Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Semen Collection and Treatment
2.2. Sperm Cryopreservation and Thawing
2.3. Acrosomal Staining
2.4. Western Blotting
2.5. Immunofluorescence Staining
2.6. Assessment of Sperm DNA Fragmentation
2.7. Mitochondrial Membrane Potential Assay
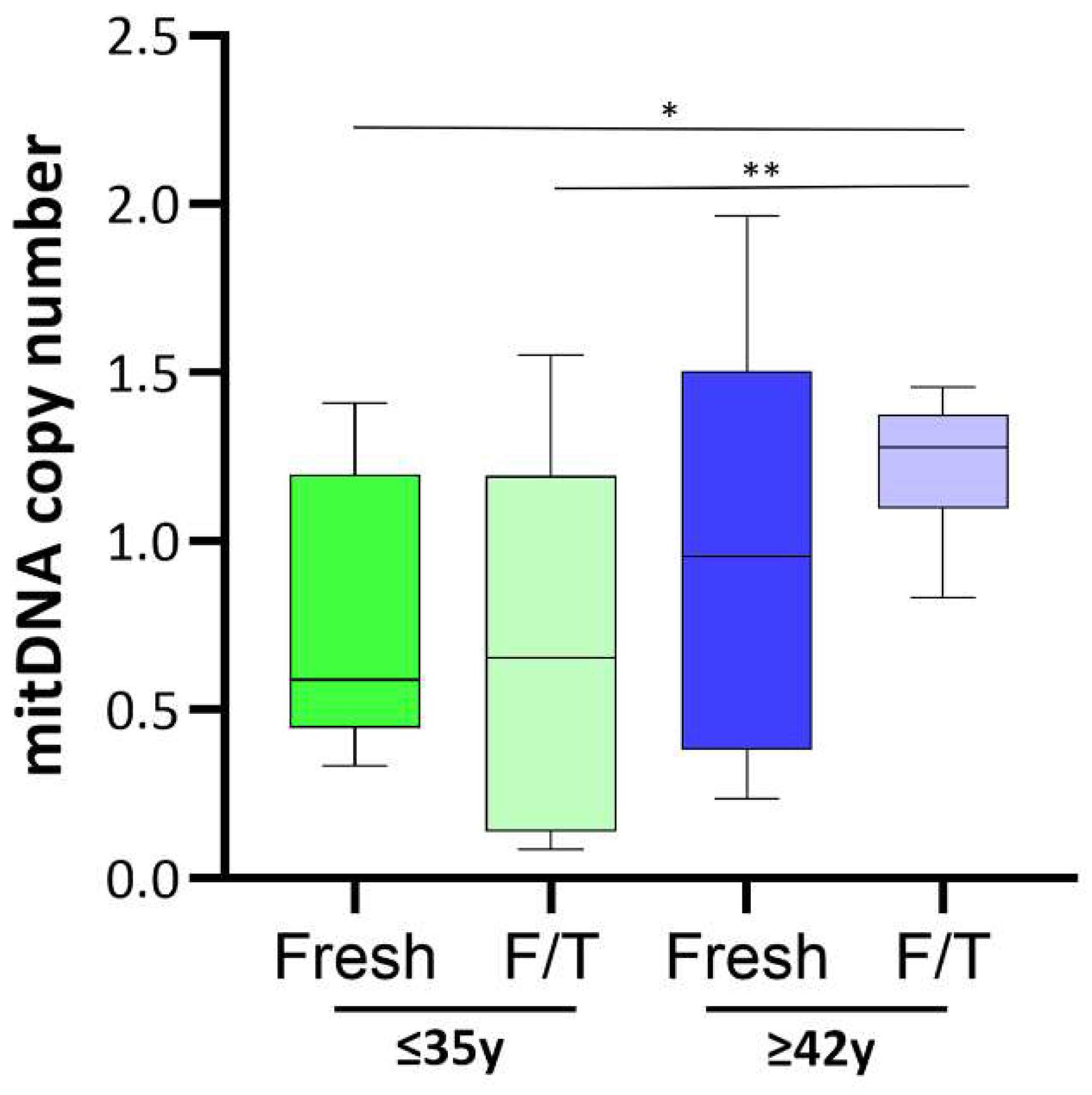
2.8. Determination of mtDNA Copy Number
2.9. Statistical Analysis
3. Results
3.1. Analysis of Sperm Parameters
3.2. DNA Fragmentation
3.3. Assessment of Mitochondrial Functionality
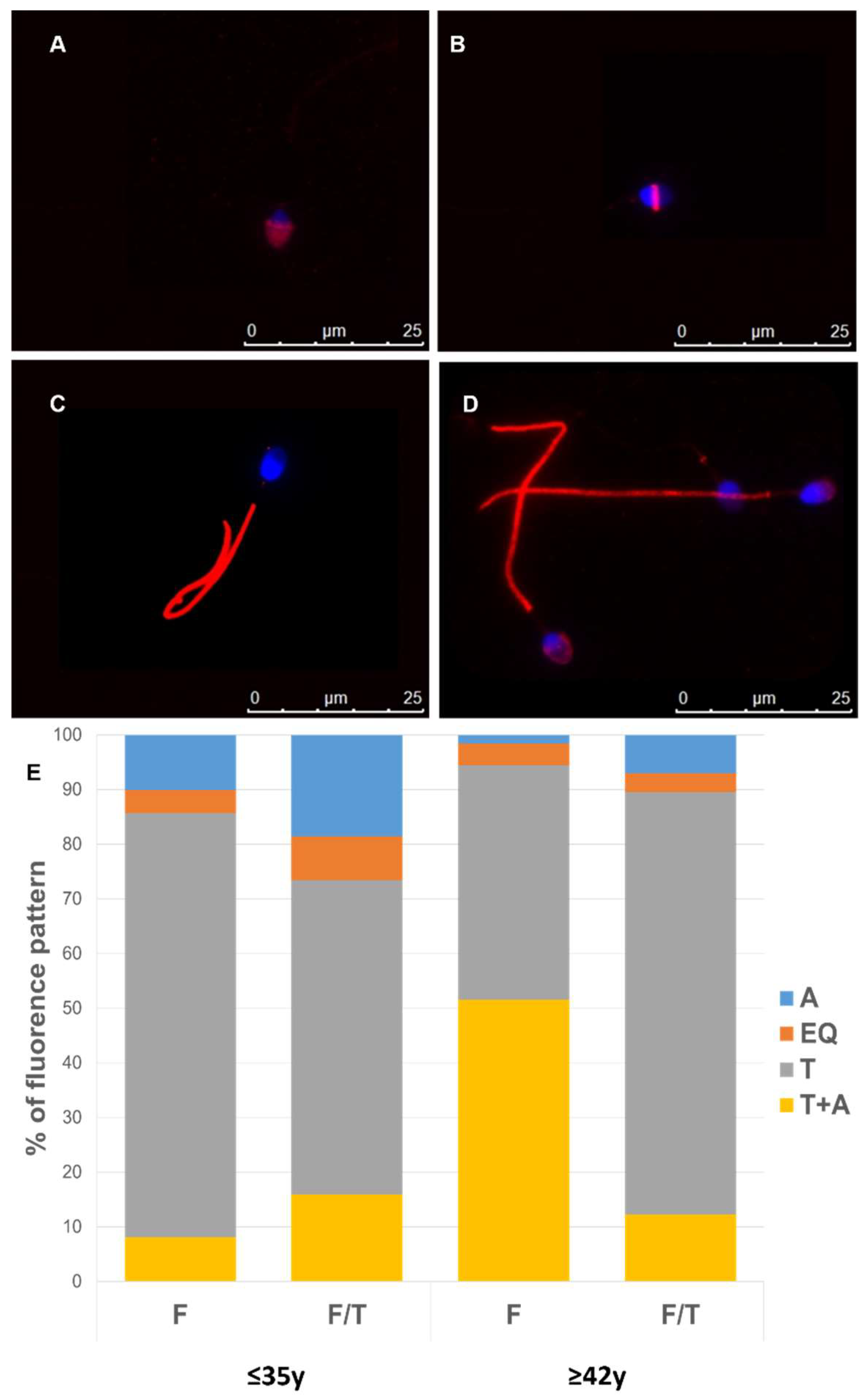
3.4. Effect of Advanced Male Age on Cryo-Capacitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in Semen Quality among 30,636 Young Chinese Men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef] [PubMed]
- Barsky, M.; Blesson, C.S. Should We Be Worried about Advanced Paternal Age? Fertil. Steril. 2020, 114, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Bahri, H.; Ben Khalifa, M.; Ben Rhouma, M.; Abidi, Z.; Abbassi, E.; Ben Rhouma, K.; Benkhalifa, M. Decline in Semen Quality of North African Men: A Retrospective Study of 20,958 Sperm Analyses of Men from Different North African Countries Tested in Tunisia over a Period of 6 Years (2013–2018). Ann. Hum. Biol. 2021, 48, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the Aging Male: A Systematic Review. Fertil. Steril. 2022, 118, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Kaarouch, I.; Bouamoud, N.; Madkour, A.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Amzazi, S.; Copin, H.; Benkhalifa, M.; et al. Paternal Age: Negative Impact on Sperm Genome Decays and IVF Outcomes after 40 Years. Mol. Reprod. Devel. 2018, 85, 271–280. [Google Scholar] [CrossRef]
- Marić, T.; Fučić, A.; Aghayanian, A. Environmental and Occupational Exposures Associated with Male Infertility. Arch. Ind. Hyg. Toxicol. 2021, 72, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Toriello, H.V.; Meck, J.M. Statement on Guidance for Genetic Counseling in Advanced Paternal Age. Genet. Med. 2008, 10, 457–460. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Staraj, S. Testicular Size: The Effects of Aging, Malnutrition, and Illness. J. Androl. 1985, 6, 144–151. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; Mckinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Brahem, S.; Mehdi, M.; Elghezal, H.; Saad, A. The Effects of Male Aging on Semen Quality, Sperm DNA Fragmentation and Chromosomal Abnormalities in an Infertile Population. Am. J. Reprod. Genet. 2011, 28, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical Relevance of Oxidative Stress in Male Factor Infertility: An Update. Am. J. Rep. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Broer, L.; Codd, V.; Nyholt, D.R.; Deelen, J.; Mangino, M.; Willemsen, G.; Albrecht, E.; Amin, N.; Beekman, M.; De Geus, E.J.C.; et al. Meta-Analysis of Telomere Length in 19 713 Subjects Reveals High Heritability, Stronger Maternal Inheritance and a Paternal Age Effect. Eur. J. Hum. Genet. 2013, 21, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F. The Origins, Patterns and Implications of Human Spontaneous Mutation. Nat. Rev. Genet. 2000, 1, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Moskovtsev, S.I.; Willis, J.; Mullen, J.B.M. Age-Related Decline in Sperm Deoxyribonucleic Acid Integrity in Patients Evaluated for Male Infertility. Fertil. Steril. 2006, 85, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Reichman, N.E.; Teitler, J.O. Paternal Age as a Risk Factor for Low Birthweight. Am. J. Public Health 2006, 96, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and the Origins of Paternal Effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Pino, V.; Sanz, A.; Valdés, N.; Crosby, J.; Mackenna, A. The Effects of Aging on Semen Parameters and Sperm DNA Fragmentation. JBRA Assist. Reprod. 2020, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, M.K.; Barut, O.; Dogan, N.T.; Hamarat, M.B.; Resim, S. At What Age Threshold Does the Decline in Semen Parameters Begin? J. Coll. Physicians Surg. Pak. 2021, 31, 4–7. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, R.; Yang, S.; Wang, Y.; Huang, Y.; Yan, L.; Jiang, H.; Qiao, J. Age-Related Changes in Human Conventional Semen Parameters and Sperm Chromatin Structure Assay-Defined Sperm DNA/Chromatin Integrity. Reprod. BioMed. Online 2021, 42, 973–982. [Google Scholar] [CrossRef]
- Das, M.; Al-Hathal, N.; San-Gabriel, M.; Phillips, S.; Kadoch, I.-J.; Bissonnette, F.; Holzer, H.; Zini, A. High Prevalence of Isolated Sperm DNA Damage in Infertile Men with Advanced Paternal Age. J. Assist. Reprod. Genet. 2013, 30, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Winkle, T.; Rosenbusch, B.; Gagsteiger, F.; Paiss, T.; Zoller, N. The Correlation between Male Age, Sperm Quality and Sperm DNA Fragmentation in 320 Men Attending a Fertility Center. J. Assist. Reprod. Genet. 2009, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huffman, A.M.; Whitcomb, B.W.; Josyula, S.; Labrie, S.; Tougias, E.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Sperm Mitochondrial DNA Measures and Semen Parameters among Men Undergoing Fertility Treatment. Reprod. BioMed. Online 2019, 38, 66–75. [Google Scholar] [CrossRef]
- Oluwayiose, O.A.; Josyula, S.; Houle, E.; Marcho, C.; Whitcomb, B.W.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Association between Sperm Mitochondarial DNA Copy Number and Nuclear DNA Methylation. Epigenomics 2020, 12, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Amor, H.; Zeyad, A.; Alkhaled, Y.; Laqqan, M.; Saad, A.; Ben Ali, H.; Hammadeh, M.E. Relationship between Nuclear DNA Fragmentation, Mitochondrial DNA Damage and Standard Sperm Parameters in Spermatozoa of Fertile and Sub-fertile Men before and after Freeze-thawing Procedure. Andrologia 2018, 50, e12998. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cryopreservation of Sperm: Effects on Chromatin and Strategies to Prevent Them. In Genetic Damage in Human Spermatozoa; Baldi, E., Muratori, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1166, pp. 149–167. ISBN 978-3-030-21663-4. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Opuwari, C.S.; Henkel, R.R. An Update on Oxidative Damage to Spermatozoa and Oocytes. BioMed Res. Int. 2016, 2016, 9540142. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Murugavel, K.; Antoine, D.; Prakash, M.A.; Saraf, K.K.; Nag, P.; Karuthadurai, T.; Kumaresan, A. The Proportion of Tyrosine Phosphorylated Spermatozoa in Cryopreserved Semen Is Negatively Related to Crossbred Bull Fertility. Theriogenology 2020, 149, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.P.; Perez Casasus, S.; Haxhiu, A.; Barbarulo, F.; Scarcella, M.; Governini, L.; Piomboni, P.; Scarica, C.; Luddi, A. Exposure to Cumulus Cell Secretome Improves Sperm Function: New Perspectives for Sperm Selection In Vitro. Cells 2023, 12, 2349. [Google Scholar] [CrossRef]
- Luongo, F.P.; Passaponti, S.; Haxhiu, A.; Raeispour, M.; Belmonte, G.; Governini, L.; Casarini, L.; Piomboni, P.; Luddi, A. Bitter Taste Receptors and Endocrine Disruptors: Cellular and Molecular Insights from an In Vitro Model of Human Granulosa Cells. IJMS 2022, 23, 15540. [Google Scholar] [CrossRef]
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 21, 2840. [Google Scholar] [CrossRef] [PubMed]
- Sati, L.; Cayli, S.; Delpiano, E.; Sakkas, D.; Huszar, G. The Pattern of Tyrosine Phosphorylation in Human Sperm in Response to Binding to Zona Pellucida or Hyaluronic Acid. Reprod. Sci. 2014, 21, 573–581. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, A.; Roberts, P.; Yap, K.; Matson, P. Development of a Simplified Method of Human Semen Storage for the Testing of Sperm DNA Fragmentation Using the Halosperm G2 Test Kit. Fertil. Steril. 2014, 102, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Ramalho-Santos, J. Assessment of Mitochondrial Potential: Implications for the Correct Monitoring of Human Sperm Function. Int. J. Androl. 2010, 33, e180–e186. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Khorram Khorshid, H.R.; Akhondi, M.M.; Mokarram, P.; Sadeghi, M.R. A Simple, Rapid and Economic Manual Method for Human Sperm DNA Extraction in Genetic and Epigenetic Studies. Middle East Fertil. Soc. J. 2018, 23, 216–219. [Google Scholar] [CrossRef]
- Duma-Pauta, J.M.; Juárez-López, N.O.; Gutiérrez-Pérez, O.; Córdova-Izquierdo, A.; Vigueras-Villaseñor, R.M.; Juárez-Mosqueda, M.d.L. Cryopreservation, in Addition to Protein Tyrosine Phosphorylation, Alters the Distribution of Phosphatidyl Inositol Bisphosphate and the Localization of Cytoskeletal and Signaling Proteins (Gelsolin, Tyrosine Kinase c-SRC and Phospholipase C-ζ) in the Perinuclear Theca of Boar Sperm. Cryobiology 2023, 113, 104589. [Google Scholar] [CrossRef]
- Zribi, N.; Feki Chakroun, N.; El Euch, H.; Gargouri, J.; Bahloul, A.; Ammar Keskes, L. Effects of Cryopreservation on Human Sperm Deoxyribonucleic Acid Integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The Causes of Reduced Fertility with Cryopreserved Semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Schuffner, A.; Morshedi, M.; Oehninger, S. Cryopreservation of Fractionated, Highly Motile Human Spermatozoa: Effect on Membrane Phosphatidylserine Externalization and Lipid Peroxidation. Hum. Reprod. 2001, 16, 2148–2153. [Google Scholar] [CrossRef]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal Age Matters: Association with Sperm Criteria’s- Spermatozoa DNA Integrity and Methylation Profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef]
- Donatti, L.M.; Martello, C.L.; Andrade, G.M.; Oliveira, N.P.; Frantz, N. Advanced Paternal Age Affects the Sperm DNA Fragmentation Index and May Lead to Lower Good-Quality Blastocysts. Reprod. Sci. 2023, 30, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Fleming, S.D.; Aitken, R.J.; De Iuliis, G.N.; Zieschang, J.-A.; Clark, A.M. Cryopreservation-Induced Human Sperm DNA Damage Is Predominantly Mediated by Oxidative Stress Rather than Apoptosis. Hum. Reprod. 2009, 24, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Sharma, R.K.; Gupta, A.K.; Grunewald, S.; Mascha, E.J.; Thomas, A.J.; Glander, H.-J.; Agarwal, A. Cryopreservation and Thawing Is Associated with Varying Extent of Activation of Apoptotic Machinery in Subsets of Ejaculated Human Spermatozoa1. Biol. Reprod. 2004, 71, 1828–1837. [Google Scholar] [CrossRef]
- Evenson, D.P.; Djira, G.; Kasperson, K.; Christianson, J. Relationships between the Age of 25,445 Men Attending Infertility Clinics and Sperm Chromatin Structure Assay (SCSA®) Defined Sperm DNA and Chromatin Integrity. Fertil. Steril. 2020, 114, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, E.; Lee-Estevez, M.; Valdebenito, I.; Watanabe, I.; Oliveira, R.P.S.; Romero, J.; Castillo, R.L.; Farías, J.G. Effects of Cryopreservation on Mitochondrial Function and Sperm Quality in Fish. Aquaculture 2019, 511, 634190. [Google Scholar] [CrossRef]
- Gonzalez, M.; Prashar, T.; Connaughton, H.; Barry, M.; Robker, R.; Rose, R. Restoring Sperm Quality Post-Cryopreservation Using Mitochondrial-Targeted Compounds. Antioxidants 2022, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Yoshida, M.; Ling, F. Mitochondrial Fusion Increases the Mitochondrial DNA Copy Number in Budding Yeast. Genes Cells 2011, 16, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Whitcomb, B.W.; Huffman, A.; Brandon, N.; Labrie, S.; Tougias, E.; Lynch, K.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Associations of Sperm Mitochondrial DNA Copy Number and Deletion Rate with Fertilization and Embryo Development in a Clinical Setting. Hum. Reprod. 2019, 34, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2021, 11, 10. [Google Scholar] [CrossRef]
- Bailey, J.; Morrier, A.; Cormier, N. Semen Cryopreservation: Successes and Persistent Problems in Farm Species. Can. J. Anim. Sci. 2003, 83, 393–401. [Google Scholar] [CrossRef]
- Benko, F.; Mohammadi-Sangcheshmeh, A.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 1: Structural, Functional, and Oxidative Similarities and Differences. PLoS ONE 2022, 17, e0276683. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Non-APA | APA | p Value |
| nº of samples | 105 | 95 | - |
| Age (years) | 27.75 ± 6.425 | 45.35 ± 3.093 | <0.0001 *** |
| BMI | 22.5 ± 2.2 | 23.2 ± 1.8 | >0.05 |
| Fresh semen | |||
| Seminal volume (mL) | 2.997 ± 1.631 | 2.25 ± 0.8775 | 0.0219 * |
| Vitality (%) | 81.18 ± 7.960 | 78.33 ± 10.60 | 0.2729 |
| Number of spermatozoa (×106)/mL | 72.83 ± 45.95 | 65.03 ± 33.69 | 0.4119 |
| Total number of spermatozoa (×106) | 200.5 ± 128.3 | 130.1 ± 70.10 | 0.0067 ** |
| Progressive motility (%) | 56.78 ± 12.23 | 50.14 ± 12.22 | 0.0231 * |
| Total motility (%) | 61.50 ± 8.856 | 57.35 ± 10.14 | 0.0666 |
| Morphologically normal sperm (%) | 9.278 ± 4.193 | 6.703 ± 2.971 | 0.0036 ** |
| Frozen semen | |||
| Motility (%) | 8.556 ± 8.801 | 5.638 ± 6.298 | 0.1249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Casasús, S.; Luongo, F.P.; Haxhiu, A.; Orini, M.; Scupoli, G.; Governini, L.; Piomboni, P.; Buratini, J.; Dal Canto, M.; Luddi, A. Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa. Cells 2024, 13, 625. https://doi.org/10.3390/cells13070625
Pérez Casasús S, Luongo FP, Haxhiu A, Orini M, Scupoli G, Governini L, Piomboni P, Buratini J, Dal Canto M, Luddi A. Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa. Cells. 2024; 13(7):625. https://doi.org/10.3390/cells13070625
Chicago/Turabian StylePérez Casasús, Silvia, Francesca Paola Luongo, Alesandro Haxhiu, Martina Orini, Giorgia Scupoli, Laura Governini, Paola Piomboni, Jose Buratini, Mariabeatrice Dal Canto, and Alice Luddi. 2024. "Paternal Age Amplifies Cryopreservation-Induced Stress in Human Spermatozoa" Cells 13, no. 7: 625. https://doi.org/10.3390/cells13070625








