Vibration Rather than Microgravity Affects Bone Metabolism in Adult Zebrafish Scale Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethic Statement
2.3. Microgravity Treatment
2.4. Samples Collection
2.5. Bone Matrix Staining
2.6. Histological and Biochemical TRAP Activity
2.7. Cortisol Quantification
2.8. Statistics
3. Results
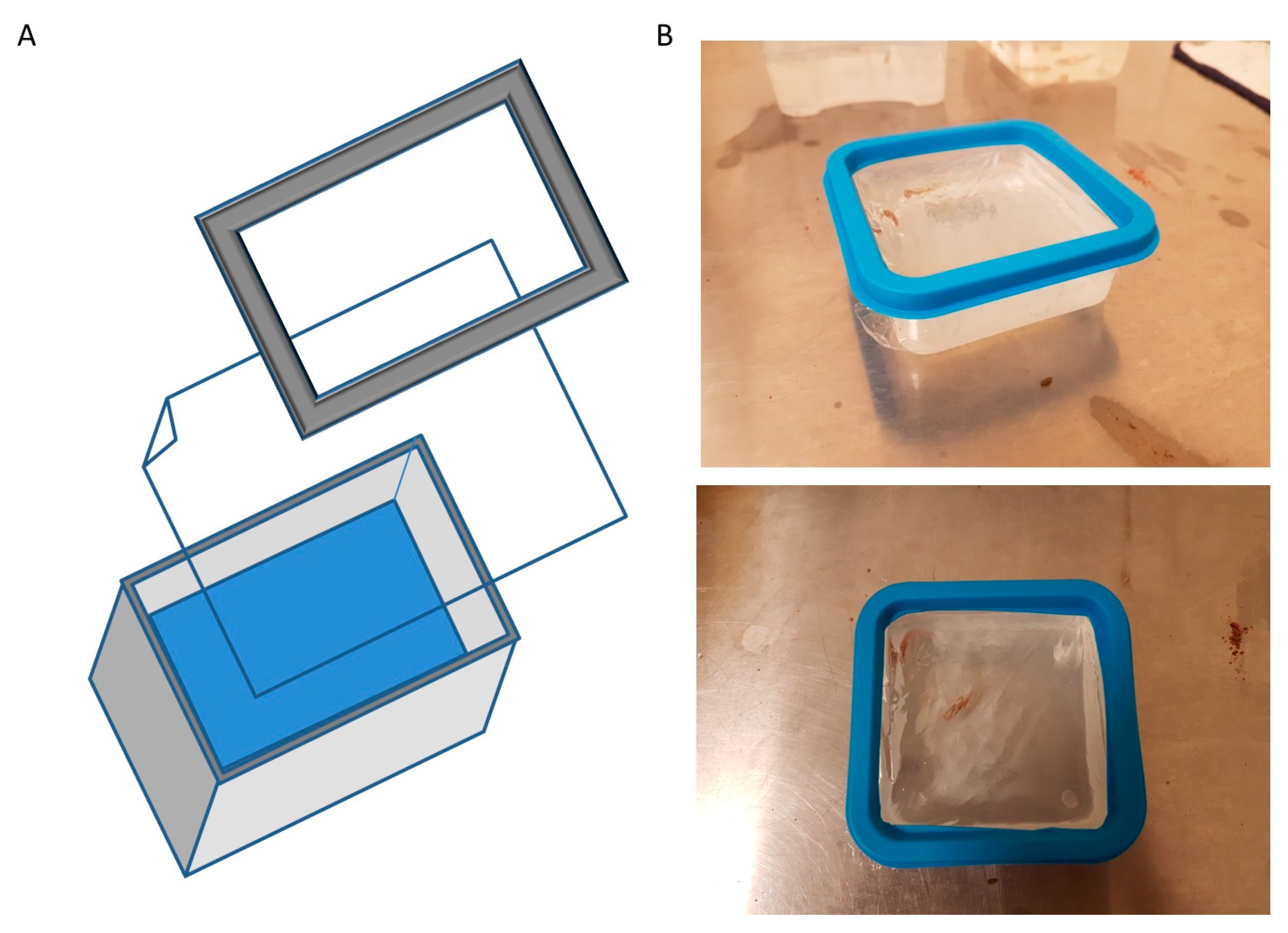
3.1. Microgravity Fish-Box
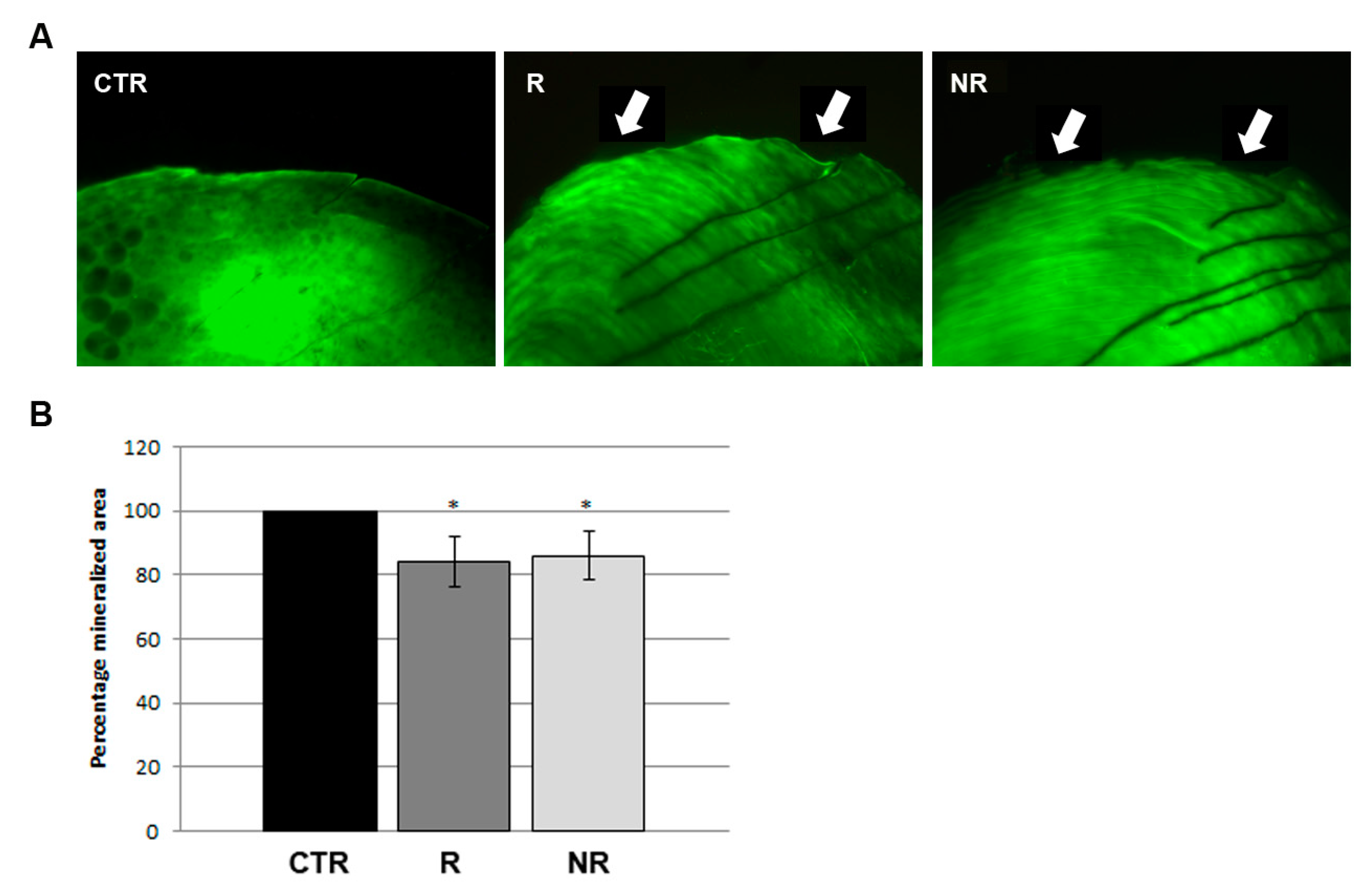
3.2. Microgravity Simulation Was Associated to Mineral Matrix Loss in Zebrafish Scale
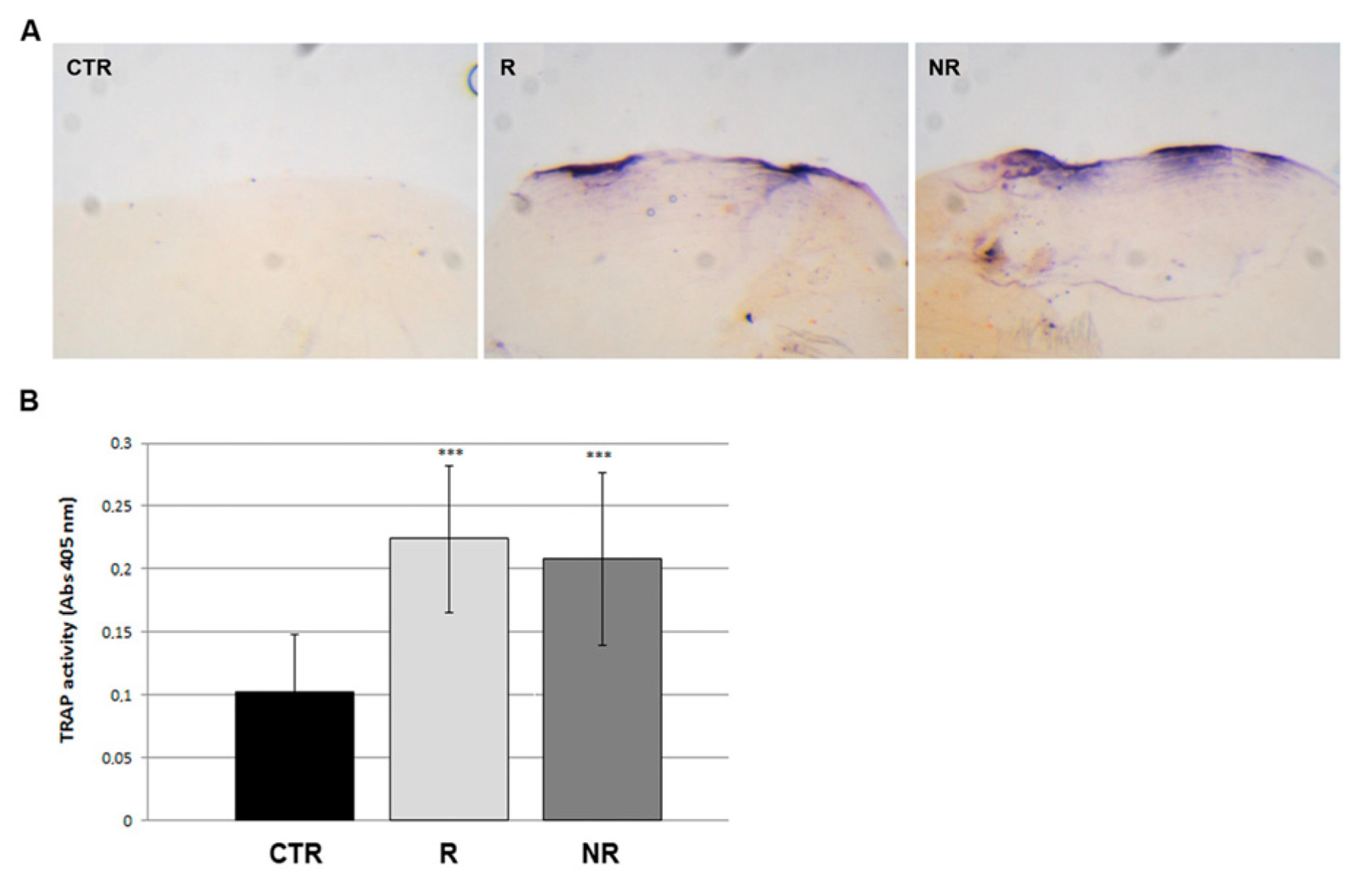
3.3. Bone Resorption Was Promoted by Osteoclast Activity in Scales after Simulated Microgravity
3.4. Bone Loss Phenotype Was Not Correlated to Cortisol Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S.V. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity 2020, 6, 13. [Google Scholar] [CrossRef]
- Montagna, G.; Pani, G.; Flinkman, D.; Cristofaro, F.; Pascucci, B.; Massimino, L.; Lamparelli, L.A.; Fassina, L.; James, P.; Coffey, E.; et al. Long-term osteogenic differentiation of human bone marrow stromal cells in simulated microgravity: Novel proteins sighted. Cell Mol. Life Sci. 2022, 79, 536. [Google Scholar] [CrossRef]
- Ren, Z.; Harriot, A.D.; Mair, D.B.; Chung, M.K.; Lee, P.H.U.; Kim, D.H. Biomanufacturing of 3D Tissue Constructs in Microgravity and their Applications in Human Pathophysiological Studies. Adv. Healthc. Mater. 2023, 12, e2300157. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading of growing rats: A model for predicting skeletal changes during space flight. Bone 1998, 22, 83S–88S. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Nday, C.M.; Frantzidis, C.; Jackson, G.; Bamidis, P.; Kourtidou-Papadeli, C. Neurophysiological changes in simulated microgravity: An animal model. Neurol. India 2019, 67 (Suppl. S2), S221–S226. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Aceto, J.; Dalcq, J.; Alestrom, P.; Nourizadeh-Lillabadi, R.; Goerlich, R.; Schiller, V.; Winkler, C.; Renn, J.; Eberius, M.; et al. Small fish species as powerful model systems to study vertebrate physiology in space. J. Gravit. Physiol. 2008, 15, 253–254. [Google Scholar]
- Rahmann, H.; Anken, R.H. Gravity related research with fishes—Perspectives in regard to the upcoming International Space Station, ISS. Space Life Sci. 2002, 30, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Slenzka, K.; Appel, R.; Rahmann, H. Development and altered gravity dependent changes in glucose-6-phosphate dehydrogenase activity in the brain of the cichlid fish Oreochromis mossambicus. Neurochem. Int. 1995, 26, 579–585. [Google Scholar] [CrossRef]
- Brungs, S.; Hauslage, J.; Hilbig, R.; Hemmersbach, R.; Anken, R. Effects of simulated weightlessness on fish otolith. Adv. Space Res. 2011, 48, 792–798. [Google Scholar] [CrossRef]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Bradamante, S.; Maier, J.A.; Alestrom, P.; van Loon, J.J.; Muller, M. Effects of microgravity simulation on zebrafish transcriptomes and bone physiology-exposure starting at 5 days post fertilization. NPJ Microgravity 2016, 2, 16010. [Google Scholar] [CrossRef]
- Renn, J.; Winkler, C.; Schartl, M.; Fischer, R.; Goerlich, R. Zebrafish and medaka as models for bone research including implications regarding space-related issues. Protoplasma 2006, 229, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Marée, R.; Dardenne, N.; Jeanray, N.; Wehenkel, L.; Aleström, P.; van Loon, J.J.; Muller, M. Zebrafish Bone and General Physiology Are Differently Affected by Hormones or Changes in Gravity. PLoS ONE 2015, 10, e0126928. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.A.; Aggleton, J.; van Loon, J.; Godivier, J.; Harniman, R.; Pei, J.; Nowlan, N.; Hammond, C. Exposure to hypergravity during zebrafish development alters cartilage material properties and strain distribution. Bone Jt. Res. 2021, 10, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Edsall, S.C. Long-Term Effects of Simulated Microgravity and Vibration Exposure on Skeletal Development in Zebrafish. Stem Cells Dev. 2018, 27, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together. Biomed. Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Borst, A.G.; Van Loon, J.W.A. Technology and developments for the random positioning machine, RPM. Microgravity Sci. Tecnol. 2009, 21, 287–292. [Google Scholar] [CrossRef]
- Brungs, S.; Hauslage, J.; Hemmersbach, R. Validation of Random Positioning Versus Clinorotation Using a Macrophage Model System. Microgravity Sci. Technol. 2019, 31, 223–230. [Google Scholar] [CrossRef]
- Pedroso, G.L.; Hammes, T.O.; Escobar, T.D.; Fracasso, L.B.; Forgiarini, L.F.; da Silveira, T.R. Blood Collection for Biochemical Analysis in Adult Zebrafish. J. Vis. Exp. 2012, 63, e3865. [Google Scholar]
- Persson, P.; Takagi, Y.; Bjornsson, B. Tartrate resistant acid phosphatase as a marker for scale resorption in rainbow trout, Oncorhynchus mykiss: Effects of estradiol-17° treatment and refeeding. Fish Physiol. Biochem. 1995, 14, 329–339. [Google Scholar] [CrossRef]
- Masukawa, M.; Ochiai, T.; Kamigaichi, S.; Ishioka, N.; Uchida, S.; Kono, Y.; Sakimura, T. NASDA next generation Aquatic Habitat for Space Shuttle and ISS. Adv. Space Res. 2003, 32, 1541–1546. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ikegame, M.; Furusawa, Y.; Tabuchi, Y.; Hatano, K.; Watanabe, K.; Kawago, U.; Hirayama, J.; Yano, S.; Sekiguchi, T.; et al. Osteoclastic and Osteoblastic Responses to Hypergravity and Microgravity: Analysis Using Goldfish Scales as a Bone Model. Zoolog. Sci. 2022, 39, 388–396. [Google Scholar] [CrossRef]
- Turko, A.J.; Kültz, D.; Fudge, D.; Croll, R.P.; Smith, F.M.; Stoyek, M.R.; Wright, P.A. Skeletal stiffening in an amphibious fish out of water is a response to increased body weight. J. Exp. Biol. 2017, 220, 3621–3631. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Rabey, K.N.; Li, Y.; Norton, J.N.; Reynolds, R.P.; Schmitt, D. Vibrating Frequency Thresholds in Mice and Rats: Implications for the Effects of Vibrations on Animal Health. Ann. Biomed. Eng. 2015, 43, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Prisby, R.D.; Lafage-Proust, M.H.; Malaval, L.; Belli, A.; Vico, L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models: What we know and what we need to know. Ageing Res. Rev. 2008, 7, 319–329. [Google Scholar] [CrossRef]
- Johanning, E. Hole-body vibration-related health disorders in occupational medicine—An international comparison. Ergonomics 2015, 58, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.D.J.; de Oliveira, R.G.; de Oliveira, L.C.; Santos-Filho, S.D.; Sá-Caputo, D.C.; Bernardo-Filho, M. Effectiveness of whole-body vibration on bone mineral density in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2022, 34, 29–52. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Li, W.; Wang, J.; Gao, L.; Li, S. The Impact of Whole-Body Vibration Training on Bone Minerals and Lean Mass in Children and Adolescents with Motor Disabilities: A Systematic Review and Meta-Analysis. Children 2022, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.E.; Wilzman, A.R.; Murdock, K.E.; Troy, K.L. Effect of External Mechanical Stimuli on Human Bone: A narrative review. Prog. Biomed. Eng. 2022, 4, 012006. [Google Scholar] [CrossRef] [PubMed]
- Jeradi, S.; Franz-Odendaal, T.A. Vibration exposure uncovers a critical early developmental window for zebrafish caudal fin development. Dev. Genes. Evol. 2022, 232, 67–79. [Google Scholar] [CrossRef]
- Yano, S.; Kitamura, K.; Satoh, Y.; Nakano, M.; Hattori, A.; Sekiguchi, T.; Ikegame, M.; Nakashima, H.; Omori, K.; Hayakawa, K.; et al. Static and dynamic hypergravity responses of osteoblasts and osteoclasts in medaka scales. Zoolog. Sci. 2013, 30, 217–223. [Google Scholar] [CrossRef]
- Tryggvason, B.V.; Duval, W.M.B.; Smith, R.W.; Rezkallah, K.S.; Varma, S.; Redden, R.F.; Herring, R.A. The vibration environment on the international space station: Its significance to fluid-based experiments. Acta Astronautica 2001, 48, 59–70. [Google Scholar] [CrossRef]
- Chyun, Y.S.; Kream, B.E.; Raisz, L.G. Cortisol decreases bone formation by inhibiting periosteal cell proliferation. Endocrinology 1984, 114, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.L.; Barr, S.I. The relationship between 24-h urinary cortisol and bone in healthy young women. Int. J. Behav. Med. 2010, 17, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Donoho, D.; Miller, K.K.; Misra, M.; Meenaghan, E.; Lydecker, J.; Wexler, T.; Herzog, D.B.; Klibanski, A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J. Clin. Endocrinol. Metab. 2009, 94, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Patel, K.T.; Guereca, G.; Pierce, L.; Herzog, D.B.; Misra, M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin. Endocrinol. 2013, 78, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Iacovelli, M.; Tsarpela, O.; Cardinale, M.; Bonifazi, M.; Tihanyi, J.; Viru, M.; De Lorenzo, A.; Viru, A. Hormonal responses to whole-body vibration in men. Eur. J. Appl. Physiol. 2000, 81, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Giunta, M.; Rigamonti, A.E.; Agosti, F.; Patrizi, A.; Compri, E.; Cardinale, M.; Sartorio, A. Combination of external load and whole body vibration potentiates the GH-releasing effect of squatting in healthy females. Horm. Metab. Res. 2013, 45, 611–616. [Google Scholar] [CrossRef]
- Iandolo, D.; Strigini, M.; Guignandon, A.; Vico, L. Osteocytes and Weightlessness. Curr. Osteoporos. Rep. 2021, 19, 626–636. [Google Scholar] [CrossRef]
- Chatani, M.; Mantoku, A.; Takeyama, K.; Abduweli, D.; Sugamori, Y.; Aoki, K.; Ohya, K.; Suzuki, H.; Uchida, S.; Sakimura, T.; et al. Microgravity promotes osteoclast activity in medaka fish reared at the international space station. Sci. Rep. 2015, 21, 14172. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Moriishi, T.; Miyazaki, T.; Iimura, T.; Hamagaki, M.; Nakane, A.; Tamamura, Y.; Komori, T.; Yamaguchi, A. Comparative morphology of the osteocyte lacunocanalicular system in various vertebrates. J. Bone Miner. Metab. 2011, 29, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Banfi, G.; Mariotti, M. Mechanical Static Force Negatively Regulates Vitality and Early Skeletal Development in Zebrafish Embryos. Appl. Sci. 2022, 12, 2912. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovali, M.; Zava, S.; Banfi, G.; Rizzo, A.M.; Mariotti, M. Vibration Rather than Microgravity Affects Bone Metabolism in Adult Zebrafish Scale Model. Cells 2024, 13, 509. https://doi.org/10.3390/cells13060509
Carnovali M, Zava S, Banfi G, Rizzo AM, Mariotti M. Vibration Rather than Microgravity Affects Bone Metabolism in Adult Zebrafish Scale Model. Cells. 2024; 13(6):509. https://doi.org/10.3390/cells13060509
Chicago/Turabian StyleCarnovali, Marta, Stefania Zava, Giuseppe Banfi, Angela Maria Rizzo, and Massimo Mariotti. 2024. "Vibration Rather than Microgravity Affects Bone Metabolism in Adult Zebrafish Scale Model" Cells 13, no. 6: 509. https://doi.org/10.3390/cells13060509
APA StyleCarnovali, M., Zava, S., Banfi, G., Rizzo, A. M., & Mariotti, M. (2024). Vibration Rather than Microgravity Affects Bone Metabolism in Adult Zebrafish Scale Model. Cells, 13(6), 509. https://doi.org/10.3390/cells13060509







