Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy
Abstract
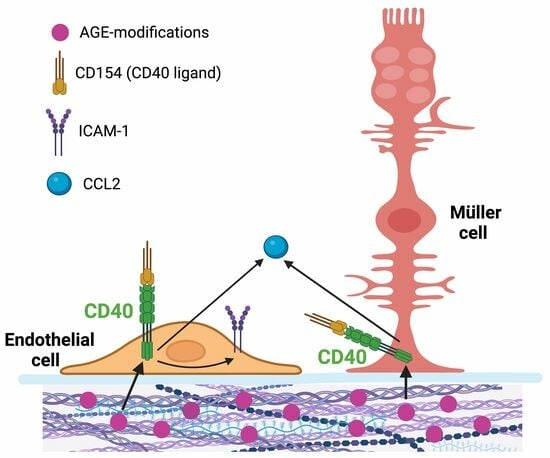
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. In Vitro Stimulation
2.3. Flow Cytometry
2.4. ELISA
2.5. Human Subjects
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Methylglyoxal-Modified Fibronectin and Albumin Upregulate CD40 Expression in Human Endothelial Cells
3.2. MGO-Modified Fibronectin Upregulates CD40 in Primary Human Retinal Müller Cells
3.3. MGO-Modified Fibronectin Enhances Pro-Inflammatory Responses Triggered by CD40 Ligation
3.4. Increased Staining with an Anti-CML Antibody in Extracellular Matrix Proteins Is Associated with Areas of Increased CD40 Expression in Endothelial and Müller Cells in the Retinas of Patients with Diabetic Retinopathy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody/Host | Company/Catalog # | Working Dilution |
|---|---|---|
| CD40/rabbit | Bioss/bs-2929R | 1:100 |
| CML/mouse | Novus/NBP3-11230 | 1:2000 |
| Fibronectin/rabbit | Bioss/bs-0666R | 1:100 |
| Laminin/rabbit | Bioss/bs-0821R | 1:50 |
| Vimentin/chicken | Novus/NB300-223 | 1:300 |
| von Willebrand factor/sheep | Genetex/GTX74137 | 1:30 |
| Alexa Fluor 488/donkey anti-sheep | JacksonImm Res/713-545-147 | 1:400 |
| Alexa Fluor 488/goat anti-chicken | JacksonImm Res/103-545-155 | 1:100 |
| Alexa Fluor 555/goat anti-mouse | Invitrogen/A32727 | 1:1000 |
| Red X/donkey anti-mouse | JacksonImm Res/715-295-151 | 1:100 |
| Alexa Fluor 647/goat anti-rabbit | JacksonImm Res/111-605-144 | 1:400 |
| Alexa Fluor 647/donkey anti rabbit | JacksonImm Res/711-605-152 | 1:1000 |
References
- Mach, F.; Schonbeck, U.; Sukhova, G.K.; Atkinson, E.; Libby, P. Reduction of atherosclerosis in mice by inhibition of CD40 signaling. Nature 1998, 394, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.-A.C.; Greene, J.A.; Okenka, G.; Miao, Y.; Sheibani, N.; Kern, T.S.; Subauste, C.S. CD40 promotes the development of early diabetic retinopathy. Diabetologia 2014, 57, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.-A.C.; Lopez Corcino, Y.; Miao, Y.; Tang, J.; Sheibani, N.; Kern, T.S.; Dubyak, G.R.; Subauste, C.S. CD40 in retinal Muller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy in mice. Diabetes 2017, 66, 483–493. [Google Scholar] [CrossRef]
- Portillo, J.C.; Yu, J.S.; Vos, S.; Bapputty, R.; Lopez Corcino, Y.; Hubal, A.; Daw, J.; Arora, S.; Sun, W.; Lu, Z.R.; et al. Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Muller cells. Diabetologia 2022, 65, 2157–2171. [Google Scholar] [CrossRef]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef]
- Vos, S.; Aaron, R.; Weng, M.; Daw, J.; Rodriguez Rivera, E.; Subauste, C.S. CD40 upregulation in the retina of patients with diabetic retinopathy: Association with TRAF2/TRAF6 upregulation and inflammatory molecule expression. Investig. Ophthalmol. Vis. Sci. 2023, 64, 17. [Google Scholar] [CrossRef]
- Greene, J.A.; Portillo, J.-A.; Lopez Corcino, Y.; Subauste, C.S. CD40-TRAF signaling upregulates CXC3L1 and TNF-a in human aortic endothelial cells but not in retinal endothelial cells. PLoS ONE 2015, 10, e0144133. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Kim, Y. Blood and Tissue Advanced Glycation End Products as Determinants of Cardiometabolic Disorders Focusing on Human Studies. Nutrients 2023, 15, 2002. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Shipanova, I.N.; Faust, F.M. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem. 1996, 271, 19338–19345. [Google Scholar] [CrossRef] [PubMed]
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in three alpha,beta-dicarbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in advanced Maillard reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef]
- Fosmark, D.S.; Torjesen, P.A.; Kilhovd, B.K.; Berg, T.J.; Sandvik, L.; Hanssen, K.F.; Agardh, C.D.; Agardh, E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2006, 55, 232–236. [Google Scholar] [CrossRef]
- Bansal, S.; Burman, A.; Tripathi, A.K. Advanced glycation end products: Key mediator and therapeutic target of cardiovascular complications in diabetes. World J. Diabetes 2023, 14, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Glenn, J.V.; Hsu, D.K.; Liu, F.T.; Gardiner, T.A.; Stitt, A.W. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp. Diabetes Res. 2007, 2007, 51837. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.A.; Stitt, A.W.; Anderson, H.R.; Archer, D.B. Selective loss of vascular smooth muscle cells in the retinal microcirculation of diabetic dogs. Br. J. Ophthalmol. 1994, 78, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and AGE-infused rats. Am. J. Pathol. 1997, 150, 523–531. [Google Scholar] [PubMed]
- Stitt, A.; Gardiner, T.A.; Alderson, N.L.; Canning, P.; Frizzell, N.; Duffy, N.; Boyle, C.; Januszewski, A.S.; Chachich, M.; Baynes, J.W.; et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes 2002, 51, 2826–2832. [Google Scholar] [CrossRef]
- Hammes, H.P.; Alt, A.; Niwa, T.; Clausen, J.T.; Bretzel, R.G.; Brownlee, M.; Schleicher, E.D. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 1999, 42, 728–736. [Google Scholar] [CrossRef]
- Choudhuri, S.; Dutta, D.; Sen, A.; Chowdhury, I.H.; Mitra, B.; Mondal, L.K.; Saha, A.; Bhadhuri, G.; Bhattacharya, B. Role of N-epsilon- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol. Vis. 2013, 19, 100–113. [Google Scholar]
- Warboys, C.M.; Toh, H.B.; Fraser, P.A. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1319–1328. [Google Scholar] [CrossRef]
- Moore, T.C.; Moore, J.E.; Kaji, Y.; Frizzell, N.; Usui, T.; Poulaki, V.; Campbell, I.L.; Stitt, A.W.; Gardiner, T.A.; Archer, D.B.; et al. The role of advanced glycation end products in retinal microvascular leukostasis. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4457–4464. [Google Scholar] [CrossRef]
- Hammes, H.P.; Martin, S.; Federlin, K.; Geisen, K.; Brownlee, M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc. Natl. Acad. Sci. USA 1991, 88, 11555–11558. [Google Scholar] [CrossRef]
- Barile, G.R.; Pachydaki, S.I.; Tari, S.R.; Lee, S.E.; Donmoyer, C.M.; Ma, W.; Rong, L.L.; Buciarelli, L.G.; Wendt, T.; Horig, H.; et al. The RAGE axis in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Kaji, Y.; Usui, T.; Ishida, S.; Yamashiro, K.; Moore, T.C.; Moore, J.; Yamamoto, Y.; Yamamoto, H.; Adamis, A.P. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Investig. Ophthalmol. Vis. Sci. 2007, 48, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Gangadhariah, M.H.; Mailankot, M.; Reneker, L.; Nagaraj, R.H. Inhibition of methylglyoxal-mediated protein modification in glyoxalase I overexpressing mouse lenses. J. Ophthalmol. 2010, 2010, 274317. [Google Scholar] [CrossRef] [PubMed]
- Tarsio, J.F.; Reger, L.A.; Furcht, L.T. Decreased interaction of fibronectin, type IV collagen, and heparin due to nonenzymatic glycation. Implications for diabetes mellitus. Biochemistry 1987, 26, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Katsuzaki, T.; Miyazaki, S.; Miyazaki, T.; Ishizaki, Y.; Hayase, F.; Tatemichi, N.; Takei, Y. Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J. Clin. Investig. 1997, 99, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Guillausseau, P.J. Diabetes, advanced glycation endproducts and vascular disease. Vasc. Med. 1998, 3, 131–137. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef]
- Ramalanjaona, G.; Kempczinski, R.F.; Rosenman, J.E.; Douville, E.C.; Silberstein, E.B. The effect of fibronectin coating on endothelial cell kinetics in polytetrafluoroethylene grafts. J. Vasc. Surg. 1986, 3, 264–272. [Google Scholar] [CrossRef]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, J.; Wiemann, S.; Hildebrandt, S.; Faissner, A. Extracellular Matrix Remodeling in the Retina and Optic Nerve of a Novel Glaucoma Mouse Model. Biology 2021, 10, 169. [Google Scholar] [CrossRef]
- Yellin, M.J.; D’Agati, V.; Parkinson, G.; Han, A.S.; Szema, A.; Baum, D.; Estes, D.; Szabolcs, M.; Chess, L. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritis. Arthritis Rheum. 1997, 40, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Bruemmer, D.; Riggers, U.; Holzmeister, J.; Grill, M.; Lippek, F.; Settmacher, U.; Regitz-Zagrosek, V.; Fleck, E.; Graf, K. Expression of CD40 in vascular smooth muscle cells and macrophages is associated with early development of human atherosclerotic lesions. Am. J. Cardiol. 2001, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, Y.; Ma, J.; Niu, L.; Tay, F.R. Clinical/Translational Aspects of Advanced Glycation End-Products. Trends Endocrinol. Metab. 2019, 30, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.J.; Yu, J.; Wang, H.J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Yamagishi, S.; Amano, S.; Inagaki, Y.; Okamoto, T.; Koga, K.; Sasaki, N.; Yamamoto, H.; Takeuchi, M.; Makita, Z. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem. Biophys. Res. Commun. 2002, 290, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Redaelli, A.; Buehler, M.J.; Vesentini, S. Age- and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in Advanced Glycation End-products. Matrix Biol. 2014, 34, 89–95. [Google Scholar] [CrossRef]
- Thompson, K.; Chen, J.; Luo, Q.; Xiao, Y.; Cummins, T.R.; Bhatwadekar, A.D. Advanced glycation end (AGE) product modification of laminin downregulates Kir4.1 in retinal Muller cells. PLoS ONE 2018, 13, e0193280. [Google Scholar] [CrossRef]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Takahashi, H.K.; Mori, S.; Wake, H.; Liu, K.; Yoshino, T.; Ohashi, K.; Tanaka, N.; Shikata, K.; Makino, H.; Nishibori, M. Advanced glycation end products subspecies-selectively induce adhesion molecule expression and cytokine production in human peripheral blood mononuclear cells. J. Pharmacol. Exp. Ther. 2009, 330, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Shikata, K.; Hironaka, K.; Kushiro, M.; Yamasaki, Y.; Sugimoto, H.; Ota, Z.; Araki, N.; Horiuchi, S. Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy. Kidney Int. 1995, 48, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Takeya, M.; Mori, T.; Araki, N.; Suzuki, H.; Horiuchi, S.; Kodama, T.; Miyauchi, Y.; Takahashi, K. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am. J. Pathol. 1995, 147, 654–667. [Google Scholar] [PubMed]
- Vlassara, H.; Brownlee, M.; Cerami, A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1981, 78, 5190–5192. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Yagihashi, S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann. N. Y. Acad. Sci. 2005, 1043, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, P.J.; Wheelock, K.M.; Howell, S.; Weil, E.J.; Tanamas, S.K.; Knowler, W.C.; Lemley, K.V.; Mauer, M.; Yee, B.; Nelson, R.G.; et al. Advanced Glycation End Products Predict Loss of Renal Function and Correlate with Lesions of Diabetic Kidney Disease in American Indians with Type 2 Diabetes. Diabetes 2016, 65, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Zhang, S.; Breidenbach, J.D.; Russell, B.H.; George, J.; Haller, S.T. CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease. J. Clin. Med. 2020, 9, 3653. [Google Scholar] [CrossRef]
- Kan, H.W.; Hsieh, J.H.; Chien, H.F.; Lin, Y.H.; Yeh, T.Y.; Chao, C.C.; Hsieh, S.T. CD40-mediated HIF-1alpha expression underlying microangiopathy in diabetic nerve pathology. Dis. Model. Mech. 2018, 11, dmm033647. [Google Scholar] [CrossRef] [PubMed]





| Diagnosis | Age/Sex | Other Clinical Information |
|---|---|---|
| Diabetic retinopathy | 58/M | Gangrene and stroke |
| Diabetic retinopathy | 71/M | Myocardial infarction, end-stage renal disease, diabetic neuropathy, gastro-intestinal bleed, and hyperlipidemia |
| Diabetic retinopathy | 73/F | Myocardial infarction, heart failure, hypertension, and hyperlipidemia |
| Diabetic retinopathy | 69/M | Myocardial infarction, ischemic cardiomyopathy, hypertension, stroke, and hyperlipidemia |
| Proliferative diabetic retinopathy | 73/M | Gastrointestinal bleed, myocardial infarction, and hyperlipidemia |
| Proliferative diabetic retinopathy | 73/M | Myocardial infarction, chronic kidney disease, hypertension, and hyperlipidemia |
| Proliferative diabetic retinopathy | 63/M | Coronary artery disease, heart failure, hypertension, and pneumonia |
| Proliferative diabetic retinopathy | 58/F | Myocardial infarction, heart failure, chronic kidney disease, and hyperlipidemia |
| Non-diabetic | 78/M | Myocardial infarction, hypertension, hyperlipidemia, and alcoholism |
| Non-diabetic | 61/M | Myocardial infarction, hyperlipidemia, and hypertension |
| Non-diabetic | 53/M | Lung cancer and hypertension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portillo, J.-A.C.; Pfaff, A.; Vos, S.; Weng, M.; Nagaraj, R.H.; Subauste, C.S. Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy. Cells 2024, 13, 429. https://doi.org/10.3390/cells13050429
Portillo J-AC, Pfaff A, Vos S, Weng M, Nagaraj RH, Subauste CS. Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy. Cells. 2024; 13(5):429. https://doi.org/10.3390/cells13050429
Chicago/Turabian StylePortillo, Jose-Andres C., Amelia Pfaff, Sarah Vos, Matthew Weng, Ram H. Nagaraj, and Carlos S. Subauste. 2024. "Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy" Cells 13, no. 5: 429. https://doi.org/10.3390/cells13050429
APA StylePortillo, J.-A. C., Pfaff, A., Vos, S., Weng, M., Nagaraj, R. H., & Subauste, C. S. (2024). Advanced Glycation End Products Upregulate CD40 in Human Retinal Endothelial and Müller Cells: Relevance to Diabetic Retinopathy. Cells, 13(5), 429. https://doi.org/10.3390/cells13050429