adulthood: Evidence from a Schizophrenia Rat Model. Mol. Psychiatry 2023, 28, 1170–1181. [Google Scholar] [CrossRef]
- Chang, J.-C.; Chao, Y.-C.; Chang, H.-S.; Wu, Y.-L.; Chang, H.-J.; Lin, Y.-S.; Cheng, W.-L.; Lin, T.-T.; Liu, C.-S. Intranasal Delivery of Mitochondria for Treatment of Parkinson’s Disease Model Rats Lesioned with 6-Hydroxydopamine. Sci. Rep. 2021, 11, 10597. [Google Scholar] [CrossRef]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef]
- Jacoby, E.; Bar-Yosef, O.; Gruber, N.; Lahav, E.; Varda-Bloom, N.; Bolkier, Y.; Bar, D.; Blumkin, M.B.-Y.; Barak, S.; Eisenstein, E.; et al. Mitochondrial Augmentation of Hematopoietic Stem Cells in Children with Single Large-Scale Mitochondrial DNA Deletion Syndromes. Sci. Transl. Med. 2022, 14, eabo3724. [Google Scholar] [CrossRef]
- Morimoto, Y.; Gamage, U.S.K.; Yamochi, T.; Saeki, N.; Morimoto, N.; Yamanaka, M.; Koike, A.; Miyamoto, Y.; Tanaka, K.; Fukuda, A.; et al. Mitochondrial Transfer into Human Oocytes Improved Embryo Quality and Clinical Outcomes in Recurrent Pregnancy Failure Cases. Int. J. Mol. Sci. 2023, 24, 2738. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous Administration of Mitochondria for Treating Experimental Parkinson’s Disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, Z.; Hou, Y.; Zhang, L.; Fu, A. Improvement of Cognitive and Motor Performance with Mitotherapy in Aged Mice. Int. J. Biol. Sci. 2020, 16, 849–858. [Google Scholar] [CrossRef]
- Gollihue, J.L.; Patel, S.P.; Eldahan, K.C.; Cox, D.H.; Donahue, R.R.; Taylor, B.K.; Sullivan, P.G.; Rabchevsky, A.G. Effects of Mitochondrial Transplantation on Bioenergetics, Cellular Incorporation, and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2018, 35, 1800–1818. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-W.; Fang, S.-Y.; Hsu, J.-Y.C.; Huang, C.-Y.; Lee, P.-H.; Huang, C.-C.; Chen, H.-F.; Lam, C.-F.; Lee, J.-S. Mitochondrial Transplantation Attenuates Neural Damage and Improves Locomotor Function After Traumatic Spinal Cord Injury in Rats. Front. Neurosci. 2022, 16, 800883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qu, D.; Xi, Z.; Huan, Y.; Zhang, K.; Yu, C.; Yang, D.; Kang, J.; Lin, W.; Wu, S.; et al. Mitochondria Transplantation Protects Traumatic Brain Injury via Promoting Neuronal Survival and Astrocytic BDNF. Transl. Res. 2021, 235, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial Dysfunction and Psychiatric Disorders. Neurochem. Res. 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Giménez-Palomo, A.; Dodd, S.; Anmella, G.; Carvalho, A.F.; Scaini, G.; Quevedo, J.; Pacchiarotti, I.; Vieta, E.; Berk, M. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front. Psychiatry 2021, 12, 546801. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Gao, C.; Xie, L.; Zhai, L.; Cui, G.; Yin, X. Mitochondrial Transplantation Attenuates Lipopolysaccharide- Induced Depression-like Behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 240–249. [Google Scholar] [CrossRef]
- Sweetat, S.; Nitzan, K.; Suissa, N.; Haimovich, Y.; Lichtenstein, M.; Zabit, S.; Benhamron, S.; Akarieh, K.; Mishra, K.; Barasch, D.; et al. The Beneficial Effect of Mitochondrial Transfer Therapy in 5XFAD Mice via Liver–Serum–Brain Response. Cells 2023, 12, 1006. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, D.; Li, Z.; Guo, H.; Wu, Y.; Feng, J. Hippocampal Mitochondrial Transplantation Alleviates Age-Associated Cognitive Decline via Enhancing Wnt Signaling and Neurogenesis. Comput. Intell. Neurosci. 2022, 2022, 9325302. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jiang, T.; Tang, W.; Ma, Z.; Pu, K.; Xu, F.; Chang, H.; Zhao, G.; Gao, W.; Li, Y.; et al. Transplantation of Platelet-Derived Mitochondria Alleviates Cognitive Impairment and Mitochondrial Dysfunction in Db/Db Mice. Clin. Sci. 2020, 134, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Javani, G.; Babri, S.; Farajdokht, F.; Ghaffari-Nasab, A.; Mohaddes, G. Mitochondrial Transplantation Improves Anxiety- and Depression-like Behaviors in Aged Stress-Exposed Rats. Mech. Ageing Dev. 2022, 202, 111632. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Tanaka, M.; Nakano, T.; Licastro, E.; Nakamura, Y.; Li, W.; Esposito, E.; Mandeville, E.T.; Chou, S.H.-Y.; Ning, M.; et al. O-GlcNAcylation Is Essential for Therapeutic Mitochondrial Transplantation. Commun. Med. 2023, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, L.; Karimipour, M.; Seyedaghamiri, F.; Abolhasanpour, N.; Sadigh-Eteghad, S.; Mahmoudi, J.; Farhoudi, M. Intranasal Administration of Mitochondria Alleviated Cognitive Impairments and Mitochondrial Dysfunction in the Photothrombotic Model of mPFC Stroke in Mice. J. Stroke Cerebrovasc. Dis. 2022, 31, 106801. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-Y.; Roan, J.-N.; Lee, J.-S.; Chiu, M.-H.; Lin, M.-W.; Liu, C.-C.; Lam, C.-F. Transplantation of Viable Mitochondria Attenuates Neurologic Injury after Spinal Cord Ischemia. J. Thorac. Cardiovasc. Surg. 2021, 161, e337–e347. [Google Scholar] [CrossRef]
- Alway, S.E.; Paez, H.G.; Pitzer, C.R.; Ferrandi, P.J.; Khan, M.M.; Mohamed, J.S.; Carson, J.A.; Deschenes, M.R. Mitochondria Transplant Therapy Improves Regeneration and Restoration of Injured Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2023, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, J.; Ni, H.; Zhang, L.; Wang, J.; Li, Y.; Jiang, W.; Wu, Z.; Zhou, M. Mitochondrial Transplantation Reduces Lower Limb Ischemia-Reperfusion Injury by Increasing Skeletal Muscle Energy and Adipocyte Browning. Mol. Ther.—Methods Clin. Dev. 2023, 31, 101152. [Google Scholar] [CrossRef]
- Nascimento-dos-Santos, G.; de-Souza-Ferreira, E.; Lani, R.; Faria, C.C.; Araújo, V.G.; Teixeira-Pinheiro, L.C.; Vasconcelos, T.; Gonçalo, T.; Santiago, M.F.; Linden, R.; et al. Neuroprotection from Optic Nerve Injury and Modulation of Oxidative Metabolism by Transplantation of Active Mitochondria to the Retina. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165686. [Google Scholar] [CrossRef]
- Strubbe-Rivera, J.O.; Schrad, J.R.; Pavlov, E.V.; Conway, J.F.; Parent, K.N.; Bazil, J.N. The Mitochondrial Permeability Transition Phenomenon Elucidated by Cryo-EM Reveals the Genuine Impact of Calcium Overload on Mitochondrial Structure and Function. Sci. Rep. 2021, 11, 1037. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Stotland, A. MitoTimer: A Novel Protein for Monitoring Mitochondrial Turnover in the Heart. J. Mol. Med. 2015, 93, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.G.; Davies, A.M. The Significance of Mitochondrial DNA Half-Life to the Lifespan of Post-Mitotic Cells; Cell Biology. bioRxiv 2020. [Google Scholar] [CrossRef]
- Krishna, S.; Arrojo, E.; Drigo, R.; Capitanio, J.S.; Ramachandra, R.; Ellisman, M.; Hetzer, M.W. Identification of Long-Lived Proteins in the Mitochondria Reveals Increased Stability of the Electron Transport Chain. Dev. Cell 2021, 56, 2952–2965.e9. [Google Scholar] [CrossRef]
- Patananan, A.N.; Sercel, A.J.; Wu, T.-H.; Ahsan, F.M.; Torres, A.; Kennedy, S.A.L.; Vandiver, A.; Collier, A.J.; Mehrabi, A.; Van Lew, J.; et al. Pressure-Driven Mitochondrial Transfer Pipeline Generates Mammalian Cells of Desired Genetic Combinations and Fates. Cell Rep. 2020, 33, 108562. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Zorzano, A. The Sensing of Mitochondrial DAMPs by Non-Immune Cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef]
- Chernyak, B.V. Mitochondrial Transplantation: A Critical Analysis. Biochemistry 2020, 85, 636–641. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Chu, Y.; Yun, Z.; Yan, Y.; Jin, J. Mitochondrial Transplantation: Opportunities and Challenges in the Treatment of Obesity, Diabetes, and Nonalcoholic Fatty Liver Disease. J. Transl. Med. 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, M.; Morra, F.; Di Meo, I.; Tiranti, V. Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1969. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, M.S.; Marciniak, C.; Eckel-Mahan, K.; McManus, M.; Crimi, M.; Waymire, K.; Lin, C.S.; Masubuchi, S.; Friend, N.; Koike, M.; et al. Heteroplasmy of Mouse mtDNA Is Genetically Unstable and Results in Altered Behavior and Cognition. Cell 2012, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, K.; Dowling, D.K.; Morrow, E.H. Mitochondrial Replacement, Evolution, and the Clinic. Science 2013, 341, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- Dobler, R.; Dowling, D.K.; Morrow, E.H.; Reinhardt, K. A Systematic Review and Meta-Analysis Reveals Pervasive Effects of Germline Mitochondrial Replacement on Components of Health. Hum. Reprod. Update 2018, 24, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Eyre-Walker, A. Mitochondrial Replacement Therapy: Are Mito-Nuclear Interactions Likely To Be a Problem? Genetics 2017, 205, 1365–1372. [Google Scholar] [CrossRef]
- Gupta, R.; Kanai, M.; Durham, T.J.; Tsuo, K.; McCoy, J.G.; Kotrys, A.V.; Zhou, W.; Chinnery, P.F.; Karczewski, K.J.; Calvo, S.E.; et al. Nuclear Genetic Control of mtDNA Copy Number and Heteroplasmy in Humans. Nature 2023, 620, 839–848. [Google Scholar] [CrossRef]
- Ulger, O.; Kubat, G.B. Therapeutic Applications of Mitochondrial Transplantation. Biochimie 2022, 195, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).