A Single-Cell Landscape of Spermioteleosis in Mice and Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Preparation for Single-Cell RNA-Sequencing
2.2.1. Generation of Epididymis Cell Suspensions
2.2.2. Generation of Testis Cell Suspensions
2.3. Analyses of Single-Cell Transcriptomes
2.4. Visualization
2.5. Testis Tissue Immunostaining
2.6. Mouse Epididymis Fluorescence In Situ Hybridization
2.7. Ethical Statements
2.8. Data Availability
3. Results
3.1. Transcriptional Atlas of the Testis and Epididymis in Mice and Pigs
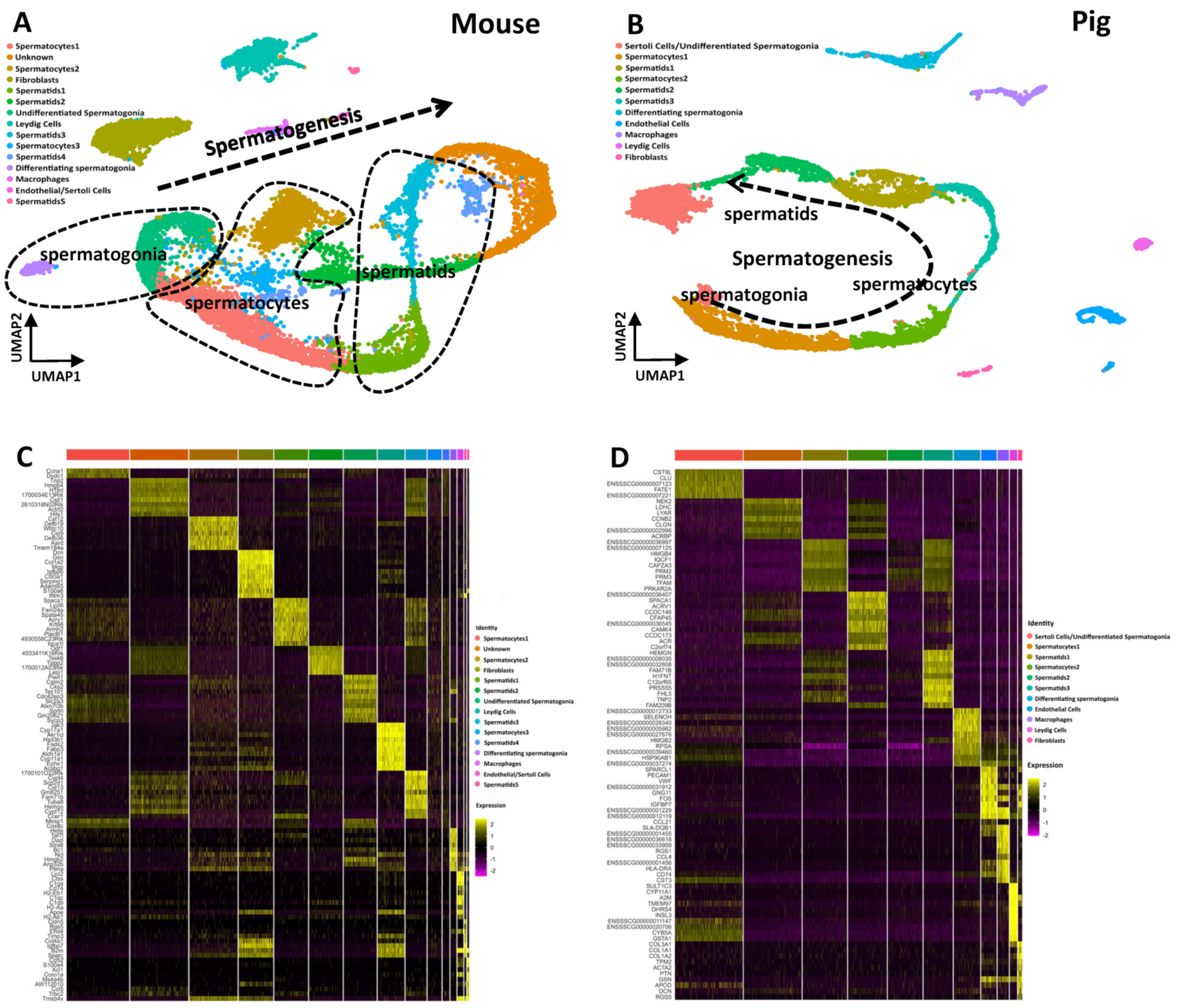
3.2. The Single-Cell Atlas of the Testis in Mice and Pigs Shows That Spermatogenesis Is a Continuous Process
3.3. Gene Expression Patterns of Testes in Mice and Pigs
3.4. Developmental Trajectory-Related Differences between Mice and Pigs during Spermatogenesis Development
3.5. Spermatogonia Development Trajectory and Gene Expression Profiles between Mice and Pigs
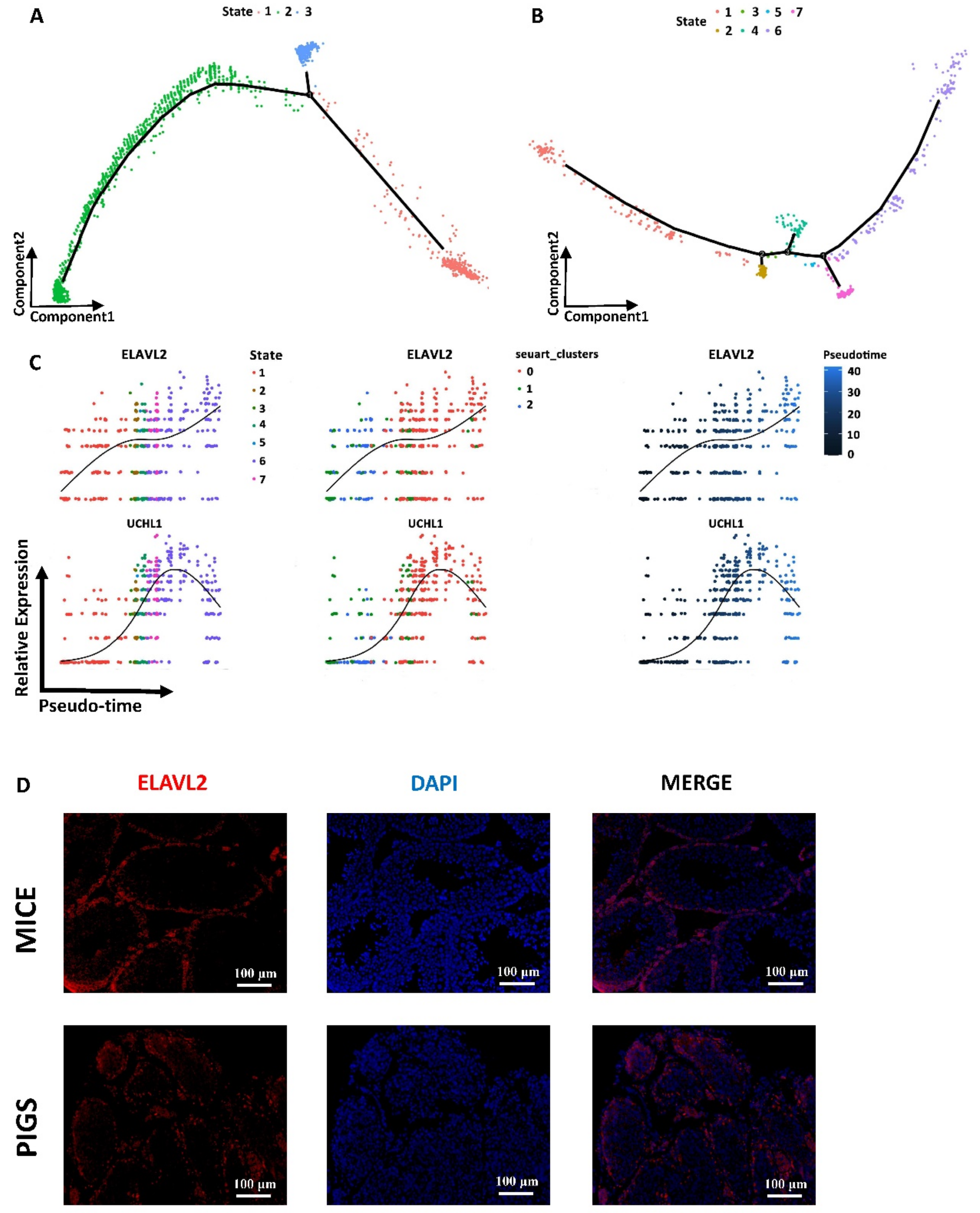
3.6. Different Dynamic Gene Expression Patterns of Spermatogonia in Mice and Pigs
3.7. Developmental Differences between Mouse and Pig Spermatocyte Clusters
3.8. Dynamic Gene Expression Patterns between Mice and Pigs
3.9. Unique Haploid Transcriptome Facilitates Spermiogenesis
3.10. Different Dynamic Gene Expression Patterns between Mouse and Pig Spermatids
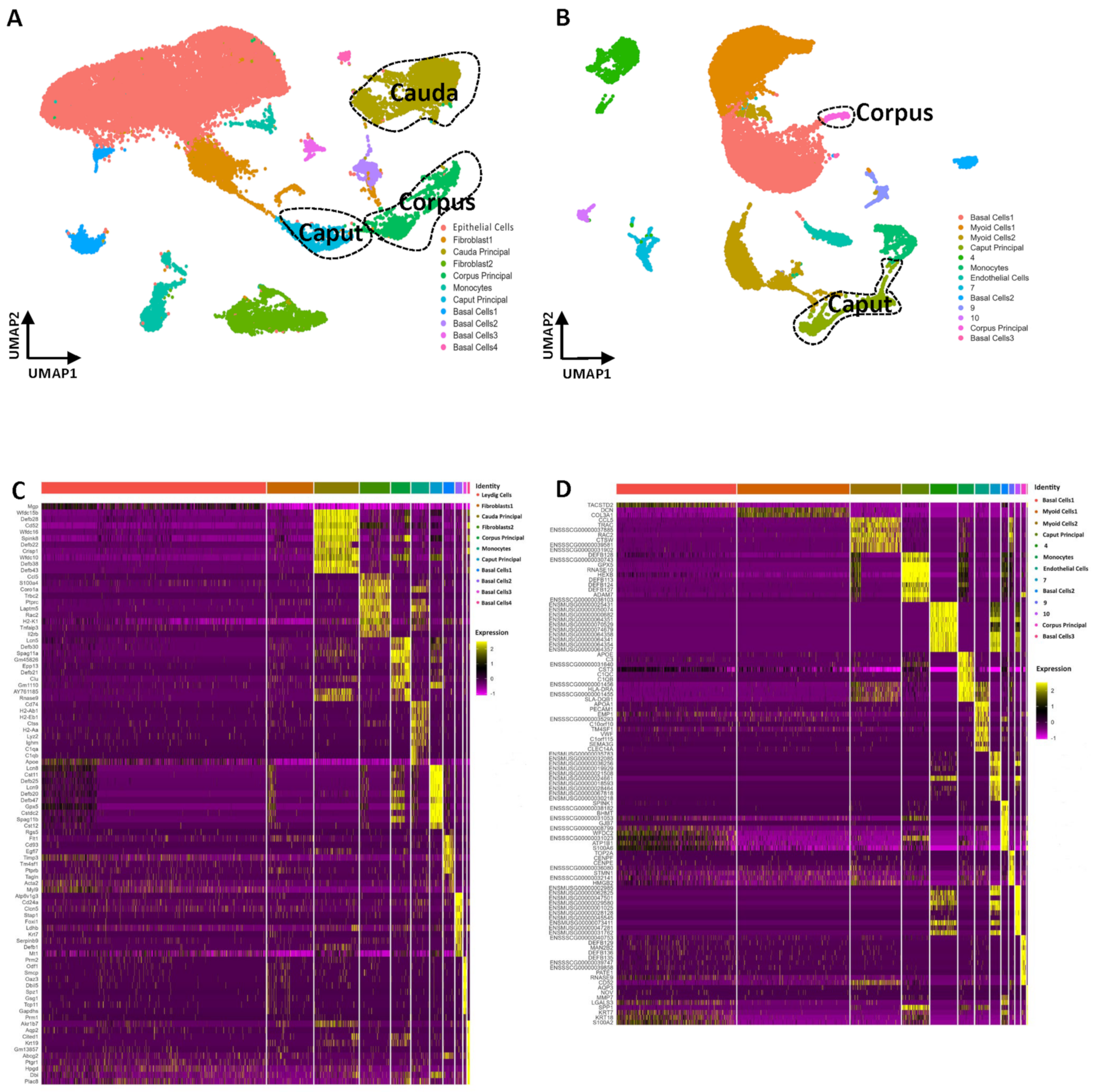
3.11. Single-Cell Transcriptomes of the Epididymis in Mice and Pigs
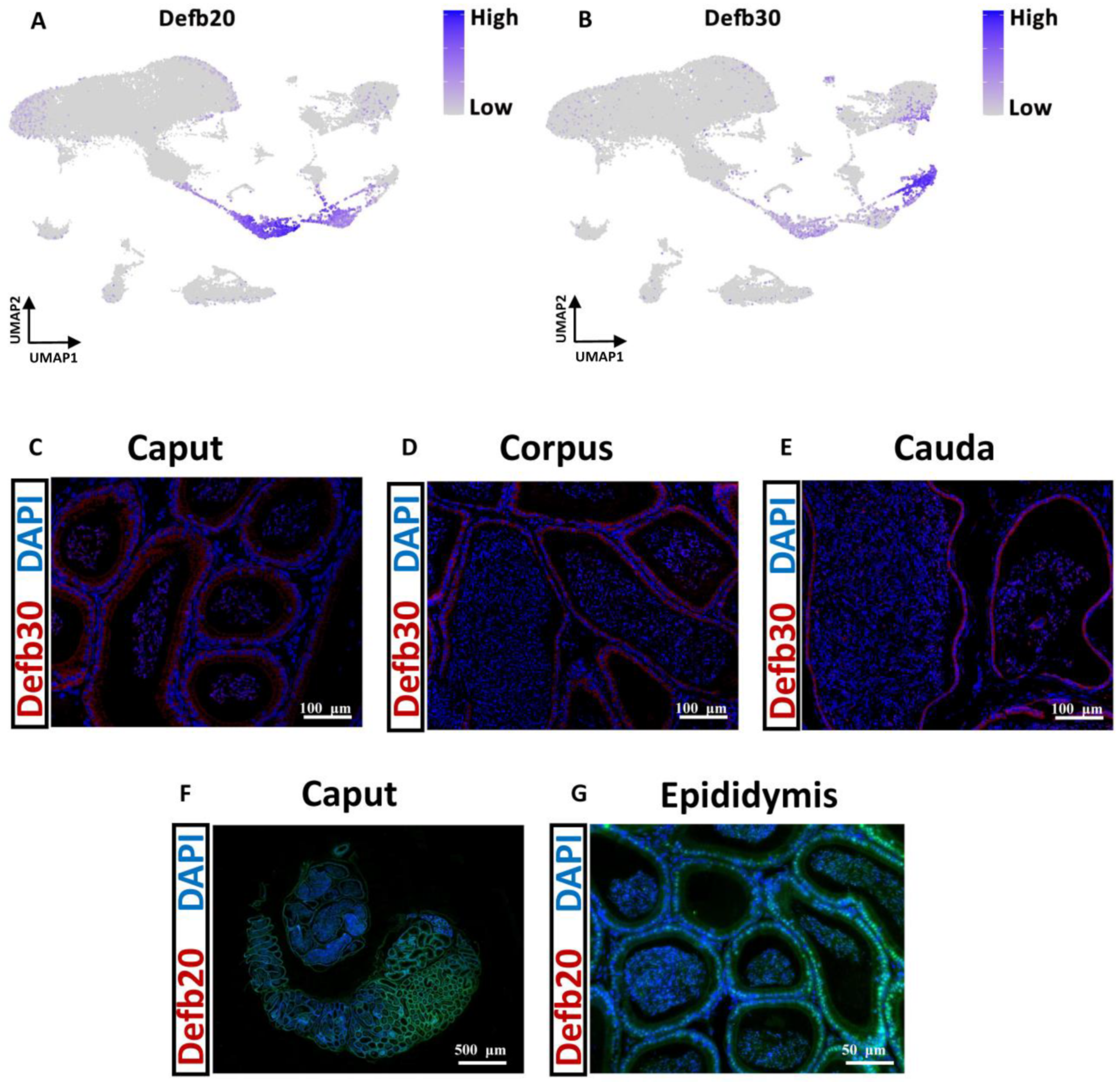
3.12. Distinctive Principal Cell Programs across the Epididymis in Mice and Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Single-cell RNA sequencing of adult mouse testes. Sci. Data 2018, 5, 180192. [Google Scholar] [CrossRef]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef]
- Cooper, T.G.; Yeung, C.H.; Fetic, S.; Sobhani, A.; Nieschlag, E. Cytoplasmic droplets are normal structures of human sperm but are not well preserved by routine procedures for assessing sperm morphology. Hum. Reprod. 2004, 19, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Axnér, E.; Linde-Forsberg, C.; Einarsson, S. Morphology and motility of spermatozoa from different regions of the epididymal duct in the domestic cat. Theriogenology 1999, 52, 767–778. [Google Scholar] [CrossRef]
- Shi, J.; Fok, K.L.; Dai, P.; Qiao, F.; Zhang, M.; Liu, H.; Sang, M.; Ye, M.; Liu, Y.; Zhou, Y.; et al. Spatio-temporal landscape of mouse epididymal cells and specific mitochondria-rich segments defined by large-scale single-cell RNA-seq. Cell Discov. 2021, 7, 34. [Google Scholar] [CrossRef]
- Rinaldi, V.D.; Donnard, E.; Gellatly, K.; Rasmussen, M.; Kucukural, A.; Yukselen, O.; Garber, M.; Sharma, U.; Rando, O.J. An atlas of cell types in the mouse epididymis and vas deferens. eLife 2020, 9, e55474. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.S.; Jelinsky, S.A.; Bang, H.J.; DiCandeloro, P.; Wilson, E.; Kopf, G.S.; Turner, T.T. The mouse epididymal transcriptome: Transcriptional profiling of segmental gene expression in the epididymis. Biol. Reprod. 2005, 73, 404–413. [Google Scholar] [CrossRef]
- Dubé, E.; Chan, P.T.; Hermo, L.; Cyr, D.G. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol. Reprod. 2007, 76, 1034–1044. [Google Scholar] [CrossRef]
- Thimon, V.; Koukoui, O.; Calvo, E.; Sullivan, R. Region-specific gene expression profiling along the human epididymis. Mol. Hum. Reprod. 2007, 13, 691–704. [Google Scholar] [CrossRef]
- Guyonnet, B.; Marot, G.; Dacheux, J.L.; Mercat, M.J.; Schwob, S.; Jaffrézic, F.; Gatti, J.L. The adult boar testicular and epididymal transcriptomes. BMC Genom. 2009, 10, 369. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Sarropoulos, I.; Marin, R.; Cardoso-Moreira, M.; Kaessmann, H. Developmental dynamics of lncRNAs across mammalian organs and species. Nature 2019, 571, 510–514. [Google Scholar] [CrossRef]
- Lau, X.; Munusamy, P.; Ng, M.J.; Sangrithi, M. Single-Cell RNA Sequencing of the Cynomolgus Macaque Testis Reveals Conserved Transcriptional Profiles during Mammalian Spermatogenesis. Dev. Cell 2020, 54, 548–566.e7. [Google Scholar] [CrossRef]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547.e12. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, M.; Liu, Z.; Liu, R.; Zheng, Y.; Yu, T.; Lv, Y.; Lu, H.; Zeng, W.; Zhang, T.; et al. Single-cell RNA-seq analysis of testicular somatic cell development in pigs. J. Genet. Genom. Yi Chuan Xue Bao 2022, 49, 1016–1028. [Google Scholar] [CrossRef]
- Rosenthal, N.; Brown, S. The mouse ascending: Perspectives for human-disease models. Nat. Cell Biol. 2007, 9, 993–999. [Google Scholar] [CrossRef]
- Hou, N.; Du, X.; Wu, S. Advances in pig models of human diseases. Anim. Models Exp. Med. 2022, 5, 141–152. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Leushkin, E.; Liechti, A.; Ovchinnikova, S.; Mößinger, K.; Brüning, T.; Rummel, C.; Grützner, F.; Cardoso-Moreira, M.; Janich, P.; et al. Transcriptome and translatome co-evolution in mammals. Nature 2020, 588, 642–647. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, B.; Narciandi, F.; O’Farrelly, C.; Kelly, A.K.; Fair, S.; Meade, K.G.; Lonergan, P. Cauda Epididymis-Specific Beta-Defensin 126 Promotes Sperm Motility but Not Fertilizing Ability in Cattle. Biol. Reprod. 2016, 95, 122. [Google Scholar] [CrossRef]
- Diao, R.; Fok, K.L.; Chen, H.; Yu, M.K.; Duan, Y.; Chung, C.M.; Li, Z.; Wu, H.; Li, Z.; Zhang, H.; et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci. Transl. Med. 2014, 6, 249ra108. [Google Scholar] [CrossRef]
- Wu, P.; Liu, T.L.; Li, L.L.; Liu, Z.P.; Tian, L.H.; Hou, Z.J. Declined expressing mRNA of beta-defensin 108 from epididymis is associated with decreased sperm motility in blue fox (Vulpes lagopus). BMC Vet. Res. 2021, 17, 12. [Google Scholar] [CrossRef]
- Li, P.; Chan, H.C.; He, B.; So, S.C.; Chung, Y.W.; Shang, Q.; Zhang, Y.D.; Zhang, Y.L. An antimicrobial peptide gene found in the male reproductive system of rats. Science 2001, 291, 1783–1785. [Google Scholar] [CrossRef]
- Zhao, Y.; Diao, H.; Ni, Z.; Hu, S.; Yu, H.; Zhang, Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell. Mol. Life Sci. CMLS 2011, 68, 697–708. [Google Scholar] [CrossRef]
- Zhou, C.X.; Zhang, Y.L.; Xiao, L.; Zheng, M.; Leung, K.M.; Chan, M.Y.; Lo, P.S.; Tsang, L.L.; Wong, H.Y.; Ho, L.S.; et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 2004, 6, 458–464. [Google Scholar] [CrossRef]
- Aram, R.; Chan, P.T.K.; Cyr, D.G. Beta-defensin126 is correlated with sperm motility in fertile and infertile men. Biol. Reprod. 2020, 102, 92–101. [Google Scholar] [CrossRef]
- Hermann, B.P.; Mutoji, K.N.; Velte, E.K.; Ko, D.; Oatley, J.M.; Geyer, C.B.; McCarrey, J.R. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol. Reprod. 2015, 92, 54. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2023. Nucleic Acids Res. 2023, 51, D18–D28. [Google Scholar] [CrossRef]
- Suzuki, H.; Ahn, H.W.; Chu, T.; Bowden, W.; Gassei, K.; Orwig, K.; Rajkovic, A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Dev. Biol. 2012, 361, 301–312. [Google Scholar] [CrossRef]
- Busada, J.T.; Velte, E.K.; Serra, N.; Cook, K.; Niedenberger, B.A.; Willis, W.D.; Goulding, E.H.; Eddy, E.M.; Geyer, C.B. Rhox13 is required for a quantitatively normal first wave of spermatogenesis in mice. Reproduction 2016, 152, 379–388. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.M.; Pouchnik, D.; Banasik, B.; McCarrey, J.R.; Small, C.; et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Q.; Li, T.; Liu, R.; Cheng, Z.; Guo, M.; Xiao, J.; Wu, D.; Zeng, W. Sertoli cell and spermatogonial development in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 45. [Google Scholar] [CrossRef]
- Barrios, F.; Filipponi, D.; Campolo, F.; Gori, M.; Bramucci, F.; Pellegrini, M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Dolci, S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J. Cell Sci. 2012, 125 Pt 6, 1455–1464. [Google Scholar]
- Xu, L.; Chang, G.; Ma, T.; Wang, H.; Chen, J.; Li, Z.; Guo, X.; Wan, F.; Ren, L.; Lu, W.; et al. Piwil1 mediates meiosis during spermatogenesis in chicken. Anim. Reprod. Sci. 2016, 166, 99–108. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, X.; Chen, C.; Diao, R.; Lai, Y.; Huang, J.; Chen, J.; Yu, Z.; Gui, Y.; Tang, A.; et al. Developmental expression and function of DKKL1/Dkkl1 in humans and mice. Reprod. Biol. Endocrinol. 2012, 10, 51. [Google Scholar] [CrossRef]
- Trovero, M.F.; Rodríguez-Casuriaga, R.; Romeo, C.; Santiñaque, F.F.; François, M.; Folle, G.A.; Benavente, R.; Sotelo-Silveira, J.R.; Geisinger, A. Revealing stage-specific expression patterns of long noncoding RNAs along mouse spermatogenesis. RNA Biol. 2020, 17, 350–365. [Google Scholar] [CrossRef]
- Küçükköse, E.; Peters, N.A.; Ubink, I.; van Keulen, V.A.M.; Daghighian, R.; Verheem, A.; Laoukili, J.; Kranenburg, O. KIT promotes tumor stroma formation and counteracts tumor-suppressive TGFβ signaling in colorectal cancer. Cell Death Dis. 2022, 13, 617. [Google Scholar] [CrossRef]
- Norambuena, P.A.; Diblík, J.; Krenkova, P.; Paulasova, P.; Macek, M.; Macek, M.S. An ADP-ribosyltransferase 3 (ART3) variant is associated with reduced sperm counts in Czech males: Case/control association study replicating results from the Japanese population. Neuro Endocrinol. Lett. 2012, 33, 48–52. [Google Scholar]
- Yoshitake, H.; Tsukamoto, H.; Maruyama-Fukushima, M.; Takamori, K.; Ogawa, H.; Araki, Y. TEX101, a germ cell-marker glycoprotein, is associated with lymphocyte antigen 6 complex locus k within the mouse testis. Biochem. Biophys. Res. Commun. 2008, 372, 277–282. [Google Scholar] [CrossRef]
- Yang, C.; Yao, C.; Ji, Z.; Zhao, L.; Chen, H.; Li, P.; Tian, R.; Zhi, E.; Huang, Y.; Han, X.; et al. RNA-binding protein ELAVL2 plays post-transcriptional roles in the regulation of spermatogonia proliferation and apoptosis. Cell Prolif. 2021, 54, e13098. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. Ubiquitin carboxy-terminal hydrolase L1-physiology and pathology. Cell Biochem. Funct. 2020, 38, 533–540. [Google Scholar] [CrossRef]
- Hickford, D.E.; Frankenberg, S.; Pask, A.J.; Shaw, G.; Renfree, M.B. DDX4 (VASA) is conserved in germ cell development in marsupials and monotremes. Biol. Reprod. 2011, 85, 733–743. [Google Scholar] [CrossRef]
- Ozaki, Y.; Saito, K.; Shinya, M.; Kawasaki, T.; Sakai, N. Evaluation of Sycp3, Plzf and Cyclin B3 expression and suitability as spermatogonia and spermatocyte markers in zebrafish. Gene Expr. Patterns GEP 2011, 11, 309–315. [Google Scholar] [CrossRef]
- Osuru, H.P.; Monroe, J.E.; Chebolu, A.P.; Akamune, J.; Pramoonjago, P.; Ranpura, S.A.; Reddi, P.P. The acrosomal protein SP-10 (Acrv1) is an ideal marker for staging of the cycle of seminiferous epithelium in the mouse. Mol. Reprod. Dev. 2014, 81, 896–907. [Google Scholar] [CrossRef]
- Reddi, P.P. Transcriptional regulation of spatiotemporal gene expression within the seminiferous epithelium: Mouse Acrv1 gene as a model. Andrology 2023, 11, 904–910. [Google Scholar] [CrossRef]
- Lee, B.; Jin, S.; Choi, H.; Kwon, J.T.; Kim, J.; Jeong, J.; Kwon, Y.I.; Cho, C. Expression and function of the testis-predominant protein LYAR in mice. Mol. Cells 2013, 35, 54–60. [Google Scholar] [CrossRef]
- Oranratanaphan, S.; Kobwitaya, K.; Termrungruanglert, W.; Triratanachat, S.; Kitkumthorn, N.; Mutirangura, A. Value of CCNA1 promoter methylation in triaging ASC-US cytology. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 473–477. [Google Scholar] [CrossRef]
- Tanaka, K.; Parvinen, M.; Nigg, E.A. The in vivo expression pattern of mouse Nek2, a NIMA-related kinase, indicates a role in both mitosis and meiosis. Exp. Cell Res. 1997, 237, 264–274. [Google Scholar] [CrossRef]
- Lin, Y.M.; Teng, Y.N.; Chung, C.L.; Tsai, W.C.; Lin, Y.H.; Lin, J.S.; Kuo, P.L. Decreased mRNA transcripts of M-phase promoting factor and its regulators in the testes of infertile men. Hum. Reprod. 2006, 21, 138–144. [Google Scholar] [CrossRef]
- Mi, Y.; Shi, Z.; Li, J. Spata19 is critical for sperm mitochondrial function and male fertility. Mol. Reprod. Dev. 2015, 82, 907–913. [Google Scholar] [CrossRef]
- Radhakrishnan, Y.; Hamil, K.G.; Yenugu, S.; Young, S.L.; French, F.S.; Hall, S.H. Identification, characterization, and evolution of a primate beta-defensin gene cluster. Genes Immun. 2005, 6, 203–210. [Google Scholar] [CrossRef]
- Yenugu, S.; Chintalgattu, V.; Wingard, C.J.; Radhakrishnan, Y.; French, F.S.; Hall, S.H. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus). Reprod. Biol. Endocrinol. RBE 2006, 4, 7. [Google Scholar] [CrossRef]
- Jin, J.; Li, X.; Ye, M.; Qiao, F.; Chen, H.; Fok, K.L. Defb19 regulates the migration of germ cell and is involved in male fertility. Cell Biosci. 2022, 12, 188. [Google Scholar] [CrossRef]
- Kaytor, M.D.; Livingston, D.M. GSG1, a yeast gene required for sporulation. Yeast 1995, 11, 1147–1155. [Google Scholar] [CrossRef]
- Catena, R.; Escoffier, E.; Caron, C.; Khochbin, S.; Martianov, I.; Davidson, I. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biol. Reprod. 2009, 80, 358–366. [Google Scholar] [CrossRef]
- Kim, C.R.; Noda, T.; Kim, H.; Kim, G.; Park, S.; Na, Y.; Oura, S.; Shimada, K.; Bang, I.; Ahn, J.Y.; et al. PHF7 Modulates BRDT Stability and Histone-to-Protamine Exchange during Spermiogenesis. Cell Rep. 2020, 32, 107950. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Pan, B.; Zheng, C.; Hong, L.; Han, W. KRT8 Serves as a Novel Biomarker for LUAD and Promotes Metastasis and EMT via NF-κB Signaling. Front. Oncol. 2022, 12, 875146. [Google Scholar] [CrossRef]
- Pan, L.; E, T.; Xu, C.; Fan, X.; Xia, J.; Liu, Y.; Liu, J.; Zhao, J.; Bao, N.; Zhao, Y.; et al. The apoptotic effects of soybean agglutinin were induced through three different signal pathways by down-regulating cytoskeleton proteins in IPEC-J2 cells. Sci. Rep. 2023, 13, 5753. [Google Scholar] [CrossRef]
- Leung, G.P.; Cheung, K.H.; Leung, C.T.; Tsang, M.W.; Wong, P.Y. Regulation of epididymal principal cell functions by basal cells: Role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol. Cell. Endocrinol. 2004, 216, 5–13. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Xie, S.; Yin, Q.; Tang, C.; Ni, Z.; Fei, J.; Zhang, Y. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of β-defensin family members on sperm maturation in rat epididymis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 1354–1363. [Google Scholar] [CrossRef]
- Zhao, W.; Quansah, E.; Yuan, M.; Gou, Q.; Mengal, K.; Li, P.; Wu, S.; Xu, C.; Yi, C.; Cai, X. Region-specific gene expression in the epididymis of Yak. Theriogenology 2019, 139, 132–146. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. S1), 1–8. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; Plath, K.; Hotaling, J.M.; Stukenborg, J.B.; Clark, A.T.; et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 2021, 28, 764–778.e4. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef]
- Lu, J.; Liao, J.; Qin, M.; Li, H.; Zhang, Q.; Chen, Y.; Cheng, J. Single-Cell RNAseq Resolve the Potential Effects of LanCL1 Gene in the Mouse Testis. Cells 2022, 11, 4135. [Google Scholar] [CrossRef]
- Tan, H.; Wang, W.; Zhou, C.; Wang, Y.; Zhang, S.; Yang, P.; Guo, R.; Chen, W.; Zhang, J.; Ye, L.; et al. Single-cell RNA-seq uncovers dynamic processes orchestrated by RNA-binding protein DDX43 in chromatin remodeling during spermiogenesis. Nat. Commun. 2023, 14, 2499. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Lei, P.; Guo, M.; Liu, R.; Wang, L.; Yu, T.; Lv, Y.; Zhang, T.; Zeng, W.; et al. Single-cell RNA-sequencing reveals the dynamic process and novel markers in porcine spermatogenesis. J. Anim. Sci. Biotechnol. 2021, 12, 122. [Google Scholar] [CrossRef]
- Ballow, D.; Meistrich, M.L.; Matzuk, M.; Rajkovic, A. Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 2006, 294, 161–167. [Google Scholar] [CrossRef]
- Mulligan, M.R.; Bicknell, L.S. The molecular genetics of nELAVL in brain development and disease. Eur. J. Hum. Genet. EJHG 2023, 31, 1209–1217. [Google Scholar] [CrossRef]
- Biswas, L.; Tyc, K.; El Yakoubi, W.; Morgan, K.; Xing, J.; Schindler, K. Meiosis interrupted: The genetics of female infertility via meiotic failure. Reproduction 2021, 161, R13–R35. [Google Scholar] [CrossRef]
- Tadokoro, Y.; Yomogida, K.; Ohta, H.; Tohda, A.; Nishimune, Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 2002, 113, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kajiura-Kobayashi, H.; Kobayashi, T.; Nagahama, Y. The cloning of cyclin B3 and its gene expression during hormonally induced spermatogenesis in the teleost, Anguilla japonica. Biochem. Biophys. Res. Commun. 2004, 323, 288–292. [Google Scholar] [CrossRef]
- Chapman, D.L.; Wolgemuth, D.J. Isolation of the murine cyclin B2 cDNA and characterization of the lineage and temporal specificity of expression of the B1 and B2 cyclins during oogenesis, spermatogenesis and early embryogenesis. Development 1993, 118, 229–240. [Google Scholar] [CrossRef]
- Yao, C.; Sun, M.; Yuan, Q.; Niu, M.; Chen, Z.; Hou, J.; Wang, H.; Wen, L.; Liu, Y.; Li, Z.; et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget 2016, 7, 2201–2219. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, C.; He, C.; Mei, C.; Liao, A.; Huang, D. The Immune Characteristics of the Epididymis and the Immune Pathway of the Epididymitis Caused by Different Pathogens. Front. Immunol. 2020, 11, 2115. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Muliawati, D.; Rizki, M.D.; Parisudha, A.; Hardiyanto, L. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod. Biol. 2020, 20, 536–540. [Google Scholar] [CrossRef]
- Florman, H.M. Sequential focal and global elevations of sperm intracellular Ca2+ are initiated by the zona pellucida during acrosomal exocytosis. Dev. Biol. 1994, 165, 152–164. [Google Scholar] [CrossRef]
- Fan, K.; Jiang, J.; Wang, Z.; Fan, R.; Yin, W.; Sun, Y.; Li, H. Expression and purification of soluble porcine cystatin 11 in Pichia pastoris. Appl. Biochem. Biotechnol. 2014, 174, 1959–1968. [Google Scholar] [CrossRef]
- Hamil, K.G.; Liu, Q.; Sivashanmugam, P.; Yenugu, S.; Soundararajan, R.; Grossman, G.; Richardson, R.T.; Zhang, Y.L.; O’Rand, M.G.; Petrusz, P.; et al. Cystatin 11: A new member of the cystatin type 2 family. Endocrinology 2002, 143, 2787–2796. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Adipocytokines and insulin resistance: The possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care 2009, 32 (Suppl. S2), S362. [Google Scholar] [CrossRef]
- Datta, R.; Podolsky, M.J.; Yang, C.D.; Alba, D.L.; Singh, S.; Koliwad, S.; Lizama, C.O.; Atabai, K. MFGE8 inhibits insulin signaling through PTP1B. bioRxiv 2023. [Google Scholar] [CrossRef]
- Castella, S.; Fouchécourt, S.; Teixeira-Gomes, A.P.; Vinh, J.; Belghazi, M.; Dacheux, F.; Dacheux, J.L. Identification of a member of a new RNase a family specifically secreted by epididymal caput epithelium. Biol. Reprod. 2004, 70, 319–328. [Google Scholar] [CrossRef]
- Penttinen, J.; Pujianto, D.A.; Sipila, P.; Huhtaniemi, I.; Poutanen, M. Discovery in silico and characterization in vitro of novel genes exclusively expressed in the mouse epididymis. Mol. Endocrinol. 2003, 17, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Lareyre, J.J.; Winfrey, V.P.; Kasper, S.; Ong, D.E.; Matusik, R.J.; Olson, G.E.; Orgebin-Crist, M.C. Gene duplication gives rise to a new 17-kilodalton lipocalin that shows epididymal region-specific expression and testicular factor(s) regulation. Endocrinology 2001, 142, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.J.; Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Muñoz, M.W.; Battistone, M.A.; Cuasnicú, P.S. Epididymal protein CRISP1 plays different roles during the fertilization process. J. Androl. 2011, 32, 672–678. [Google Scholar] [CrossRef]
- Ernesto, J.I.; Weigel Muñoz, M.; Battistone, M.A.; Vasen, G.; Martínez-López, P.; Orta, G.; Figueiras-Fierro, D.; De la Vega-Beltran, J.L.; Moreno, I.A.; Guidobaldi, H.A.; et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 2015, 210, 1213–1224. [Google Scholar] [CrossRef]
- Schwaab, V.; Faure, J.; Dufaure, J.P.; Drevet, J.R. GPx3: The plasma-type glutathione peroxidase is expressed under androgenic control in the mouse epididymis and vas deferens. Mol. Reprod. Dev. 1998, 51, 362–372. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.-M.; Fan, C.-Q.; Zhang, G.-L. A Single-Cell Landscape of Spermioteleosis in Mice and Pigs. Cells 2024, 13, 563. https://doi.org/10.3390/cells13070563
Liu M-M, Fan C-Q, Zhang G-L. A Single-Cell Landscape of Spermioteleosis in Mice and Pigs. Cells. 2024; 13(7):563. https://doi.org/10.3390/cells13070563
Chicago/Turabian StyleLiu, Meng-Meng, Chu-Qi Fan, and Guo-Liang Zhang. 2024. "A Single-Cell Landscape of Spermioteleosis in Mice and Pigs" Cells 13, no. 7: 563. https://doi.org/10.3390/cells13070563




