Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
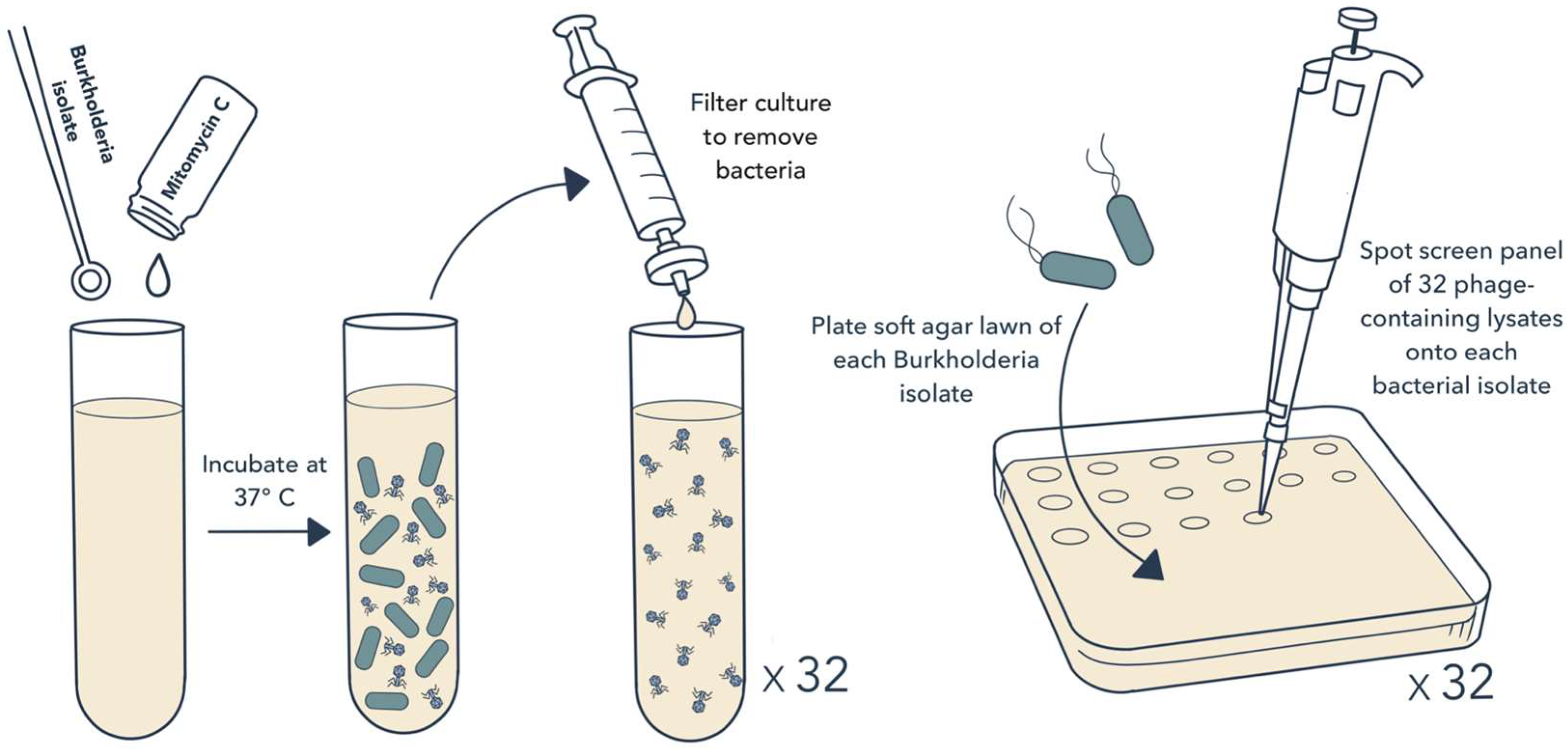
2.1. Bacterial Isolates and Induction of Prophage Release
2.2. Isolation of Phages and Host Range Testing
2.3. Whole Genome Sequencing and Analysis
2.4. EM Imaging
2.5. Statistical Analysis
3. Results
3.1. Prophage Induction and Isolation
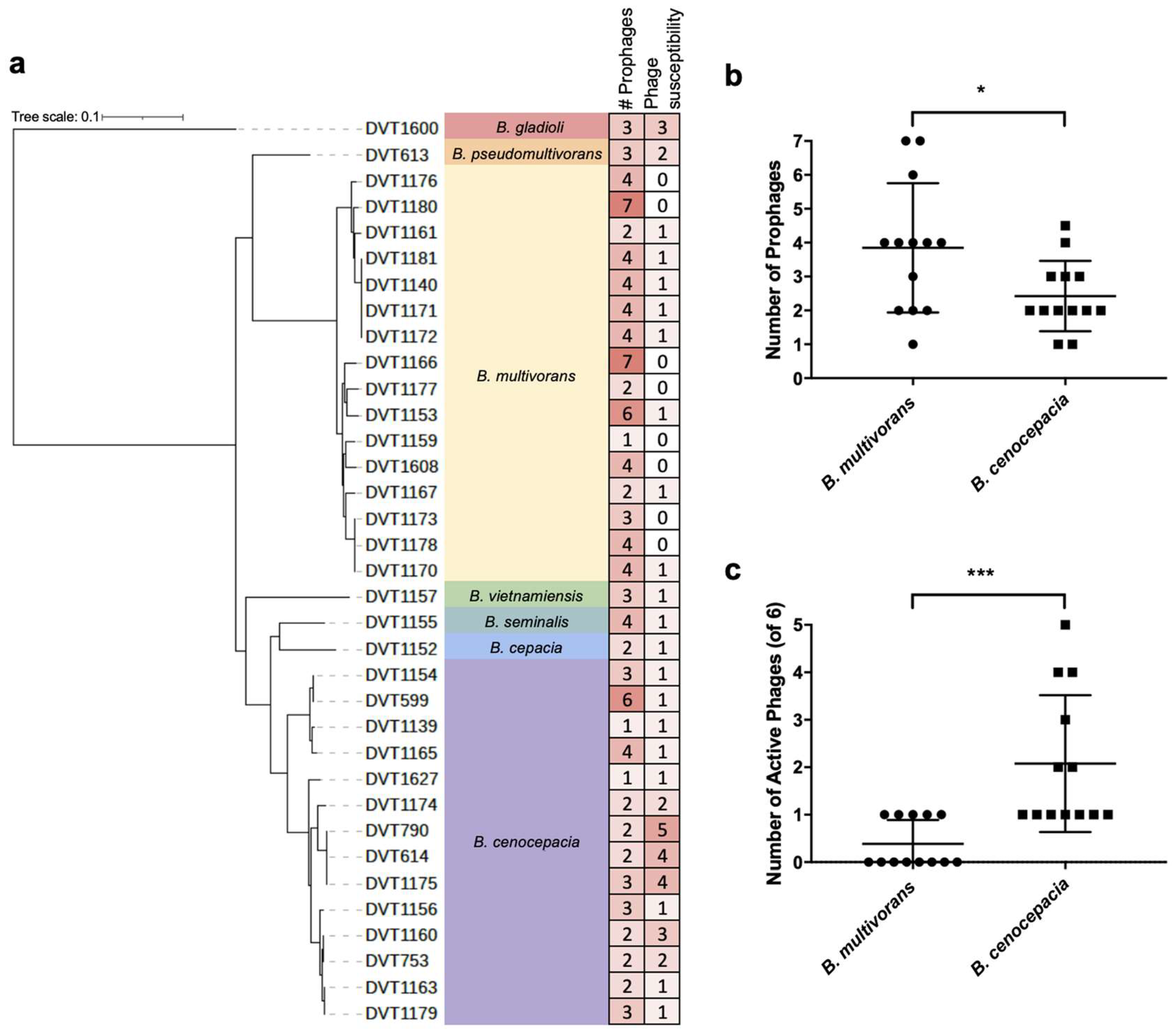
3.2. Whole Genome Sequencing of Burkholderia spp. Clinical Isolates
3.3. Phage Host-Range Screening
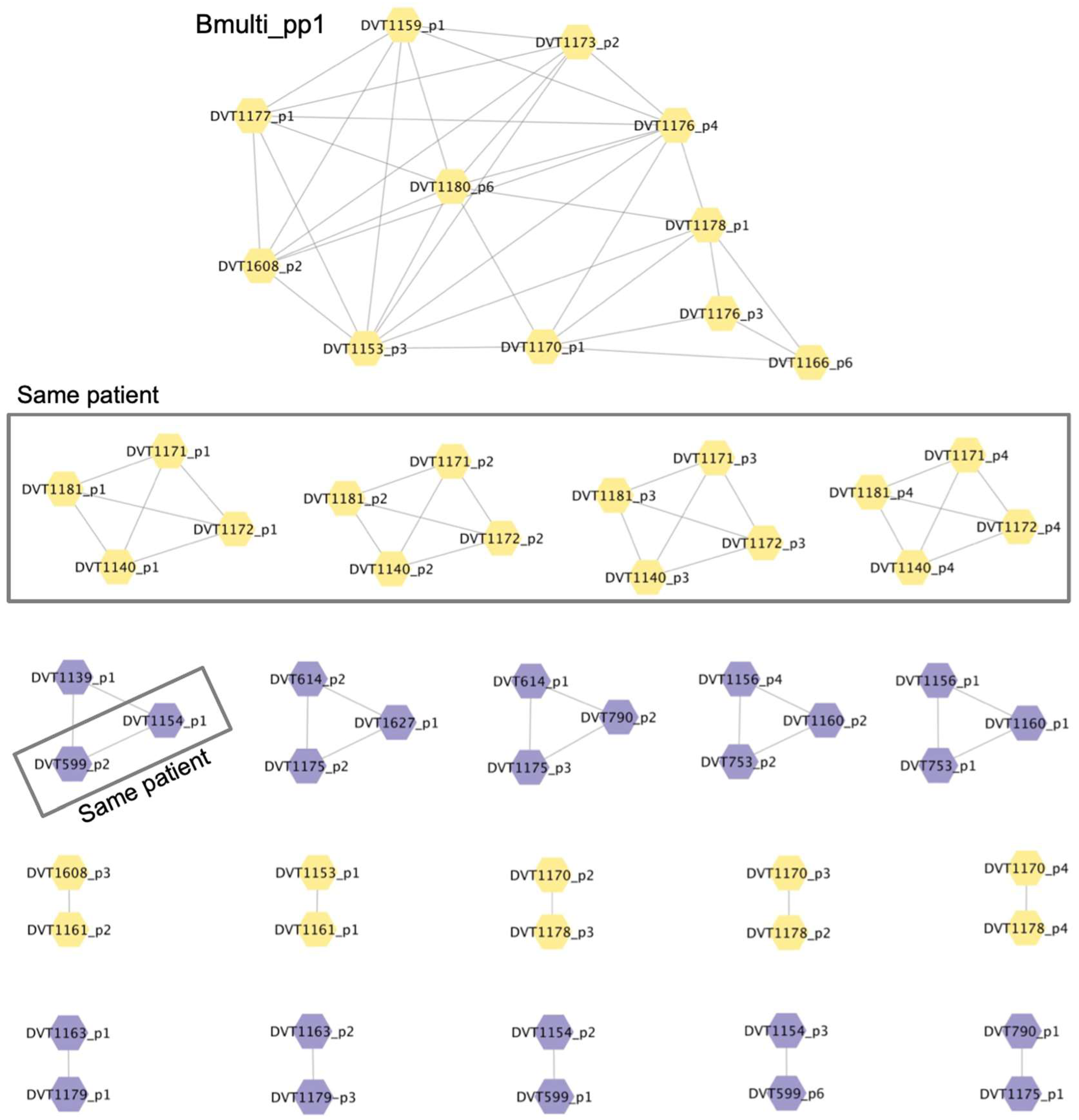
3.4. Whole Genome Sequencing of Phages
3.5. Analysis of Prophage Carriage in Burkholderia spp. Clinical Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular Signatures and Phylogenomic Analysis of the Genus Burkholderia: Proposal for Division of This Genus into the Emended Genus Burkholderia Containing Pathogenic Organisms and a New Genus Paraburkholderia Gen. Nov. Harboring Environmental Species. Front. Genet. 2014, 5, 429. [Google Scholar] [CrossRef]
- Coenye, T.; Vandamme, P.; Govan, J.R.W.; LiPuma, J.J. Taxonomy and Identification of the Burkholderia cepacia Complex. J. Clin. Microbiol. 2001, 39, 3427–3436. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The Multifarious, Multireplicon Burkholderia cepacia Complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef]
- Somayaji, R.; Yau, Y.C.W.; Tullis, E.; LiPuma, J.J.; Ratjen, F.; Waters, V. Clinical Outcomes Associated with Burkholderia cepacia Complex Infection in Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Lauman, P.; Dennis, J.J. Advances in Phage Therapy: Targeting the Burkholderia cepacia Complex. Viruses 2021, 13, 1331. [Google Scholar] [CrossRef] [PubMed]
- LiPuma, J.J.; Spilker, T.; Gill, L.H.; Campbell, P.W., 3rd; Liu, L.; Mahenthiralingam, E. Disproportionate Distribution of Burkholderia cepacia Complex Species and Transmissibility Markers in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2001, 164, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.; Deretic, V. Microbial Pathogenesis in Cystic Fibrosis: Mucoid Pseudomonas Aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Dodd, M.; Webb, A. Burkholderia cepacia: Current Clinical Issues, Environmental Controversies and Ethical Dilemmas. Eur. Respir. J. 2001, 17, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Branstetter, J.W.; Yarbrough, A.; Poole, C. Management of Cepacia Syndrome with a Combination of Intravenous and Inhaled Antimicrobials in a Non-Cystic Fibrosis Pediatric Patient. J. Pediatr. Pharmacol. Ther. 2020, 25, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Zahariadis, G.; Levy, M.H.; Burns, J.L. Cepacia-Like Syndrome Caused by Burkholderia multivorans. Can. J. Infect. Dis. 2003, 14, 123–125. [Google Scholar] [PubMed]
- Burns, J.L.; Wadsworth, C.D.; Barry, J.J.; Goodall, C.P. Nucleotide Sequence Analysis of a Gene from Burkholderia (Pseudomonas) cepacia Encoding an Outer Membrane Lipoprotein Involved in Multiple Antibiotic Resistance. Antimicrob. Agents Chemother. 1996, 40, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Peptide Antibiotics. Lancet 1997, 349, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Kidd, T.J.; Williams, B. Combination Aerosol Therapy to Treat Burkholderia cepacia Complex. Eur. Respir. J. 2005, 26, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered Bacteriophages for Treatment of a Patient with a Disseminated Drug-Resistant Mycobacterium Abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e0207121. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early Clinical Experience of Bacteriophage Therapy in 3 Lung Transplant Recipients. Am. J. Transplant. 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Smith, M. Salt in My Soul: An Unfinished Life; Random House: New York, NY, USA, 2019. [Google Scholar]
- Haidar, G.; Chan, B.K.; Cho, S.; Kramer, K.H.; Nordstrom, H.R.; Wallace, N.R.; Stellfox, M.E.; Holland, M.; Kline, E.G.; Kozar, J.M.; et al. Phage Therapy in a Lung Transplant Recipient with Cystic Fibrosis Infected with Multidrug-Resistant Burkholderia Multivorans. Transpl. Infect. Dis. 2023, 25, e14041. [Google Scholar] [CrossRef]
- Roszniowski, B.; Latka, A.; Maciejewska, B.; Vandenheuvel, D.; Olszak, T.; Briers, Y.; Holt, G.S.; Valvano, M.A.; Lavigne, R.; Smith, D.L.; et al. The Temperate Burkholderia Phage Ap3 of the Peduovirinae Shows Efficient Antimicrobial Activity against B. Cenocepacia of the Iiia Lineage. Appl. Microbiol. Biotechnol. 2017, 101, 1203–1216. [Google Scholar] [CrossRef]
- Seed, K.D.; Dennis, J.J. Experimental Bacteriophage Therapy Increases Survival of Galleria Mellonella Larvae Infected with Clinically Relevant Strains of the Burkholderia cepacia Complex. Antimicrob. Agents Chemother. 2009, 53, 2205–2208. [Google Scholar] [CrossRef]
- Carmody, L.A.; Gill, J.J.; Summer, E.J.; Sajjan, U.S.; Gonzalez, C.F.; Young, R.F.; LiPuma, J.J. Efficacy of Bacteriophage Therapy in a Model of Burkholderia cenocepacia Pulmonary Infection. J. Infect. Dis. 2010, 201, 264–271. [Google Scholar] [CrossRef]
- Semler, D.D.; Goudie, A.D.; Finlay, W.H.; Dennis, J.J. Aerosol Phage Therapy Efficacy in Burkholderia cepacia Complex Respiratory Infections. Antimicrob. Agents Chemother. 2014, 58, 4005–4013. [Google Scholar] [CrossRef]
- Roszniowski, B.; McClean, S.; Drulis-Kawa, Z. Burkholderia cenocepacia Prophages-Prevalence, Chromosome Location and Major Genes Involved. Viruses 2018, 10, 297. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Garlena, R.A.; Russell, D.A.; Aull, H.G.; Mahalingam, V.; Divens, A.M.; Guerrero-Bustamante, C.A.; Zack, K.M.; Abad, L.; et al. Mycobacterium Abscessus Strain Morphotype Determines Phage Susceptibility, the Repertoire of Therapeutically Useful Phages, and Phage Resistance. mBio 2021, 12. [Google Scholar] [CrossRef]
- Raya, R.R.; H’Bert, M. Isolation of Phage Via Induction of Lysogens. Methods Mol. Biol. 2009, 501, 23–32. [Google Scholar]
- Sundermann, A.J.; Chen, J.; Kumar, P.; Ayres, A.M.; Cho, S.T.; Ezeonwuka, C.; Griffith, M.P.; Miller, J.K.; Mustapha, M.M.; Pasculle, A.W.; et al. Whole-Genome Sequencing Surveillance and Machine Learning of the Electronic Health Record for Enhanced Healthcare Outbreak Detection. Clin. Infect. Dis. 2022, 75, 476–482. [Google Scholar] [CrossRef]
- Russell, D.A.; Clokie, M.R.J.; Kropinski, A.M.; Lavigne, R. Bacteriophages: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. Spades: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The Rast Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. Raxml Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez, R.L.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput Ani Analysis of 90k Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Marcu, A.; Liang, Y.; Wishart, D.S. Phast, Phaster and Phastest: Tools for Finding Prophage in Bacterial Genomes. Brief. Bioinform. 2017, 20, 1560–1567. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Mahony, J.; Hanemaaijer, L.; Kouwen, T.R.H.M.; Neve, H.; MacSharry, J.; van Sinderen, D. Detecting Lactococcus Lactis Prophages by Mitomycin C-Mediated Induction Coupled to Flow Cytometry Analysis. Front. Microbiol. 2017, 8, 1343. [Google Scholar] [CrossRef]
- Otsuji, N.; Sekiguchi, M.; Iijima, T.; Takagi, Y. Induction of Phage Formation in the Lysogenic Escherichia Coli K-12 by Mitomycin C. Nature 1959, 184 (Suppl. S14), 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Bondy-Denomy, J.; Qian, J.; Westra, E.R.; Buckling, A.; Guttman, D.S.; Davidson, A.R.; Maxwell, K.L. Prophages Mediate Defense against Phage Infection through Diverse Mechanisms. ISME J. 2016, 10, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Jacobs-Sera, D.; Bustamante, C.A.G.; Garlena, R.A.; Mavrich, T.N.; Pope, W.H.; Reyes, J.C.C.; Russell, D.A.; Adair, T.; Alvey, R.; et al. Prophage-Mediated Defence against Viral Attack and Viral Counter-Defence. Nat. Microbiol. 2017, 2, 16251. [Google Scholar] [CrossRef]
- Harrison, E.; Brockhurst, M.A. Ecological and Evolutionary Benefits of Temperate Phage: What Does or Doesn’t Kill You Makes You Stronger. BioEssays 2017, 39, 1700112. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in Nature: Mechanisms, Impact and Ecology of Temperate Phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef]
- Tomasz, M. Mitomycin C: Small, Fast and Deadly (but Very Selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar] [CrossRef]
- Lynch, K.H.; Seed, K.D.; Stothard, P.; Dennis, J.J. Inactivation of Burkholderia cepacia Complex Phage Ks9 Gp41 Identifies the Phage Repressor and Generates Lytic Virions. J. Virol. 2010, 84, 1276–1288. [Google Scholar] [CrossRef]
- Yao, G.; Le, T.; Korn, A.M.; Peterson, H.N.; Liu, M.; Gonzalez, C.F.; Gill, J.J. Phage Milagro: A Platform for Engineering a Broad Host Range Virulent Phage for Burkholderia. J. Virol. 2023, 97, e0085023. [Google Scholar] [CrossRef]
- Lauman, P.; Dennis, J.J. Synergistic Interactions among Burkholderia cepacia Complex-Targeting Phages Reveal a Novel Therapeutic Role for Lysogenization-Capable Phages. Microbiol. Spectr. 2023, 11, e0443022. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Fatima, R.; Hynes, A.P. Temperate Phage-Antibiotic Synergy Eradicates Bacteria through Depletion of Lysogens. Cell Rep. 2021, 35, 109172. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D.; Dennis, J.J. Isolation and Characterization of Bacteriophages of the Burkholderia cepacia Complex. FEMS Microbiol. Lett. 2005, 251, 273–280. [Google Scholar] [CrossRef]
- Langley, R.; Kenna, D.T.; Vandamme, P.; Ure, R.; Govan, J.R.W. Lysogeny and Bacteriophage Host Range within the Burkholderia cepacia Complex. J. Med. Microbiol. 2003, 52 Pt 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Weiser, R.; Yap, Z.L.; Otter, A.; Jones, B.V.; Salvage, J.; Parkhill, J.; Mahenthiralingam, E. A Novel Inducible Prophage from Burkholderia Vietnamiensis G4 Is Widely Distributed across the Species and Has Lytic Activity against Pathogenic Burkholderia. Viruses 2020, 12, 601. [Google Scholar] [CrossRef]


| Phage ID | Source Isolate | Source Species | Length (bp) | GC % | Predicted Genus 1 | NCBI Similar Phage 2 |
|---|---|---|---|---|---|---|
| BCC02/03/04 | DVT1180 | B. multivorans | 34,126 | 64.2 | Peduovirinae; Kisquinquevirus | Burkholderia Phage KS5 (GU911303.1) |
| BCC05/06 | DVT1166 | B. multivorans | 30,957 | 62.8 | Peduoviridae; Duodecimduovirus | Burkholderia Phage phiE12-2 (NC_009236.1) |
| BCC07 | DVT1155 | B. seminalis | 38,216 | 66.7 | Peduoviridae; Aptresvirus | Burkholderia Phage Mana (NC_055863.1) |
| BCC08 | DVT1155 | B. seminalis | 28,709 | 63.7 | Peduoviridae; Kayeltresvirus | Burkholderia Phage KL3 (GU911304.1) |
| Bch7 | Env 3 | - | 68,166 | 54.7 | Bcepfunavirus | Burkholderia Phage Maja (MT708549.1) |
| DSMZ 107315 | Env 3 | - | 22,967 | 61.5 | Peduovirinae; Kisquattuordecimvirus | Burkholderia Phage FLC5 (NC_055722.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordstrom, H.R.; Griffith, M.P.; Rangachar Srinivasa, V.; Wallace, N.R.; Li, A.; Cooper, V.S.; Shields, R.K.; Van Tyne, D. Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential. Cells 2024, 13, 428. https://doi.org/10.3390/cells13050428
Nordstrom HR, Griffith MP, Rangachar Srinivasa V, Wallace NR, Li A, Cooper VS, Shields RK, Van Tyne D. Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential. Cells. 2024; 13(5):428. https://doi.org/10.3390/cells13050428
Chicago/Turabian StyleNordstrom, Hayley R., Marissa P. Griffith, Vatsala Rangachar Srinivasa, Nathan R. Wallace, Anna Li, Vaughn S. Cooper, Ryan K. Shields, and Daria Van Tyne. 2024. "Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential" Cells 13, no. 5: 428. https://doi.org/10.3390/cells13050428
APA StyleNordstrom, H. R., Griffith, M. P., Rangachar Srinivasa, V., Wallace, N. R., Li, A., Cooper, V. S., Shields, R. K., & Van Tyne, D. (2024). Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential. Cells, 13(5), 428. https://doi.org/10.3390/cells13050428









